AMPA and NMDA Receptors in Hippocampus of Rats with Fluoride-Induced Cognitive Decline
Abstract
1. Introduction
2. Results
2.1. Brain F- Content
2.2. Influence of Excessive F- Consumption on Memory and Spatial Learning
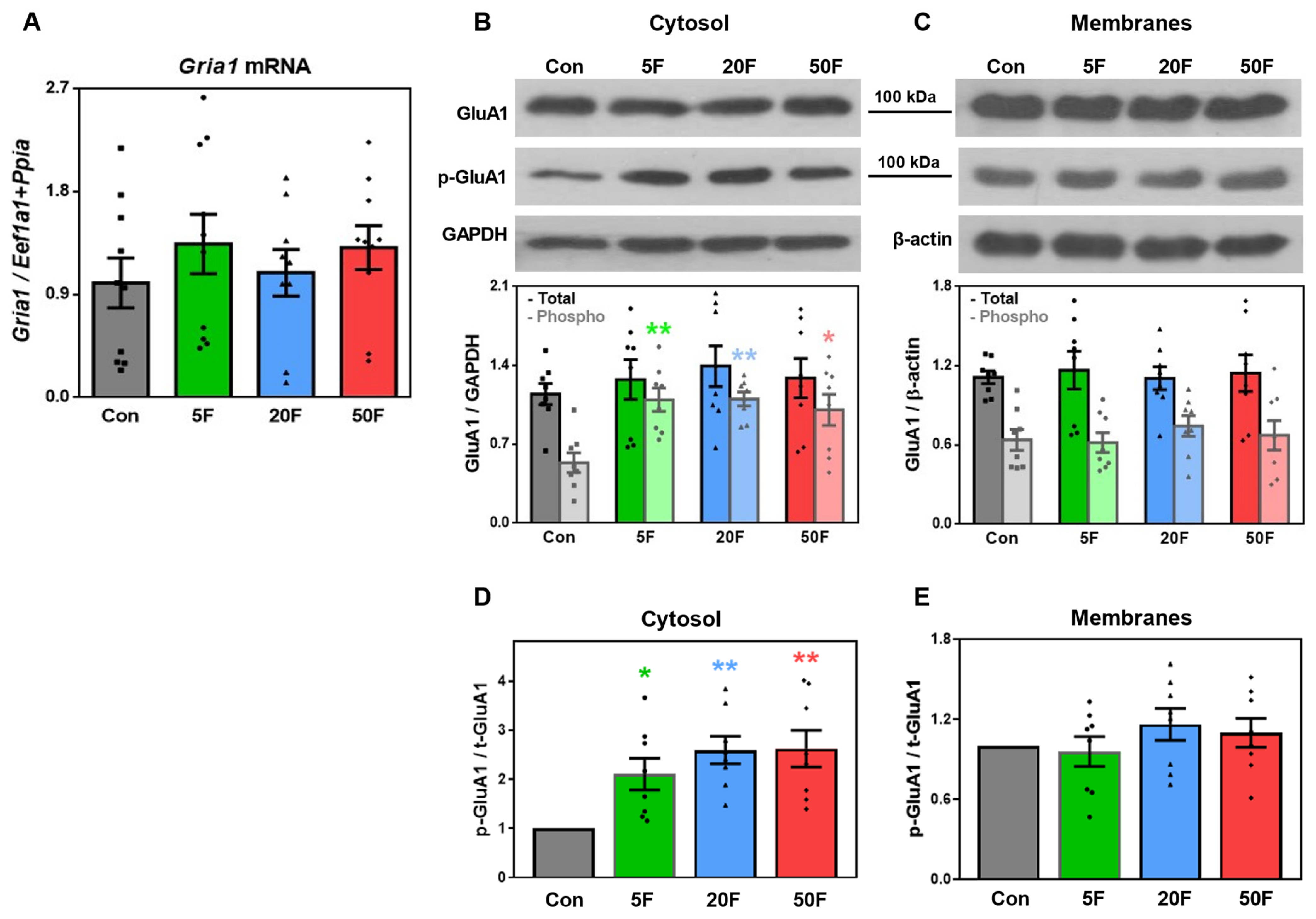
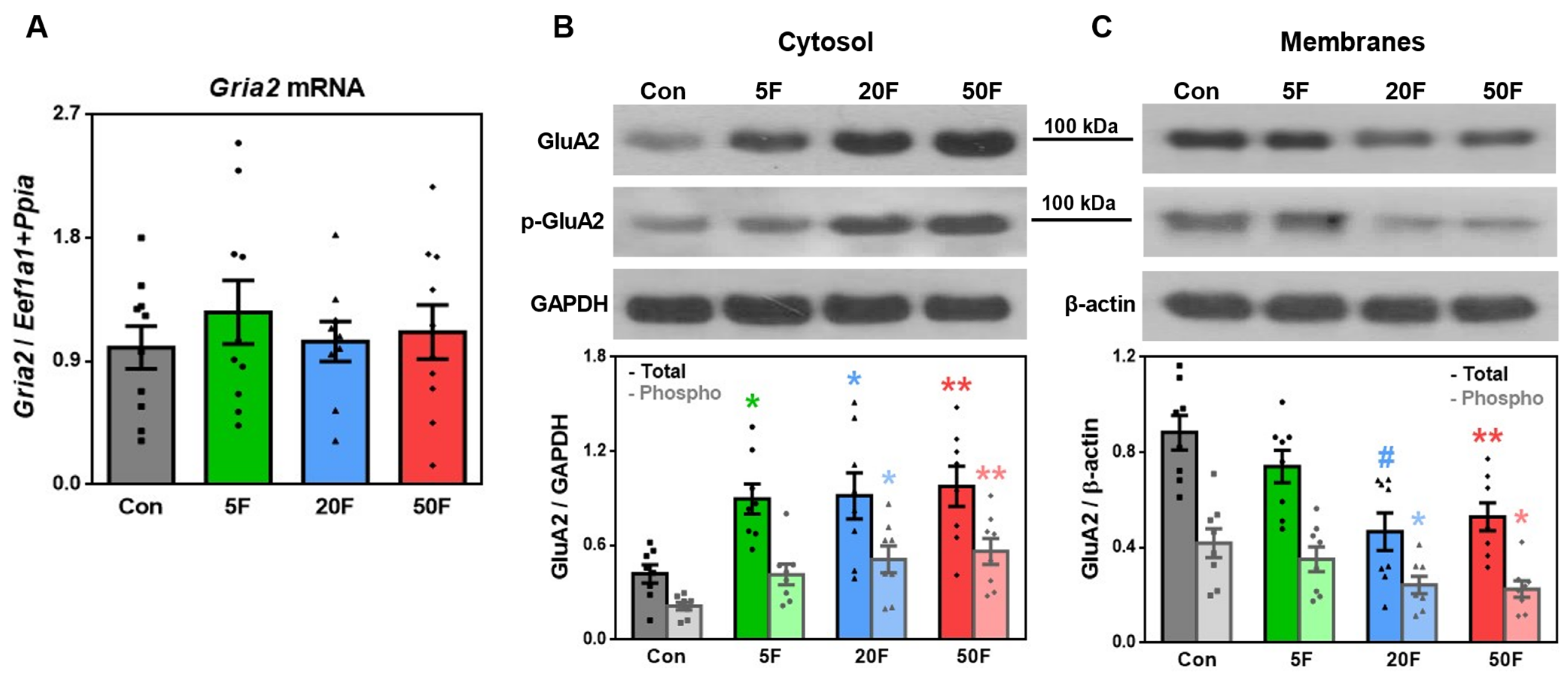
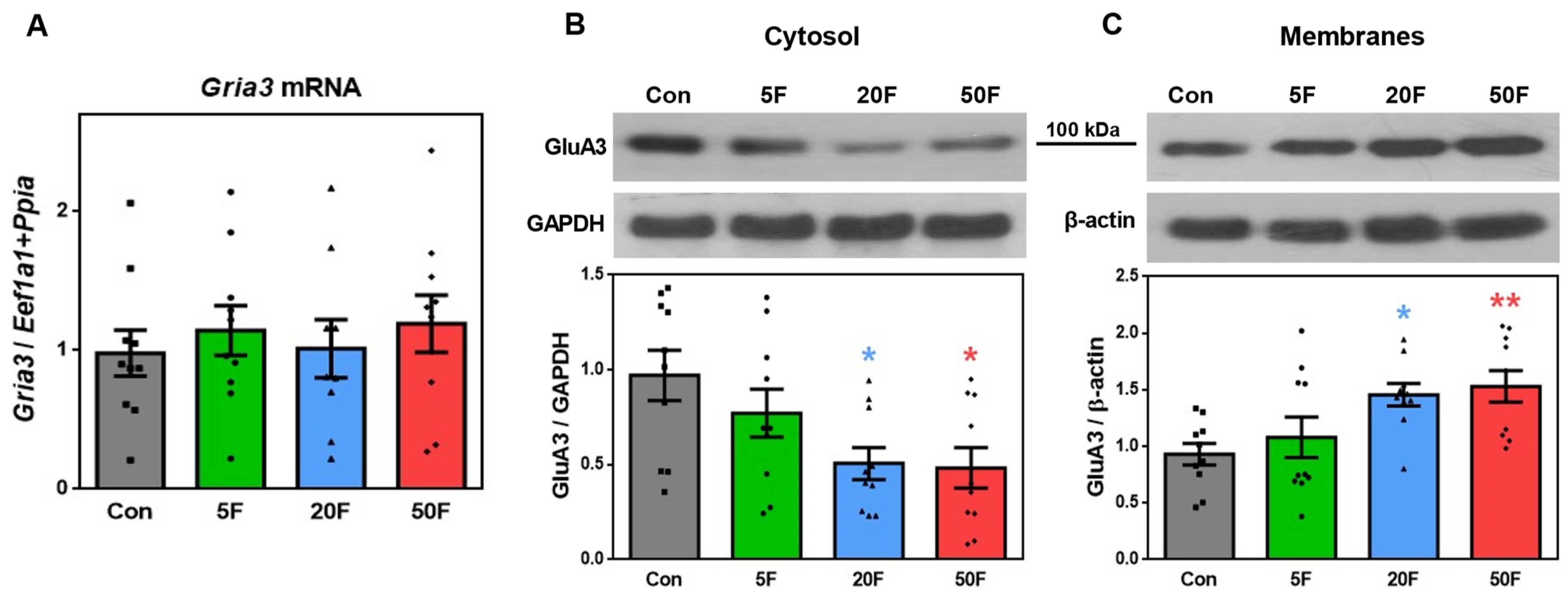
2.3. Effect of Fluoride on Expression of Subunits Composing AMPA Receptors in Rat Hippocampus
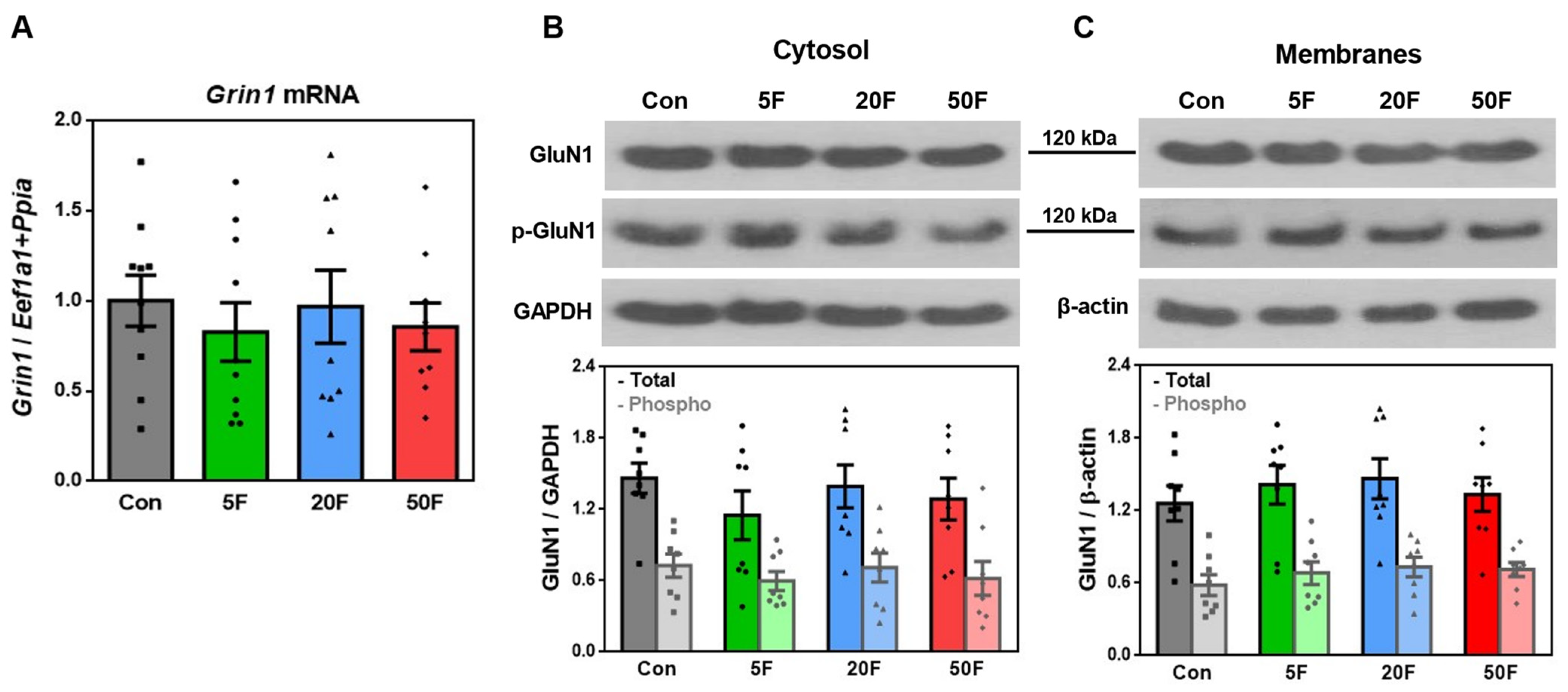
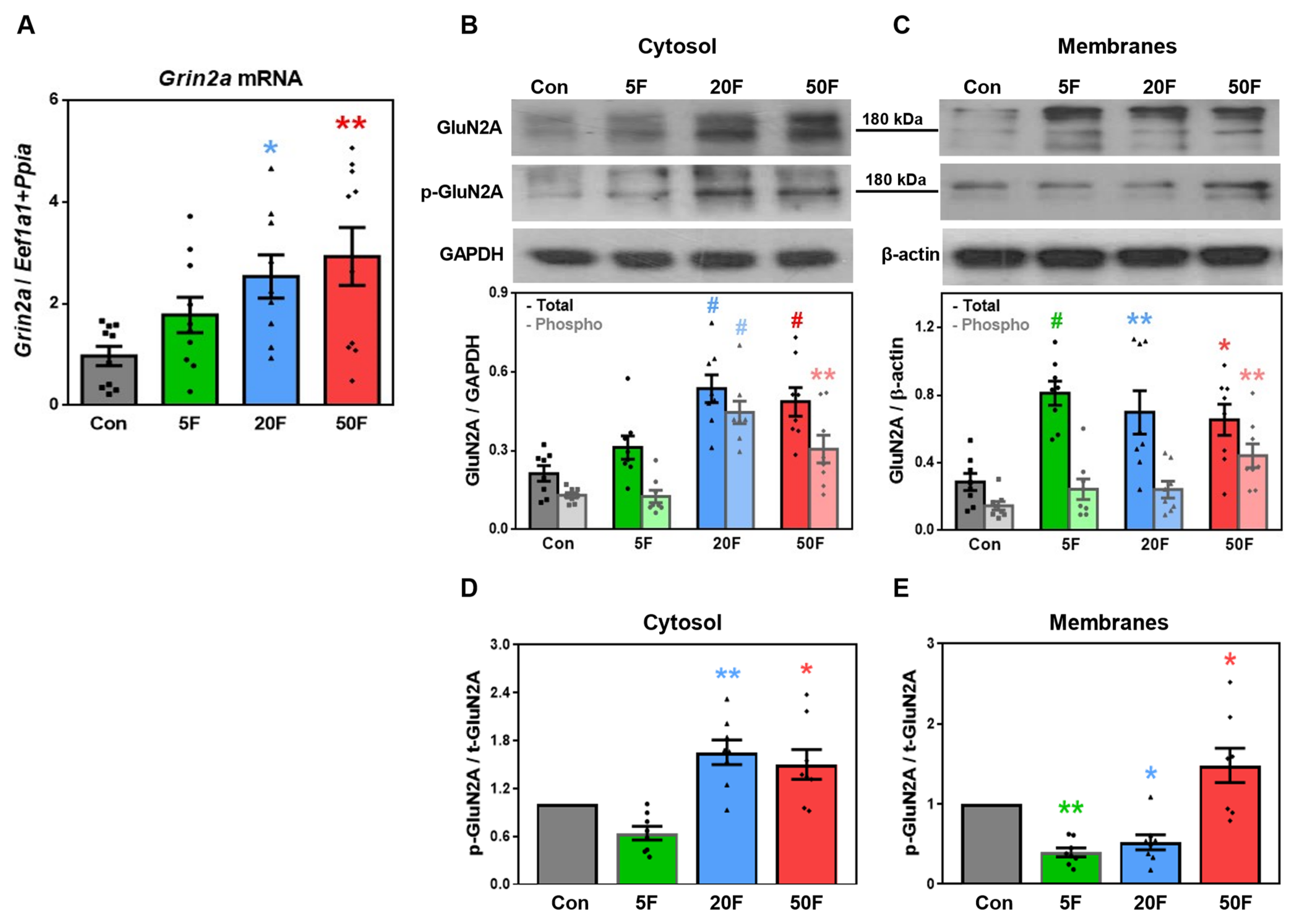
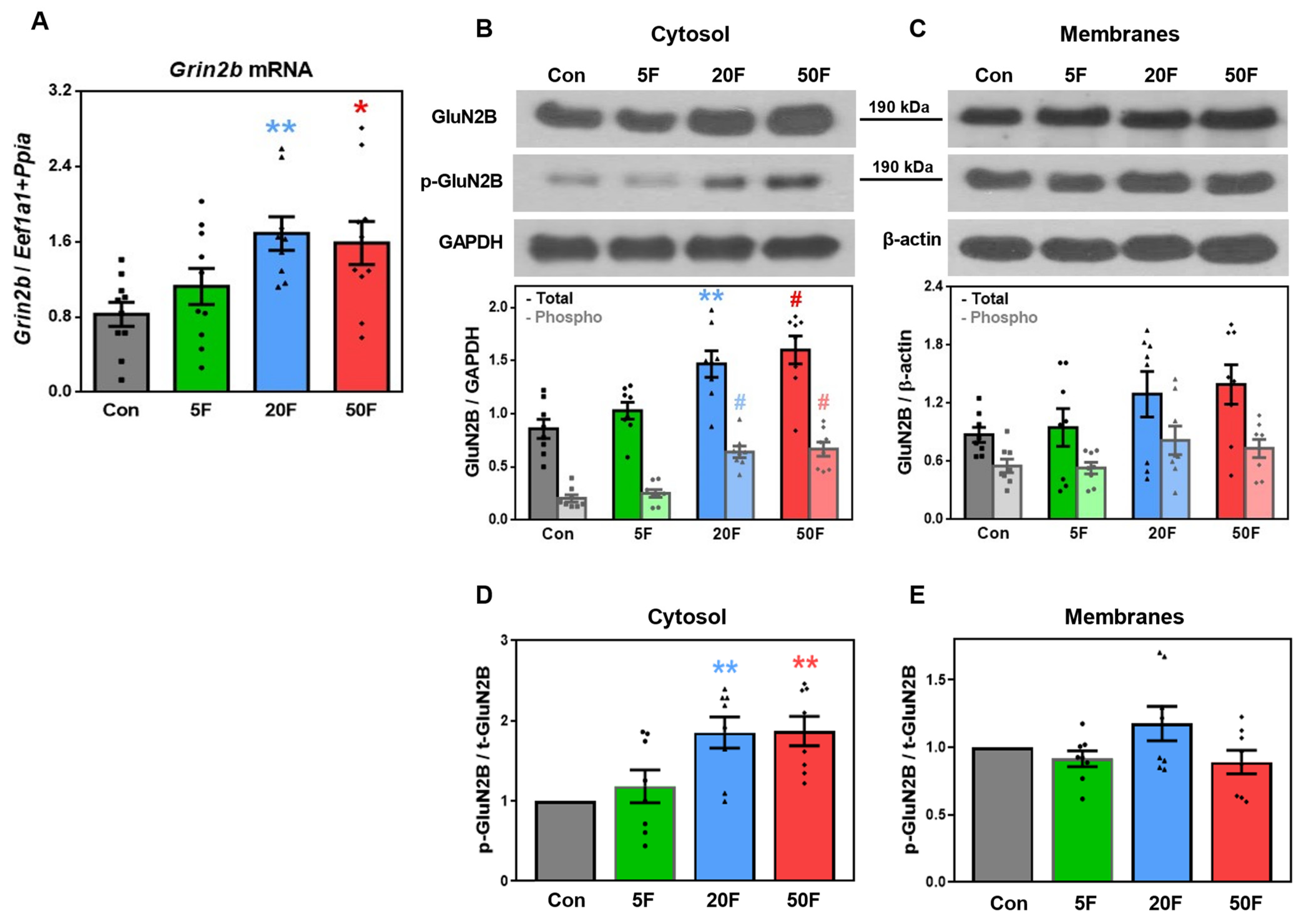
2.4. Effect of Fluoride on Expression of Subunits Composing NMDA Receptors in Rat Hippocampus
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. F- Determination
4.3. Examination of Cognitive Capacities
4.3.1. Novel Object Recognition Test (NOR)
4.3.2. Y-Maze Test
4.3.3. Morris Water Maze Test
4.4. mRNA Extraction and cDNA Synthesis
4.5. Quantitative Real-Time PCR
4.6. Western Blotting Analysis
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Johnston, N.R.; Strobel, S.A. Principles of fluoride toxicity and the cellular response: A review. Arch. Toxicol. 2020, 94, 1051–1069. [Google Scholar] [CrossRef] [PubMed]
- Lubojanski, A.; Piesiak-Panczyszyn, D.; Zakrzewski, W.; Dobrzynski, W.; Szymonowicz, M.; Rybak, Z.; Mielan, B.; Wiglusz, R.J.; Watras, A.; Dobrzynski, M. The Safety of Fluoride Compounds and Their Effect on the Human Body—A Narrative Review. Materials 2023, 16, 1242. [Google Scholar] [CrossRef]
- Bailey, K.; Chilton, J.; Dahi, E.; Lennon, M.; Jackson, P.; Fawell, J. (Eds.) Fluoride in Drinking Water; WHO (World Health Organization), WHO Press: Geneva, Switzerland, 2006; pp. 37–39. [Google Scholar]
- Till, C.; Green, R. Controversy: The evolving science of fluoride: When new evidence doesn’t conform with existing beliefs. Pediatr. Res. 2020, 90, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Agalakova, N.I.; Nadei, O.V. Inorganic fluoride and functions of brain. Crit. Rev. Toxicol. 2020, 50, 28–46. [Google Scholar] [CrossRef] [PubMed]
- Adkins, E.A.; Brunst, K.J. Impacts of Fluoride Neurotoxicity and Mitochondrial Dysfunction on Cognition and Mental Health: A Literature Review. Int. J. Environ. Res. Public Health 2021, 18, 12884. [Google Scholar] [CrossRef] [PubMed]
- Ottappilakkil, H.; Babu, S.; Balasubramanian, S.; Manoharan, S.; Perumal, E. Fluoride Induced Neurobehavioral Impairments in Experimental Animals: A Brief Review. Biol. Trace Element Res. 2022, 201, 1214–1236. [Google Scholar] [CrossRef]
- Duan, Q.; Jiao, J.; Chen, X.; Wang, X. Association between water fluoride and the level of children’s intelligence: A dose–response meta-analysis. Public Health 2017, 154, 87–97. [Google Scholar] [CrossRef]
