Urothelial Urinary Bladder Cancer Is Characterized by Stage-Dependent Aberrations in Metabolism of Bioactive Sphingolipids
Abstract
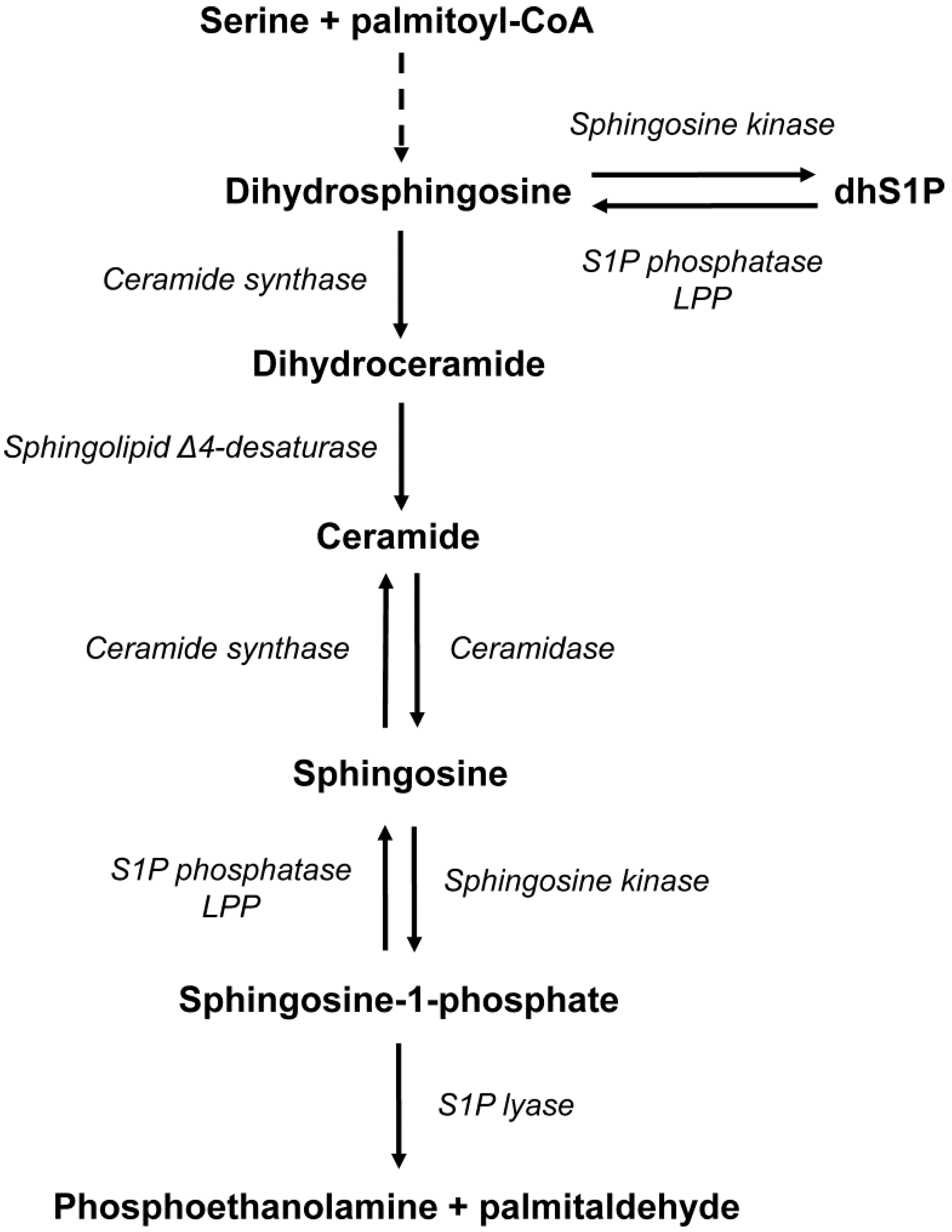
1. Introduction
2. Results
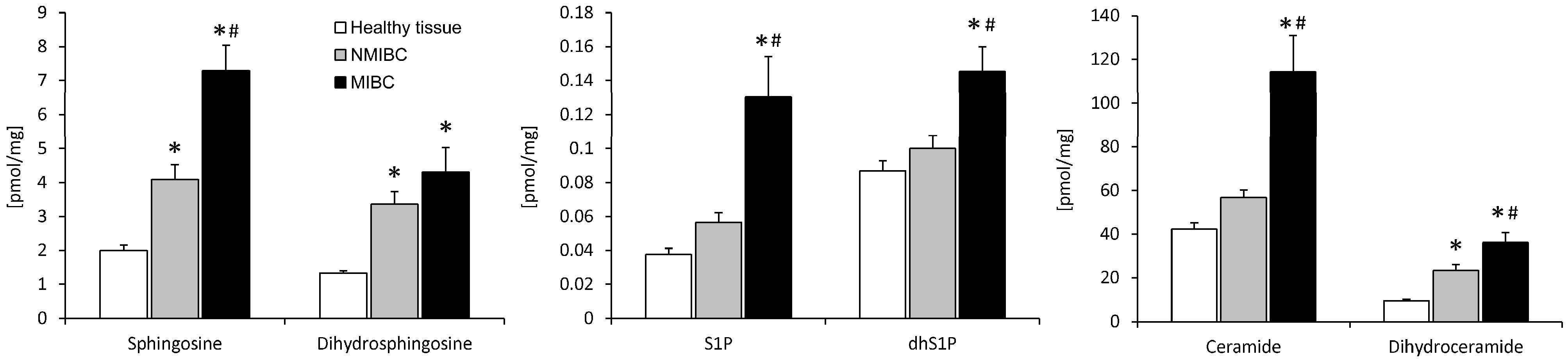
2.1. Sphingolipid Content
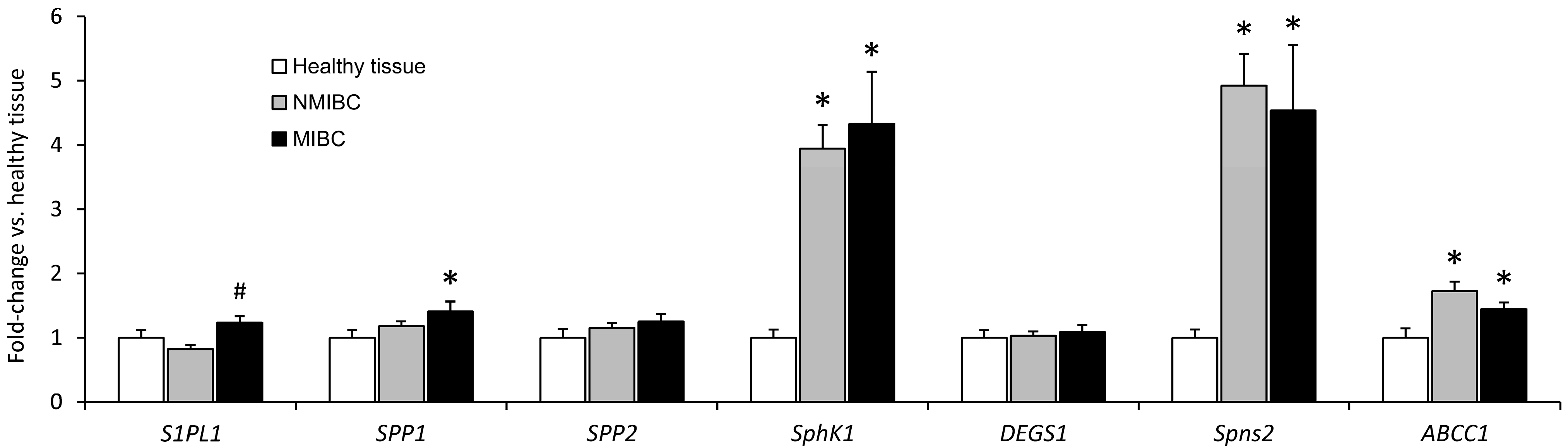
2.2. Expression of mRNA
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Sphingolipid Analysis
4.3. Real-Time PCR
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ohanian, J.; Ohanian, V. Sphingolipids in mammalian cell signalling. Cell. Mol. Life Sci. 2001, 58, 2053–2068. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Sanllehi, P.; Abad, J.L.; Casas, J.; Delgado, A. Inhibitors of sphingosine-1-phosphate metabolism (sphingosine kinases and sphingosine-1-phosphate lyase). Chem. Phys. Lipids. 2016, 197, 69–81. [Google Scholar] [CrossRef]
- Takabe, K.; Paugh, S.W.; Milstien, S.; Spiegel, S. “Inside-out” signaling of sphingosine-1-phosphate: Therapeutic targets. Pharmacol. Rev. 2008, 60, 181–195. [Google Scholar] [CrossRef]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef]
- Nganga, R.; Oleinik, N.; Ogretmen, B. Mechanisms of Ceramide-Dependent Cancer Cell Death. Adv. Cancer Res. 2018, 140, 1–25. [Google Scholar]
- Wang, P.; Yuan, Y.; Lin, W.; Zhong, H.; Xu, K.; Qi, X. Roles of sphingosine-1-phosphate signaling in cancer. Cancer Cell Int. 2019, 19, 295. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Liu, H.; Geng, M. Targeting sphingosine-1-phosphate signaling for cancer therapy. Sci. China Life Sci. 2017, 60, 585–600. [Google Scholar] [CrossRef]
- Sedic, M.; Grbcic, P.; Pavelic, S.K. Bioactive Sphingolipids as Biomarkers Predictive of Disease Severity and Treatment Response in Cancer: Current Status and Translational Challenges. Anticancer Res. 2019, 39, 41–56. