Various Strategies of Tendon Stem/Progenitor Cell Reprogramming for Tendon Regeneration
Abstract
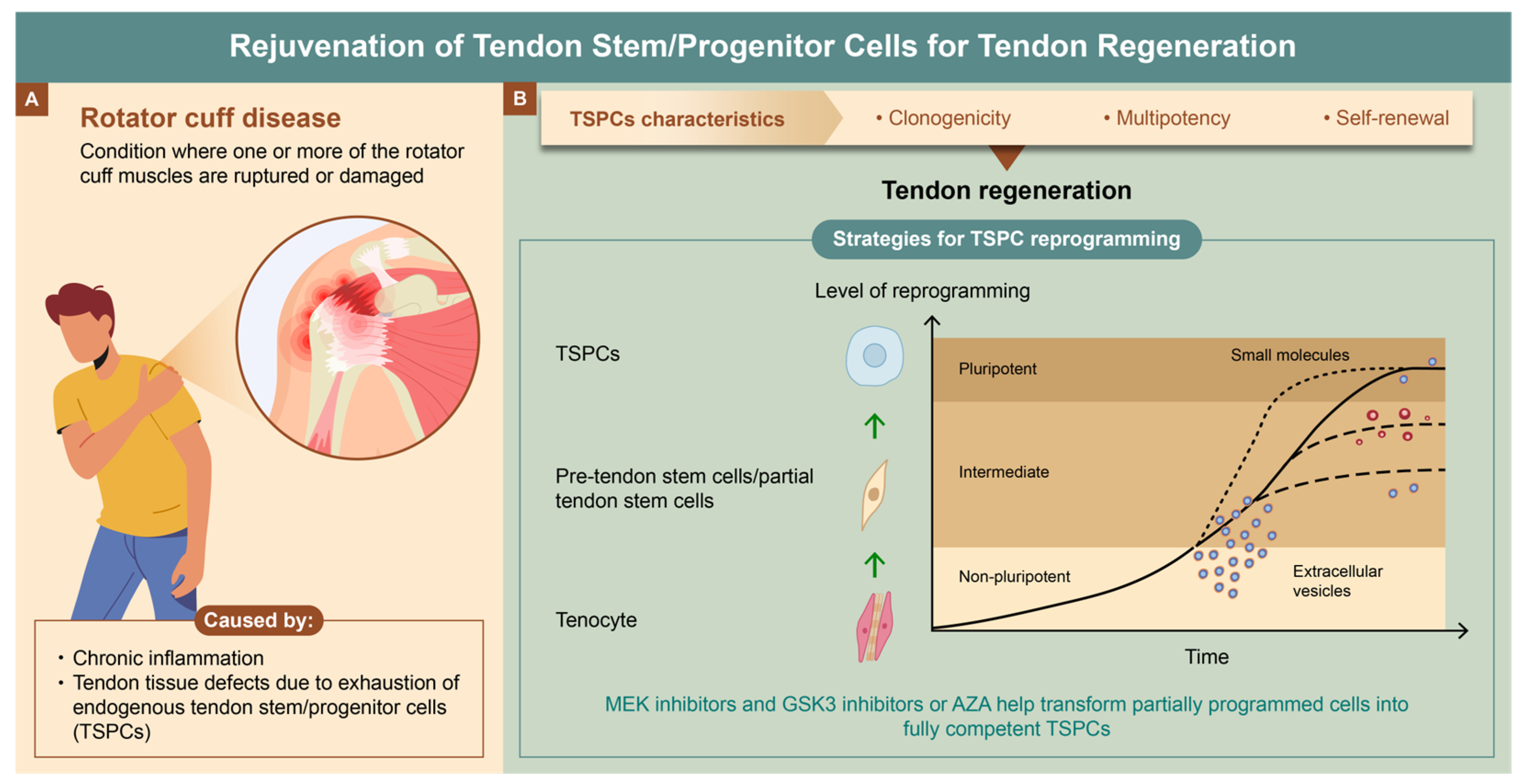
1. Introduction
2. Causes of Rotator Cuff Disease
3. Identification and Characterization of Tenocytes and TSPCs
4. Novel TSPC Markers
5. Various Strategies for TSPC Reprogramming
5.1. Part A: Transcription Factors
5.2. Part B: Small Molecules
5.3. Part C: Extracellular Vesicles
5.4. Part D: Fetal MSCs
6. Discussion
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urwin, M.; Symmons, D.; Allison, T.; Brammah, T.; Busby, H.; Roxby, M.; Simmons, A.; Williams, G. Estimating the burden of musculoskeletal disorders in the community: The comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Ann. Rheum. Dis. 1998, 57, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Chard, M.D.; Hazleman, B.L. Shoulder disorders in the elderly (a hospital study). Ann. Rheum. Dis. 1987, 46, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Chard, M.D.; Hazleman, R.; Hazleman, B.L.; King, R.H.; Reiss, B.B. Shoulder disorders in the elderly: A community survey. Arthritis Rheum. 1991, 34, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Park, J.H.; Cheong, H.K. Prevalence of musculoskeletal symptoms related with activities of daily living and contributing factors in Korean adults. J. Prev. Med. Public Health 2013, 46, 39–49. [Google Scholar] [CrossRef]
- Thorpe, C.T.; Screen, H.R. Tendon structure and composition. Adv. Exp. Med. Biol. 2016, 920, 3–10. [Google Scholar]
- Kannus, P. Structure of the tendon connective tissue. Scand. J. Med. Sci. Sports 2000, 10, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.M.; Zhang, L.; et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Q.; Wang, K.; Ma, C.; Liu, H.; Liu, Y.; Guan, W. Isolation, culture and biological characteristics of multipotent porcine tendon-derived stem cells. Int. J. Mol. Med. 2018, 41, 3611–3619. [Google Scholar] [CrossRef]
- Rui, Y.F.; Lui, P.P.; Li, G.; Fu, S.C.; Lee, Y.W.; Chan, K.M. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng. Part A 2010, 16, 1549–1558. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.H. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet. Disord. 2010, 11, 10. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Akinbiyi, T.; Xu, L.; Ramcharan, M.; Leong, D.J.; Ros, S.J.; Colvin, A.C.; Schaffler, M.B.; Majeska, R.J.; Flatow, E.L.; et al. Tendon-derived stem/progenitor cell aging: Defective self-renewal and altered fate. Aging Cell 2010, 9, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Rui, Y.F.; Lui, P.P.; Wong, Y.M.; Tan, Q.; Chan, K.M. Altered fate of tendon-derived stem cells isolated from a failed tendon-healing animal model of tendinopathy. Stem Cells Dev. 2013, 22, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Dunkman, A.A.; Buckley, M.R.; Mienaltowski, M.J.; Adams, S.M.; Thomas, S.J.; Satchell, L.; Kumar, A.; Pathmanathan, L.; Beason, D.P.; Iozzo, R.V.; et al. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol. 2013, 32, 3–13. [Google Scholar] [CrossRef]
- Thornton, G.M.; Lemmex, D.B.; Ono, Y.; Beach, C.J.; Reno, C.R.; Hart, D.A.; Lo, I.K. Aging affects mechanical properties and lubricin/PRG4 gene expression in normal ligaments. J. Biomech. 2015, 48, 3306–3311. [Google Scholar] [CrossRef]
