A Prospective Observational Study on Analyzing Lung Cancer Gene Mutation Variant Allele Frequency (VAF) and Its Correlation with Treatment Efficacy
Abstract
1. Introduction
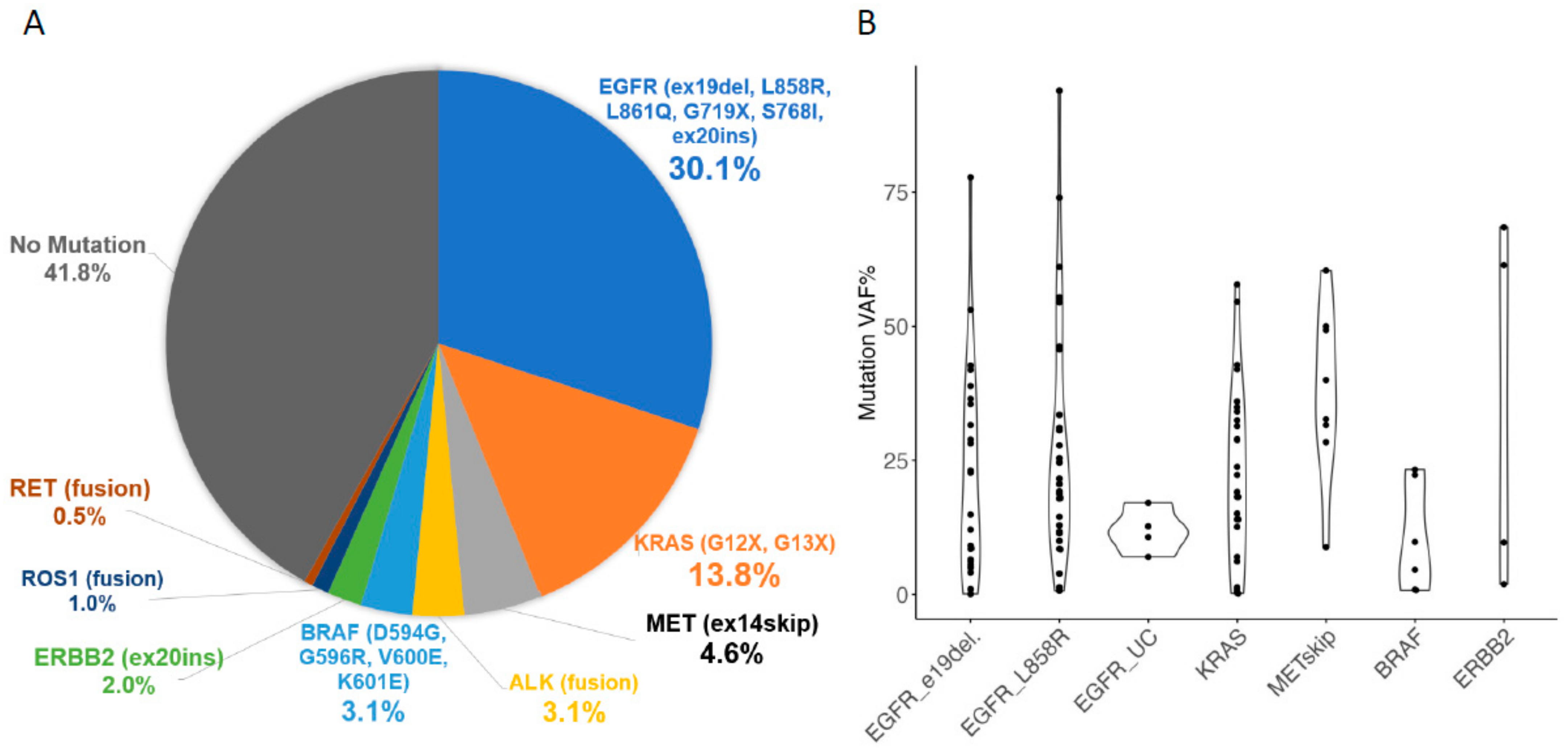
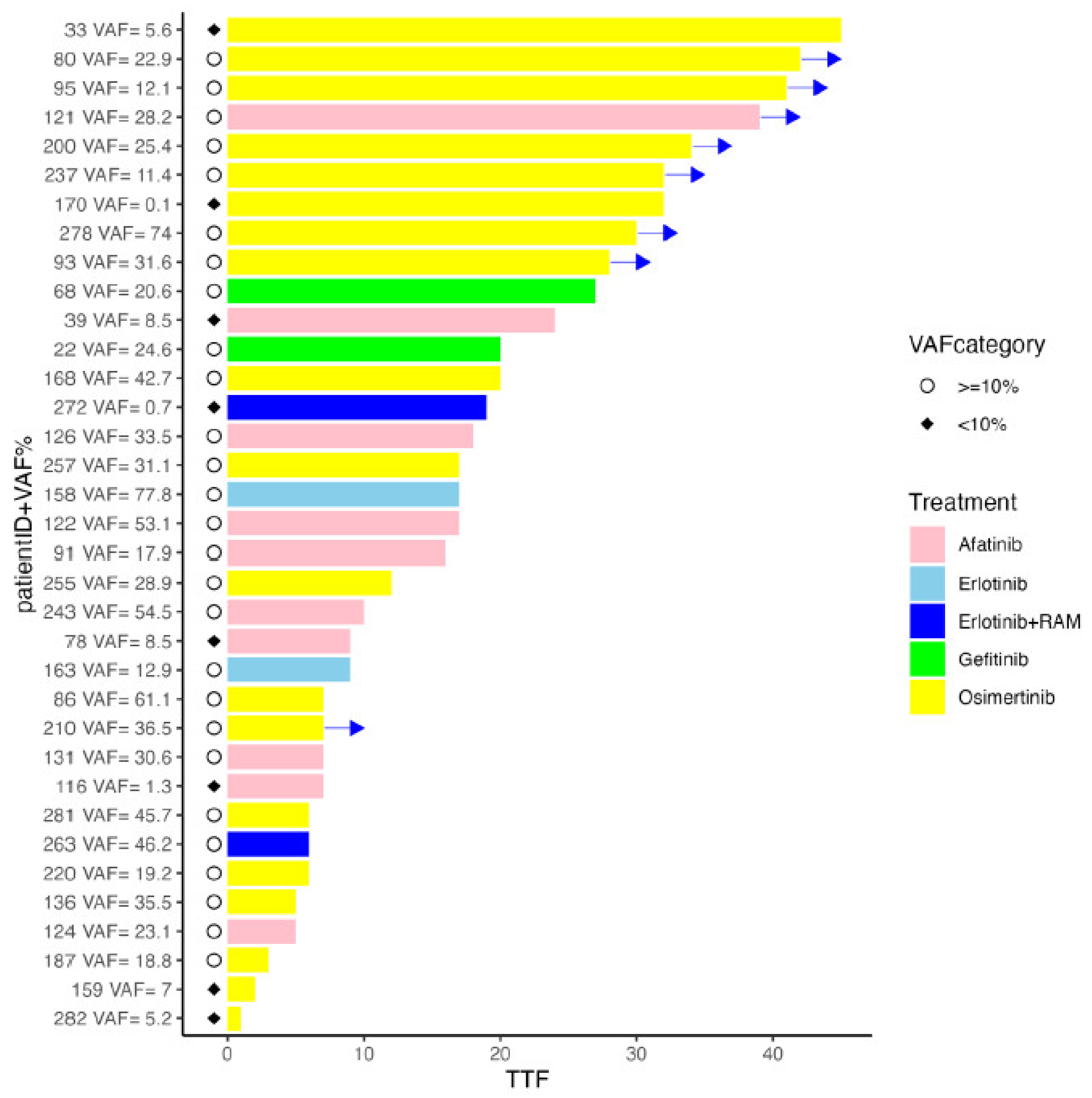
2. Results
3. Discussion
4. Materials and Methods
4.1. Aim and Study Design
4.2. Patient Selection
4.3. Diagnostic Procedures
4.4. Cytology Specimen Collection
4.5. Mutation VAF Analysis Using Lung Cancer Compact PanelTM (LCCP)
4.6. Outcome Assessments: Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kris, M.G.; Johnson, B.E.; Berry, L.D.; Kwiatkowski, D.J.; Iafrate, A.J.; Wistuba, I.I.; Varella-Garcia, M.; Franklin, W.A.; Aronson, S.L.; Su, P.-F.; et al. Using Multiplexed Assays of Oncogenic Drivers in Lung Cancers to Select Targeted Drugs. JAMA 2014, 311, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, V.; Tarazona, N.; Cejalvo, J.M.; Lombardi, P.; Huerta, M.; Roselló, S.; Fleitas, T.; Roda, D.; Cervantes, A. Personalized Medicine: Recent Progress in Cancer Therapy. Cancers 2020, 12, 1009. [Google Scholar] [CrossRef] [PubMed]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.; Barlesi, F.; Lolkema, M.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- FDA. Summary of safety and effectiveness data for Oncomine Dx Target Test. 2017. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160045B.pdf (accessed on 27 March 2022).