- Strunecka, A.; Strunecky, O. Chronic Fluoride Exposure and the Risk of Autism Spectrum Disorder. Int. J. Environ. Res. Public Health 2019, 16, 3431. [Google Scholar] [CrossRef]
- Grandjean, P. Developmental fluoride neurotoxicity: An updated review. Environ. Health 2019, 18, 110. [Google Scholar] [CrossRef]
- Veneri, F.; Vinceti, M.; Generali, L.; Giannone, M.E.; Mazzoleni, E.; Birnbaum, L.S.; Consolo, U.; Filippini, T. Fluoride exposure and cognitive neurodevelopment: Systematic review and dose-response meta-analysis. Environ. Res. 2023, 221, 115239. [Google Scholar] [CrossRef]
- Żwierełło, W.; Maruszewska, A.; Skórka-Majewicz, M.; Gutowska, I. Fluoride in the Central Nervous System and Its Potential Influence on the Development and Invasiveness of Brain Tumours—A Research Hypothesis. Int. J. Mol. Sci. 2023, 24, 1558. [Google Scholar] [CrossRef] [PubMed]
- Guth, S.; Hüser, S.; Roth, A.; Degen, G.; Diel, P.; Edlund, K.; Eisenbrand, G.; Engel, K.-H.; Epe, B.; Grune, T.; et al. Toxicity of fluoride: Critical evaluation of evidence for human developmental neurotoxicity in epidemiological studies, animal experiments and in vitro analyses. Arch. Toxicol. 2020, 94, 1375–1415. [Google Scholar] [CrossRef] [PubMed]
- Miranda, G.H.N.; Alvarenga, M.O.P.; Ferreira, M.K.M.; Puty, B.; Bittencourt, L.O.; Fagundes, N.C.F.; Pessan, J.P.; Buzalaf, M.A.R.; Lima, R.R. A systematic review and meta-analysis of the association between fluoride exposure and neurological disorders. Sci. Rep. 2021, 11, 22659. [Google Scholar] [CrossRef]
- Nadei, O.V.; Ivanova, T.I.; Sufieva, D.A.; Agalakova, N.I. Morphological Changes of the Rat Hippocampal Neurons Following Excessive Fluoride Consumption. J. Anat. Histopathol. 2020, 9, 53–60. [Google Scholar] [CrossRef]
- Nadei, O.V.; Khvorova, I.A.; Agalakova, N.I. Cognitive Decline of Rats with Chronic Fluorosis Is Associated with Alterations in Hippocampal Calpain Signaling. Biol. Trace Element Res. 2019, 197, 495–506. [Google Scholar] [CrossRef]
- Vani, M.L.; Reddy, K.P. Effects of fluoride accumulation on some enzymes of brain and gastrocnemius muscle of mice. Fluoride 2000, 33, 17–26. [Google Scholar]
- Jiang, S.; Su, J.; Yao, S.; Zhang, Y.; Cao, F.; Wang, F.; Wang, H.; Li, J.; Xi, S. Fluoride and Arsenic Exposure Impairs Learning and Memory and Decreases mGluR5 Expression in the Hippocampus and Cortex in Rats. PLoS ONE 2014, 9, e96041. [Google Scholar] [CrossRef]
- Reddy, Y.P.; Tiwari, S.; Tomar, L.K.; Desai, N.; Sharma, V.K. Fluoride-Induced Expression of Neuroinflammatory Markers and Neurophysiological Regulation in the Brain of Wistar Rat Model. Biol. Trace Element Res. 2020, 199, 2621–2626. [Google Scholar] [CrossRef]
- Niu, R.; Sun, Z.; Cheng, Z.; Li, Z.; Wang, J. Decreased learning ability and low hippocampus glutamate in offspring rats exposed to fluoride and lead. Environ. Toxicol. Pharmacol. 2009, 28, 254–258. [Google Scholar] [CrossRef]
