Joint Toxicity and Interaction of Carbon-Based Nanomaterials with Co-Existing Pollutants in Aquatic Environments: A Review
Abstract
1. Introduction
2. Carbon-Based Nanomaterials: History, Risks, and Challenges
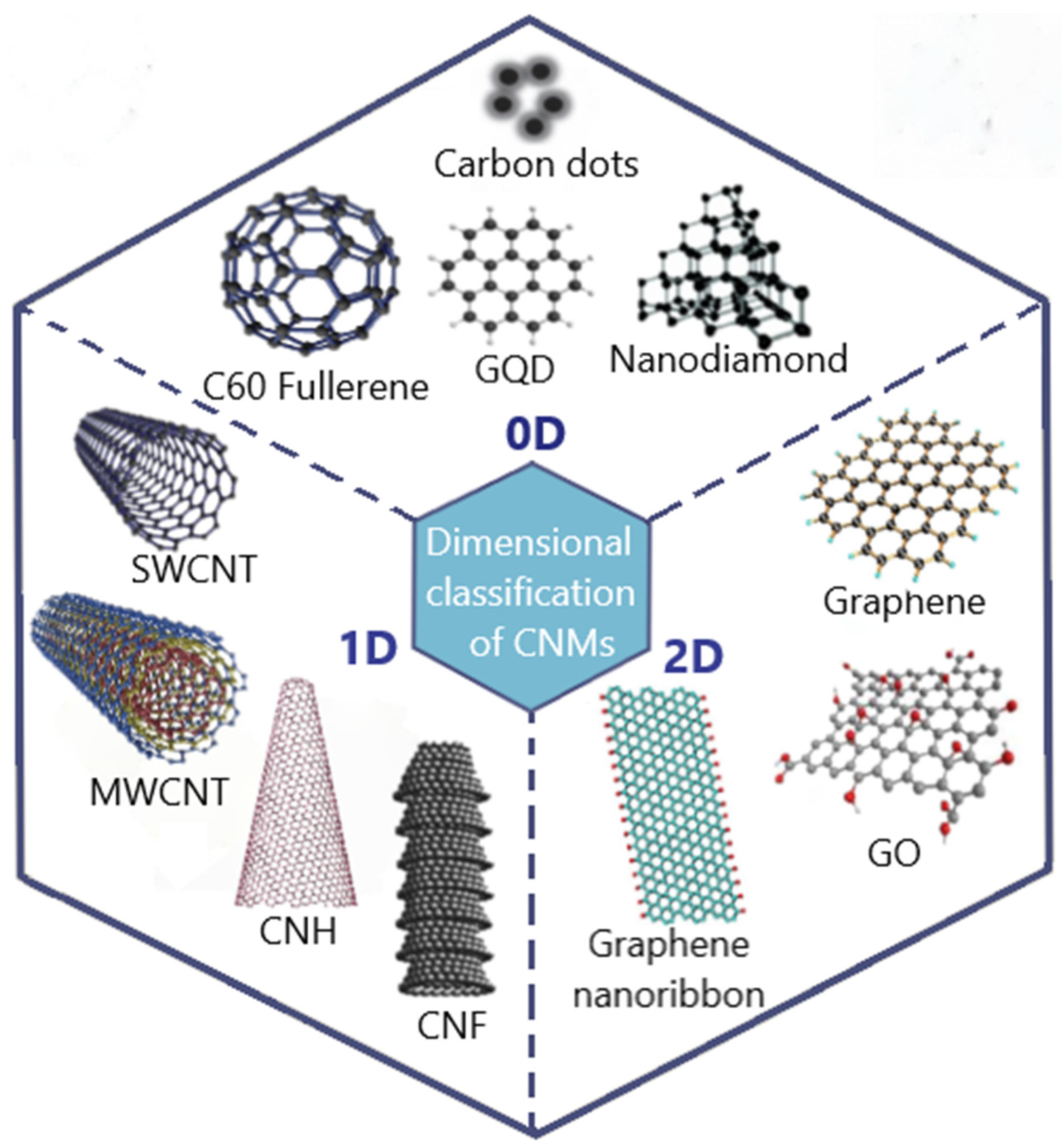
2.1. History, Classification, and Application of Carbon-Based Nanomaterials
2.2. Environmental Safety and Risk Assessment of Carbon-Based Nanomaterials
3. Predictive Models for Mixture Toxicity Assessment
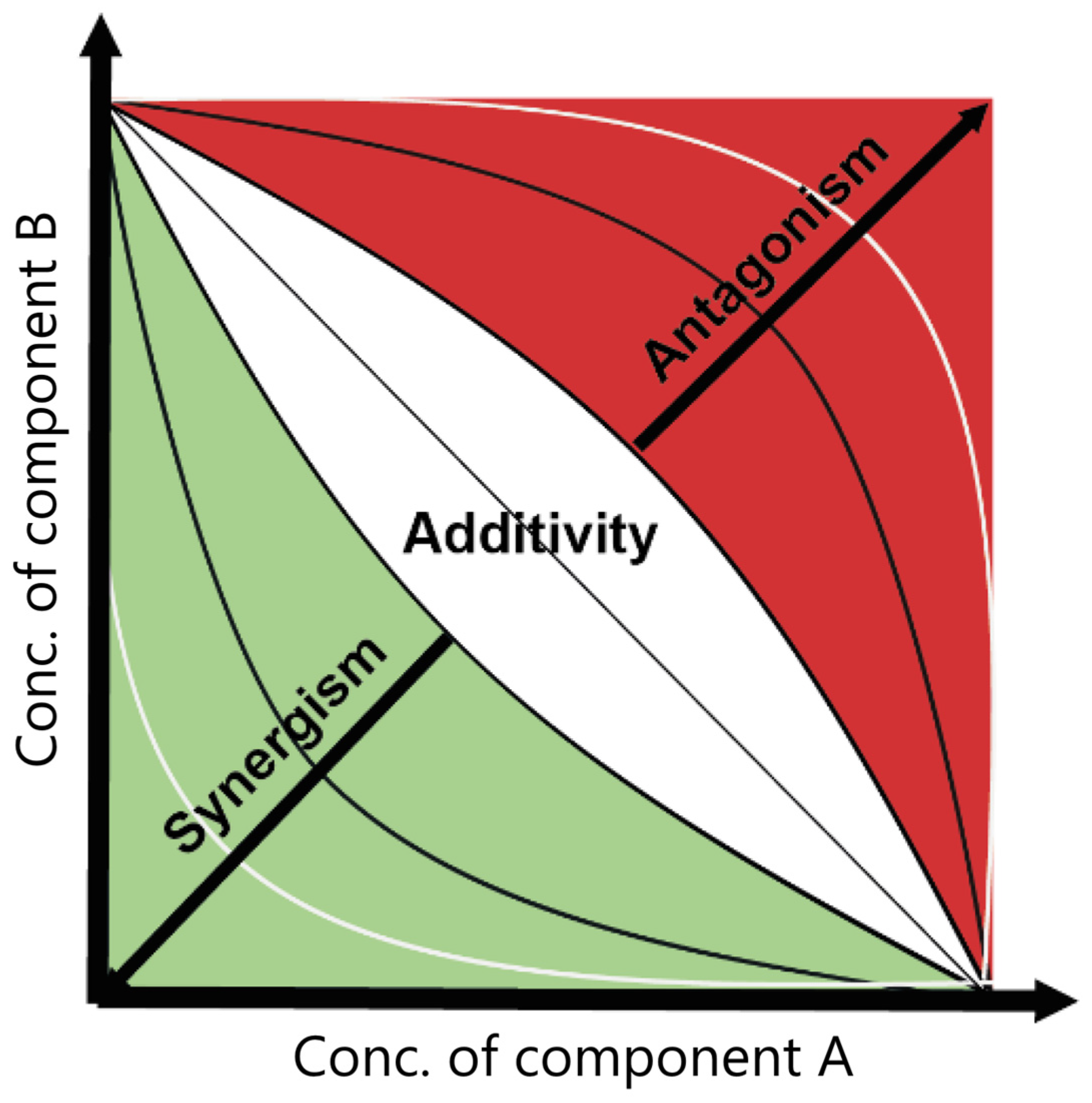
3.1. The Main Principles of Mixture Toxicity Modeling
3.2. Applicability of Joint Toxicity Models for Carbon-Based Nanomaterials
4. Current Available Data of CNMs Interaction and Joint Toxicity with Emerging Aquatic Pollutants
4.1. Joint Toxicity of CNMs with Heavy Metals and Metal-Based Nanoparticles
4.2. Joint Toxicity of CNMs with Pesticides
4.3. Joint Toxicity of CNMs with Organic Contaminants, Including Hydrocarbons
4.4. Joint Toxicity of CNMs with Other Co-Contaminants
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Marchi, L.; Neto, V.; Pretti, C.; Figueira, E.; Chiellini, F.; Morelli, A.; Soares, A.M.; Freitas, R. Toxic effects of multi-walled carbon nanotubes on bivalves: Comparison between functionalized and nonfunctionalized nanoparticles. Sci. Total Environ. 2018, 622, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Freixa, A.; Acuña, V.; Sanchís, J.; Farré, M.; Barceló, D.; Sabater, S. Ecotoxicological effects of carbon based nanomaterials in aquatic organisms. Sci. Total Environ. 2018, 619, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Pikula, K.; Johari, S.A.; Golokhvast, K. Colloidal Behavior and Biodegradation of Engineered Carbon-Based Nanomaterials in Aquatic Environment. Nanomaterials 2022, 12, 4149. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Madima, N.; Mishra, S.; Inamuddin, I.; Mishra, A. Carbon-based nanomaterials for remediation of organic and inorganic pollutants from wastewater: A review. Environ. Chem. Lett. 2020, 18, 1169–1191. [Google Scholar] [CrossRef]
- Chung, J.H.; Hasyimah, N.; Hussein, N. Application of carbon nanotubes (CNTs) for remediation of emerging pollutants-a review. Trop. Aquat. Soil Pollut. 2022, 2, 13–26. [Google Scholar] [CrossRef]
- Dubey, R.; Dutta, D.; Sarkar, A.; Chattopadhyay, P. Functionalized carbon nanotubes: Synthesis, properties and applications in water purification, drug delivery, and material and biomedical sciences. Nanoscale Adv. 2021, 3, 5722–5744. [Google Scholar] [CrossRef]
- Mottier, A.; Mouchet, F.; Pinelli, E.; Gauthier, L.; Flahaut, E. Environmental impact of engineered carbon nanoparticles: From releases to effects on the aquatic biota. Curr. Opin. Biotechnol. 2017, 46, 1–6. [Google Scholar] [CrossRef]
- Laux, P.; Riebeling, C.; Booth, A.M.; Brain, J.D.; Brunner, J.; Cerrillo, C.; Creutzenberg, O.; Estrela-Lopis, I.; Gebel, T.; Johanson, G. Challenges in characterizing the environmental fate and effects of carbon nanotubes and inorganic nanomaterials in aquatic systems. Environ. Sci. Nano 2018, 5, 48–63. [Google Scholar] [CrossRef]
- Petersen, E.J.; Zhang, L.; Mattison, N.T.; O’Carroll, D.M.; Whelton, A.J.; Uddin, N.; Nguyen, T.; Huang, Q.; Henry, T.B.; Holbrook, R.D. Potential release pathways, environmental fate, and ecological risks of carbon nanotubes. Environ. Sci. Technol. 2011, 45, 9837–9856. [Google Scholar] [CrossRef]
- Kahru, A.; Dubourguier, H.-C. From ecotoxicology to nanoecotoxicology. Toxicology 2010, 269, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I. Nanomaterial and nanoparticle: Origin and activity. In Nanoscience and Plant–Soil Systems; Springer: Cham, Switzerland, 2017; pp. 71–112. [Google Scholar]
- Hochella, M.F., Jr.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363, eaau8299. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K. Engineered Nanoparticles: Structure, Properties and Mechanisms of Toxicity; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Barhoum, A.; García-Betancourt, M.L.; Jeevanandam, J.; Hussien, E.A.; Mekkawy, S.A.; Mostafa, M.; Omran, M.M.; Abdalla, M.S.; Bechelany, M. Review on natural, incidental, bioinspired, and engineered nanomaterials: History, definitions, classifications, synthesis, properties, market, toxicities, risks, and regulations. Nanomaterials 2022, 12, 177. [Google Scholar] [CrossRef]
- Smalley, R.E. Discovering the fullerenes. Rev. Mod. Phys. 1997, 69, 723. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.-E.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Neto, A.C.; Guinea, F.; Peres, N.M.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The structure of suspended graphene sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef]
- Jović, D.; Jaćević, V.; Kuča, K.; Borišev, I.; Mrdjanovic, J.; Petrovic, D.; Seke, M.; Djordjevic, A. The puzzling potential of carbon nanomaterials: General properties, application, and toxicity. Nanomaterials 2020, 10, 1508. [Google Scholar] [CrossRef]
- Rizwan, M.; Shoukat, A.; Ayub, A.; Razzaq, B.; Tahir, M.B. Types and classification of nanomaterials. In Nanomaterials: Synthesis, Characterization, Hazards and Safety; Elsevier: Amsterdam, The Netherlands, 2021; pp. 31–54. [Google Scholar]
- Reckmeier, C.; Schneider, J.; Susha, A.; Rogach, A. Luminescent colloidal carbon dots: Optical properties and effects of doping. Opt. Express 2016, 24, A312–A340. [Google Scholar] [CrossRef] [PubMed]
- Bandaru, P.R. Electrical properties and applications of carbon nanotube structures. J. Nanosci. Nanotechnol. 2007, 7, 1239–1267. [Google Scholar] [CrossRef] [PubMed]
- Anzar, N.; Hasan, R.; Tyagi, M.; Yadav, N.; Narang, J. Carbon nanotube-A review on Synthesis, Properties and plethora of applications in the field of biomedical science. Sens. Int. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Raphey, V.; Henna, T.; Nivitha, K.; Mufeedha, P.; Sabu, C.; Pramod, K. Advanced biomedical applications of carbon nanotube. Mater. Sci. Eng. C 2019, 100, 616–630. [Google Scholar] [CrossRef]
- Karousis, N.; Suarez-Martinez, I.; Ewels, C.P.; Tagmatarchis, N. Structure, properties, functionalization, and applications of carbon nanohorns. Chem. Rev. 2016, 116, 4850–4883. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Colombo, L.; Gellert, P.; Schwab, M.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Jiříčková, A.; Jankovský, O.; Sofer, Z.; Sedmidubský, D. Synthesis and applications of graphene oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef]
- Service, R.F. Is nanotechnology dangerous? Science 2000, 290, 1526–1527. [Google Scholar] [CrossRef]
- Maynard, A.D.; Aitken, R.J.; Butz, T.; Colvin, V.; Donaldson, K.; Oberdörster, G.; Philbert, M.A.; Ryan, J.; Seaton, A.; Stone, V. Safe handling of nanotechnology. Nature 2006, 444, 267–269. [Google Scholar] [CrossRef]
- Donaldson, K.; Stone, V.; Tran, C.L.; Kreyling, W.; Borm, P.J.A. Nanotoxicology. Occup. Environ. Med. 2004, 61, 727–728. [Google Scholar] [CrossRef]