- Conboy, I.M.; Conboy, M.J.; Smythe, G.M.; Rando, T.A. Notch-mediated restoration of regenerative potential to aged muscle. Science 2003, 302, 1575–1577. [Google Scholar] [CrossRef]
- Conboy, I.M.; Rando, T.A. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev. Cell 2002, 3, 397–409. [Google Scholar] [CrossRef]
- Iakova, P.; Awad, S.S.; Timchenko, N.A. Aging reduces proliferative capacities of liver by switching pathways of EBPα growth arrest. Cell 2003, 113, 495–506. [Google Scholar] [CrossRef]
- Sano, H.; Ishii, H.; Yeadon, A.; Backman, D.S.; Brunet, J.A.; Uhthoff, H.K. Degeneration at the insertion weakens the tensile strength of the supraspinatus tendon: A comparative mechanical and histologic study of the bone-tendon complex. J. Orthop. Res. 1997, 15, 719–726. [Google Scholar] [CrossRef]
- Dakin, S.G.; Martinez, F.O.; Yapp, C.; Wells, G.; Oppermann, U.; Dean, B.J.; Smith, R.D.; Wheway, K.; Watkins, B.; Roche, L.; et al. Inflammation activation and resolution in human tendon disease. Sci. Transl. Med. 2015, 7, 311ra173. [Google Scholar] [CrossRef]
- Abraham, A.C.; Shah, S.A.; Thomopoulos, S. Targeting inflammation in rotator cuff tendon degeneration and repair. Tech. Shoulder Elb. Surg. 2017, 18, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Camernik, K.; Mihelic, A.; Mihalic, R.; Haring, G.; Herman, S.; Marolt Presen, D.; Janez, A.; Trebse, R.; Marc, J.; Zupan, J. Comprehensive analysis of skeletal muscle- and bone-derived mesenchymal stem/stromal cells in patients with osteoarthritis and femoral neck fracture. Stem Cell. Res. Ther. 2020, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Howell, K.; Chien, C.; Bell, R.; Laudier, D.; Tufa, S.F.; Keene, D.R.; Andarawis-Puri, N.; Huang, A.H. Novel model of tendon regeneration reveals distinct cell mechanisms underlying regenerative and fibrotic tendon healing. Sci. Rep. 2017, 7, 45238. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Angele, P.; Jarvinen, T.A.H.; Docheva, D. Rescue plan for Achilles: Therapeutics steering the fate and functions of stem cells in tendon wound healing. Adv. Drug Deliv. Rev. 2018, 129, 352–375. [Google Scholar] [CrossRef]
- Kohler, J.; Popov, C.; Klotz, B.; Alberton, P.; Prall, W.C.; Haasters, F.; Muller-Deubert, S.; Ebert, R.; Klein-Hitpass, L.; Jakob, F.; et al. Uncovering the cellular and molecular changes in tendon stem/progenitor cells attributed to tendon aging and degeneration. Aging Cell 2013, 12, 988–999. [Google Scholar] [CrossRef]
- Li, Y.; Dai, G.; Shi, L.; Lin, Y.; Chen, M.; Li, G.; Rui, Y. The potential roles of tendon stem/progenitor cells in tendon aging. Curr. Stem Cell Res. Ther. 2019, 14, 34–42. [Google Scholar] [CrossRef]
- Walia, B.; Huang, A.H. Tendon stem progenitor cells: Understanding the biology to inform therapeutic strategies for tendon repair. J. Orthop. Res. 2019, 37, 1270–1280. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Y.; Liu, W.; Shan, Q.; Buonocore, S.D.; Cui, L. Bridging tendon defects using autologous tenocyte engineered tendon in a hen model. Plast. Reconstr. Surg. 2002, 110, 1280–1289. [Google Scholar]
- Wang, B.; Liu, W.; Zhang, Y.; Jiang, Y.; Zhang, W.J.; Zhou, G.; Cui, L.; Cao, Y. Engineering of extensor tendon complex by an ex vivo approach. Biomaterials 2008, 29, 2954–2961. [Google Scholar] [CrossRef]
- Chen, B.; Wang, B.; Zhang, W.J.; Zhou, G.; Cao, Y.; Liu, W. In vivo tendon engineering with skeletal muscle derived cells in a mouse model. Biomaterials 2012, 33, 6086–6097. [Google Scholar] [CrossRef]