- González-Alfonso, W.L.; Pavel, P.; Karina, H.-M.; Del Razo, L.M.; Sanchez-Peña, L.C.; Zepeda, A.; Gonsebatt, M.E. Chronic exposure to inorganic arsenic and fluoride induces redox imbalance, inhibits the transsulfuration pathway, and alters glutamate receptor expression in the brain, resulting in memory impairment in adult male mouse offspring. Arch. Toxicol. 2023, 97, 2371–2383. [Google Scholar] [CrossRef]
- Kim, J.-H.; Marton, J.; Ametamey, S.M.; Cumming, P. A Review of Molecular Imaging of Glutamate Receptors. Molecules 2020, 25, 4749. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Dey, A.; Gopalakrishnan, A.V.; Swati, K.; Ojha, S.; Prakash, A.; Kumar, D.; Ambasta, R.K.; Jha, N.K.; Jha, S.K.; et al. Glutamatergic neurotransmission: A potential pharmacotherapeutic target for the treatment of cognitive disorders. Ageing Res. Rev. 2023, 85, 101838. [Google Scholar] [CrossRef] [PubMed]
- Diering, G.H.; Huganir, R.L. The AMPA Receptor Code of Synaptic Plasticity. Neuron 2018, 100, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Purkey, A.M.; Dell’acqua, M.L. Phosphorylation-Dependent Regulation of Ca2+-Permeable AMPA Receptors During Hippocampal Synaptic Plasticity. Front. Synaptic Neurosci. 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Italia, M.; Ferrari, E.; Di Luca, M.; Gardoni, F. GluA3-containing AMPA receptors: From physiology to synaptic dysfunction in brain disorders. Neurobiol. Dis. 2021, 161, 105539. [Google Scholar] [CrossRef]
- Chater, T.E.; Goda, Y. The Shaping of AMPA Receptor Surface Distribution by Neuronal Activity. Front. Synaptic Neurosci. 2022, 14, 833782. [Google Scholar] [CrossRef]
- Hanley, J.G. Subunit-specific trafficking mechanisms regulating the synaptic expression of Ca2+-permeable AMPA receptors. Semin. Cell Dev. Biol. 2014, 27, 14–22. [Google Scholar] [CrossRef]
- Hardt, O.; Nader, K.; Wang, Y.-T. GluA2-dependent AMPA receptor endocytosis and the decay of early and late long-term potentiation: Possible mechanisms for forgetting of short- and long-term memories. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130141. [Google Scholar] [CrossRef]
- Wu, Q.-L.; Gao, Y.; Li, J.-T.; Ma, W.-Y.; Chen, N.-H. The Role of AMPARs Composition and Trafficking in Synaptic Plasticity and Diseases. Cell. Mol. Neurobiol. 2022, 42, 2489–2504. [Google Scholar] [CrossRef]
- Chen, S.-R.; Zhou, H.-Y.; Byun, H.S.; Pan, H.-L. Nerve Injury Increases GluA2-Lacking AMPA Receptor Prevalence in Spinal Cords: Functional Significance and Signaling Mechanisms. J. Pharmacol. Exp. Ther. 2013, 347, 765–772. [Google Scholar] [CrossRef]
