Understanding Endothelial Dysfunction and Its Role in Ischemic Stroke After the Outbreak of Recanalization Therapies
Abstract
1. Introduction
2. The Endothelium and Its Components
2.1. Endothelial Cells
2.2. Nitric Oxide
2.3. ENOS Enzyme
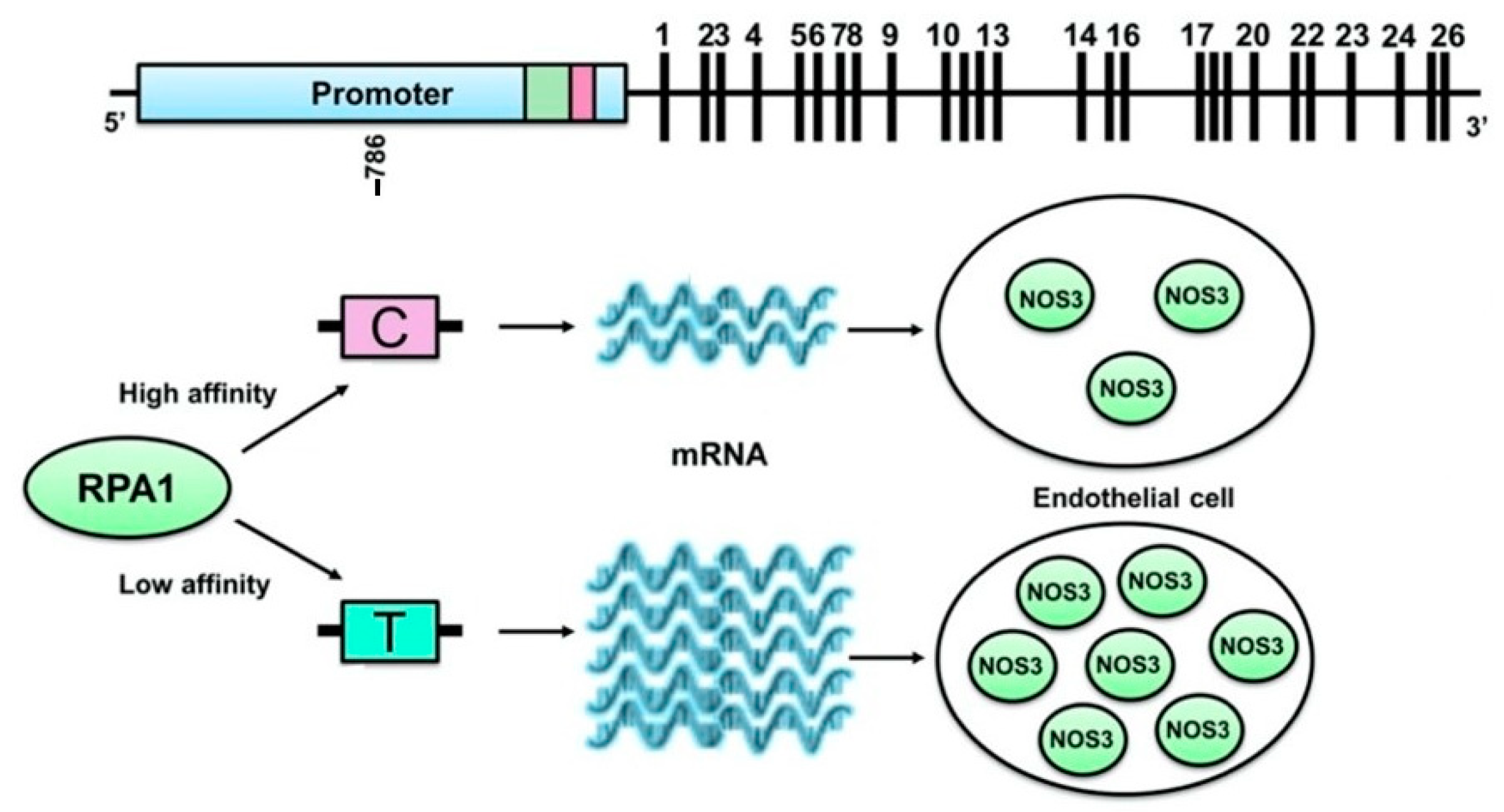
2.4. Other Participants in the Endothelial Function
2.5. Cerebral Endothelium
3. Endothelial Dysfunction (ED)
4. Endothelial Function and Stroke
4.1. Physiopathology of Stroke and Endothelial Function
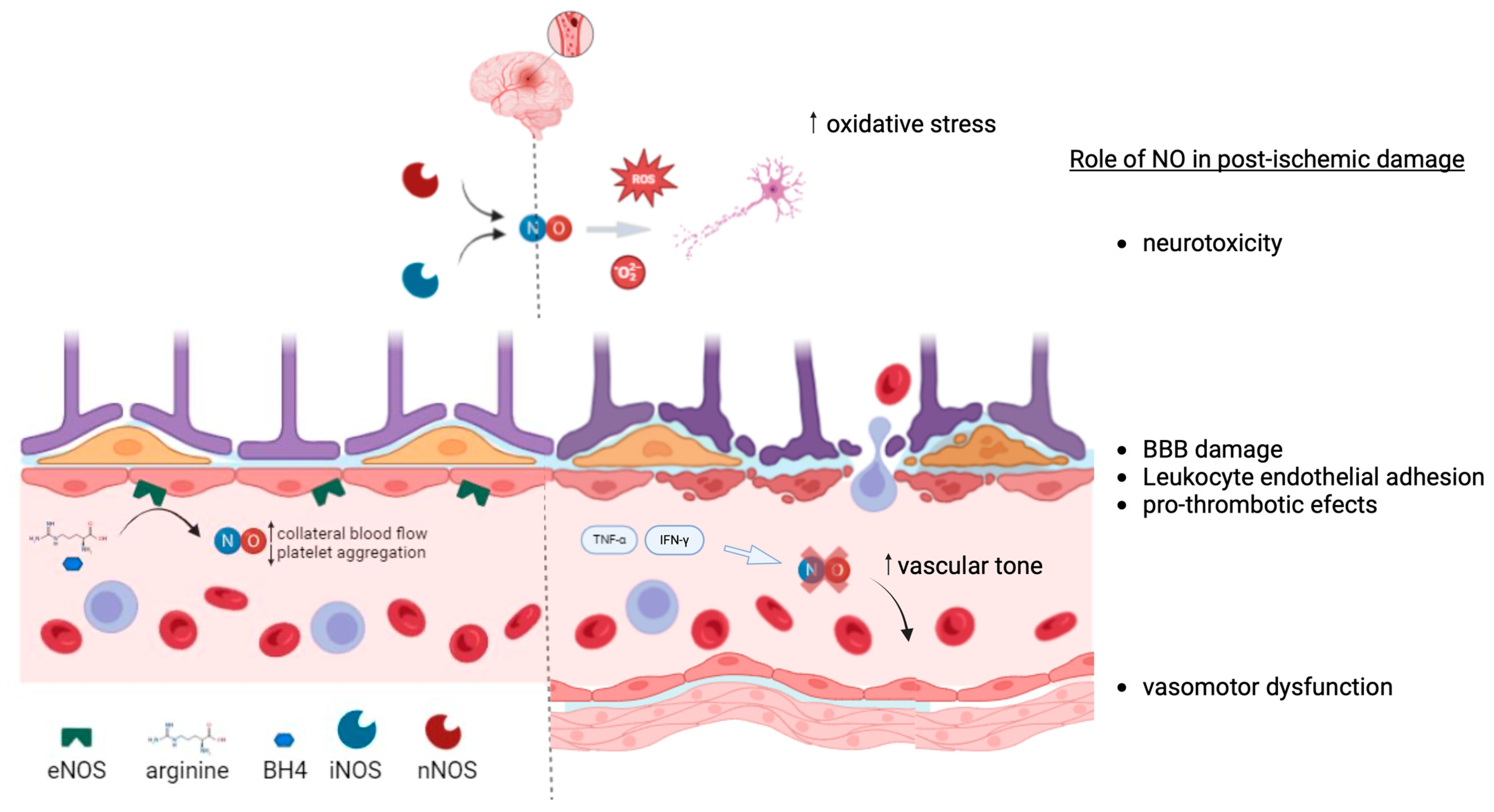
4.2. Endothelial Dysfunction in Ischemic Stroke
4.2.1. The Role of Endothelial Dysfunction in Stroke Initiation
4.2.2. Endothelial Dysfunction in Stroke Subtypes
4.2.3. Endothelial Dysfunction and Stroke Severity and Prognosis
4.3. Reperfusion Therapies and Endothelial Dysfunction
4.4. Collateral Response to Stroke and Endothelial Dysfunction
4.5. Blood–Brain Barrier Disruption, Endothelium, and the Risk of Hemorrhagic Transformation After Stroke
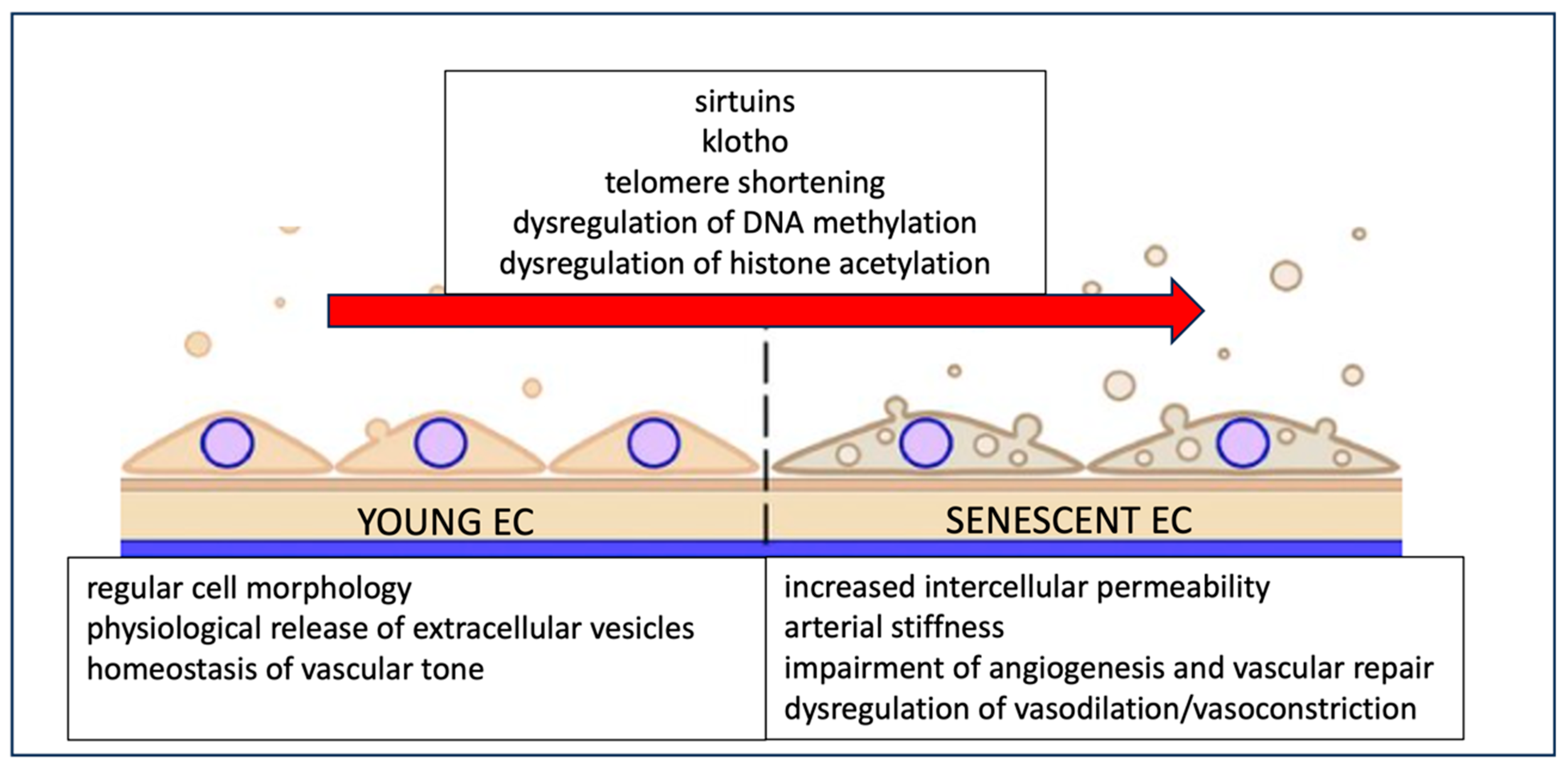
4.6. Age, Stroke, and Endothelial Cell Senescence
5. Therapeutic Approaches Regarding Endothelial Dysfunction and Stroke
- -
- Medical interventions:
- -
- Non-pharmacological strategies
- -
- Cell based therapies
- -
- Lifestyle modification
- -
- Combination therapies
6. Future Perspectives and Unanswered Questions in the Endothelial Dysfunction–Stroke Relationship
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feigin, V.L.; Abate, M.D.; Abate, Y.H.; Abd ElHafeez, S.; Abd-Allah, F.; Abdelalim, A.; Abdelkader, A.; Abdelmasseh, M.; Abd-Elsalam, S.; Abdi, P.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 973–1003. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef] [PubMed]
- Sturtzel, C. Endothelial Cells. Adv. Exp. Med. Biol. 2017, 1003, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Roquer, J.; Segura, T.; Serena, J.; Castillo, J. Endothelial dysfunction, vascular disease and stroke: The ARTICO study. Cerebrovasc. Dis. 2009, 27 (Suppl. S1), 25–37. [Google Scholar] [CrossRef]
- Fisher, M.; Saver, J.L. Future directions of acute ischaemic stroke therapy. Lancet Neurol. 2015, 14, 758–767. [Google Scholar] [CrossRef]
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef]
- Testa, U.; Castelli, G.; Pelosi, E. Role of endothelial progenitor cells in vascular development, homestatic maintenance of blood vessels and in injury-mediated reparative response. Stem Cell Investig. 2020, 7, 7. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK26848 (accessed on 4 September 2024).