- Lui, P.P.; Chan, K.M. Tendon-derived stem cells (TDSCs): From basic science to potential roles in tendon pathology and tissue engineering applications. Stem Cell Rev. Rep. 2011, 7, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Mienaltowski, M.J.; Adams, S.M.; Birk, D.E. Regional differences in stem cell/progenitor cell populations from the mouse achilles tendon. Tissue Eng. Part A 2013, 19, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Docheva, D.; Muller, S.A.; Majewski, M.; Evans, C.H. Biologics for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef]
- Tempfer, H.; Wagner, A.; Gehwolf, R.; Lehner, C.; Tauber, M.; Resch, H.; Bauer, H.C. Perivascular cells of the supraspinatus tendon express both tendon- and stem cell-related markers. Histochem. Cell Biol. 2009, 131, 733–741. [Google Scholar] [CrossRef]
- Dex, S.; Lin, D.; Shukunami, C.; Docheva, D. Tenogenic modulating insider factor: Systematic assessment on the functions of tenomodulin gene. Gene 2016, 587, 1–17. [Google Scholar] [CrossRef]
- Lovati, A.B.; Corradetti, B.; Lange Consiglio, A.; Recordati, C.; Bonacina, E.; Bizzaro, D.; Cremonesi, F. Characterization and differentiation of equine tendon-derived progenitor cells. J. Biol. Regul. Homeost. Agents 2011, 25, S75–S84. [Google Scholar]
- Yang, J.; Zhao, Q.; Wang, K.; Liu, H.; Ma, C.; Huang, H.; Liu, Y. Isolation and biological characterization of tendon-derived stem cells from fetal bovine. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 846–856. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, F.Y.; Tarafder, S.; Kao, K.; Jun, Y.; Yang, G.; Mao, J.J. Harnessing endogenous stem/progenitor cells for tendon regeneration. J. Clin. Invest. 2015, 125, 2690–2701. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.H.; Kim, O.S.; Park, E.Y.; Kim, B.J.; Lee, J.H.; Kang, S.B.; Lee, J.H.; Han, H.S.; Rhee, S.H.; Yoon, K.S. Fetal mesenchymal stem cells derived from human umbilical cord sustain primitive characteristics during extensive expansion. Cell Tissue Res. 2008, 334, 423–433. [Google Scholar] [CrossRef]
- Staverosky, J.A.; Pryce, B.A.; Watson, S.S.; Schweitzer, R. Tubulin polymerization-promoting protein family member 3, Tppp3, is a specific marker of the differentiating tendon sheath and synovial joints. Dev. Dyn. 2009, 238, 685–692. [Google Scholar] [CrossRef]
- Harvey, T.; Flamenco, S.; Fan, C.M. A Tppp3+Pdgfra+ tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis. Nat. Cell Biol. 2019, 21, 1490–1503. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Hu, J.J.; Yang, L.; Zheng, Z.F.; An, C.R.; Wu, B.B.; Zhang, C.; Shen, W.L.; Liu, H.H.; Chen, J.L.; et al. Single-cell analysis reveals a nestin+ tendon stem/progenitor cell population with strong tenogenic potentiality. Sci. Adv. 2016, 2, e1600874. [Google Scholar] [CrossRef]
- Lin, D.; Alberton, P.; Caceres, M.D.; Volkmer, E.; Schieker, M.; Docheva, D. Tenomodulin is essential for prevention of adipocyte accumulation and fibrovascular scar formation during early tendon healing. Cell Death Dis. 2017, 8, e3116. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Ohnishi, K.; Semi, K.; Yamamoto, T.; Shimizu, M.; Tanaka, A.; Mitsunaga, K.; Okita, K.; Osafune, K.; Arioka, Y.; Maeda, T.; et al. Premature termination of reprogramming in vivo leads to cancer development through altered epigenetic regulation. Cell 2014, 156, 663–677. [Google Scholar] [CrossRef]