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Knapp, P.; Baranowski, M.; Knapp, M.; Zabielski, P.; Blachnio-Zabielska, A.U.; Gorski, J. Altered sphingolipid metabolism in human endometrial cancer. Prostaglandins Other Lipid Mediat. 2010, 92, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Mlynarczyk, G.; Miklosz, A.; Suchanski, J.; Reza, S.; Romanowicz, L.; Sobolewski, K.; Chabowski, A.; Baranowski, M. Grade-dependent changes in sphingolipid metabolism in clear cell renal cell carcinoma. J. Cell. Biochem. 2022, 123, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Tong, H.; Li, T.; Cao, H.; Zhu, J.; Yin, S.; He, W. SPHK1 contributes to cisplatin resistance in bladder cancer cells via the NONO/STAT3 axis. Int. J. Mol. Med. 2021, 48, 204. [Google Scholar] [CrossRef]
- Meng, X.D.; Zhou, Z.S.; Qiu, J.H.; Shen, W.H.; Wu, Q.; Xiao, J. Increased SPHK1 expression is associated with poor prognosis in bladder cancer. Tumour Biol. 2014, 35, 2075–2080. [Google Scholar] [CrossRef]
- Li, L.; Wang, D.; Xin, S.; Ren, X.; Zhang, J. Sphingosine Kinase 1 Acts as a Hypoxia-Upregulated Oncogene to Regulate Cell Invasion and Resistance to NK Cell Killing in Bladder Carcinoma Cells. Ann. Clin. Lab. Sci. 2022, 52, 763–771. [Google Scholar]
- Wollny, T.; Wnorowska, U.; Piktel, E.; Suprewicz, L.; Krol, G.; Gluszek, K.; Gozdz, S.; Kopczynski, J.; Bucki, R. Sphingosine-1-Phosphate-Triggered Expression of Cathelicidin LL-37 Promotes the Growth of Human Bladder Cancer Cells. Int. J. Mol. Sci. 2022, 23, 7443. [Google Scholar] [CrossRef]
- Tang, C.; Wu, Y.; Wang, X.; Chen, K.; Tang, Z.; Guo, X. LncRNA MAFG-AS1 regulates miR-125b-5p/SphK1 axis to promote the proliferation, migration, and invasion of bladder cancer cells. Hum. Cell. 2021, 34, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Duan, P.; Zhu, H.; Rao, D. miR-613 inhibits bladder cancer proliferation and migration through targeting SphK1. Am. J. Transl. Res. 2017, 9, 1213–1221. [Google Scholar]