- Oberdorster, G.; Oberdorster, E.; Oberdorster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Oberdorster, G.; Oberdorster, E.; Oberdorster, J. Concepts of nanoparticle dose metric and response metric. Environ. Health Perspect. 2007, 115, A290. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Laux, P.; Luch, A.; Sudrik, C.; Wiehr, S.; Wild, A.M.; Santomauro, G.; Bill, J.; Sitti, M. Review of emerging concepts in nanotoxicology: Opportunities and challenges for safer nanomaterial design. Toxicol. Mech. Methods 2019, 29, 378–387. [Google Scholar] [CrossRef]
- Hong, H.; Part, F.; Nowack, B. Prospective Dynamic and Probabilistic Material Flow Analysis of Graphene-Based Materials in Europe from 2004 to 2030. Environ. Sci. Technol. 2022, 56, 13798–13809. [Google Scholar] [CrossRef]
- Sun, T.Y.; Gottschalk, F.; Hungerbühler, K.; Nowack, B. Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ. Pollut. 2014, 185, 69–76. [Google Scholar] [CrossRef]
- Saxena, P.; Sangela, V.; Ranjan, S.; Dutta, V.; Dasgupta, N.; Phulwaria, M.; Rathore, D.S. Aquatic nanotoxicology: Impact of carbon nanomaterials on algal flora. Energy Ecol. Environ. 2020, 5, 240–252. [Google Scholar] [CrossRef]
- Malhotra, N.; Villaflores, O.B.; Audira, G.; Siregar, P.; Lee, J.-S.; Ger, T.-R.; Hsiao, C.-D. Toxicity studies on graphene-based nanomaterials in aquatic organisms: Current understanding. Molecules 2020, 25, 3618. [Google Scholar] [CrossRef]
- Gottschalk, F.; Kost, E.; Nowack, B. Engineered nanomaterials in water and soils: A risk quantification based on probabilistic exposure and effect modeling. Environ. Toxicol. Chem. 2013, 32, 1278–1287. [Google Scholar] [CrossRef]
- Coll, C.; Notter, D.; Gottschalk, F.; Sun, T.; Som, C.; Nowack, B. Probabilistic environmental risk assessment of five nanomaterials (nano-TiO2, nano-Ag, nano-ZnO, CNT, and fullerenes). Nanotoxicology 2016, 10, 436–444. [Google Scholar] [CrossRef]
- Rodea-Palomares, I.; González-Pleiter, M.; Martín-Betancor, K.; Rosal, R.; Fernández-Piñas, F. Additivity and interactions in ecotoxicity of pollutant mixtures: Some patterns, conclusions, and open questions. Toxics 2015, 3, 342–369. [Google Scholar] [CrossRef]
- Loewe, S.T. Effect of combinations: Mathematical basis of problem. Arch. Exp. Pathol. Pharmakol. 1926, 114, 313–326. [Google Scholar] [CrossRef]
- Bliss, C.I. The toxicity of poisons applied jointly 1. Ann. Appl. Biol. 1939, 26, 585–615. [Google Scholar] [CrossRef]
- Backhaus, T. Medicines, shaken and stirred: A critical review on the ecotoxicology of pharmaceutical mixtures. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130585. [Google Scholar] [CrossRef] [PubMed]
- Kamo, M. Mathematical Models for Chemical Mixtures. In Theories in Ecological Risk Assessment; Springer: Berlin/Heidelberg, Germany, 2023; pp. 151–182. [Google Scholar]
- Junghans, M.; Backhaus, T.; Faust, M.; Scholze, M.; Grimme, L. Application and validation of approaches for the predictive hazard assessment of realistic pesticide mixtures. Aquat. Toxicol. 2006, 76, 93–110. [Google Scholar] [CrossRef]
- Cokol, M.; Chua, H.N.; Tasan, M.; Mutlu, B.; Weinstein, Z.B.; Suzuki, Y.; Nergiz, M.E.; Costanzo, M.; Baryshnikova, A.; Giaever, G. Systematic exploration of synergistic drug pairs. Mol. Syst. Biol. 2011, 7, 544. [Google Scholar] [CrossRef]
- Warne, M.S.J.; Hawker, D.W. The number of components in a mixture determines whether synergistic and antagonistic or additive toxicity predominate: The funnel hypothesis. Ecotoxicol. Environ. Saf. 1995, 31, 23–28. [Google Scholar] [CrossRef]
- LeBlanc, G.A.; Wang, G. Chemical mixtures: Greater-than-additive effects? Environ. Health Perspect. 2006, 114, A517–A518. [Google Scholar] [CrossRef][Green Version]
- Liu, J.; Liu, X.; Song, Q.; Compson, Z.G.; LeRoy, C.J.; Luan, F.; Wang, H.; Hu, Y.; Yang, Q. Synergistic effects: A common theme in mixed-species litter decomposition. New Phytol. 2020, 227, 757–765. [Google Scholar] [CrossRef]
- Schindler, M. Modeling synergistic effects by using general Hill-type response surfaces describing drug interactions. Sci. Rep. 2022, 12, 10524. [Google Scholar] [CrossRef]
- Calzetta, L.; Koziol-White, C. Pharmacological interactions: Synergism, or not synergism, that is the question. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100046. [Google Scholar] [CrossRef]
- Chatterjee, M.; Roy, K. Recent advances on modelling the toxicity of environmental pollutants for risk assessment: From single pollutants to mixtures. Curr. Pollut. Rep. 2022, 8, 81–97. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S. State of the art in the application of QSAR techniques for predicting mixture toxicity in environmental risk assessment. SAR QSAR Environ. Res. 2015, 26, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, F.; Wang, S.; Peijnenburg, W.J. Assessment and prediction of joint algal toxicity of binary mixtures of graphene and ionic liquids. Chemosphere 2017, 185, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, L. An insight into the combined toxicity of 3,4-dichloroaniline with two-dimensional nanomaterials: From classical mixture theory to structure-activity relationship. Int. J. Mol. Sci. 2023, 24, 3723. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Vijver, M.G.; Peijnenburg, W.J. Prediction of the joint toxicity of multiple engineered nanoparticles: The integration of classic mixture models and in silico methods. Chem. Res. Toxicol. 2020, 34, 176–178. [Google Scholar] [CrossRef]
- Trinh, T.X.; Seo, M.; Yoon, T.H.; Kim, J. Developing random forest based QSAR models for predicting the mixture toxicity of TiO2 based nano-mixtures to Daphnia magna. NanoImpact 2022, 25, 100383. [Google Scholar] [CrossRef]
- Apul, O.G.; Wang, Q.; Shao, T.; Rieck, J.R.; Karanfil, T. Predictive model development for adsorption of aromatic contaminants by multi-walled carbon nanotubes. Environ. Sci. Technol. 2013, 47, 2295–2303. [Google Scholar] [CrossRef]
- Lata, S.; Vikas. Concentration-dependent adsorption of organic contaminants by graphene nanosheets: Quantum-mechanical models. J. Mol. Model. 2021, 27, 48. [Google Scholar] [CrossRef]
- Alloway, B.J. Heavy metals and metalloids as micronutrients for plants and animals. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Springer: Dordrecht, The Netherlands, 2013; pp. 195–209. [Google Scholar]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Baby, J.; Raj, J.S.; Biby, E.T.; Sankarganesh, P.; Jeevitha, M.; Ajisha, S.; Rajan, S.S. Toxic effect of heavy metals on aquatic environment. Int. J. Biol. Chem. Sci. 2010, 4, 939–952. [Google Scholar] [CrossRef]
- Brezonik, P.L.; King, S.O.; Mach, C.E. The influence of water chemistry on trace metal bioavailability and toxicity to aquatic organisms. In Metal Ecotoxicology Concepts and Applications; CRC Press: Boca Raton, FL, USA, 2020; pp. 1–31. [Google Scholar]
- Singh, S.; Kapoor, D.; Khasnabis, S.; Singh, J.; Ramamurthy, P.C. Mechanism and kinetics of adsorption and removal of heavy metals from wastewater using nanomaterials. Environ. Chem. Lett. 2021, 19, 2351–2381. [Google Scholar] [CrossRef]