- Yoder, M.C. Human endothelial progenitor cells. Cold Spring Harb. Perspect. Med. 2012, 2, a006692. [Google Scholar] [CrossRef]
- Miki, N.; Kawabe, Y.; Kuriyama, K. Activation of cerebral guanylate cyclase by nitric oxide. Biochem. Biophys. Res. Commun. 1977, 75, 851–856. [Google Scholar] [CrossRef]
- Faraci, F.M.; Heistad, D.D. Regulation of the cerebral circulation: Role of endothelium and potassium channels. Physiol. Rev. 1998, 78, 53–97. [Google Scholar] [CrossRef]
- Miller, A.A.; Budzyn, K.; Sobey, C.G. Vascular dysfunction in cerebrovascular disease: Mechanisms and therapeutic intervention. Clin. Sci. 2010, 119, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Cobb, F.R.; Kraus, W.E.; Gow, A.J. Total nitrogen oxide following exercise testing reflects endothelial function and discriminates health status. Free Radic. Biol. Med. 2006, 41, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [CrossRef] [PubMed]
- Fish, J.E.; Marsden, P.A. Endothelial nitric oxide synthase: Insight into cell-specific gene regulation in the vascular endothelium. Cell. Mol. Life Sci. CMLS 2006, 63, 144–162. [Google Scholar] [CrossRef]
- Albrecht, E.W.J.A.; Stegeman, C.A.; Heeringa, P.; Henning, R.H.; van Goor, H. Protective role of endothelial nitric oxide synthase. J. Pathol. 2003, 199, 8–17. [Google Scholar] [CrossRef]
- Atochin, D.N.; Huang, P.L. Endothelial nitric oxide synthase transgenic models of endothelial dysfunction. Pflugers Arch. 2010, 460, 965–974. [Google Scholar] [CrossRef]
- Marsden, P.A.; Heng, H.H.; Scherer, S.W.; Stewart, R.J.; Hall, A.V.; Shi, X.M.; Tsui, L.C.; Schappert, K.T. Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J. Biol. Chem. 1993, 268, 17478–17488. [Google Scholar] [CrossRef]
- Cooke, G.E.; Doshi, A.; Binkley, P.F. Endothelial nitric oxide synthase gene: Prospects for treatment of heart disease. Pharmacogenomics 2007, 8, 1723–1734. [Google Scholar] [CrossRef]
- Metzger, I.F.; Sertório, J.T.C.; Tanus-Santos, J.E. Modulation of nitric oxide formation by endothelial nitric oxide synthase gene haplotypes. Free Radic. Biol. Med. 2007, 43, 987–992. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, X.; Wu, W.; Zhang, D. Endothelial NO synthase gene polymorphisms and risk of ischemic stroke in Asian population: A meta-analysis. PLoS ONE 2013, 8, e60472. [Google Scholar] [CrossRef]
- Nakayama, M.; Yasue, H.; Yoshimura, M.; Shimasaki, Y.; Kugiyama, K.; Ogawa, H.; Motoyama, T.; Saito, Y.; Ogawa, Y.; Miyamoto, Y.; et al. T-786-->C mutation in the 5’-flanking region of the endothelial nitric oxide synthase gene is associated with coronary spasm. Circulation 1999, 99, 2864–2870. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Saito, Y.; Nakayama, M.; Shimasaki, Y.; Yoshimura, T.; Yoshimura, M.; Harada, M.; Kajiyama, N.; Kishimoto, I.; Kuwahara, K.; et al. Replication protein A1 reduces transcription of the endothelial nitric oxide synthase gene containing a -786T-->C mutation associated with coronary spastic angina. Hum. Mol. Genet. 2000, 9, 2629–2637. [Google Scholar] [CrossRef] [PubMed]
- Nagassaki, S.; Sertório, J.T.C.; Metzger, I.F.; Bem, A.F.; Rocha, J.B.T.; Tanus-Santos, J.E. eNOS gene T-786C polymorphism modulates atorvastatin-induced increase in blood nitrite. Free Radic. Biol. Med. 2006, 41, 1044–1049. [Google Scholar] [CrossRef]
- Oliveira-Paula, G.H.; Lacchini, R.; Tanus-Santos, J.E. Endothelial nitric oxide synthase: From biochemistry and gene structure to clinical implications of NOS3 polymorphisms. Gene 2016, 575, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Salvi, E.; Kuznetsova, T.; Thijs, L.; Lupoli, S.; Stolarz-Skrzypek, K.; D’Avila, F.; Tikhonoff, V.; De Astis, S.; Barcella, M.; Seidlerová, J.; et al. Target sequencing, cell experiments, and a population study establish endothelial nitric oxide synthase (eNOS) gene as hypertension susceptibility gene. Hypertension 2013, 62, 844–852. [Google Scholar] [CrossRef]
- Lacchini, R.; Silva, P.S.; Tanus-Santos, J.E. A pharmacogenetics-based approach to reduce cardiovascular mortality with the prophylactic use of statins. Basic Clin. Pharmacol. Toxicol. 2010, 106, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Paula, G.H.; Lacchini, R.; Tanus-Santos, J.E. Clinical and pharmacogenetic impact of endothelial nitric oxide synthase polymorphisms on cardiovascular diseases. Nitric Oxide 2017, 63, 39–51. [Google Scholar] [CrossRef]
- Grosse, G.M.; Schwedhelm, E.; Worthmann, H.; Choe, C.-U. Arginine Derivatives in Cerebrovascular Diseases: Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2020, 21, 1798. [Google Scholar] [CrossRef]
- Pretnar-Oblak, J.; Sabovic, M.; Zaletel, M. Associations between systemic and cerebral endothelial impairment determined by cerebrovascular reactivity to L-arginine. Endothelium 2007, 14, 73–80. [Google Scholar] [CrossRef]
- Chia, P.Y.; Teo, A.; Yeo, T.W. Overview of the Assessment of Endothelial Function in Humans. Front. Med. 2020, 7, 542567. [Google Scholar] [CrossRef]
- Flammer, A.J.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfield, J.; Ganz, P.; Hamburg, N.M.; Lüscher, T.F.; Shechter, M.; Taddei, S.; et al. The Assessment of Endothelial Function: From Research into Clinical Practice. Circulation 2012, 126, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Moens, A.L.; Goovaerts, I.; Claeys, M.J.; Vrints, C.J. Flow-mediated vasodilation: A diagnostic instrument, or an experimental tool? Chest 2005, 127, 2254–2263. [Google Scholar] [CrossRef]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Gokce, N.; Keaney, J.F.; Hunter, L.M.; Watkins, M.T.; Menzoian, J.O.; Vita, J.A. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: A prospective study. Circulation 2002, 105, 1567–1572. [Google Scholar] [CrossRef]
- Yeboah, J.; Crouse, J.R.; Hsu, F.-C.; Burke, G.L.; Herrington, D.M. Brachial Flow-Mediated Dilation Predicts Incident Cardiovascular Events in Older Adults: The Cardiovascular Health Study. Circulation 2007, 115, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Axtell, A.L.; Gomari, F.A.; Cooke, J.P. Assessing endothelial vasodilator function with the Endo-PAT 2000. J. Vis. Exp. JoVE 2010, 44, 2167. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Kwon, T.-G.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prognostic Value of Flow-Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2015, 4, e002270. [Google Scholar] [CrossRef]
- Vagal, A.S.; Leach, J.L.; Fernandez-Ulloa, M.; Zuccarello, M. The Acetazolamide Challenge: Techniques and Applications in the Evaluation of Chronic Cerebral Ischemia. Am. J. Neuroradiol. 2009, 30, 876–884. [Google Scholar] [CrossRef]
- Pretnar-Oblak, J. Cerebral endothelial function determined by cerebrovascular reactivity to L-arginine. BioMed Res. Int. 2014, 2014, 601515. [Google Scholar] [CrossRef]