- Sakaguchi, T.; Iketani, A.; Furuhashi, K.; Nakamura, Y.; Suzuki, Y.; Ito, K.; Fujiwara, K.; Nishii, Y.; Katsuta, K.; Taguchi, O.; et al. Comparison of the analytical performance between the Oncomine Dx Target Test and a conventional single gene test for epidermal growth factor receptor mutation in non-small cell lung cancer. Thorac. Cancer 2020, 12, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Kunimasa, K.; Matsumoto, S.; Kawamura, T.; Inoue, T.; Tamiya, M.; Kanzaki, R.; Maniwa, T.; Okami, J.; Honma, K.; Goto, K.; et al. Clinical application of the AMOY 9-in-1 panel to lung cancer patients. Lung Cancer 2023, 179, 107190. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, K.; Iinuma, M.; Shinozaki, Y.; Nishine, H.; Inoue, T.; Mineshita, M. A case of advanced adenocarcinoma genetically confirmed with EGFR/BRAF co-mutation in both primary and metastatic lesions. Ther. Adv. Med. Oncol. 2021, 13, 17588359211053420. [Google Scholar] [CrossRef] [PubMed]
- Ariyasu, R.; Nishikawa, S.; Uchibori, K.; Oh-Hara, T.; Yoshizawa, T.; Dotsu, Y.; Koyama, J.; Saiki, M.; Sonoda, T.; Kitazono, S.; et al. High ratio of T790M to EGFR activating mutations correlate with the osimertinib response in non-small-cell lung cancer. Lung Cancer 2018, 117, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, Y.; Tian, P.; Wang, W.; Wang, K.; Chuai, S.; Li, Y.; Zhao, S.; Wang, Y.; Li, W. Low T790M relative allele frequency indicates concurrent resistance mechanisms and poor responsiveness to osimertinib. Transl. Lung Cancer Res. 2020, 9, 1952–1962. [Google Scholar] [CrossRef] [PubMed]
- Buder, A.; Hochmair, M.J.; Filipits, M. The Allele Frequency of EGFR Mutations Predicts Survival in Advanced EGFR T790M-Positive Non-small Cell Lung Cancer Patients Treated with Osimertinib. Target. Oncol. 2021, 16, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Okami, J.; Nakamura, H.; Honma, K.; Sato, Y.; Nakamura, S.; Kukita, Y.; Nakatsuka, S.-I.; Higashiyama, M. Analytical Performance of a Highly Sensitive System to Detect Gene Variants Using Next-Generation Sequencing for Lung Cancer Companion Diagnostics. Diagnostics 2023, 13, 1476. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, K.; Kida, H.; Handa, H.; Inoue, T.; Saji, H.; Koike, J.; Nakamura, S.; Sato, Y.; Ueda, Y.; Suzuki, F.; et al. A Prospective Validation Study of Lung Cancer Gene Panel Testing Using Cytological Specimens. Cancers 2022, 14, 3784. [Google Scholar] [CrossRef] [PubMed]
- Questionnaire Survey on the Appropriateness of Biomarker Testing Based on EGFR Gene Mutation Rates (First Report). Available online: https://www.haigan.gr.jp/uploads/files/EGFR%E9%81%BA%E4%BC%9D%E5%AD%90%E5%A4%89%E7%95%B0%E6%A4%9C%E5%87%BA%E7%8E%87%E3%82%A2%E3%83%B3%E3%82%B1%E3%83%BC%E3%83%88%E3%81%AE%E5%A0%B1%E5%91%8AVer.1.pdf (accessed on 12 August 2024).
- Reportable Variants for Each Companion Diagnostic method (Created in April 2024). Available online: https://www.haigan.gr.jp/Uploads/files/%28%E4%BB%98%E8%A1%A8%29%20%E5%90%84%E3%82%B3%E3%83%B3%E3%83%91%E3%83%8B%E3%82%AA%E3%83%B3%E8%A8%BA%E6%96%AD%E6%B3%95%E3%81%AB%E3%81%8A%E3%81%91%E3%82%8B%E5%A0%B1%E5%91%8A%E5%AF%BE%E8%B1%A1%E3%83%90%E3%83%AA%E3%82%A2%E3%83%B3%E3%83%88%20%282024%E5%B9%B44%E6%9C%88%E4%BD%9C%E6%88%90%29%282%29.pdf (accessed on 12 August 2024).