- Maksimova, Y.G. Microorganisms and Carbon Nanotubes: Interaction and Applications (Review). Appl. Biochem. Microbiol. 2019, 55, 1–12. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Osibote, O.A. Carbon derived nanomaterials for the sorption of heavy metals from aqueous solution: A review. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100578. [Google Scholar] [CrossRef]
- Bhattacharjee, T.; Konwar, A.; Boruah, J.S.; Chowdhury, D.; Majumdar, G. A sustainable approach for heavy metal remediation from water using carbon dot based composites: A review. J. Hazard. Mater. Adv. 2023, 10, 100295. [Google Scholar] [CrossRef]
- Baby, R.; Saifullah, B.; Hussein, M.Z. Carbon nanomaterials for the treatment of heavy metal-contaminated water and environmental remediation. Nanoscale Res. Lett. 2019, 14, 341. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J. The application of graphene-based materials for the removal of heavy metals and radionuclides from water and wastewater. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1042–1105. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Tan, L.; Wang, J. Combined toxicities of copper nanoparticles with carbon nanotubes on marine microalgae Skeletonema costatum. Environ. Sci. Pollut. Res. 2018, 25, 13127–13133. [Google Scholar] [CrossRef]
- Pikula, K.; Chaika, V.; Zakharenko, A.; Savelyeva, A.; Kirsanova, I.; Anisimova, A.; Golokhvast, K. Toxicity of Carbon, Silicon, and Metal-Based Nanoparticles to the Hemocytes of Three Marine Bivalves. Animals 2020, 10, 827. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Li, S.; Chang, J. Acute toxicity, respiratory reaction, and sensitivity of three cyprinid fish species caused by exposure to four heavy metals. PLoS ONE 2013, 8, e65282. [Google Scholar] [CrossRef]
- Satoh, A.; Vudikaria, L.Q.; Kurano, N.; Miyachi, S. Evaluation of the sensitivity of marine microalgal strains to the heavy metals, Cu, As, Sb, Pb and Cd. Environ. Int. 2005, 31, 713–722. [Google Scholar] [CrossRef]
- Chiellini, C.; Guglielminetti, L.; Pistelli, L.; Ciurli, A. Screening of trace metal elements for pollution tolerance of freshwater and marine microalgal strains: Overview and perspectives. Algal Res. 2020, 45, 101751. [Google Scholar] [CrossRef]
- Pikula, K.; Johari, S.A.; Santos-Oliveira, R.; Golokhvast, K. The Comparative Toxic Impact Assessment of Carbon Nanotubes, Fullerene, Graphene, and Graphene Oxide on Marine Microalgae Porphyridium purpureum. Toxics 2023, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Pikula, K.; Johari, S.A.; Santos-Oliveira, R.; Golokhvast, K. Toxicity and Biotransformation of Carbon-Based Nanomaterials in Marine Microalgae Heterosigma akashiwo. Int. J. Mol. Sci. 2023, 24, 10020. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.R.; Salimian, M.; Ferro, M.; Marques, P.A.; Goncalves, G.; Titus, E.; Domingues, I. Biochemical and behavioral responses of zebrafish embryos to magnetic graphene/nickel nanocomposites. Ecotoxicol. Environ. Saf. 2019, 186, 109760. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Pavlaki, M.D.; Loureiro, S.; Martins, R.; Tyler, C.R.; Mansouri, B.; Kharkan, J.; Shekari, H. Co-exposure of zinc oxide nanoparticles and multi-layer graphenes in blackfish (Capoeta fusca): Evaluation of lethal, behavioural, and histopathological effects. Ecotoxicology 2022, 31, 425–439. [Google Scholar] [CrossRef]
- de Medeiros, A.M.Z.; Côa, F.; Alves, O.L.; Martinez, D.S.T.; Barbieri, E. Metabolic effects in the freshwater fish Geophagus iporangensis in response to single and combined exposure to graphene oxide and trace elements. Chemosphere 2020, 243, 125316. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Yuan, P.; Wu, Z.; Wang, Z.; Wu, W. Graphene oxide promoted chromium uptake by zebrafish embryos with multiple effects: Adsorption, bioenergetic flux and metabolism. Sci. Total Environ. 2022, 802, 149914. [Google Scholar] [CrossRef]
- Jurgelėnė, Ž.; Montvydienė, D.; Šemčuk, S.; Stankevičiūtė, M.; Sauliutė, G.; Pažusienė, J.; Morkvėnas, A.; Butrimienė, R.; Jokšas, K.; Pakštas, V. The impact of co-treatment with graphene oxide and metal mixture on Salmo trutta at early development stages: The sorption capacity and potential toxicity. Sci. Total Environ. 2022, 838, 156525. [Google Scholar] [CrossRef]
- Gao, X.; Ren, H.; Huang, Y.; Li, Y.; Shen, J. Influence of multi-walled carbon nanotubes on the toxicity of ZnO nanoparticles in the intestinal histopathology, apoptosis, and microbial community of common carp. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 276, 109790. [Google Scholar] [CrossRef]
- Morozesk, M.; Franqui, L.S.; Pinheiro, F.C.; Nóbrega, J.A.; Martinez, D.S.; Fernandes, M.N. Effects of multiwalled carbon nanotubes co-exposure with cadmium on zebrafish cell line: Metal uptake and accumulation, oxidative stress, genotoxicity and cell cycle. Ecotoxicol. Environ. Saf. 2020, 202, 110892. [Google Scholar] [CrossRef]
- Britto, R.S.; Nascimento, J.P.; Serode, T.; Santos, A.P.; Soares, A.M.; Figueira, E.; Furtado, C.; Lima-Ventura, J.; Monserrat, J.M.; Freitas, R. The effects of co-exposure of graphene oxide and copper under different pH conditions in Manila clam Ruditapes philippinarum. Environ. Sci. Pollut. Res. 2020, 27, 30945–30956. [Google Scholar] [CrossRef] [PubMed]
- de Melo, C.B.; Côa, F.; Alves, O.L.; Martinez, D.S.T.; Barbieri, E. Co-exposure of graphene oxide with trace elements: Effects on acute ecotoxicity and routine metabolism in Palaemon pandaliformis (shrimp). Chemosphere 2019, 223, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.S.T.; Da Silva, G.H.; de Medeiros, A.M.Z.; Khan, L.U.; Papadiamantis, A.G.; Lynch, I. Effect of the albumin corona on the toxicity of combined graphene oxide and cadmium to daphnia magna and integration of the datasets into the nanocommons knowledge base. Nanomaterials 2020, 10, 1936. [Google Scholar] [CrossRef]
- Wang, X.; Qu, R.; Liu, J.; Wei, Z.; Wang, L.; Yang, S.; Huang, Q.; Wang, Z. Effect of different carbon nanotubes on cadmium toxicity to Daphnia magna: The role of catalyst impurities and adsorption capacity. Environ. Pollut. 2016, 208, 732–738. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, X.; Bai, L.; Quan, X. Effects of nanomaterials on metal toxicity: Case study of graphene family on Cd. Ecotoxicol. Environ. Saf. 2020, 194, 110448. [Google Scholar] [CrossRef]
- Hu, C.; Hu, N.; Li, X.; Zhao, Y. Graphene oxide alleviates the ecotoxicity of copper on the freshwater microalga Scenedesmus obliquus. Ecotoxicol. Environ. Saf. 2016, 132, 360–365. [Google Scholar] [CrossRef]
- Zhao, J.; Ning, F.; Cao, X.; Yao, H.; Wang, Z.; Xing, B. Photo-transformation of graphene oxide in the presence of co-existing metal ions regulated its toxicity to freshwater algae. Water Res. 2020, 176, 115735. [Google Scholar] [CrossRef]