- Karlsson, W.K.; Sørensen, C.G.; Kruuse, C. l-arginine and l-NMMA for assessing cerebral endothelial dysfunction in ischaemic cerebrovascular disease: A systematic review. Clin. Exp. Pharmacol. Physiol. 2017, 44, 13–20. [Google Scholar] [CrossRef]
- Pretnar-Oblak, J.; Sabovic, M.; Vidmar, G.; Zaletel, M. Evaluation of L-arginine reactivity in comparison with flow-mediated dilatation and intima-media thickness. Ultrasound Med. Biol. 2007, 33, 1546–1551. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Huang, P.L.; Ma, J.; Meng, W.; Ayata, C.; Fishman, M.C.; Moskowitz, M.A. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J. Cereb. Blood Flow Metab. 1996, 16, 981–987. [Google Scholar] [CrossRef]
- Iadecola, C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997, 20, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, M.J.L.; Huang, Z.; Ferrante, R.J.; Sasamata, M.; Molliver, M.E.; Snyder, S.H.; Moskowitz, M.A. Neuronal Nitric Oxide Synthase Activation and Peroxynitrite Formation in Ischemic Stroke Linked to Neural Damage. J. Neurosci. 1999, 19, 5910–5918. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, D.; Kendall, M.J. Nitric oxide in acute ischaemic stroke: A target for neuroprotection. J. Neurol. Neurosurg. Psychiatry 1999, 67, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Ohkubo, T.; Asano, Y.; Hattori, K.; Shimazu, T.; Yamazato, M.; Nagoya, H.; Kato, Y.; Araki, N. Nitric Oxide Production during Cerebral Ischemia and Reperfusion in eNOS- and nNOS-Knockout Mice. Curr. Neurovasc. Res. 2010, 7, 23–31. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Rundek, T.; Hundle, R.; Ratchford, E.; Ramas, R.; Sciacca, R.; Di Tullio, M.R.; Boden-Albala, B.; Miyake, Y.; Elkind, M.S.V.; Sacco, R.L.; et al. Endothelial dysfunction is associated with carotid plaque: A cross-sectional study from the population based Northern Manhattan Study. BMC Cardiovasc. Disord. 2006, 6, 35. [Google Scholar] [CrossRef][Green Version]
- Celermajer, D.S.; Sorensen, K.E.; Gooch, V.M.; Spiegelhalter, D.J.; Miller, O.I.; Sullivan, I.D.; Lloyd, J.K.; Deanfield, J.E. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992, 340, 1111–1115. [Google Scholar] [CrossRef]
- Kozera, G.M.; Dubaniewicz, M.; Zdrojewski, T.; Madej-Dmochowska, A.; Mielczarek, M.; Wojczal, J.; Chwojnicki, K.; Swierblewska, E.; Schminke, U.; Wyrzykowski, B.; et al. Cerebral vasomotor reactivity and extent of white matter lesions in middle-aged men with arterial hypertension: A pilot study. Am. J. Hypertens. 2010, 23, 1198–1203. [Google Scholar] [CrossRef][Green Version]
- Kumar, A.; Misra, S.; Kumar, P.; Prasad, K.; Pandit, A.K.; Chakravarty, K.; Kathuria, P.; Gulati, A. Association between endothelial nitric oxide synthase gene polymorphisms and risk of ischemic stroke: A meta-analysis. Neurol. India 2017, 65, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Anlıaçık, S.Ö.; Tokgöz, S.; Zamani, A.G.; Yıldırım, M.S.; İyisoy, M.S. Investigation of the relationship between ischemic stroke and endothelial nitric oxide synthase gene polymorphisms [G894T, intron 4 VNTR and T786C]. Turk. J. Med. Sci. 2019, 49, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Stenborg, A.; Terent, A.; Lind, L. Endothelium-dependent vasodilatation in forearm is impaired in stroke patients. J. Intern. Med. 2006, 259, 569–575. [Google Scholar] [CrossRef]
- Zvan, B.; Zaletel, M.; Pogacnik, T.; Kiauta, T. Testing of cerebral endothelium function with L-arginine after stroke. Int. Angiol. 2002, 21, 256–259. [Google Scholar]
- Scherbakov, N.; Sandek, A.; Martens-Lobenhoffer, J.; Kung, T.; Turhan, G.; Liman, T.; Ebinger, M.; von Haehling, S.; Bode-Böger, S.M.; Endres, M.; et al. Endothelial dysfunction of the peripheral vascular bed in the acute phase after ischemic stroke. Cerebrovasc. Dis. 2012, 33, 37–46. [Google Scholar] [CrossRef]
- Kozuka, K.; Kohriyama, T.; Nomura, E.; Ikeda, J.; Kajikawa, H.; Nakamura, S. Endothelial markers and adhesion molecules in acute ischemic stroke--sequential change and differences in stroke subtype. Atherosclerosis 2002, 161, 161–168. [Google Scholar] [CrossRef]
- Knottnerus, I.L.H.; Ten Cate, H.; Lodder, J.; Kessels, F.; van Oostenbrugge, R.J. Endothelial dysfunction in lacunar stroke: A systematic review. Cerebrovasc. Dis. 2009, 27, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, S.F.; Doubal, F.N.; Shuler, K.; Wardlaw, J.M. A Systematic Review of Dynamic Cerebral and Peripheral Endothelial Function in Lacunar Stroke Versus Controls. Stroke 2010, 41, e434–e442. [Google Scholar] [CrossRef]
- Micieli, G.; Bosone, D.; Zappoli, F.; Marcheselli, S.; Argenteri, A.; Nappi, G. Vasomotor response to CO2 and L-Arginine in patients with severe internal carotid artery stenosis; pre- and post-surgical evaluation with transcranial Doppler. J. Neurol. Sci. 1999, 163, 153–158. [Google Scholar] [CrossRef]
- Chlumský, I.; Charvát, J. Endothelial dysfunction, distensibility and intima-media thickness and aetiology of stroke. J. Int. Med. Res. 2005, 33, 555–561. [Google Scholar] [CrossRef]
- Zupan, M.; Šabović, M.; Zaletel, M.; Popovič, K.Š.; Žvan, B. The presence of cerebral and/or systemic endothelial dysfunction in patients with leukoaraiosis—A case control pilot study. BMC Neurol. 2015, 15, 158. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Hunt, B.J.; O’Sullivan, M.; Parmar, K.; Bamford, J.M.; Briley, D.; Brown, M.M.; Thomas, D.J.; Markus, H.S. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain J. Neurol. 2003, 126, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Ruchoux, M.M.; Maurage, C.A. CADASIL: Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. J. Neuropathol. Exp. Neurol. 1997, 56, 947–964. [Google Scholar] [CrossRef] [PubMed]
- Chabriat, H.; Joutel, A.; Dichgans, M.; Tournier-Lasserve, E.; Bousser, M.-G. Cadasil. Lancet Neurol. 2009, 8, 643–653. [Google Scholar] [CrossRef]
- De Boer, I.; Stam, A.H.; Buntinx, L.; Zielman, R.; van der Steen, I.; van den Maagdenberg, A.M.J.M.; de Koning, E.J.P.; Ferrari, M.D.; de Hoon, J.N.; Terwindt, G.M. RVCL-S and CADASIL display distinct impaired vascular function. Neurology 2018, 91, e956–e963. [Google Scholar] [CrossRef]
- Santos-García, D.; Blanco, M.; Serena, J.; Arias, S.; Millán, M.; Rodríguez-Yáñez, M.; Leira, R.; Dávalos, A.; Castillo, J. Brachial arterial flow mediated dilation in acute ischemic stroke. Eur. J. Neurol. 2009, 16, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Santos-García, D.; Blanco, M.; Serena, J.; Rodríguez-Yáñez, M.; Leira, R.; Castillo, J. Impaired brachial flow-mediated dilation is a predictor of a new-onset vascular event after stroke. Cerebrovasc. Dis. 2011, 32, 155–162. [Google Scholar] [CrossRef]