- Mosteiro, L.; Pantoja, C.; Alcazar, N.; Marion, R.M.; Chondronasiou, D.; Rovira, M.; Fernandez-Marcos, P.J.; Munoz-Martin, M.; Blanco-Aparicio, C.; Pastor, J.; et al. Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science 2016, 354, aaf4445. [Google Scholar] [CrossRef] [PubMed]
- Papp, B.; Plath, K. Epigenetics of reprogramming to induced pluripotency. Cell 2013, 152, 1324–1343. [Google Scholar] [CrossRef] [PubMed]
- Cevallos, R.R.; Edwards, Y.J.K.; Parant, J.M.; Yoder, B.K.; Hu, K. Human transcription factors responsive to initial reprogramming predominantly undergo legitimate reprogramming during fibroblast conversion to iPSCs. Sci. Rep. 2020, 10, 19710. [Google Scholar] [CrossRef]
- Saitoh, I.; Sato, M.; Kiyokawa, Y.; Inada, E.; Iwase, Y.; Ibano, N.; Noguchi, H. Induced Tissue-Specific Stem Cells (iTSCs): Their generation and possible use in regenerative medicine. Pharmaceutics 2021, 13, 780. [Google Scholar] [CrossRef]
- Sarkar, T.J.; Quarta, M.; Mukherjee, S.; Colville, A.; Paine, P.; Doan, L.; Tran, C.M.; Chu, C.R.; Horvath, S.; Qi, L.S.; et al. Transient non-integrative expression of nuclear reprogramming factors promotes multifaceted amelioration of aging in human cells. Nat. Commun. 2020, 11, 1545. [Google Scholar] [CrossRef]
- Racila, D.; Winter, M.; Said, M.; Tomanek-Chalkley, A.; Wiechert, S.; Eckert, R.L.; Bickenbach, J.R. Transient expression of OCT4 is sufficient to allow human keratinocytes to change their differentiation pathway. Gene Ther. 2011, 18, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Ring, K.L.; Tong, L.M.; Balestra, M.E.; Javier, R.; Andrews-Zwilling, Y.; Li, G.; Walker, D.; Zhang, W.R.; Kreitzer, A.C.; Huang, Y. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell 2012, 11, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Nemajerova, A.; Kim, S.Y.; Petrenko, O.; Moll, U.M. Two-factor reprogramming of somatic cells to pluripotent stem cells reveals partial functional redundancy of Sox2 and Klf4. Cell Death Differ. 2012, 19, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shi, Y.; Ding, S. A chemical approach to stem-cell biology and regenerative medicine. Nature 2008, 453, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Ng, J.H.; Heng, J.C.; Ng, H.H. Molecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cells. Cell Stem Cell 2009, 4, 301–312. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Yin, X.; Yang, W.; Du, Y.; Hou, P.; Ge, J.; Liu, C.; Zhang, W.; Zhang, X.; et al. Generation of iPSCs from mouse fibroblasts with a single gene, Oct4, and small molecules. Cell Res. 2011, 21, 196–204. [Google Scholar] [CrossRef]
- Hou, P.; Li, Y.; Zhang, X.; Liu, C.; Guan, J.; Li, H.; Zhao, T.; Ye, J.; Yang, W.; Liu, K.; et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science 2013, 341, 651–654. [Google Scholar] [CrossRef]
- Yang, J.H.; Petty, C.A.; Dixon-McDougall, T.; Lopez, M.V.; Tyshkovskiy, A.; Maybury-Lewis, S.; Tian, X.; Ibrahim, N.; Chen, Z.; Griffin, P.T.; et al. Chemically induced reprogramming to reverse cellular aging. Aging 2023, 15, 5966–5989. [Google Scholar] [CrossRef]
- Li, X.; Liu, D.; Ma, Y.; Du, X.; Jing, J.; Wang, L.; Xie, B.; Sun, D.; Sun, S.; Jin, X.; et al. Direct reprogramming of fibroblasts via a chemically induced XEN-like state. Cell Stem Cell 2017, 21, 264–273.e7. [Google Scholar] [CrossRef]