- Yin, J.; Dong, Z.; Liu, Y.; Wang, H.; Li, A.; Zhuo, Z.; Feng, W.; Fan, W. Toxicity of reduced graphene oxide modified by metals in microalgae: Effect of the surface properties of algal cells and nanomaterials. Carbon 2020, 169, 182–192. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, F.; Vijver, M.G.; Peijnenburg, W.J. Graphene nanoplatelets and reduced graphene oxide elevate the microalgal cytotoxicity of nano-zirconium oxide. Chemosphere 2021, 276, 130015. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, X.; Tan, L.; Zhao, T.; Ni, Z.; Zhang, N.; Wang, J. Single and combined nanotoxicity of ZnO nanoparticles and graphene quantum dots against the microalga Heterosigma akashiwo. Environ. Sci. Nano 2022, 9, 3094–3109. [Google Scholar]
- Zhu, X.; Tan, L.; Zhao, T.; Huang, W.; Guo, X.; Wang, J.; Wang, J. Alone and combined toxicity of ZnO nanoparticles and graphene quantum dots on microalgae Gymnodinium. Environ. Sci. Pollut. Res. 2022, 29, 47310–47322. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, W.; Xu, Y.; Hu, N.; Ma, J.; Cao, W.; Sun, S.; Hu, C.; Zhao, Y.; Huang, Q. Effects of carbon nanotubes on the toxicities of copper, cadmium and zinc toward the freshwater microalgae Scenedesmus obliquus. Aquat. Toxicol. 2020, 224, 105504. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Gong, J.; Cao, W.; Chen, Z.; Huang, D.; Ye, J.; Cai, Z. The combined toxicity and mechanism of multi-walled carbon nanotubes and nano copper oxide toward freshwater algae: Tetradesmus obliquus. J. Environ. Sci. 2022, 112, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ren, X.; Wu, J.; Hayat, T.; Alsaedi, A.; Cheng, C.; Chen, C. Graphene oxide interactions with co-existing heavy metal cations: Adsorption, colloidal properties and joint toxicity. Environ. Sci. Nano 2018, 5, 362–371. [Google Scholar] [CrossRef]
- Wang, F.; Yao, J.; Liu, H.; Liu, R.; Chen, H.; Yi, Z.; Yu, Q.; Ma, L.; Xing, B. Cu and Cr enhanced the effect of various carbon nanotubes on microbial communities in an aquatic environment. J. Hazard. Mater. 2015, 292, 137–145. [Google Scholar] [CrossRef]
- Ye, N.; Wang, Z.; Wang, S.; Peijnenburg, W.J. Toxicity of mixtures of zinc oxide and graphene oxide nanoparticles to aquatic organisms of different trophic level: Particles outperform dissolved ions. Nanotoxicology 2018, 12, 423–438. [Google Scholar] [CrossRef]
- Thapar, A.; Zalawadia, A.; Pokharkar, O.V.; Satam, S.S. Classification of pesticides and its damaging effects: A review. Biolife 2016, 4, 13–24. [Google Scholar]
- Yadav, G.K.; Ahmaruzzaman, M. Recent advances in the development of nanocomposites for effective removal of pesticides from aqueous stream. J. Nanopart. Res. 2021, 23, 213. [Google Scholar] [CrossRef]
- Kumar, R.; Sankhla, M.S.; Kumar, R.; Sonone, S.S. Impact of pesticide toxicity in aquatic environment. Biointerface Res. Appl. Chem. 2021, 11, 10131–10140. [Google Scholar]
- Grung, M.; Lin, Y.; Zhang, H.; Steen, A.O.; Huang, J.; Zhang, G.; Larssen, T. Pesticide levels and environmental risk in aquatic environments in China—A review. Environ. Int. 2015, 81, 87–97. [Google Scholar] [CrossRef]
- Hegde, V.; Bhat, M.P.; Lee, J.-H.; Kurkuri, M.D.; Kim, C.S.; Lee, K.-H. Carbon-based nanomaterials: Multifaceted role in agrochemical recognition, remediation, and release. Nano Today 2024, 57, 102388. [Google Scholar] [CrossRef]
- Liu, J.; Luo, Y.; Jiang, X.; Sun, G.; Song, S.; Yang, M.; Shen, J. Enhanced and sustained pesticidal activity of a graphene-based pesticide delivery system against the diamondback moth Plutella xylostella. Pest Manag. Sci. 2022, 78, 5358–5365. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, J.; Wang, Z.; Li, C.; Li, X. Crop-safe pyraclostrobin-loaded multiwalled carbon nanotube delivery systems: Higher fungicidal activity and lower acute toxicity. ACS Agric. Sci. Technol. 2022, 2, 534–545. [Google Scholar] [CrossRef]
- Zou, W.; Zhang, X.; Ouyang, S.; Hu, X.; Zhou, Q. Graphene oxide nanosheets mitigate the developmental toxicity of TDCIPP in zebrafish via activating the mitochondrial respiratory chain and energy metabolism. Sci. Total Environ. 2020, 727, 138486. [Google Scholar] [CrossRef]
- Zhao, T.; Ren, B.; Zhao, Y.; Chen, H.; Wang, J.; Li, Y.; Liang, H.; Li, L.; Liang, H. Multi-walled carbon nanotubes impact on the enantioselective bioaccumulation and toxicity of the chiral insecticide bifenthrin to zebrafish (Danio rerio). Chemosphere 2022, 294, 133690. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, X.; Ren, X.; Duan, X. Antagonistic effects of multi-walled carbon nanotubes and BDE-47 in zebrafish (Danio rerio): Oxidative stress, apoptosis and DNA damage. Aquat. Toxicol. 2020, 225, 105546. [Google Scholar] [CrossRef]
- Campos-Garcia, J.; Martinez, D.S.T.; Alves, O.L.; Leonardo, A.F.G.; Barbieri, E. Ecotoxicological effects of carbofuran and oxidised multiwalled carbon nanotubes on the freshwater fish Nile tilapia: Nanotubes enhance pesticide ecotoxicity. Ecotoxicol. Environ. Saf. 2015, 111, 131–137. [Google Scholar] [CrossRef]
- Li, F.; Meng, X.; Wang, X.; Ji, C.; Wu, H. Graphene-triphenyl phosphate (TPP) co-exposure in the marine environment: Interference with metabolism and immune regulation in mussel Mytilus galloprovincialis. Ecotoxicol. Environ. Saf. 2021, 227, 112904. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Meng, X.; Xia, C.; Ji, C.; Wu, H. Abnormality of mussel in the early developmental stages induced by graphene and triphenyl phosphate: In silico toxicogenomic data-mining, in vivo, and toxicity pathway-oriented approach. Aquat. Toxicol. 2023, 263, 106674. [Google Scholar] [CrossRef]
- Meng, X.; Li, F.; Wang, X.; Liu, J.; Ji, C.; Wu, H. Toxicological effects of graphene on mussel Mytilus galloprovincialis hemocytes after individual and combined exposure with triphenyl phosphate. Mar. Pollut. Bull. 2020, 151, 110838. [Google Scholar] [CrossRef]
- de Paula, T.N.M.; Vendemiatti, J.A.S.; Camparotto, N.G.; Toledo, B.; Oliveira, Á.C.; Neves, T.F.; Umbuzeiro, G.A.; Prediger, P. Behavior of two classes of organic contaminants in the presence of graphene oxide: Ecotoxicity, physicochemical characterization and theoretical calculations. Sci. Total Environ. 2022, 822, 153515. [Google Scholar] [CrossRef] [PubMed]
- Martín-de-Lucía, I.; Gonçalves, S.F.; Leganés, F.; Fernández-Piñas, F.; Rosal, R.; Loureiro, S. Combined toxicity of graphite-diamond nanoparticles and thiabendazole to Daphnia magna. Sci. Total Environ. 2019, 688, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Alves, K.V.B.; Martinez, D.S.T.; Alves, O.L.; Barbieri, E. Co-exposure of carbon nanotubes with carbofuran pesticide affects metabolic rate in Palaemon pandaliformis (shrimp). Chemosphere 2022, 288, 132359. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Zhu, Y.; Hou, J.; White, J.C.; Gardea-Torresdey, J.L.; Lin, D. Antagonistic toxicity of carbon nanotubes and pentachlorophenol to Escherichia coli: Physiological and transcriptional responses. Carbon 2019, 145, 658–667. [Google Scholar] [CrossRef]
- Deng, R.; Yang, K.; Lin, D. Pentachlorophenol and ciprofloxacin present dissimilar joint toxicities with carbon nanotubes to Bacillus subtilis. Environ. Pollut. 2021, 270, 116071. [Google Scholar] [CrossRef]