- Castillo, J.; Rama, R.; Dávalos, A. Nitric oxide-related brain damage in acute ischemic stroke. Stroke 2000, 31, 852–857. [Google Scholar] [CrossRef]
- Hill, J.M.; Zalos, G.; Halcox, J.P.J.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 2003, 348, 593–600. [Google Scholar] [CrossRef]
- Ghani, U.; Shuaib, A.; Salam, A.; Nasir, A.; Shuaib, U.; Jeerakathil, T.; Sher, F.; O’Rourke, F.; Nasser, A.M.; Schwindt, B.; et al. Endothelial progenitor cells during cerebrovascular disease. Stroke 2005, 36, 151–153. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Zhang, L.; Jiang, Q.; Chopp, M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ. Res. 2002, 90, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, J.; He, G.; Qu, F.; Zheng, M. Mobilization of endothelial progenitor cell in patients with acute ischemic stroke. Neurol. Sci. 2018, 39, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Sobrino, T.; Hurtado, O.; Moro, M.A.; Rodríguez-Yáñez, M.; Castellanos, M.; Brea, D.; Moldes, O.; Blanco, M.; Arenillas, J.F.; Leira, R.; et al. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke 2007, 38, 2759–2764. [Google Scholar] [CrossRef] [PubMed]
- Kashiwazaki, D.; Akioka, N.; Kuwayama, N.; Hayashi, T.; Noguchi, K.; Tanaka, K.; Kuroda, S. Involvement of circulating endothelial progenitor cells in carotid plaque growth and vulnerability. J. Neurosurg. 2016, 125, 1549–1556. [Google Scholar] [CrossRef]
- Sashindranath, M.; Nandurkar, H.H. Endothelial Dysfunction in the Brain: Setting the Stage for Stroke and Other Cerebrovascular Complications of COVID-19. Stroke 2021, 52, 1895–1904. [Google Scholar] [CrossRef]
- Haffke, M.; Freitag, H.; Rudolf, G.; Seifert, M.; Doehner, W.; Scherbakov, N.; Hanitsch, L.; Wittke, K.; Bauer, S.; Konietschke, F.; et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2022, 20, 138. [Google Scholar] [CrossRef]
- Powers, W.J.; Derdeyn, C.P.; Biller, J.; Coffey, C.S.; Hoh, B.L.; Jauch, E.C.; Johnston, K.C.; Johnston, S.C.; Khalessi, A.A.; Kidwell, C.S.; et al. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients with Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2015, 46, 3020–3035. [Google Scholar] [CrossRef]
- Nogueira, R.G.; Gupta, R.; Jovin, T.G.; Levy, E.I.; Liebeskind, D.S.; Zaidat, O.O.; Rai, A.; Hirsch, J.A.; Hsu, D.P.; Rymer, M.M.; et al. Predictors and clinical relevance of hemorrhagic transformation after endovascular therapy for anterior circulation large vessel occlusion strokes: A multicenter retrospective analysis of 1122 patients. J. Neurointerv. Surg. 2015, 7, 16–21. [Google Scholar] [CrossRef]
- Chamorro, Á.; Meisel, A.; Planas, A.M.; Urra, X.; van de Beek, D.; Veltkamp, R. The immunology of acute stroke. Nat. Rev. Neurol. 2012, 8, 401–410. [Google Scholar] [CrossRef]
- Khatri, R.; McKinney, A.M.; Swenson, B.; Janardhan, V. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology 2012, 79, S52–S57. [Google Scholar] [CrossRef]
- Renú, A.; Amaro, S.; Laredo, C.; Román, L.S.; Llull, L.; Lopez, A.; Urra, X.; Blasco, J.; Oleaga, L.; Chamorro, Á. Relevance of blood-brain barrier disruption after endovascular treatment of ischemic stroke: Dual-energy computed tomographic study. Stroke 2015, 46, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Doostkam, S.; Reinhard, M.; Ivanovas, V.; Taschner, C.A. Immunohistochemical Analysis of Thrombi Retrieved During Treatment of Acute Ischemic Stroke: Does Stent-Retriever Cause Intimal Damage? Stroke 2013, 44, 1720–1722. [Google Scholar] [CrossRef]
- Sheth, S.A.; Liebeskind, D.S. Imaging Evaluation of Collaterals in the Brain: Physiology and Clinical Translation. Curr. Radiol. Rep. 2014, 2, 29. [Google Scholar] [CrossRef]
- Ichijo, M.; Miki, K.; Ishibashi, S.; Tomita, M.; Kamata, T.; Fujigasaki, H.; Mizusawa, H. Posterior cerebral artery laterality on magnetic resonance angiography predicts long-term functional outcome in middle cerebral artery occlusion. Stroke 2013, 44, 512–515. [Google Scholar] [CrossRef]
- Liebeskind, D.S. Collateral circulation. Stroke 2003, 34, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Seckin, S.; Emrah, B.; Biyik, I.; Emre, A.; Burak, T.; Azmi, S.; Omer, C.; Sinan, D. 786T/c endothelial nitric oxide synthase gene polymorphism and coronary collateral circulation. Adv. Hyg. Exp. Med. 2016, 70, 80–85. [Google Scholar] [CrossRef]
- Beard, D.J.; Li, Z.; Schneider, A.M.; Couch, Y.; Cipolla, M.J.; Buchan, A.M. Rapamycin Induces an eNOS (Endothelial Nitric Oxide Synthase) Dependent Increase in Brain Collateral Perfusion in Wistar and Spontaneously Hypertensive Rats. Stroke 2020, 51, 2834–2843. [Google Scholar] [CrossRef]
- De la Riva, P.; Rodríguez-Antigüedad, J.; Gómez, V.; Arenaza, G.; Gorostidi, A.; Díez, N.; de Arce, A.; Martínez-Zabaleta, M.; González, F.; Luttich, A.; et al. Endothelial NO synthase 786T/T polymorphism increases hemorrhagic transformation after endovascular thrombectomy. Nitric Oxide Biol. Chem. 2022, 129, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Aroor, A.R.; Jia, C.; Sowers, J.R. Endothelial cell senescence in aging-related vascular dysfunction. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2019, 1865, 1802–1809. [Google Scholar] [CrossRef]
- Kamper, A.M.; Spilt, A.; de Craen, A.J.M.; van Buchem, M.A.; Westendorp, R.G.J.; Blauw, G.J. Basal cerebral blood flow is dependent on the nitric oxide pathway in elderly but not in young healthy men. Exp. Gerontol. 2004, 39, 1245–1248. [Google Scholar] [CrossRef]
- Garry, P.S.; Ezra, M.; Rowland, M.J.; Westbrook, J.; Pattinson, K.T.S. The role of the nitric oxide pathway in brain injury and its treatment--from bench to bedside. Exp. Neurol. 2015, 263, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Ankolekar, S.; Fuller, M.; Cross, I.; Renton, C.; Cox, P.; Sprigg, N.; Siriwardena, A.N.; Bath, P.M. Feasibility of an ambulance-based stroke trial, and safety of glyceryl trinitrate in ultra-acute stroke: The rapid intervention with glyceryl trinitrate in Hypertensive Stroke Trial (RIGHT, ISRCTN66434824). Stroke 2013, 44, 3120–3128. [Google Scholar] [CrossRef] [PubMed]
- Bath, P.M.; Pathansali, R.; Iddenden, R.; Bath, F.J. The effect of transdermal glyceryl trinitrate, a nitric oxide donor, on blood pressure and platelet function in acute stroke. Cerebrovasc. Dis. 2001, 11, 265–272. [Google Scholar] [CrossRef] [PubMed]
- RIGHT-2 Investigators. Prehospital transdermal glyceryl trinitrate in patients with ultra-acute presumed stroke (RIGHT-2): An ambulance-based, randomised, sham-controlled, blinded, phase 3 trial. Lancet 2019, 393, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Havard, D. Rapid Intervention with Glyceryl Trinitrate in Hypertensive Stroke Trial-2. Available online: http://www.isrctn.com/ISRCTN26986053 (accessed on 4 January 2024).