- Shi, Y.; Do, J.T.; Desponts, C.; Hahm, H.S.; Scholer, H.R.; Ding, S. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell 2008, 2, 525–528. [Google Scholar] [CrossRef]
- Shi, Y.; Desponts, C.; Do, J.T.; Hahm, H.S.; Scholer, H.R.; Ding, S. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell 2008, 3, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, T.S.; Hanna, J.; Zhang, X.; Ku, M.; Wernig, M.; Schorderet, P.; Bernstein, B.E.; Jaenisch, R.; Lander, E.S.; Meissner, A. Dissecting direct reprogramming through integrative genomic analysis. Nature 2008, 454, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, D.; Maehr, R.; Guo, W.; Eijkelenboom, A.; Snitow, M.; Chen, A.E.; Melton, D.A. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat. Biotechnol. 2008, 26, 795–797. [Google Scholar] [CrossRef]
- Huangfu, D.; Osafune, K.; Maehr, R.; Guo, W.; Eijkelenboom, A.; Chen, S.; Muhlestein, W.; Melton, D.A. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat. Biotechnol. 2008, 26, 1269–1275. [Google Scholar] [CrossRef]
- Silva, J.; Barrandon, O.; Nichols, J.; Kawaguchi, J.; Theunissen, T.W.; Smith, A. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008, 6, e253. [Google Scholar] [CrossRef]
- Li, W.; Wei, W.; Zhu, S.; Zhu, J.; Shi, Y.; Lin, T.; Hao, E.; Hayek, A.; Deng, H.; Ding, S. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell 2009, 4, 16–19. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, T.; Guan, J.; Zhang, X.; Fu, Y.; Ye, J.; Zhu, J.; Meng, G.; Ge, J.; Yang, S.; et al. A XEN-like state bridges somatic cells to pluripotency during chemical reprogramming. Cell 2015, 163, 1678–1691. [Google Scholar] [CrossRef]
- Guan, J.; Wang, G.; Wang, J.; Zhang, Z.; Fu, Y.; Cheng, L.; Meng, G.; Lyu, Y.; Zhu, J.; Li, Y.; et al. Chemical reprogramming of human somatic cells to pluripotent stem cells. Nature 2022, 605, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Conboy, I.M.; Conboy, M.J.; Wagers, A.J.; Girma, E.R.; Weissman, I.L.; Rando, T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005, 433, 760–764. [Google Scholar] [CrossRef]
- Liu, S.; Mahairaki, V.; Bai, H.; Ding, Z.; Li, J.; Witwer, K.W.; Cheng, L. Highly purified human extracellular vesicles produced by stem cells alleviate aging cellular phenotypes of senescent human cells. Stem Cells 2019, 37, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Liu, S.; Lim, M.; Zhao, S.; Cui, K.; Zhang, K.; Wang, L.; Ji, Q.; Han, Z.; et al. Embryonic stem cell-derived extracellular vesicles enhance the therapeutic effect of mesenchymal stem cells. Theranostics 2019, 9, 6976–6990. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Cheng, J.; Shi, W.; Ren, B.; Zhao, F.; Shi, Y.; Yang, P.; Duan, X.; Zhang, J.; Fu, X.; et al. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater. 2020, 106, 328–341. [Google Scholar] [CrossRef]
- Khanh, V.C.; Yamashita, T.; Ohneda, K.; Tokunaga, C.; Kato, H.; Osaka, M.; Hiramatsu, Y.; Ohneda, O. Rejuvenation of mesenchymal stem cells by extracellular vesicles inhibits the elevation of reactive oxygen species. Sci. Rep. 2020, 10, 17315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhu, J.; Ma, Q.; Zhao, Y.; Wang, Y.; Hu, X.; Chen, J.; Zhu, W.; Han, Z.; Yu, H. Exosomes derived from human umbilical cord MSCs rejuvenate aged MSCs and enhance their functions for myocardial repair. Stem Cell Res. Ther. 2020, 11, 273. [Google Scholar] [CrossRef]