- Gu, X.; Jiang, Y.; Xue, W.; Song, C.; Wang, Y.; Liu, Y.; Cui, B. SPNS2 promotes the malignancy of colorectal cancer cells via regulating Akt and ERK pathway. Clin. Exp. Pharmacol. Physiol. 2019, 46, 861–871. [Google Scholar] [CrossRef]
- Fang, L.; Hou, J.; Cao, Y.; Shan, J.J.; Zhao, J. Spinster homolog 2 in cancers, its functions and mechanisms. Cell. Signal. 2021, 77, 109821. [Google Scholar] [CrossRef]
- Chen, C.L.; Meng, E.; Wu, S.T.; Lai, H.F.; Lu, Y.S.; Yang, M.H.; Tsao, C.W.; Kao, C.C.; Chiu, Y.L. Targeting S1PR1 May Result in Enhanced Migration of Cancer Cells in Bladder Carcinoma. Cancers 2021, 13, 4474. [Google Scholar] [CrossRef] [PubMed]
- Palangi, A.; Shakhssalim, N.; Parvin, M.; Bayat, S.; Allameh, A. Differential expression of S1P receptor subtypes in human bladder transitional cell carcinoma. Clin. Transl. Oncol. 2019, 21, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Go, H.; Kim, P.J.; Jeon, Y.K.; Cho, Y.M.; Kim, K.; Park, B.H.; Ku, J.Y. Sphingosine-1-phosphate receptor 1 (S1PR1) expression in non-muscle invasive urothelial carcinoma: Association with poor clinical outcome and potential therapeutic target. Eur. J. Cancer 2015, 51, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.N.; Zhang, H.; Zhang, L.; Cai, T.T.; Huang, D.J.; He, J.; Ni, H.H.; Zhou, F.J.; Zhang, X.S.; Li, J. Sphingosine 1 phosphate receptor-1 (S1P1) promotes tumor-associated regulatory T cell expansion: Leading to poor survival in bladder cancer. Cell Death Dis. 2019, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Bettiga, A.; Aureli, M.; Colciago, G.; Murdica, V.; Moschini, M.; Luciano, R.; Canals, D.; Hannun, Y.; Hedlund, P.; Lavorgna, G.; et al. Bladder cancer cell growth and motility implicate cannabinoid 2 receptor-mediated modifications of sphingolipids metabolism. Sci. Rep. 2017, 7, 42157. [Google Scholar] [CrossRef]