- Singh, A.; Dhau, J.S.; Kumar, R. Application of carbon-based nanomaterials for removal of hydrocarbons. In New Frontiers of Nanomaterials in Environmental Science; Springer: Singapore, 2021; pp. 205–227. [Google Scholar]
- Nagy, A.S.; Simon, G.; Szabó, J.; Vass, I. Polycyclic aromatic hydrocarbons in surface water and bed sediments of the Hungarian upper section of the Danube River. Environ. Monit. Assess. 2013, 185, 4619–4631. [Google Scholar] [CrossRef]
- Shi, W.; Xu, M.; Liu, Q.; Xie, S. Polycyclic aromatic hydrocarbons in seawater, surface sediment, and marine organisms of Haizhou Bay in Yellow Sea, China: Distribution, source apportionment, and health risk assessment. Mar. Pollut. Bull. 2022, 174, 113280. [Google Scholar] [CrossRef]
- Ighariemu, V.; Belonwu, D.C.; Wegwu, M.O. Level of petroleum hydrocarbons in water and sediments of ikoli creek Bayelsa state Nigeria. Toxicol. Environ. Health Sci. 2019, 11, 114–119. [Google Scholar] [CrossRef]
- Bina, B.; Amin, M.M.; Rashidi, A.; Pourzamani, H. Water and wastewater treatment from BTEX by carbon nanotubes and Nano-Fe. Water Resour. 2014, 41, 719–727. [Google Scholar] [CrossRef]
- Chin, C.-J.M.; Shih, L.-C.; Tsai, H.-J.; Liu, T.-K. Adsorption of o-xylene and p-xylene from water by SWCNTs. Carbon 2007, 45, 1254–1260. [Google Scholar] [CrossRef]
- Lu, C.; Su, F.; Hu, S. Surface modification of carbon nanotubes for enhancing BTEX adsorption from aqueous solutions. Appl. Surf. Sci. 2008, 254, 7035–7041. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Chen, B. Adsorption of polycyclic aromatic hydrocarbons by graphene and graphene oxide nanosheets. Environ. Sci. Technol. 2014, 48, 4817–4825. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Haftka, J.J.-H.; Sinnige, T.L.; Hermens, J.L.; Chen, W. Adsorption of polar, nonpolar, and substituted aromatics to colloidal graphene oxide nanoparticles. Environ. Pollut. 2014, 186, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yang, J.; Wang, R.; Xiao, B.; Liu, Q.; Sun, B.; Wang, X.; Zhu, L. Graphene oxide enhanced the endocrine disrupting effects of bisphenol A in adult male zebrafish: Integrated deep learning and metabolomics studies. Sci. Total Environ. 2022, 809, 151103. [Google Scholar] [CrossRef]
- Li, Y.; Men, B.; He, Y.; Xu, H.; Liu, M.; Wang, D. Effect of single-wall carbon nanotubes on bioconcentration and toxicity of perfluorooctane sulfonate in zebrafish (Danio rerio). Sci. Total Environ. 2017, 607, 509–518. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Z. Individual and binary exposure of embryonic zebrafish (Danio rerio) to single-walled and multi-walled carbon nanotubes in the absence and presence of dissolved organic matter. Sci. Total Environ. 2023, 903, 166458. [Google Scholar] [CrossRef]
- Linard, E.N.; van den Hurk, P.; Karanfil, T.; Apul, O.G.; Klaine, S.J. Influence of carbon nanotubes on the bioavailability of fluoranthene. Environ. Toxicol. Chem. 2015, 34, 658–666. [Google Scholar] [CrossRef]
- Meng, X.; Li, F.; Wang, X.; Liu, J.; Ji, C.; Wu, H. Combinatorial immune and stress response, cytoskeleton and signal transduction effects of graphene and triphenyl phosphate (TPP) in mussel Mytilus galloprovincialis. J. Hazard. Mater. 2019, 378, 120778. [Google Scholar] [CrossRef]
- González-Soto, N.; Blasco, N.; Irazola, M.; Bilbao, E.; Guilhermino, L.; Cajaraville, M.P. Fate and effects of graphene oxide alone and with sorbed benzo (a) pyrene in mussels Mytilus galloprovincialis. J. Hazard. Mater. 2023, 452, 131280. [Google Scholar] [CrossRef]
- Barranger, A.; Rance, G.A.; Aminot, Y.; Dallas, L.J.; Sforzini, S.; Weston, N.J.; Lodge, R.W.; Banni, M.; Arlt, V.M.; Moore, M.N. An integrated approach to determine interactive genotoxic and global gene expression effects of multiwalled carbon nanotubes (MWCNTs) and benzo [a] pyrene (BaP) on marine mussels: Evidence of reverse ‘Trojan Horse’effects. Nanotoxicology 2019, 13, 1324–1343. [Google Scholar] [CrossRef]
- Al-Subiai, S.N.; Arlt, V.M.; Frickers, P.E.; Readman, J.W.; Stolpe, B.; Lead, J.R.; Moody, A.J.; Jha, A.N. Merging nano-genotoxicology with eco-genotoxicology: An integrated approach to determine interactive genotoxic and sub-lethal toxic effects of C60 fullerenes and fluoranthene in marine mussels, Mytilus sp. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 745, 92–103. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, J.; Li, Y.; Cao, X.; Guo, C.; Xu, L.; Wang, Z.; Feng, J.; Yi, H.; Xing, B. Humic acid mitigated toxicity of graphene-family materials to algae through reducing oxidative stress and heteroaggregation. Environ. Sci. Nano 2019, 6, 1909–1920. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Liang, D.; Liu, Y.; Zhao, Q.; Huang, P.; Li, X.; Fan, W. Accumulation, transformation and subcellular distribution of arsenite associated with five carbon nanomaterials in freshwater zebrafish specific-tissues. J. Hazard. Mater. 2021, 415, 125579. [Google Scholar] [CrossRef]
- Wang, X.; Dong, Z.; Zhao, Q.; Li, C.; Fan, W.-H. Alleviative effects of C60 fullerene nanoparticles on arsenate transformation and toxicity to Danio rerio. Sci. Total Environ. 2024, 947, 174765. [Google Scholar] [CrossRef]
- Cao, X.; Ma, C.; Zhao, J.; Musante, C.; White, J.C.; Wang, Z.; Xing, B. Interaction of graphene oxide with co-existing arsenite and arsenate: Adsorption, transformation and combined toxicity. Environ. Int. 2019, 131, 104992. [Google Scholar] [CrossRef]
- Martín-de-Lucía, I.; Campos-Mañas, M.C.; Agüera, A.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Combined toxicity of graphene oxide and wastewater to the green alga Chlamydomonas reinhardtii. Environ. Sci. Nano 2018, 5, 1729–1744. [Google Scholar] [CrossRef]
- You, M.; You, X.; Yang, X.; Hu, J.; Sun, W. Adsorption of antibiotics onto graphene oxide imparts their antagonistic effects on Synechocystis sp.: Model development and proteomic analysis. Environ. Sci. Nano 2022, 9, 243–253. [Google Scholar] [CrossRef]
- Zhao, J.; Liang, Y.; Jin, L.; Zhang, H.; Shi, B.; Tang, X. Joint Toxic Action and Metabolic Mechanisms of Graphene Nanomaterial Mixtures in Microcystis Aeruginosa. Pol. J. Environ. Stud. 2023, 32, 1447–1458. [Google Scholar] [CrossRef]
- You, M.; You, X.; Hu, J.; Yang, X.; Sun, W. Carbon nanotubes influence the toxic effects of chloramphenicol and tetracycline on cyanobacterium Synechocystis sp. in different ways. Environ. Sci. Nano 2021, 8, 634–646. [Google Scholar] [CrossRef]
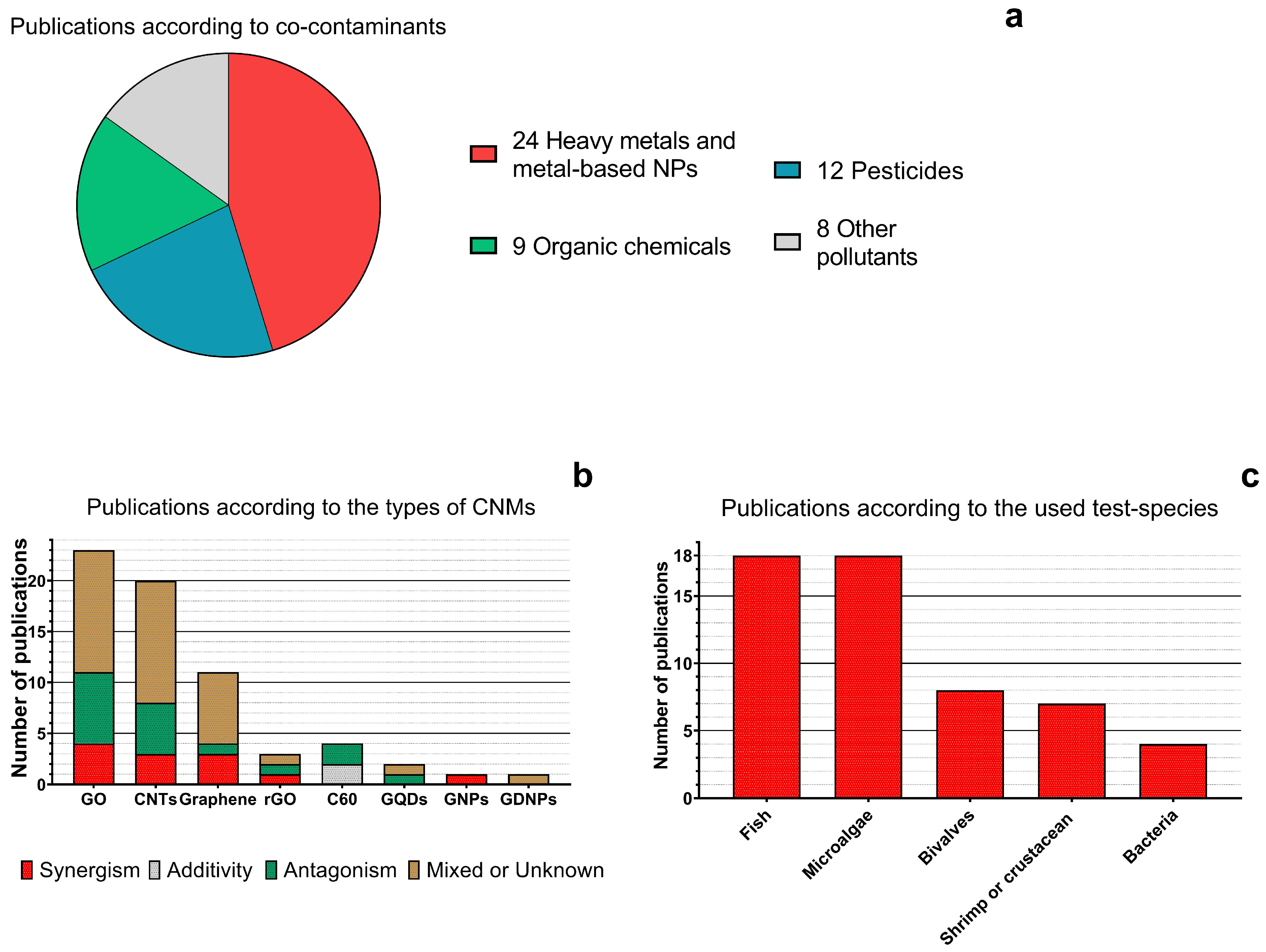
| № | CNMs | Co-Contaminant | Species | Toxicity Endpoints | Observed Effects | Reference |
|---|---|---|---|---|---|---|
| Studies used fish as a test-model | ||||||
| 1. | GN | Two types of nanocomposites with Ni | Danio rerio | 3 h, 144 h post-fertilization embryo toxicity test, biochemical response, locomotor behavior assay, bioaccumulation | neither of two GN/Ni nanocomposites presented lethal or developmental effects in zebrafish; both nanocomposites reduced the locomotion of zebrafish larvae; the differences in biochemical response were mostly associated with shape of nanoparticles than with their size | Almeida et al., 2019 [80] |