- van der Windt, D.A.; Koes, B.W.; Boeke, A.J.; Deville, W.; De Jong, B.A.; Bouter, L.M. Shoulder disorders in general practice: Prognostic indicators of outcome. Br. J. Gen. Pract. 1996, 46, 519–523. [Google Scholar]
- Yao, X.; Wei, W.; Wang, X.; Chenglin, L.; Bjorklund, M.; Ouyang, H. Stem cell derived exosomes: microRNA therapy for age-related musculoskeletal disorders. Biomaterials 2019, 224, 119492. [Google Scholar] [CrossRef]
- Yu, H.; Yuan, Y.; Shen, H.; Cheng, T. Hematopoietic stem cell exhaustion impacted by p18 INK4C and p21 Cip1/Waf1 in opposite manners. Blood 2006, 107, 1200–1206. [Google Scholar] [CrossRef]
- Singh, S.; Jakubison, B.; Keller, J.R. Protection of hematopoietic stem cells from stress-induced exhaustion and aging. Curr. Opin. Hematol. 2020, 27, 225–231. [Google Scholar] [CrossRef]
- Hashimoto, M.; Kamphorst, A.O.; Im, S.J.; Kissick, H.T.; Pillai, R.N.; Ramalingam, S.S.; Araki, K.; Ahmed, R. CD8 T cell exhaustion in chronic infection and cancer: Opportunities for interventions. Annu. Rev. Med. 2018, 69, 301–318. [Google Scholar] [CrossRef]
- Monsel, A.; Zhu, Y.G.; Gennai, S.; Hao, Q.; Liu, J.; Lee, J.W. Cell-based therapy for acute organ injury: Preclinical evidence and ongoing clinical trials using mesenchymal stem cells. Anesthesiology 2014, 121, 1099–1121. [Google Scholar] [CrossRef] [PubMed]
- Revuelta, M.; Matheu, A. Autophagy in stem cell aging. Aging Cell 2017, 16, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Heinemeier, K.M.; Schjerling, P.; Heinemeier, J.; Magnusson, S.P.; Kjaer, M. Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb 14C. FASEB J. 2013, 27, 2074–2079. [Google Scholar] [CrossRef] [PubMed]
- Heinemeier, K.M.; Schjerling, P.; Ohlenschlaeger, T.F.; Eismark, C.; Olsen, J.; Kjaer, M. Carbon-14 bomb pulse dating shows that tendinopathy is preceded by years of abnormally high collagen turnover. FASEB J. 2018, 32, 4763–4775. [Google Scholar] [CrossRef] [PubMed]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef]
- Weiss, C.; Kornicka-Grabowska, K.; Mularczyk, M.; Siwinska, N.; Marycz, K. Extracellular microvesicles (MV’s) isolated from 5-azacytidine-and-resveratrol-treated cells improve viability and ameliorate endoplasmic reticulum stress in metabolic syndrome derived mesenchymal stem cells. Stem Cell Rev. Rep. 2020, 16, 1343–1355. [Google Scholar] [CrossRef]

| No. | Author /Year/Journal | Title | Chemical Used | Details | Yamanaka Factor Used | Species and Cell Type | Inference | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Shi et al./2008a/Cell Stem Cell | A combined chemical and genetic approach for the generation of induced pluripotent stem cells | BIX01294 | G9a histone methyltransferase inhibitor | OK | mouse neural progenitor cells (mNPCs) | OK+BIX01294 enhances efficiency ~1.5 times more than OSKM and ~8 times more than OK; BIX01294 is able to replace S and M. | [60] |
| BIX01294 | G9a histone methyltransferase inhibitor | KSM | fetal neural progenitor cells (fNPCs) | BIX01294 is able to replace O in NPC reprogramming but with extremely low efficiency. | ||||
| 2 | Shi et al./2008b/Cell Stem Cell | Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds | BIX01294 | G9a histone methyltransferase inhibitor | OK | mouse embryonic fibroblasts (MEFs) | OK+BIX01294 enhances efficiency ~5 times more than OK and is able to replace S. | [61] |