- Chen, S.-R.; Zhang, J.; Chen, H.; Pan, H.-L. Streptozotocin-Induced Diabetic Neuropathic Pain Is Associated with Potentiated Calcium-Permeable AMPA Receptor Activity in the Spinal Cord. J. Pharmacol. Exp. Ther. 2019, 371, 242–249. [Google Scholar] [CrossRef]
- Liu, B.; Liao, M.; Mielke, J.G.; Ning, K.; Chen, Y.; Li, L.; El-Hayek, Y.H.; Gomez, E.; Zukin, R.S.; Fehlings, M.G.; et al. Ischemic Insults Direct Glutamate Receptor Subunit 2-Lacking AMPA Receptors to Synaptic Sites. J. Neurosci. 2006, 26, 5309–5319. [Google Scholar] [CrossRef]
- Babaei, P. NMDA and AMPA receptors dysregulation in Alzheimer’s disease. Eur. J. Pharmacol. 2021, 908, 174310. [Google Scholar] [CrossRef] [PubMed]
- Martín-Belmonte, A.; Aguado, C.; Alfaro-Ruíz, R.; Itakura, M.; Moreno-Martínez, A.E.; de la Ossa, L.; Molnár, E.; Fukazawa, Y.; Luján, R. Age-Dependent Shift of AMPA Receptors From Synapses to Intracellular Compartments in Alzheimer’s Disease: Immunocytochemical Analysis of the CA1 Hippocampal Region in APP/PS1 Transgenic Mouse Model. Front. Aging Neurosci. 2020, 12, 577996. [Google Scholar] [CrossRef]
- Alfaro-Ruiz, R.; Aguado, C.; Martín-Belmonte, A.; Moreno-Martínez, A.E.; Merchán-Rubira, J.; Hernández, F.; Ávila, J.; Fukazawa, Y.; Luján, R. Alteration in the Synaptic and Extrasynaptic Organization of AMPA Receptors in the Hippocampus of P301S Tau Transgenic Mice. Int. J. Mol. Sci. 2022, 23, 13527. [Google Scholar] [CrossRef] [PubMed]
- Kuniishi, H.; Yamada, D.; Wada, K.; Yamada, M.; Sekiguchi, M. Stress induces insertion of calcium-permeable AMPA receptors in the OFC–BLA synapse and modulates emotional behaviours in mice. Transl. Psychiatry 2020, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, K.; Tani, H.; Nakajima, S.; Nagai, N.; Koizumi, T.; Miyazaki, T.; Mimura, M.; Takahashi, T.; Uchida, H. AMPA receptors in schizophrenia: A systematic review of postmortem studies on receptor subunit expression and binding. Schizophr. Res. 2022, 243, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Reinders, N.R.; Pao, Y.; Renner, M.C.; da Silva-Matos, C.M.; Lodder, T.R.; Malinow, R.; Kessels, H.W. Amyloid-β effects on synapses and memory require AMPA receptor subunit GluA. Proc. Natl. Acad. Sci. USA 2016, 113, E6526–E6534. [Google Scholar] [CrossRef]
- Berchtold, N.C.; Sabbagh, M.N.; Beach, T.G.; Kim, R.C.; Cribbs, D.H.; Cotman, C.W. Brain gene expression patterns differentiate mild cognitive impairment from normal aged and Alzheimer’s disease. Neurobiol. Aging 2014, 35, 1961–1972. [Google Scholar] [CrossRef]
- Peng, S.-X.; Pei, J.; Rinaldi, B.; Chen, J.; Ge, Y.-H.; Jia, M.; Wang, J.; Delahaye-Duriez, A.; Sun, J.-H.; Zang, Y.-Y.; et al. Dysfunction of AMPA receptor GluA3 is associated with aggressive behavior in human. Mol. Psychiatry 2022, 27, 4092–4102. [Google Scholar] [CrossRef]