- Koyanagi, S.; Kuga, M.; Soeda, S.; Hosoda, Y.; Yokomatsu, T.; Takechi, H.; Akiyama, T.; Shibuya, S.; Shimeno, H. Elevation of de novo ceramide synthesis in tumor masses and the role of microsomal dihydroceramide synthase. Int. J. Cancer 2003, 105, 1–6. [Google Scholar] [CrossRef]
- Carton, J.M.; Uhlinger, D.J.; Batheja, A.D.; Derian, C.; Ho, G.; Argenteri, D.; D’Andrea, M.R. Enhanced serine palmitoyltransferase expression in proliferating fibroblasts, transformed cell lines, and human tumors. J. Histochem. Cytochem. 2003, 51, 715–726. [Google Scholar] [CrossRef]
- Paner, G.P.; Stadler, W.M.; Hansel, D.E.; Montironi, R.; Lin, D.W.; Amin, M.B. Updates in the Eighth Edition of the Tumor-Node-Metastasis Staging Classification for Urologic Cancers. Eur. Urol. 2018, 73, 560–569. [Google Scholar] [CrossRef]
- Knapp, M.; Lisowska, A.; Zabielski, P.; Musial, W.; Baranowski, M. Sustained decrease in plasma sphingosine-1-phosphate concentration and its accumulation in blood cells in acute myocardial infarction. Prostaglandins Other Lipid Mediat. 2013, 106, 53–61. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.Q.; Jin, G.; Wu, S.; Cui, J.; Wang, R.F. Selection of reference genes for gene expression studies in human bladder cancer using SYBR-Green quantitative polymerase chain reaction. Oncol. Lett. 2017, 14, 6001–6011. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
| Tumor Stage | NMIBC (n = 24) | MIBC (n = 24) |
|---|---|---|
| Sex (males/females) | 19/5 | 21/3 |
| Age (years) | 68.8 ± 2.1 | 69.7 ± 1.8 |
| BMI (kg/m2) | 28.5 ± 0.9 | 27.9 ± 0.6 |
| Distant metastasis (%) | 0 | 0 |
| Nodal invasion (%) | 0 | 0 |
| Hyperthyroidism (%) | 8.3 | 4.2 |
| Arterial hypertension (%) | 62.5 | 29.2 |
| Hypercholesterolemia (%) | 29 | 50 |
| Hypertriglyceridemia (%) | 29 | 50 |
| Type 2 diabetes (%) | 8.3 | 4.2 |
| Coronary disease (%) | 0 | 4.2 |
| Atrial fibrillation (%) | 16.7 | 0 |
| Gene | GenBank Accession No. | Forward Primer | Reverse Primer |