| 2. | Multilayer GN | ZnO | Capoeta fusca | 96 h LC50, histopathological and behavioral effects | synergistic at 96 h acute exposure, antagonistic effect on the histopathological and behavioral disorders | Sayadi et al., 2022 [81] |
| 3. | GO | Zn, Cd | Geophagus iporangensis | 24 h metabolic rate, ammonia excretion | GO intensified metabolic rise and ammonia excretion in fish caused by Zn, co-exposure of GO and Cd only decreased metabolic rate and did not affect ammonia excretion | Medeiros et al., 2020 [82] |
| 4. | GO | Cr6+ | Danio rerio embryos | 48 h exposure, embryo-larval toxicity, bioaccumulation, ROS generation, metabolic changes | co-exposure increased lipid peroxidation in embryos compare to single exposure; GO adsorbed Cr6+ ions and enhanced contact between adsorbed Cr6+ and chorions; sharp edges of GO also facilitated Cr6+ uptake by embryos | Chen et al., 2022 [83] |
| 5. | GO | Mixture of Cr, Cu, Ni, and Zn | Salmo trutta (embryos and larvae) | Bioaccumulation, survival, heart rate, genotoxicity, cytotoxicity, metallothionein levels, lipid peroxidation | single and joint exposure had no impact on embryos survival, but lethality of the metal mixture on larvae was nullified in co-exposure with GO; the chorion of embryos was more attracted to GO than external tissues of larvae | Jurgelėnė et al., 2022 [84] |
| 6. | MWCNTs | ZnO NPs | Cyprinus carpio | 4 weeks of exposure, histopathology, bioaccumulation | antagonistic effect at the low level of MWCNTs and synergetic effect at the high level of MWCNTs; MWCNTs significantly decreased ZnO accumulation in the intestine after four weeks of exposure | Gao et al., 2024 [85] |
| 7. | O-MWCNTs | Cd | Danio rerio liver cell line | 24 h exposure, DNA comet assay, ROS generation, enzyme activity | synergistic effect; co-exposure increased the Cd content in the cells; two different exposure protocols tested, FBS serum in the culture medium changed the uptake of metal into cells | Morozesk et al., 2020 [86] |
| Studies used mussels or clams as test-model | ||||||
| 8. | GO | Cu | Ruditapes philippinarum | 29-day exposure, metabolism, and oxidative stress-related parameters | demonstrated the dependence of the toxic response on pH; low pH showed increased electron transport system and glutathione-S-transferase activities and reduced glutathione levels under pollutants co-exposure | Britto et al., 2020 [87] |
| Studies used shrimp or crustacean as a test-model | ||||||
| 9. | GO | Zn, Cd | Palaemon pandaliformis | 96 h LC50, routine metabolism (oxygen consumption and ammonia excretion) | GO increased the toxicity of Zn and Cd and impaired the routine metabolism of P. pandaliformis | Batista de Melo et al., 2019 [88] |
| 10. | GO | Cd2+ and BSA (for albumin corona formation) | Daphnia magna | 48 h EC50 (immobilization) | antagonistic effect; bare GO reduced cadmium toxicity by 110%, albumin coronated GO reduced cadmium toxicity by 238%, albumin corona formation dramatically increased colloidal stability of GO and adsorption capacity of Cd2+ | Martinez et al., 2020 [89] |
| 11. | SWCNTs, MWCNTs, OH-MWCNTs, COOH-MWCNTs | Cd | Daphnia magna | 24 h LC50 (immobilization), bioaccumulation | all used CNTs enhanced the toxicity of Cd; the toxicity-increasing effect of SWCNTs and MWCNTs was mainly caused by catalyst impurities, while OH-MWCNTs and COOH-MWCNTs enhanced joint toxicity due to the greater adsorption of Cd | Wang et al., 2016 [90] |
| Studies used microalgae as a test-model | ||||||
| 12. | GN, GO, GN-H | Cd | Scenedesmus obliquus | 72 h EC50 (growth rate), Chl-a synthesis, cytotoxicity | GN and GO enhanced the toxicity of Cd at all the used concentrations, while GN-H enhanced the toxicity of Cd only at the lowest used concentration (0.1 mg/L); the influence of graphene family NMs on the acute toxicity of Cd was in the order of GO > GN > GN-H (at GNMs concentration 0.1 mg/L to 1 mg/L) | Zhang et al., 2020 [91] |
| 13. | GO | Cu2+ | Scenedesmus obliquus | 96 h EC50 (growth rate inhibition) 12 d subacute toxicity test | antagonistic effects; GO reduced the toxicity of Cu even at low and environmentally relevant concentrations (1 mg/L) | Hu et al., 2016 [92] |
| 14. | GO | Cu2+ | Chlorella pyrenoidosa | 72 h EC50 (growth rate inhibition), ROS generation | antagonistic effect; pristine GO and Cu2+ ions had significantly higher toxic effect than the same chemicals after 8 days of sunlight irradiation; Cu2+ ions suppressed the photo-transformation of GO, Cu2+ ions formed Cu-based nanoparticles on the photo-transformed GO | Zhao et al., 2020 [93] |
| 15. | rGO | nanocomposites with Au, Ag, Pd, Fe3O4, Co3O4, SnO2 | Chlamydomonas reinhardtii, Scenedesmus obliquus | 96 h acute exposure, ROS quenching, proteomic analysis, membrane damage | microalgae with more hydrophobic cell surfaces had more metal ion adsorption, rGO nanocomposites with more heterointerfaces were more prone to induce cellular oxidative stress and membrane damage | Yin et al., 2020 [94] |
| 16. | GNPs, rGO | nZrO2 | Chlorella pyrenoidosa | 72h EC10, EC50 (growth rate inhibition) ROS generation, cellular membrane functional changes | synergistic effect; rGO increased the cytotoxicity and intracellular ROS accumulation to a higher extent than GNPs | Wang et al., 2021 [95] |
| 17. | GQDs | ZnO | Heterosigma akashiwo | 96 h EC50 (growth rate) | antagonistic effect at low concentrations, and synergistic effect at high concentrations; adsorption of released Zn2+ ions on GQDs | Wang et al., 2022 [96] |
| 18. | GQDs | ZnO | Gymnodinium sp. | 96 h EC50 (growth rate inhibition), ROS generation | antagonistic effect, due to aggregation and sedimentation interaction between nanoparticles; ZnO alone had no negative effect on the algae growth, while GQDs revealed dose-dependent growth rate inhibition | Zhu et al., 2022 [97] |
| 19. | CNTs | CuO | Skeletonema costatum | 96 h exposure, chlorophyll and photosynthetic efficiency (ΦPSII) | antagonistic effect caused by adsorption of Cu2+ on CNTs and aggregation between nano-Cu and CNTs | Zhang et al., 2018 [73] |
| 20. | CNTs | Cu, Cd, Zn | Scenedesmus obliquus | 96 h EC10, EC50 (growth rate inhibition); 8 d exposure, biochemical response, photosynthetic activity | antagonistic effect caused by inhibition of metal uptake by co-exposure with CNTs; CNTs in single exposure enhanced the photosynthetic activity of S. obliquus | Sun et al., 2020 [98] |
| 21. | MWCNTs | CuO | Scenedesmus obliquus | 96 h EC50 (growth rate inhibition) ROS generation, cell membrane damage | MWCNTs were significantly more toxic than CuO NPs; at lower concentrations, CuO reduced cell membrane damage and ROS level caused by MWCNTs; highest concentrations of MWCNTs and CuO synergistically enhanced the ROS level | Fang et al., 2022 [99] |
| Studies used bacteria as test-model | ||||||
| 22. | GO | Cd2+, Co2+, Zn2+ | Escherichia coli, Staphylococcus aureus | 24 h acute exposure | an antagonistic effect caused by metal ions adsorption on GO, an increase in the zeta potential and the size of GO aggregates, and a decrease in the sharpness of GO edge | Gao et al., 2018 [100] |