- Yang, L.; Jin, P.; Wang, X.; Zhou, Q.; Lin, X.; Xi, S. Fluoride activates microglia, secretes inflammatory factors and influences synaptic neuron plasticity in the hippocampus of rats. NeuroToxicology 2018, 69, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, Y.; Xue, X.; Niu, R.; Wang, J. Maternal fluoride exposure during gestation and lactation decreased learning and memory ability, and glutamate receptor mRNA expressions of mouse pups. Hum. Exp. Toxicol. 2017, 37, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Roche, K.W. Posttranslational regulation of AMPA receptor trafficking and function. Curr. Opin. Neurobiol. 2012, 22, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Guo, M.-L.; Jin, D.-Z.; Xue, B.; Fibuch, E.E.; Mao, L.-M. Roles of subunit phosphorylation in regulating glutamate receptor function. Eur. J. Pharmacol. 2013, 728, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Corti, E.; Duarte, C.B. The role of post-translational modifications in synaptic AMPA receptor activity. Biochem. Soc. Trans. 2023, 51, 315–330. [Google Scholar] [CrossRef]
- Crombag, H.S.; Sutton, J.M.; Takamiya, K.; Holland, P.C.; Gallagher, M.; Huganir, R.L. A role for alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid GluR1 phosphorylation in the modulatory effects of appetitive reward cues on goal-directed behavior. Eur. J. Neurosci. 2008, 27, 3284–3291. [Google Scholar] [CrossRef]
- Caudal, D.; Godsil, B.P.; Mailliet, F.; Bergerot, D.; Jay, T.M. Acute Stress Induces Contrasting Changes in AMPA Receptor Subunit Phosphorylation within the Prefrontal Cortex, Amygdala and Hippocampus. PLoS ONE 2010, 5, e15282. [Google Scholar] [CrossRef]
- Caudal, D.; Rame, M.; Jay, T.M.; Godsil, B.P. Dynamic Regulation of AMPAR Phosphorylation In Vivo Following Acute Behavioral Stress. Cell. Mol. Neurobiol. 2016, 36, 1331–1342. [Google Scholar] [CrossRef]
- Lee, M.T.; Peng, W.-H.; Kan, H.-W.; Wu, C.-C.; Wang, D.-W.; Ho, Y.-C. Neurobiology of Depression: Chronic Stress Alters the Glutamatergic System in the Brain—Focusing on AMPA Receptor. Biomedicines 2022, 10, 1005. [Google Scholar] [CrossRef]
- Zhao, Y.-B.; Hou, X.-F.; Li, X.; Zhu, L.-S.; Zhu, J.; Ma, G.-R.; Liu, Y.-X.; Miao, Y.-C.; Zhou, Q.-Y.; Xu, L.; et al. Early Memory Impairment is Accompanied by Changes in GluA1/p-GluA1 in APP/PS1 Mice. Curr. Alzheimer Res. 2022, 19, 667–673. [Google Scholar] [CrossRef]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Gardoni, F.; Di Luca, M. Protein-protein interactions at the NMDA receptor complex: From synaptic retention to synaptonuclear protein messengers. Neuropharmacology 2021, 190, 108551. [Google Scholar] [CrossRef]