| BayK8644 | L-type calcium channel agonist | OK | MEFs | OK+BIX01294+BayK8644 enhances efficiency ~15 times more than OK. | ||||
| RG108 | DNA methyltransferase (DNMT) inhibitor | OK | MEFs | OK+BIX01294+RG108 enhances reprogramming efficiency ~30 times more than OK. | ||||
| 3 | Mikkelsen et al./2008/Nature | Dissecting direct reprogramming through integrative genomic analysis | AZA | DNMT inhibitor | OSKM | MEFs | AZA treatment during days 8–10 resulted in a ~4-fold increase in efficiency compared with untreated controls. | [62] |
| 4 | Huangfu et al./2008a/Nature Biotechnology | Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds | VPA | histone deacetylase (HDAC) inhibitor | OSKM | MEFs | More than 100-fold increase in efficiency with OSKM. | [63] |
| AZA | DNMT inhibitor | OSK | MEFs | ~3-fold increase in efficiency with OSK. | ||||
| VPA | HDAC inhibitor | OSK | MEFs | ~50-fold increase in efficiency with OSK. | ||||
| Dexamethasone (dex) | synthetic glucocorticoid | OSKM | MEFs | Improved the effect of 5′-azaC by 2.6-fold when used in combination, even though dex alone had no significant effect. | ||||
| TSA | HDAC inhibitor | OSKM | MEFs | ~15-fold increase in efficiency with OSKM. | ||||
| SAHA | HDAC inhibitor | OSKM | MEFs | ~2-fold increase in efficiency with OSKM. | ||||
| 5 | Huangfu et al./2008b/Nature Biotechnology | Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2 | VPA | HDAC inhibitor | OSK | human fibroblasts | 10- to 20-fold increase compared with OSK (reprogramming efficiency 1.1%). | [64] |
| VPA | HDAC inhibitor | OS | human fibroblasts | VPA is able to replace K and M (reprogramming efficiency 0.001%). | ||||
| 6 | Silva et al./2008/PLOS Biology | Promotion of reprogramming to ground state pluripotency by signal inhibition | PD0325901 + CHIR99021 (2i) | inhibitors of MEK and GSK3, respectively | OK | MEFs | Together with LIF, it promotes ground state pluripotency in OK pre-iPSCs | [65] |
| 7 | Li W et al./2009/Cell Stem Cell | Generation of rat and human-induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors | PD0325901 + CHIR99021 (2i) + A-83-01 | Inhibitors of MEK, GSK3, and TGF-b1(ALK5), respectively | OSK | rat liver epithelial cells | Together with LIF and 2i, they generate mESC-like rat iPSCs | [66] |
| PD0325901 + CHIR99021 (2i) + A-83-01 | inhibitor of MEK, GSK3, and TGF-b1(ALK5) respectively | OSK | human fibroblasts | Together with LIF and 2i, they generate mESC-like human iPSCs | ||||
| 8 | Li et al./2011/Cell Research | Generation of iPSCs from mouse fibroblasts with a single gene, Oct4, and small molecules | VC6T | VPA, CHIR99021, 616452, tranylcypromine | O | mouse fibroblasts | A specific chemical combination that is sufficient to permit reprogramming from mouse embryonic and adult fibroblasts in the presence of a single transcription factor (Oct4) within 20 days, replacing Sox2, Klf4, and c-Myc. | [56] |
| 9 | Hou et al./2013/Science | Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds | VC6TF | VPA, CHIR99021, 616452, tranylcypromine, forskolin | None | Oct4 promoter-driven GFP expression (OG)-MEFs | A GFP-positive cluster was generated using VC6TF on day 20 (D20) after chemical treatment. The expression of two pluripotency-related genes, Sall4 and Sox2, and the expression of several extraembryonic endoderm (XEN) markers, Gata4, Gata6, and Sox17 were significantly induced by VC6TF. | [57] |