| 23. | MWCNTs, COOH-MWCNTs, OH-MWCNTs, NH2-MWCNTs, SWCNTs | Cu, Cr | microbial communities with dominant Bacillus sp. and Acidithiobacillus sp. | 40 d exposure, population quantitation, microbial community structure, metal ions sorption | co-exposure with metals decreased bacteria population after 10 d exposure, while after 40 d CNTs with Cu, increased bacterial cell number;carboxyl- and hydroxyl-CNTs exhibited more toxicity than pristine SWCNTs, MWCNTs, and amino-functionalized MWCNTs | Wang et al., 2015 [101] |
| Studies used multispecies test-model | ||||||
| 24. | GO | ZnO | Scenedesmus obliquus, Daphnia magna, Danio rerio | EC/LC10, EC/LC50 (algae: 96 h growth rate; daphnids: 48 h immobilization; fish: 96 h lethality) | the joint effects of ZnO NPs and GO NPs were additive to S. obliquus and D. magna but antagonistic to D. rerio. The impact of Zn2+-ions was limited due to the adsorption to the GO NPs | Ye et al., 2018 [102] |
| № | CNMs | Co-Contaminant | Species | Toxicity Endpoints | Observed Effects | Reference |
|---|---|---|---|---|---|---|
| Studies used fish as a test-model | ||||||
| 1. | GO | TDCIPP | Danio rerio | 3 d, 7 d developmental toxicity, mitochondrial function, proteomic assays | antagonistic effect on the developmental toxicity (malformation, mortality, and heart rate), GO co-exposure promoted activation of the energy metabolisms in zebrafish and mitigated the adverse effects induced by TDCIPP | Zou et al., 2020 [110] |
| 2. | MWCNTs, COOH-MWCNTs | bifenthrin | Danio rerio | 42 d experiment (28 d exposure phase and 14 d elimination phase), gene expression, bioaccumulation | MWCNTs and COOH-MWCNTs increased the impact of bifenthrin on zebrafish; the genes related to immunity, endocrine activity, and neurotoxicity showed enantioselective expression in different zebrafish tissues; sex-specific differences were observed | Zhao et al., 2022 [111] |
| 3. | MWCNTs | BDE-47 | Danio rerio | 2 h embryo, 96 h LC50; embryonic development, oxidative stress, apoptosis, DNA damage | antagonistic effect, BDE-47 induced development inhibition, oxidative stress, and apoptosis in zebrafish; MWCNTs limited bioavailability of BDE-47, the levels of oxidative stress biomarkers, apoptosis, and DNA damage decreased in the presence of MWCNTs | Wang et al., 2020 [112] |
| 4. | HNO3–MWCNT | carbofuran | Oreochromis niloticus | 96 h LC50; oxygen consumption, swimming behavior | synergistic effect, HNO3–MWCNT more than five-fold increased the acute toxicity of carbofuran; co-exposure caused a decrease in both oxygen consumption and swimming capacity | Campos-Garcia et al., 2015 [113] |
| Studies used mussels or clams as test-model | ||||||
| 5. | GN | TPP | Mytilus galloprovincialis | computational toxicology and multi-omics technology | the down-regulated genes in graphene + TPP treatment were mainly associated with oxidative stress and energy metabolism; metabolic response indicated disturbances in energy metabolism and osmotic regulation under co-exposure | Li et al., 2021 [114] |
| 6. | GN | TPP | Mytilus galloprovincialis | embryo exposure, in silico toxicogenomic, metabolic pathway analysis, oxidative stress, developmental abnormality | authors established a conceptual framework of developmental abnormality; co-exposure induced significant transcriptional inhibition, disturbed morphology and physiological parameters, increased deformity and mortality to induce the developmental abnormality | Wang et al., 2023 [115] |
| 7. | GN | TPP | Mytilus galloprovincialis hemocytes | hematotoxicity, genotoxicity, oxidative stress | GN exposure caused oxidative stress and DNA damage in the hemocytes and these effects were significantly reduced after combined exposure with TPP; the up-regulated genes in the co-exposure group were mainly associated with reduced apoptosis and DNA damage | Meng et al., 2020 [116] |
| Studies used shrimp or crustacean as a test-model | ||||||
| 8. | GO | PYR, LCT | Daphnia similis | 48 h EC10, EC50 (immobilization), uptake | synergistic effect, Trojan horse effect; GO increased toxicity up to 83% for PYR and 47% for LCT, pesticide adsorption on GO led to the stabilization of the suspensions; properties of the organic toxicants can influence the stability of graphene oxide suspensions and plays a fundamental role in the modulation of their toxicity | de Paula et al., 2022 [117] |
| 9. | GDNPs | TBZ | Daphnia magna | EC50 (48 h, immobilization) | synergism at low concentrations (probably the ‘Trojan horse’ effect) and antagonism at high GDNPs doses caused by aggregation of GDNPs and reducing the bioavailability of adsorbed TBZ | Martín-de-Lucía et al., 2019 [118] |
| 10. | HNO3-MWCNT | carbofuran | Palaemon pandaliformis | 24 h exposure, metabolic rate (oxygen consumption), and ammonia excretion | higher increase in metabolic rate and ammonia excretion after co-exposure (probably additive effect) | Alves et al., 2022 [119] |
| Studies used bacteria as test-model | ||||||
| 11. | CNTs | PCP | Escherichia coli | Bacterial growth inhibition, cell morphology changes, oxidative stress, transcriptional changes, bioaccumulation | antagonistic toxicity; PCP decreased CNT bioaccumulation; CNTs attenuated the PCP-induced disturbances of gene expression in biosynthetic, protein metabolic, and small molecule metabolic processes | Deng et al., 2019 [120] |
| 12. | O-CNTs | PCP, CIP | Bacillus subtilis | 3 h EC50 (bacterial growth), ROS generation, metabolomic response | additive effect with hydrophobic PCP and synergistic effect with hydrophilic antibiotic CIP because of ‘Trojan horse effect’; CNTs, PCP, and CIP had similar influences on the contents of fatty acids, amino acids, glycerol, galactosamine, and small molecular acids in bacteria | Deng et al., 2021 [121] |
| № | CNMs | Co-Contaminant | Species | Toxicity Endpoints | Observed Effects | Reference |
| Studies used fish as a test-model | ||||||
| 1. | GO | BPA | Danio rerio embryo, larvae, and adult male fish | 7 d exposure, deep neural network modeling, molecular docking analysis, metabolic pathway analysis | GO enhanced the endocrine disruption effects of BPA in the adult zebrafish by the significant reduction in testosterone and follicle-stimulating hormone levels, and lowering spermatozoa; co-exposure caused disturbance in three additional metabolic pathways and stronger perturbations on carbohydrate, lipid, and amino acid metabolism in adult fish; the opposite effect observed in zebrafish embryo and larvae | Chen et al., 2022 [131] |
| 2. | SWCNT | PFOS | Danio rerio | 24, 48, 72, and 96 h exposure, bioaccumulation, AChE activity, ROS generation, antioxidation enzymes | enhanced the injury effect of PFOS on ROS, SOD, CAT, and AChE activity; PFOS was adsorbed by SWCNT, which reduced the bioconcentration in zebrafish tissue and enhanced that in skin | Li et al., 2017 [132] |