- Cercato, M.C.; Vázquez, C.A.; Kornisiuk, E.; Aguirre, A.I.; Colettis, N.; Snitcofsky, M.; Jerusalinsky, D.A.; Baez, M.V. GluN1 and GluN2A NMDA Receptor Subunits Increase in the Hippocampus during Memory Consolidation in the Rat. Front. Behav. Neurosci. 2017, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Franchini, L.; Carrano, N.; Di Luca, M.; Gardoni, F. Synaptic GluN2A-Containing NMDA Receptors: From Physiology to Pathological Synaptic Plasticity. Int. J. Mol. Sci. 2020, 21, 1538. [Google Scholar] [CrossRef] [PubMed]
- Holehonnur, R.; Phensy, A.J.; Kim, L.J.; Milivojevic, M.; Vuong, D.; Daison, D.K.; Alex, S.; Tiner, M.; Jones, L.E.; Kroener, S.; et al. Increasing the GluN2A/GluN2B Ratio in Neurons of the Mouse Basal and Lateral Amygdala Inhibits the Modification of an Existing Fear Memory Trace. J. Neurosci. 2016, 36, 9490–9504. [Google Scholar] [CrossRef] [PubMed]
- Acutain, M.F.; Luft, J.G.; Vazquez, C.A.; Popik, B.; Cercato, M.C.; Epstein, A.; Salvetti, A.; Jerusalinsky, D.A.; Alvares, L.d.O.; Baez, M.V. Reduced Expression of Hippocampal GluN2A-NMDAR Increases Seizure Susceptibility and Causes Deficits in Contextual Memory. Front. Neurosci. 2021, 15, 644100. [Google Scholar] [CrossRef] [PubMed]
- Oka, M.; Ito, K.; Koga, M.; Kusumi, I. Changes in subunit composition of NMDA receptors in animal models of schizophrenia by repeated administration of methamphetamine. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 103, 109984. [Google Scholar] [CrossRef]
- Sabo, S.L.; Lahr, J.M.; Offer, M.; LA Weekes, A.; Sceniak, M.P. GRIN2B-related neurodevelopmental disorder: Current understanding of pathophysiological mechanisms. Front. Synaptic Neurosci. 2023, 14, 1090865. [Google Scholar] [CrossRef]
- Sivakumar, S.; Ghasemi, M.; Schachter, S.C. Targeting NMDA Receptor Complex in Management of Epilepsy. Pharmaceuticals 2022, 15, 1297. [Google Scholar] [CrossRef]
- Zubareva, O.E.; Kovalenko, A.A.; Kalemenev, S.V.; Schwarz, A.P.; Karyakin, V.B.; Zaitsev, A.V. Alterations in mRNA expression of glutamate receptor subunits and excitatory amino acid transporters following pilocarpine-induced seizures in rats. Neurosci. Lett. 2018, 686, 94–100. [Google Scholar] [CrossRef]
- Postnikova, T.Y.; Zubareva, O.E.; Kovalenko, A.A.; Kim, K.K.; Magazanik, L.G.; Zaitsev, A.V. Status epilepticus impairs synaptic plasticity in rat hippocampus and is followed by changes in expression of NMDA receptors. Biochemistry 2017, 82, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chang, L.; Song, Y.; Gao, X.; Roselli, F.; Liu, J.; Zhou, W.; Fang, Y.; Ling, W.; Li, H.; et al. Astrocytic GluN2A and GluN2B Oppose the Synaptotoxic Effects of Amyloid-β1-40 in Hippocampal Cells. J. Alzheimer’s Dis. 2016, 54, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Song, Y.; Chen, X.; Zhang, W.; Zhang, G.; Li, H.; Chang, L.; Wu, Y. Knockdown of astrocytic Grin2a aggravates