- Van Den Berg, S.A.; Uniken Venema, S.M.; Reinink, H.; Hofmeijer, J.; Schonewille, W.J.; Miedema, I.; Fransen, P.S.S.; O Pruissen, D.M.; Raaijmakers, T.W.M.; Van Dijk, G.W.; et al. Prehospital transdermal glyceryl trinitrate in patients with presumed acute stroke (MR ASAP): An ambulance-based, multicentre, randomised, open-label, blinded endpoint, phase 3 trial. Lancet Neurol. 2022, 21, 971–981. [Google Scholar] [CrossRef]
- Cheng, Z.; Gao, J.; Ding, Y.; Pang, Q.; Rajah, G.B.; Geng, X. Arterial Glyceryl Trinitrate in Acute Ischemic Stroke After Thrombectomy for Neuroprotection (AGAIN): A Pilot Randomized Controlled Trial. Neurotherapeutics 2023, 20, 1746–1754. [Google Scholar] [CrossRef]
- Cai, L.; Ding, Y.; Rajah, G.; Tong, Y.; Duan, H.; Han, Z.; Gao, J.; Cheng, Z.; Xin, R.; Jiang, S.; et al. Rapid Intravenous Glyceryl Trinitrate in Ischemic Damage (RIGID): A potential neuroprotection strategy for acute ischemic stroke (AIS) patients. Neurotherapeutics 2024, 21, e00365. [Google Scholar] [CrossRef]
- Morikawa, E.; Rosenblatt, S.; Moskowitz, M.A. L-Arginine dilates rat pial arterioles by nitric oxide-dependent mechanisms and increases blood flow during focal cerebral ischaemia. Br. J. Pharmacol. 1992, 107, 905–907. [Google Scholar] [CrossRef]
- Morikawa, E.; Huang, Z.; Moskowitz, M.A. L-arginine decreases infarct size caused by middle cerebral arterial occlusion in SHR. Am. J. Physiol.-Heart Circ. Physiol. 1992, 263, H1632–H1635. [Google Scholar] [CrossRef]
- Zhao, X.; Ross, M.E.; Iadecola, C. l-Arginine increases ischemic injury in wild-type mice but not in iNOS-deficient mice. Brain Res. 2003, 966, 308–311. [Google Scholar] [CrossRef]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Bendall, J.K.; Douglas, G.; McNeill, E.; Channon, K.M.; Crabtree, M.J. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid. Redox Signal. 2014, 20, 3040–3077. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Hamadate, N.; Matsuzaki, T.; Sakanashi, M.; Nakasone, J.; Sakanashi, M.; Tsutsui, M.; Sakanashi, M. Improvement of impaired endothelial function by tetrahydrobiopterin in stroke-prone spontaneously hypertensive rats. Eur. J. Pharmacol. 2010, 631, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, L.; Zhang, Z.; Wang, Y.; Lu, M.; LaPointe, M.; Chopp, M. A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats. Ann. Neurol. 2001, 50, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ladecola, C. Nitroprusside improves blood flow and reduces brain damage after focal ischemia. NeuroReport 1993, 4, 559–562. [Google Scholar] [CrossRef]
- Salom, J.B.; Ortí, M.; Centeno, J.M.; Torregrosa, G.; Alborch, E. Reduction of infarct size by the NO donors sodium nitroprusside and spermine/NO after transient focal cerebral ischemia in rats. Brain Res. 2000, 865, 149–156. [Google Scholar] [CrossRef]
- Zhang, F.; White, J.G.; Iadecola, C. Nitric Oxide Donors Increase Blood Flow and Reduce Brain Damage in Focal Ischemia: Evidence That Nitric Oxide is Beneficial in the Early Stages of Cerebral Ischemia. J. Cereb. Blood Flow Metab. 1994, 14, 217–226. [Google Scholar] [CrossRef]
- Zhang, F.; Iadecola, C. Reduction of Focal Cerebral Ischemic Damage by Delayed Treatment with Nitric Oxide Donors. J. Cereb. Blood Flow Metab. 1994, 14, 574–580. [Google Scholar] [CrossRef]
- Zhuang, P.; Ji, H.; Zhang, Y.; Min, Z.; Ni, Q.; You, R. ZJM-289, a novel nitric oxide donor, alleviates the cerebral ischaemic–reperfusion injury in rats. Clin. Exp. Pharmacol. Physiol. 2010, 37, e121–e127. [Google Scholar] [CrossRef]
- Martínez-Murillo, R.; Fernández, A.P.; Serrano, J.; Rodrigo, J.; Salas, E.; Mourelle, M.; Martínez, A. The nitric oxide donor LA 419 decreases brain damage in a focal ischemia model. Neurosci. Lett. 2007, 415, 149–153. [Google Scholar] [CrossRef]
- Serrano, J.; Fernández, A.; Martínez-Murillo, R.; Alonso, D.; Rodrigo, J.; Salas, E.; Mourelle, M.; Martínez, A. The nitric oxide donor LA 419 decreases ischemic brain damage. Int. J. Mol. Med. 2007, 19, 229–236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feng, S.; Yang, Q.; Liu, M.; Li, W.; Yuan, W.; Zhang, S.; Wu, B.; Li, J. Edaravone for acute ischaemic stroke. Cochrane Database Syst. Rev. 2011, 12, CD007230. [Google Scholar] [CrossRef]
- Liao, J.K.; Laufs, U. Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 89–118. [Google Scholar] [CrossRef] [PubMed]
- Pretnar-Oblak, J.; Sebestjen, M.; Sabovic, M. Statin treatment improves cerebral more than systemic endothelial dysfunction in patients with arterial hypertension. Am. J. Hypertens. 2008, 21, 674–678. [Google Scholar] [CrossRef]
- Khalil, F.; Badshah, M.; Sathyanarayanan, S.P.; Amin, N. Statins Therapy and Intracranial Hemorrhage. S. D. Med. J. S. D. State Med. Assoc. 2023, 76, 170–173. [Google Scholar]
- Wierońska, J.M.; Cieślik, P.; Kalinowski, L. Nitric Oxide-Dependent Pathways as Critical Factors in the Consequences and Recovery after Brain Ischemic Hypoxia. Biomolecules 2021, 11, 1097. [Google Scholar] [CrossRef]
- Hosseini, M.B.; Saver, J.L. Mechanisms of action of acute and subacute sphenopalatine ganglion stimulation for ischemic stroke. Int. J. Stroke 2020, 15, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, N.M.; Saver, J.L.; Diener, H.C.; Gorelick, P.B.; Shuaib, A.; Solberg, Y.; Thackeray, L.; Savic, M.; Janelidze, T.; Zarqua, N.; et al. An injectable implant to stimulate the sphenopalatine ganglion for treatment of acute ischaemic stroke up to 24 h from onset (ImpACT-24B): An international, randomised, double-blind, sham-controlled, pivotal trial. Lancet 2019, 394, 219–229. [Google Scholar] [CrossRef]
- Sheng, R.; Chen, C.; Chen, H.; Yu, P. Repetitive transcranial magnetic stimulation for stroke rehabilitation: Insights into the molecular and cellular mechanisms of neuroinflammation. Front. Immunol. 2023, 14, 1197422. [Google Scholar] [CrossRef]