| 3. | SWCNTs, MWCNTs | a mixture of different-type CNTs, NOM | Danio rerio | 96 h survival, embryo development, oxidative stress, transcriptional effects | embryonic chorions had a stronger barrier to the mixed-type CNTs than to the single-type CNTs, but the presence of NOM weakened this barrier; NOM reduced the antioxidant activity and the expression of genes involved in the antioxidant pathway | Lu and Wang 2023 [133] |
| 4. | MWCNTs | fluoranthene and NOM | Pimephales promelas | 16 h exposure, bioavailability, bioaccumulation | bioavailability of fluoranthene was reduced after adsorption to MWNTs, from 60% to 90% of the fluoranthene was adsorbed to the MWNTs; fluoranthene was not desorbed from ingested MWCNTs; NOM influenced the adsorption of fluoranthene to MWNTs | Linard et al., 2014 [134] |
| Studies used mussels or clams as test-model | ||||||
| 5. | GN | TPP | Mytilus galloprovincialis | 7 d exposure, gene expression, enzyme activity | TPP adsorption on GN could inhibit the surface activity of GN and reduce tissue damage and oxidative stress; GN in single up-regulated exposure the expression of the stress response, cytoskeleton, and reproductive genes, but these genes were significantly down-regulated after combined exposure | Meng et al., 2019 [135] |
| 6. | GO | B[a]P | Mytilus galloprovincialis | 7 d exposure, bioaccumulation, hemocyte response, enzyme activities in tissues, histopathology | higher joint toxicity due to the “Trojan horse” effect, but bioaccumulation of BaP was reduced by GO nanoplatelets | González-Soto et al., 2023 [136] |
| 7. | C60 | B[a]P | Mytilus galloprovincialis | 72 h exposure, genotoxic and proteomic response | the antagonistic effect at the genotoxic and proteomic level was observed based on a single concentration of C60 (further study is needed); co-exposure caused no difference in bioaccumulation and no Trojan horse effects | Barranger et al., 2019 [137] |
| 8. | C60 | fluoranthene | Mytilus sp. | 72 h exposure, oxidative stress, genotoxicity, histopathology, physiological effects | co-exposure had rather additive than synergistic effects; co-exposure enhanced the levels of DNA strand breaks and elevated total glutathione levels indicating oxidative stress | Al-Subiai et al., 2012 [138] |
| Studies used microalgae as a test-model | ||||||
| 9. | GN, GO, rGO | HA | Chlorella pyrenoidosa | ROS generation | antagonism between HA and all the three types of NMs; the degree of antagonism followed the order rGO > GO > GN; HA reduced membrane damage and in microalgae and NMs–algae heteroaggregation (for rGO and G) | Zhao et al., 2019 [139] |
| № | CNMs | Co-Contaminant | Species | Toxicity Endpoints | Observed Effects | Reference |
|---|---|---|---|---|---|---|
| Studies used fish as a test-model | ||||||
| 1. | C60, SWCNTs, MWCNTs, GO, GN | As (III) | Danio rerio | 96 h acute exposure, As accumulation, biochemical responses | GO and GN elevated accumulation and toxicity of As (III) in D. rerio, while the effect was marginal for co-exposure to SWCNTs, MWCNTs, and C60 | Wang et al., 2021 [140] |
| 2. | C60, SWCNTs, MWCNTs, GO, GN | As (V) | Danio rerio | 96 h acute exposure, As accumulation, biochemical responses | C60 reduced the toxicity of As(V) probably due to coating As(V) ion channels and inhibition of total As accumulation; MWCNTs demonstrated a similar C60 effect, while accumulation and toxicity of As(V) had little or no change in the presence of SWCNTs, GO and GN | Wang et al., 2024 [141] |
| Studies used microalgae and cyanobacteria as test-model | ||||||
| 3. | GN, GO | Five ionic liquids | Scenedesmus obliquus | EC10, EC50 (96 h growth rate inhibition) | additive effect at low concentrations of the mixtures but antagonistic at high concentrations; a combination of GO with ionic liquids had more severe joint toxicity than the binary mixtures with GN; the mechanism of the joint toxicity may be associated with the adsorption capability of the graphenes for the ionic liquids | Wang et al., 2017 [57] |
| 4. | GO | As (III), As (V) | Chlorella pyrenoidosa | 72 h EC50 (growth rate inhibition), ROS generation, membrane damage | a synergistic toxic effect between GO and As (III, V); even at environmental concentrations of As (III, V), the adsorption capacity of GO for As (III) was higher than As (V) | Cao et al., 2019 [142] |
| 5. | GO | biologically treated wastewater | Chlamydomonas reinhardtii | 72 h-EC50 (growth rate), esterase activity, cytoplasmic membrane potential, ROS generation | the antagonistic effect; joint exposure significantly reduced cytotoxicity due to the adsorption of toxic chemicals on the surface of GO nanoparticles and to the higher aggregation of GO in wastewater | Martín-de-Lucía et al., 2018 [143] |
| 6. | GO | FLO, ETM, OFL, CTC | Synechocystis sp. | 96 h exposure, ROS quenching, membrane permeability, malondialdehyde analysis, proteomic analysis | additive effect with FLO, antagonistic effect with ETM, OFL, and CTC; combined exposure groups revealed increased membrane permeability due to down-regulation of the proteins related to perceiving and transmitting the signals of hyperosmotic stress | You et al., 2022 [144] |
| 7. | GO | GOQD, C-SWCNT | Microcystis aeruginosa | 72 h, 7 d growth inhibition, ROS generation, metabolomic response | antagonistic action of the GO+C-SWCNT mixtures and synergistic action for the GO+GOQD mixture; a hormetic effect on microalgae proliferation was observed for GOQD and the GO+GOQD mixture | Zhao et al., 2023 [145] |
| 8. | CNTs | CAP, TC | Synechocystis sp. | 96 h acute exposure, ROS generation | additive effect in CNTs+CAP co-exposure; CNTs mitigated the inhibition effect of CAP on protein biosynthesis, while CAP enhanced the up-regulation of proteins induced by CNTs; antagonistic effect in CNTs+TC exposure due to the strong adsorption and catalytic degradation of TC by CNTs | You et al., 2021 [146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pikula, K.; Johari, S.A.; Santos-Oliveira, R.; Golokhvast, K. Joint Toxicity and Interaction of Carbon-Based Nanomaterials with Co-Existing Pollutants in Aquatic Environments: A Review. Int. J. Mol. Sci. 2024, 25, 11798. https://doi.org/10.3390/ijms252111798
Pikula K, Johari SA, Santos-Oliveira R, Golokhvast K. Joint Toxicity and Interaction of Carbon-Based Nanomaterials with Co-Existing Pollutants in Aquatic Environments: A Review. International Journal of Molecular Sciences. 2024; 25(21):11798. https://doi.org/10.3390/ijms252111798
Chicago/Turabian StylePikula, Konstantin, Seyed Ali Johari, Ralph Santos-Oliveira, and Kirill Golokhvast. 2024. "Joint Toxicity and Interaction of Carbon-Based Nanomaterials with Co-Existing Pollutants in Aquatic Environments: A Review" International Journal of Molecular Sciences 25, no. 21: 11798. https://doi.org/10.3390/ijms252111798
APA StylePikula, K., Johari, S. A., Santos-Oliveira, R., & Golokhvast, K. (2024). Joint Toxicity and Interaction of Carbon-Based Nanomaterials with Co-Existing Pollutants in Aquatic Environments: A Review. International Journal of Molecular Sciences, 25(21), 11798. https://doi.org/10.3390/ijms252111798