β-amyloid-induced memory and cognitive deficits through regulating nerve growth factor. Aging Cell 2021, 20, e13437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, X.; Du, Z.; Mao, X.; Gao, R.; Chen, Z.; Wang, H.; Zhang, G.; Zhang, N.; Li, H.; et al. Knockdown of astrocytic Grin2a exacerbated sleep deprivation-induced cognitive impairments and elevation of amyloid-beta. Sleep Med. 2022, 100, 280–290. [Google Scholar] [CrossRef]
- Wei, N.; Dong, Y.-T.; Deng, J.; Wang, Y.; Qi, X.-L.; Yu, W.-F.; Xiao, Y.; Zhou, J.-J.; Guan, Z.-Z. Changed expressions of N-methyl-d-aspartate receptors in the brains of rats and primary neurons exposed to high level of fluoride. J. Trace Elements Med. Biol. 2017, 45, 31–40. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, L.; Yin, Z.; Song, X.; Ruan, T.; Hua, L.; Liu, J.; Wang, J.; Ning, H.; Ge, Y.; et al. Fluoride-induced alterations of synapse-related proteins in the cerebral cortex of ICR offspring mouse brain. Chemosphere 2018, 201, 874–883. [Google Scholar] [CrossRef]
- Pupo, G.; Gouverneur, V. Hydrogen Bonding Phase-Transfer Catalysis with Alkali Metal Fluorides and Beyond. J. Am. Chem. Soc. 2022, 144, 5200–5213. [Google Scholar] [CrossRef]
- Qin, J.; Chai, G.; Brewer, J.M.; Lovelace, L.L.; Lebioda, L. Fluoride Inhibition of Enolase: Crystal Structure and Thermodynamics. Biochemistry 2005, 45, 793–800. [Google Scholar] [CrossRef]
- Agalakova, N.I.; Gusev, G.P. Molecular Mechanisms of Cytotoxicity and Apoptosis Induced by Inorganic Fluoride. ISRN Cell Biol. 2012, 2012, 403835. [Google Scholar] [CrossRef]
- Yildiz-Unal, A.; Korulu, S.; Karabay, A. Neuroprotective strategies against calpain-mediated neurodegeneration. Neuropsychiatr. Dis. Treat. 2015, 11, 297–310. [Google Scholar] [CrossRef]
- Zhou, G.; Hu, Y.; Wang, A.; Guo, M.; Du, Y.; Gong, Y.; Ding, L.; Feng, Z.; Hou, X.; Xu, K.; et al. Fluoride Stimulates Anxiety- and Depression-like Behaviors Associated with SIK2-CRTC1 Signaling Dysfunction. J. Agric. Food Chem. 2021, 69, 13618–13627. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Feng, Z.; Gao, M.; Wang, A.; Yan, X.; Chen, R.; Liu, B.; Yu, F.; Ba, Y.; Zhou, G. Impaired neurogenesis induced by fluoride via the Notch1 signaling and effects of carvacrol intervention. Environ. Pollut. 2024, 356, 124371. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Laboratory Animals; The National Academies Press: Washington, DC, USA, 1995; pp. 12–79. [Google Scholar] [CrossRef]