- Zong, X.; Li, Y.; Liu, C.; Qi, W.; Han, D.; Tucker, L.; Dong, Y.; Hu, S.; Yan, X.; Zhang, Q. Theta-burst transcranial magnetic stimulation promotes stroke recovery by vascular protection and neovascularization. Theranostics 2020, 10, 12090–12110. [Google Scholar] [CrossRef]
- Dirnagl, U.; Becker, K.; Meisel, A. Preconditioning and tolerance against cerebral ischaemia: From experimental strategies to clinical use. Lancet Neurol. 2009, 8, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, A.; Yano, S.; Morioka, M.; Hamada, J.; Ushio, Y.; Takeuchi, Y.; Fukunaga, K. Up-Regulation of Endothelial Nitric Oxide Synthase via Phosphatidylinositol 3-Kinase Pathway Contributes to Ischemic Tolerance in the CA1 Subfield of Gerbil Hippocampus. J. Cereb. Blood Flow Metab. 2004, 24, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Park, E.-M.; Zhou, P.; Frys, K.; Ross, M.E.; Iadecola, C. Obligatory role of inducible nitric oxide synthase in ischemic preconditioning. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2005, 25, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Atochin, D.N.; Clark, J.; Demchenko, I.T.; Moskowitz, M.A.; Huang, P.L. Rapid cerebral ischemic preconditioning in mice deficient in endothelial and neuronal nitric oxide synthases. Stroke 2003, 34, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Yokomizo, S.; Kopp, T.; Roessing, M.; Morita, A.; Lee, S.; Cho, S.; Ogawa, E.; Komai, E.; Inoue, K.; Fukushi, M.; et al. Near-Infrared II Photobiomodulation Preconditioning Ameliorates Stroke Injury via Phosphorylation of eNOS. Stroke 2024, 55, 1641–1649. [Google Scholar] [CrossRef]
- Kadir, R.R.A.; Alwjwaj, M.; Bayraktutan, U. Treatment with outgrowth endothelial cells protects cerebral barrier against ischemic injury. Cytotherapy 2022, 24, 489–499. [Google Scholar] [CrossRef]
- Moubarik, C.; Guillet, B.; Youssef, B.; Codaccioni, J.-L.; Piercecchi, M.-D.; Sabatier, F.; Lionel, P.; Dou, L.; Foucault-Bertaud, A.; Velly, L.; et al. Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Rev. Rep. 2011, 7, 208–220. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Zhang, R.; Zhang, S.; Feng, H.; Kong, Z.; Aiziretiaili, N.; Luo, Z.; Cai, Q.; Hong, Y.; et al. Adiponectin-Transfected Endothelial Progenitor Cells Have Protective Effects After 2-Hour Middle-Cerebral Artery Occlusion in Rats with Type 2 Diabetes Mellitus. Front. Neurol. 2021, 12, 630681. [Google Scholar] [CrossRef]
- Li, Y.; Chang, S.; Li, W.; Tang, G.; Ma, Y.; Liu, Y.; Yuan, F.; Zhang, Z.; Yang, G.-Y.; Wang, Y. cxcl12-engineered endothelial progenitor cells enhance neurogenesis and angiogenesis after ischemic brain injury in mice. Stem Cell Res. Ther. 2018, 9, 139. [Google Scholar] [CrossRef]
- Carenza, E.; Barceló, V.; Morancho, A.; Levander, L.; Boada, C.; Laromaine, A.; Roig, A.; Montaner, J.; Rosell, A. In vitro angiogenic performance and in vivo brain targeting of magnetized endothelial progenitor cells for neurorepair therapies. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 225–234. [Google Scholar] [CrossRef]
- Fang, J.; Guo, Y.; Tan, S.; Li, Z.; Xie, H.; Chen, P.; Wang, K.; He, Z.; He, P.; Ke, Y.; et al. Autologous Endothelial Progenitor Cells Transplantation for Acute Ischemic Stroke: A 4-Year Follow-Up Study. Stem Cells Transl. Med. 2019, 8, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Rosell, A.; Morancho, A.; Navarro-Sobrino, M.; Martínez-Saez, E.; Hernández-Guillamon, M.; Lope-Piedrafita, S.; Barceló, V.; Borrás, F.; Penalba, A.; García-Bonilla, L.; et al. Factors secreted by endothelial progenitor cells enhance neurorepair responses after cerebral ischemia in mice. PLoS ONE 2013, 8, e73244. [Google Scholar] [CrossRef] [PubMed]
- Mizuma, A.; Yamashita, T.; Kono, S.; Nakayama, T.; Baba, Y.; Itoh, S.; Asakura, K.; Niimi, Y.; Asahi, T.; Kanemaru, K.; et al. Phase II Trial of Intravenous Low-Dose Granulocyte Colony-Stimulating Factor in Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2016, 25, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Yoshizumi, M. Exercise and endothelial function: Role of endothelium-derived nitric oxide and oxidative stress in healthy subjects and hypertensive patients. Pharmacol. Ther. 2004, 102, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Chen, Y.; Zhen, K.; Ren, S.; Lv, Y.; Yu, L. Effect of continuous aerobic exercise on endothelial function: A systematic review and meta-analysis of randomized controlled trials. Front. Physiol. 2023, 14, 1043108. [Google Scholar] [CrossRef]
- Goto, C.; Higashi, Y.; Kimura, M.; Noma, K.; Hara, K.; Nakagawa, K.; Kawamura, M.; Chayama, K.; Yoshizumi, M.; Nara, I. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: Role of endothelium-dependent nitric oxide and oxidative stress. Circulation 2003, 108, 530–535. [Google Scholar] [CrossRef]
- Sabouri, M.; Amirshaghaghi, F.; Hesari, M.M. High-intensity interval training improves the vascular endothelial function comparing moderate-intensity interval training in overweight or obese adults: A meta-analysis. Clin. Nutr. ESPEN 2023, 53, 100–106. [Google Scholar] [CrossRef]
- Iwamoto, E.; Bock, J.M.; Casey, D.P. High-Intensity Exercise Enhances Conduit Artery Vascular Function in Older Adults. Med. Sci. Sports Exerc. 2018, 50, 124–130. [Google Scholar] [CrossRef]
- Fatima, K.; Rashid, A.M.; Memon, U.A.A.; Fatima, S.S.; Javaid, S.S.; Shahid, O.; Zehri, F.; Obaid, M.A.; Ahmad, M.; Almas, T.; et al. Mediterranean Diet and its Effect on Endothelial Function: A Meta-analysis and Systematic Review. Ir. J. Med. Sci. 2023, 192, 105–113. [Google Scholar] [CrossRef]
- Blumenthal, J.A.; Hinderliter, A.L.; Smith, P.J.; Mabe, S.; Watkins, L.L.; Craighead, L.; Ingle, K.; Tyson, C.; Lin, P.-H.; Kraus, W.E.; et al. Effects of Lifestyle Modification on Patients with Resistant Hypertension: Results of the TRIUMPH Randomized Clinical Trial. Circulation 2021, 144, 1212–1226. [Google Scholar] [CrossRef]