| VC6TFZ | VPA, CHIR99021, 616452, tranylcypromine, forskolin, DZNep | None | OG-MEFs | Morphology of a compact, epithelioid, GFP-positive colony on day 32 (D32) after treatment | ||||
| VC6TFZ with 2i-medium | VC6TFZ + 2i-medium | None | OG-MEFs | 2i-competent, ESC-like, and GFP-positive cells obtained as chemically induced pluripotent stem cells (CiPSCs). | ||||
| 10 | Zhao et al./2015/Cell | A XEN-like state bridges somatic cells to pluripotency during chemical reprogramming | VC6TFZASD with N2B27-2iL | VPA, CHIR99021, 616452, tranylcypromine, forskolin, DZNep, AM580, SGC0946, 5-aza-dC + N2B27-2i medium + LIF | None | MEFs | The XEN-like state allows us to identify small-molecule boosters and establish a robust chemical reprogramming system with a yield ~1000-fold greater than that of the previously reported protocol. | [67] |
| 11 | Li X et al./2017/Cell Stem Cell | Direct reprogramming of fibroblasts via achemically induced XEN-like state | VC6TFAE | VPA, TD114-2/CHIR99021, 616452, tranylcypromine, forskolin, AM580, EPZ004777 | None | MEFs, mouse postnatal fibroblasts (NBFs), and mouse adult lung fibroblasts (MAFs) | Functional neurons and hepatocytes can be induced from fibroblasts via a chemically induced and highly expandable XEN-like state, bypassing the pluripotent stage.Chemical induction increases the expression of XEN master genes (Gata4, Sall4, Sox17, and Gata6). | [59] |
| 12 | Guan et al./2022/Nature | Chemical reprogramming of human somatic cells to pluripotent stem cells | C6NYSA | CHIR99021, 616452, TTNPB, Y27632, SAG, ABT869 | None | human embryonic fibroblasts (HEFs) | A cocktail of small molecules (CHIR99021, 616452, and TTNPB) converts human fibroblasts into epithelial-like cells. Additional small molecules (Y27632, ABT869, and SAG) further promoted the formation of epithelial-like cells. | [68] |
| 13 | Yang et al./2023/Aging | Chemically induced reprogramming to reverse cellular aging | VC6TF | VPA, CHIR99021, 616452, tranylcypromine, forskolin | None | mouse fibroblasts | Rejuvenation through age reversal can be achieved not only genetically but also chemically.Within a week, a cocktail of six chemicals succeeded in restoring the whole-genome transcriptional profile characteristic of youth and reversed transcriptional age without compromising cellular identity. | [58] |
| C6NYSA | CHIR99021, 616452, TTNPB, Y27632, SAG, ABT869 | None | human fibroblasts |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, S.Y. Various Strategies of Tendon Stem/Progenitor Cell Reprogramming for Tendon Regeneration. Int. J. Mol. Sci. 2024, 25, 11745. https://doi.org/10.3390/ijms252111745
Ahn SY. Various Strategies of Tendon Stem/Progenitor Cell Reprogramming for Tendon Regeneration. International Journal of Molecular Sciences. 2024; 25(21):11745. https://doi.org/10.3390/ijms252111745
Chicago/Turabian StyleAhn, Sung Yong. 2024. "Various Strategies of Tendon Stem/Progenitor Cell Reprogramming for Tendon Regeneration" International Journal of Molecular Sciences 25, no. 21: 11745. https://doi.org/10.3390/ijms252111745
APA StyleAhn, S. Y. (2024). Various Strategies of Tendon Stem/Progenitor Cell Reprogramming for Tendon Regeneration. International Journal of Molecular Sciences, 25(21), 11745. https://doi.org/10.3390/ijms252111745