- Lyaruu, D.M.; Bronckers, A.L.J.J.; Santos, F.; Mathias, R.; DenBesten, P. The effect of fluoride on enamel and dentin formation in the uremic rat incisor. Pediatr. Nephrol. 2008, 23, 1973–1979. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ekstrand, J.J. Relationship between Fluoride in the Drinking Water and the Plasma Fluoride Concentration in Man. Caries Res. 1978, 12, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.; Do, L.; Mueller, U.; Baines, J.; Foley, M.; Peres, M. Understanding Optimum Fluoride Intake from Population-Level Evidence. Adv. Dent. Res. 2018, 29, 144–156. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2011, 13, 93–110. [Google Scholar] [CrossRef]
- Ukkirapandian, K.; Elumalai, K.; Udaykumar, K.P.; Vp, S.; Rangasmy, M. Behavioral and Biochemical Assays for Autism Models of Wistar Rats. Cureus 2024, 16, e52066. [Google Scholar] [CrossRef]
- Hernández-Mercado, K.; Zepeda, A. Morris Water Maze and Contextual Fear Conditioning Tasks to Evaluate Cognitive Functions Associated with Adult Hippocampal Neurogenesis. Front. Neurosci. 2022, 15, 782947. [Google Scholar] [CrossRef]
- Nadei, O.V.; Agalakova, N.I. Optimal Reference Genes for RT-qPCR Experiments in Hippocampus and Cortex of Rats Chronically Exposed to Excessive Fluoride. Biol. Trace Element Res. 2023, 202, 199–209. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


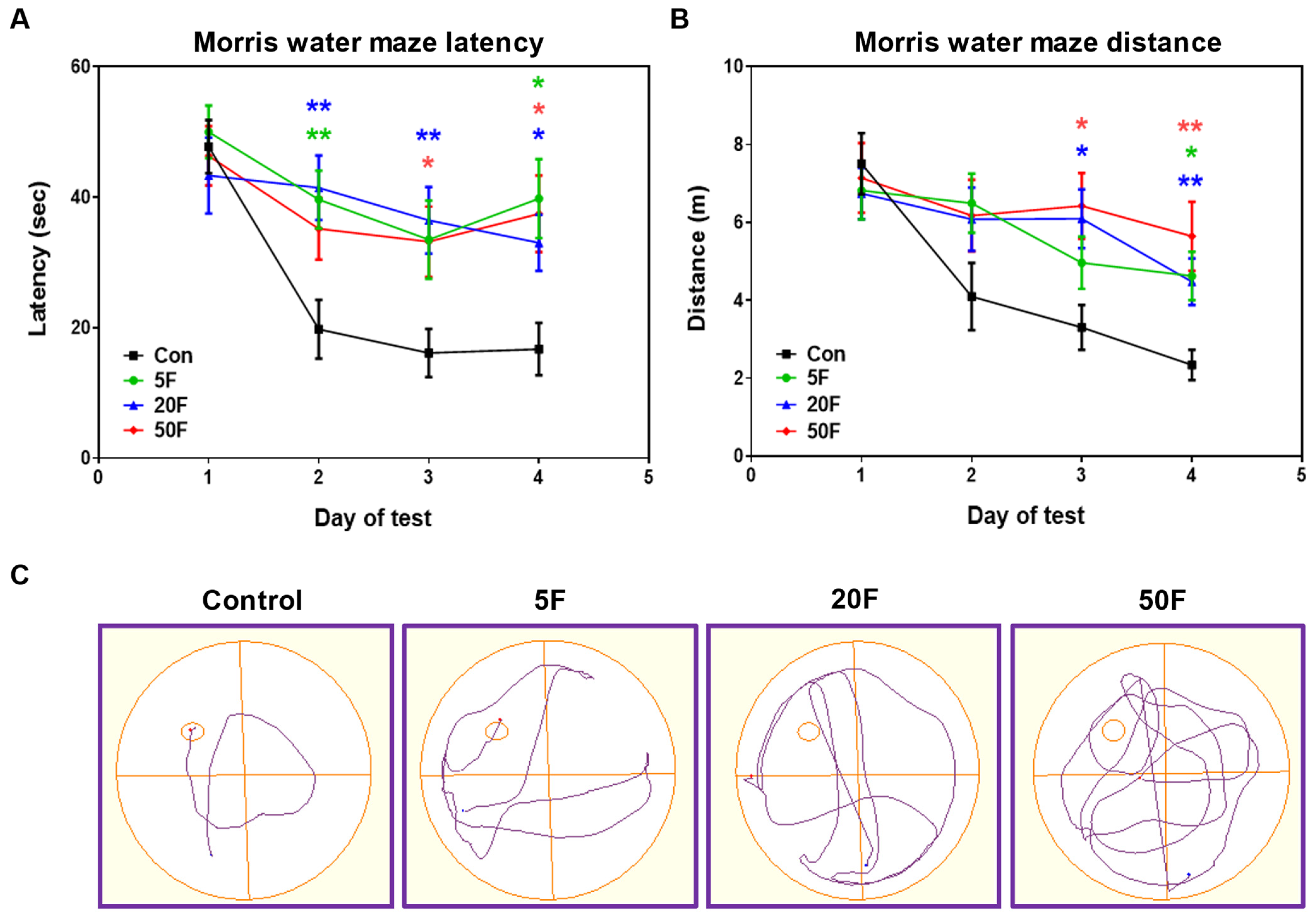
| Control | 5F | 20F | 50F | |
|---|---|---|---|---|
| Time to first entry to target quadrant (s) | 5.5 ± 0.6 | 7.6 ± 0.9 * | 8.8 ± 1.0 * | 8.1 ± 0.9 * |
| Time spent in target quadrant (s) | 17.8 ± 0.9 | 15.9 ± 1.7 | 14.9 ± 1.1 * | 16.8 ± 1.6 |
| Total distance (m) | 7.1 ± 0.6 | 5.8 ± 0.6 | 5.7 ± 0.6 | 6.9 ± 0.7 |
| Mean speed (m/s) | 0.12 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 | 0.12 ± 0.01 |
| Distance in target quadrant (m) | 1.6 ± 0.1 | 1.0 ± 0.1 ** | 1.2 ± 0.2 * | 1.3 ± 0.2 * |
| Distance in opposite zone (m) | 1.3 ± 0.2 | 1.7 ± 0.1 | 2.1 ± 0.3 * | 2.6 ± 0.3 ** |
| Number of entries to target quadrant | 4.5 ± 0.4 | 3.3 ± 0.3 * | 4.2 ± 0.5 | 4.5 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadei, O.V.; Agalakova, N.I. AMPA and NMDA Receptors in Hippocampus of Rats with Fluoride-Induced Cognitive Decline. Int. J. Mol. Sci. 2024, 25, 11796. https://doi.org/10.3390/ijms252111796
Nadei OV, Agalakova NI. AMPA and NMDA Receptors in Hippocampus of Rats with Fluoride-Induced Cognitive Decline. International Journal of Molecular Sciences. 2024; 25(21):11796. https://doi.org/10.3390/ijms252111796
Chicago/Turabian StyleNadei, Olga Vladimirovna, and Natalia Ivanovna Agalakova. 2024. "AMPA and NMDA Receptors in Hippocampus of Rats with Fluoride-Induced Cognitive Decline" International Journal of Molecular Sciences 25, no. 21: 11796. https://doi.org/10.3390/ijms252111796
APA StyleNadei, O. V., & Agalakova, N. I. (2024). AMPA and NMDA Receptors in Hippocampus of Rats with Fluoride-Induced Cognitive Decline. International Journal of Molecular Sciences, 25(21), 11796. https://doi.org/10.3390/ijms252111796