- Dickinson, K.M.; Clifton, P.M.; Keogh, J.B. A reduction of 3 g/day from a usual 9 g/day salt diet improves endothelial function and decreases endothelin-1 in a randomised cross_over study in normotensive overweight and obese subjects. Atherosclerosis 2014, 233, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, K.M.; Keogh, J.B.; Clifton, P.M. Effects of a low-salt diet on flow-mediated dilatation in humans. Am. J. Clin. Nutr. 2009, 89, 485–490. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, L.; Cappuccio, F.P.; Masulli, M.; La Fata, E.; Rendina, D.; Galletti, F. Effect of Potassium Supplementation on Endothelial Function: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients 2023, 15, 853. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.R.; Zuelch, M.L.; Smiljanec, K.; Mbakwe, A.U.; Axler, M.R.; Witman, M.A.; Lennon, S.L. Arterial Stiffness and Endothelial Function are Comparable in Young Healthy Vegetarians and Omnivores. Nutr. Res. 2022, 105, 163–172. [Google Scholar] [CrossRef]
- Byrne, J.; Murphy, C.; Keogh, J.B.; Clifton, P.M. The Effect of Magnesium Supplementation on Endothelial Function: A Randomised Cross-Over Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 8169. [Google Scholar] [CrossRef]
- Theodoridis, X.; Chourdakis, M.; Papaemmanouil, A.; Chaloulakou, S.; Georgakou, A.V.; Chatzis, G.; Triantafyllou, A. The Effect of Diet on Vascular Aging: A Narrative Review of the Available Literature. Life 2024, 14, 267. [Google Scholar] [CrossRef]
- Sasaki, S.; Higashi, Y.; Nakagawa, K.; Kimura, M.; Noma, K.; Sasaki, S.; Hara, K.; Matsuura, H.; Goto, C.; Oshima, T.; et al. A low-calorie diet improves endothelium-dependent vasodilation in obese patients with essential hypertension. Am. J. Hypertens. 2002, 15, 302–309. [Google Scholar] [CrossRef]
- Jamialahmadi, T.; Alidadi, M.; Atkin, S.L.; Kroh, M.; Almahmeed, W.; Moallem, S.A.; Al-Rasadi, K.; Rodriguez, J.H.; Santos, R.D.; Ruscica, M.; et al. Effect of Bariatric Surgery on Flow-Mediated Vasodilation as a Measure of Endothelial Function: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 4054. [Google Scholar] [CrossRef]
- Okuyama, N.; Fukumoto, K.; Takemoto, Y.; Yamauchi, T.; Makuuchi, A.; Namikawa, H.; Toyoda, H.; Tochino, Y.; Izumiya, Y.; Fukuda, D.; et al. Effects of smoking cessation on endothelial function as assessed by flow-mediated total dilation. Cardiovasc. Ultrasound 2024, 22, 11. [Google Scholar] [CrossRef]
- Johnson, H.M.; Gossett, L.K.; Piper, M.E.; Aeschlimann, S.E.; Korcarz, C.E.; Baker, T.B.; Fiore, M.C.; Stein, J.H. Effects of smoking and smoking cessation on endothelial function: 1-year outcomes from a randomized clinical trial. J. Am. Coll. Cardiol. 2010, 55, 1988–1995. [Google Scholar] [CrossRef]
| Vascular Bed | Technique | Stimulus (Examples) | Advantages | Disadvantages | Studies in Stroke |
|---|---|---|---|---|---|
| Systemic— brachial artery | FMD | Reactive hyperemia Nytroglicerin ACh | Non-invasive inexpensive | Interobserver and intraobserver variability Lack of standarization | [37,54,56,58,59,61,62,66,67,68] |
| Systemic— microvascular circulation | Disposable pletismographic probes | Reactive hiperemia Capsaicin | Reproducibility Easy, automated | Expensive | [66] |
| Venous occlusion pletismography | Ach | Contralateral arm as control Dose response relationship | Invasive Time consuming | -- | |
| Cerebral circulation | Transcranial doppler sonography | L-Arginine l-NMA Acetazolamide | Inexpensive Reproducible Availability | CO2 dependent Intravenous infusion Adverse events to drugs | [49,58,59,60,62] |
| PET, SPECT, Xe-CT, perfusion CT, perfusion MRI | L-Arginine l-NMA Acetazolamide | Quantitative Regionally specific information | Expensive Not widely accesible | -- | |
| Coronary circulation | Doppler wires | Ach Adenosine Papaverine | Direct assessment of the coronary circulation | Invasive Expensive Time intensive Limited to those undergoing coronary angiography | -- |
| Medical Interventions | Non-Pharmacological Strategies | Cell Based Therapies | Lifestyle Modifications |
|---|---|---|---|
| Glyceryl trinitrate | Sphenopalatine ganglion (SPG) stimulation | Administration of EPCs | Physical exercise |
| L-arginine | Repetitive transcranial magnetic stimulation | EPC-derived exosomes and secretomes | Dietary patterns |
| BH4 | Ischemic preconditioning | Salt reduction | |
| Statins | Near-infrared laser treatment | Body weight reduction | |
| NOS enzyme inhibitors | Smoking cessation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Riva, P.; Marta-Enguita, J.; Rodríguez-Antigüedad, J.; Bergareche, A.; de Munain, A.L. Understanding Endothelial Dysfunction and Its Role in Ischemic Stroke After the Outbreak of Recanalization Therapies. Int. J. Mol. Sci. 2024, 25, 11631. https://doi.org/10.3390/ijms252111631
de la Riva P, Marta-Enguita J, Rodríguez-Antigüedad J, Bergareche A, de Munain AL. Understanding Endothelial Dysfunction and Its Role in Ischemic Stroke After the Outbreak of Recanalization Therapies. International Journal of Molecular Sciences. 2024; 25(21):11631. https://doi.org/10.3390/ijms252111631
Chicago/Turabian Stylede la Riva, Patricia, Juan Marta-Enguita, Jon Rodríguez-Antigüedad, Alberto Bergareche, and Adolfo López de Munain. 2024. "Understanding Endothelial Dysfunction and Its Role in Ischemic Stroke After the Outbreak of Recanalization Therapies" International Journal of Molecular Sciences 25, no. 21: 11631. https://doi.org/10.3390/ijms252111631
APA Stylede la Riva, P., Marta-Enguita, J., Rodríguez-Antigüedad, J., Bergareche, A., & de Munain, A. L. (2024). Understanding Endothelial Dysfunction and Its Role in Ischemic Stroke After the Outbreak of Recanalization Therapies. International Journal of Molecular Sciences, 25(21), 11631. https://doi.org/10.3390/ijms252111631