- Yatabe, Y.; Kerr, K.M.; Utomo, A.; Rajadurai, P.; Tran, V.K.; Du, X.; Chou, T.-Y.; Enriquez, M.L.D.; Lee, G.K.; Iqbal, J.; et al. EGFR Mutation Testing Practices within the Asia Pacific Region: Results of a Multicenter Diagnostic Survey. J. Thorac. Oncol. 2015, 10, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; González, Á.; Tomás, A.J.C.; Fariñas, S.C.; Ferrero, M.; Mirda, D.; Sirera, R.; Jantus-Lewintre, E.; Camps, C. A profile on cobas® EGFR Mutation Test v2 as companion diagnostic for first-line treatment of patients with non-small cell lung cancer. Expert. Rev. Mol. Diagn. 2020, 20, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Gefitinib or Chemotherapy for Non–Small-Cell Lung Cancer with Mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef] [PubMed]
- Mitsudomi, T.; Morita, S.; Yatabe, Y.; Negoro, S.; Okamoto, I.; Tsurutani, J.; Seto, T.; Satouchi, M.; Tada, H.; Hirashima, T.; et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2009, 11, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as 1st line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomized phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef] [PubMed]
| Patient Charasteristics | n = 196 | (%) | Pathological Diagnosis | Cases | (%) |
|---|---|---|---|---|---|
| Sex | Adenocarcinoma | 154 | (78.6) | ||
| Male | 71 | (36.2) | Squamous cell carcinoma | 33 | (16.8) |
| Female | 125 | (63.7) | Not otherwise specified, other | 9 | (4.6) |
| Median age (range) | 72 | (26–90) | LCCP Mutation Detection | Cases | |
| EGFR | 59 | ||||
| KRAS G12X G13X | 27 | ||||
| Diagnostic Procedure | ALK | 6 | |||
| EBUS-TBB | 135 | (68.9) | BRAF | 6 | |
| EBUS-TBNA, EUS-FNA | 25 | (12.8) | MET exon14 skipping | 9 | |
| CT/US-guided puncture | 15 | (7.7) | RET | 1 | |
| Pleural effusion | 5 | (2.6) | ERBB2 | 4 | |
| Other | 16 | (8.2) | ROS1 | 2 |
| Sample ID | Histological Type | EGFR Variant (ODxTT, AmoyMulti, Cobas Not Supported) | Mutation Call | VAF Percentage |
|---|---|---|---|---|
| 60 | Ad | ODxTT-, Amoy-, Cobas+ | EGFR_p.E746_T751delinsIP | 8.6 |
| 67 | Ad | ODxTT-, Amoy-, Cobas+ | EGFR_p.E746_T751delinsIP | 1 |
| 110 | Ad | ODxTT+, Amoy-, Cobas+ | EGFR_p.A767_V769dup | 12.7 |
| 134 | Ad | ODxTT-, Amoy-, Cobas- | EGFR_p.E746_A750delinsQP | 6.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinozaki, Y.; Morikawa, K.; Kida, H.; Handa, H.; Saji, H.; Nakamura, S.; Sato, Y.; Ueda, Y.; Suzuki, F.; Matoba, R.; et al. A Prospective Observational Study on Analyzing Lung Cancer Gene Mutation Variant Allele Frequency (VAF) and Its Correlation with Treatment Efficacy. Int. J. Mol. Sci. 2024, 25, 11694. https://doi.org/10.3390/ijms252111694
Shinozaki Y, Morikawa K, Kida H, Handa H, Saji H, Nakamura S, Sato Y, Ueda Y, Suzuki F, Matoba R, et al. A Prospective Observational Study on Analyzing Lung Cancer Gene Mutation Variant Allele Frequency (VAF) and Its Correlation with Treatment Efficacy. International Journal of Molecular Sciences. 2024; 25(21):11694. https://doi.org/10.3390/ijms252111694
Chicago/Turabian StyleShinozaki, Yusuke, Kei Morikawa, Hirotaka Kida, Hiroshi Handa, Hisashi Saji, Seiji Nakamura, Yoshiharu Sato, Yumi Ueda, Fumihiko Suzuki, Ryo Matoba, and et al. 2024. "A Prospective Observational Study on Analyzing Lung Cancer Gene Mutation Variant Allele Frequency (VAF) and Its Correlation with Treatment Efficacy" International Journal of Molecular Sciences 25, no. 21: 11694. https://doi.org/10.3390/ijms252111694
APA StyleShinozaki, Y., Morikawa, K., Kida, H., Handa, H., Saji, H., Nakamura, S., Sato, Y., Ueda, Y., Suzuki, F., Matoba, R., & Mineshita, M. (2024). A Prospective Observational Study on Analyzing Lung Cancer Gene Mutation Variant Allele Frequency (VAF) and Its Correlation with Treatment Efficacy. International Journal of Molecular Sciences, 25(21), 11694. https://doi.org/10.3390/ijms252111694