|---|---|---|---|
| SphK1 | NM_001142601.1 | 5′-CCTACTTGGTATATGTGCC-3′ | 5′-TCGCTAACCATCAATTCC-3′ |
| S1PL1 | NM_003901.3 | 5′-CAGAGTCAAGCCAAGGAT-3′ | 5′-GTATGGAGCAGCAATAAGC-3′ |
| SPP1 | NM_030791.4 | 5′-CATCATCATCGGGCTTCATT-3′ | 5′-TAGTATCTCGGCTGTGTCTC-3′ |
| SPP2 | NM_001320834.1 | 5′-TACGGCTGTCTTGCTACTACC-3′ | 5′-ACCACGGACGACCAATGA-3′ |
| ABCC1 | NM_004996.4 | 5′-CGGTGAAGGTTGTGTACTC-3′ | 5′-CCTCCTCATTCGCATCCA-3′ |
| Spns2 | NM_001124758.3 | 5′-ACACTGTCTCACTGTCTCG-3′ | 5′-TGATGCCAGCTTGTCAGA-3′ |
| RPL13A | NM_012423.4 | 5′-CTATGACCAATAGGAAGAGCAACC-3′ | 5′-GCAGAGTATATGACCAGGTGGAA-3′ |
| DEGS1 | NM_001321541.2 | 5′-CAAACATTCCAAACCAGCGAT-3′ | 5′-GCAGTTGCATTAACCACTCAA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Młynarczyk, G.; Mikłosz, A.; Chabowski, A.; Baranowski, M. Urothelial Urinary Bladder Cancer Is Characterized by Stage-Dependent Aberrations in Metabolism of Bioactive Sphingolipids. Int. J. Mol. Sci. 2024, 25, 11889. https://doi.org/10.3390/ijms252211889
Młynarczyk G, Mikłosz A, Chabowski A, Baranowski M. Urothelial Urinary Bladder Cancer Is Characterized by Stage-Dependent Aberrations in Metabolism of Bioactive Sphingolipids. International Journal of Molecular Sciences. 2024; 25(22):11889. https://doi.org/10.3390/ijms252211889
Chicago/Turabian StyleMłynarczyk, Grzegorz, Agnieszka Mikłosz, Adrian Chabowski, and Marcin Baranowski. 2024. "Urothelial Urinary Bladder Cancer Is Characterized by Stage-Dependent Aberrations in Metabolism of Bioactive Sphingolipids" International Journal of Molecular Sciences 25, no. 22: 11889. https://doi.org/10.3390/ijms252211889
APA StyleMłynarczyk, G., Mikłosz, A., Chabowski, A., & Baranowski, M. (2024). Urothelial Urinary Bladder Cancer Is Characterized by Stage-Dependent Aberrations in Metabolism of Bioactive Sphingolipids. International Journal of Molecular Sciences, 25(22), 11889. https://doi.org/10.3390/ijms252211889







