Optimisation, Synthesis, and Characterisation of ZnO Nanoparticles Using Leonotis ocymifolia (L. ocymifolia) Leaf Extracts for Antibacterial and Photodegradation Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Qualitative Analysis of L.O. Extracts
2.2. Optimisation of Synthesis Parameters
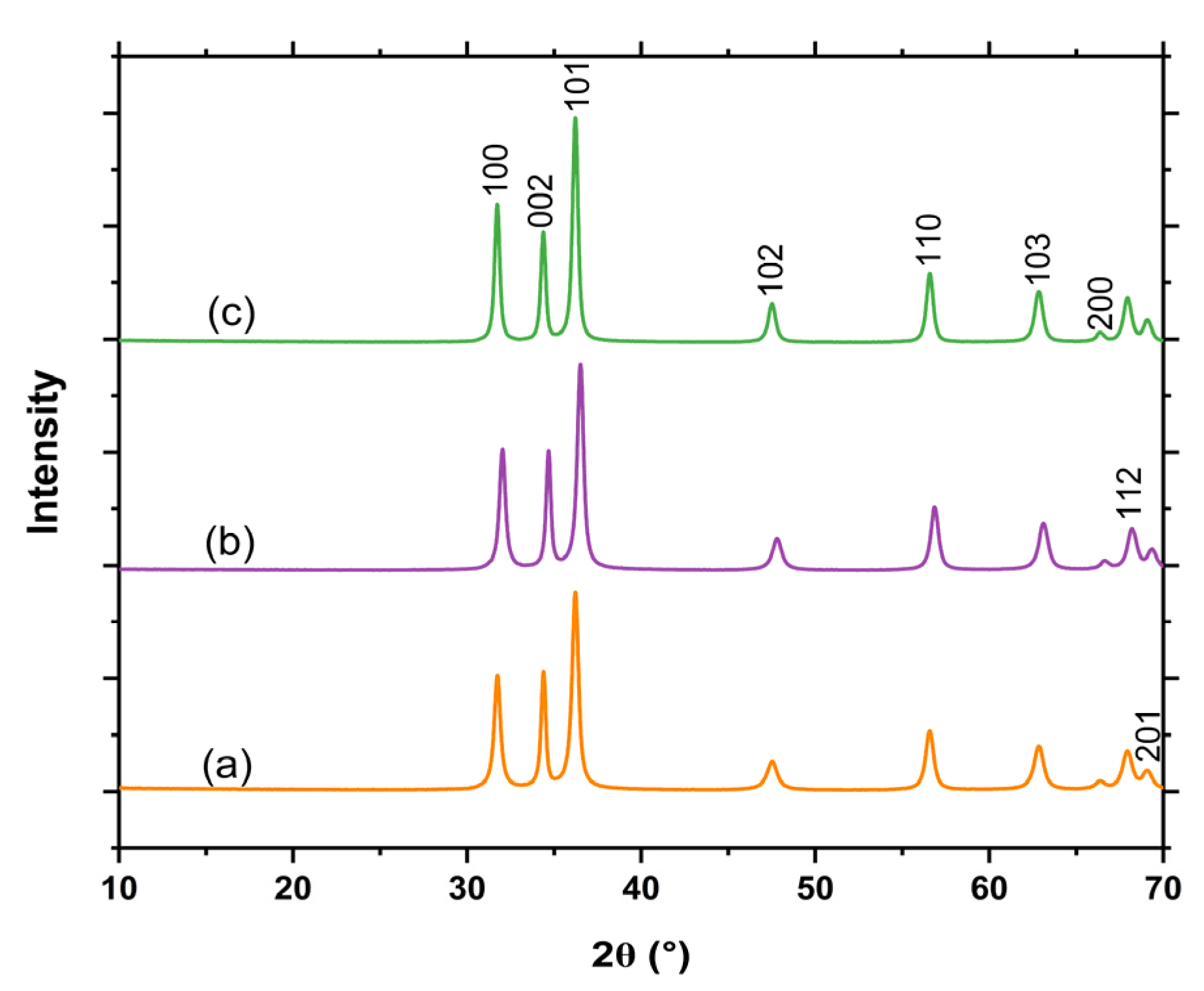
2.3. X-Ray Diffraction (XRD) Analysis
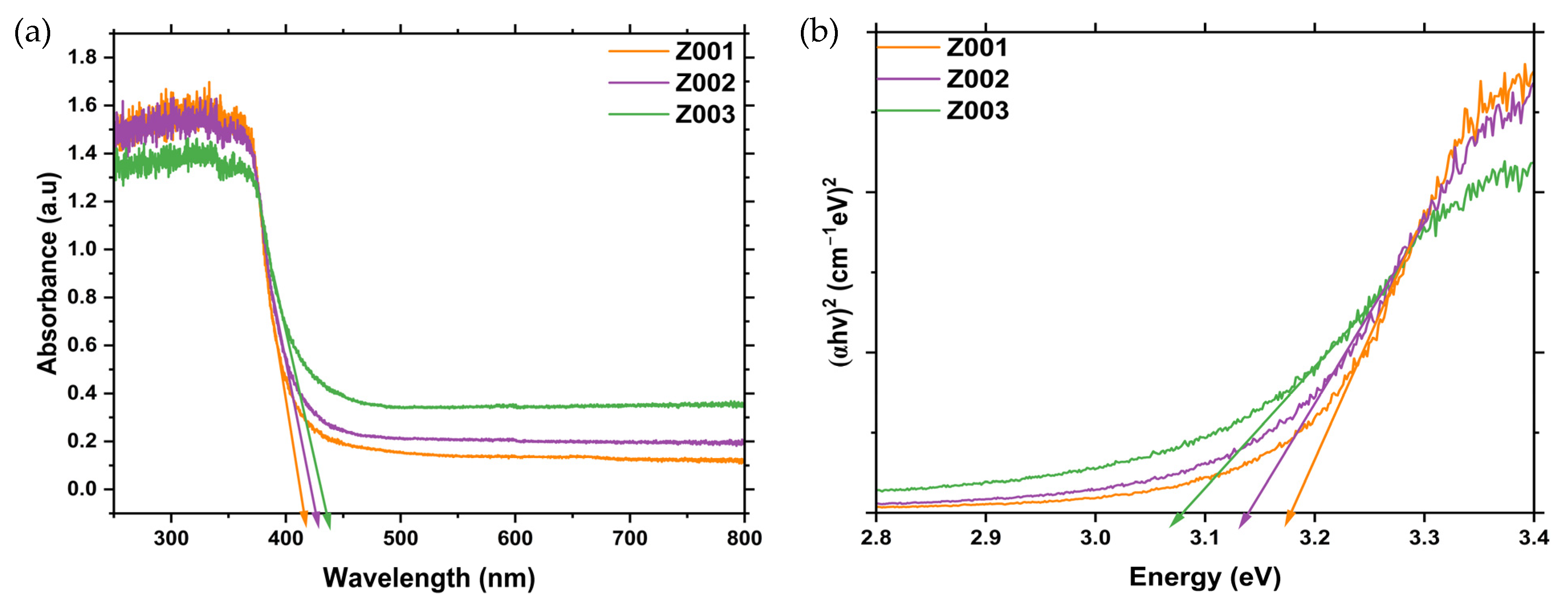
2.4. UV-Vis-Diffuse Reflectance Spectroscopy (DRS)
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
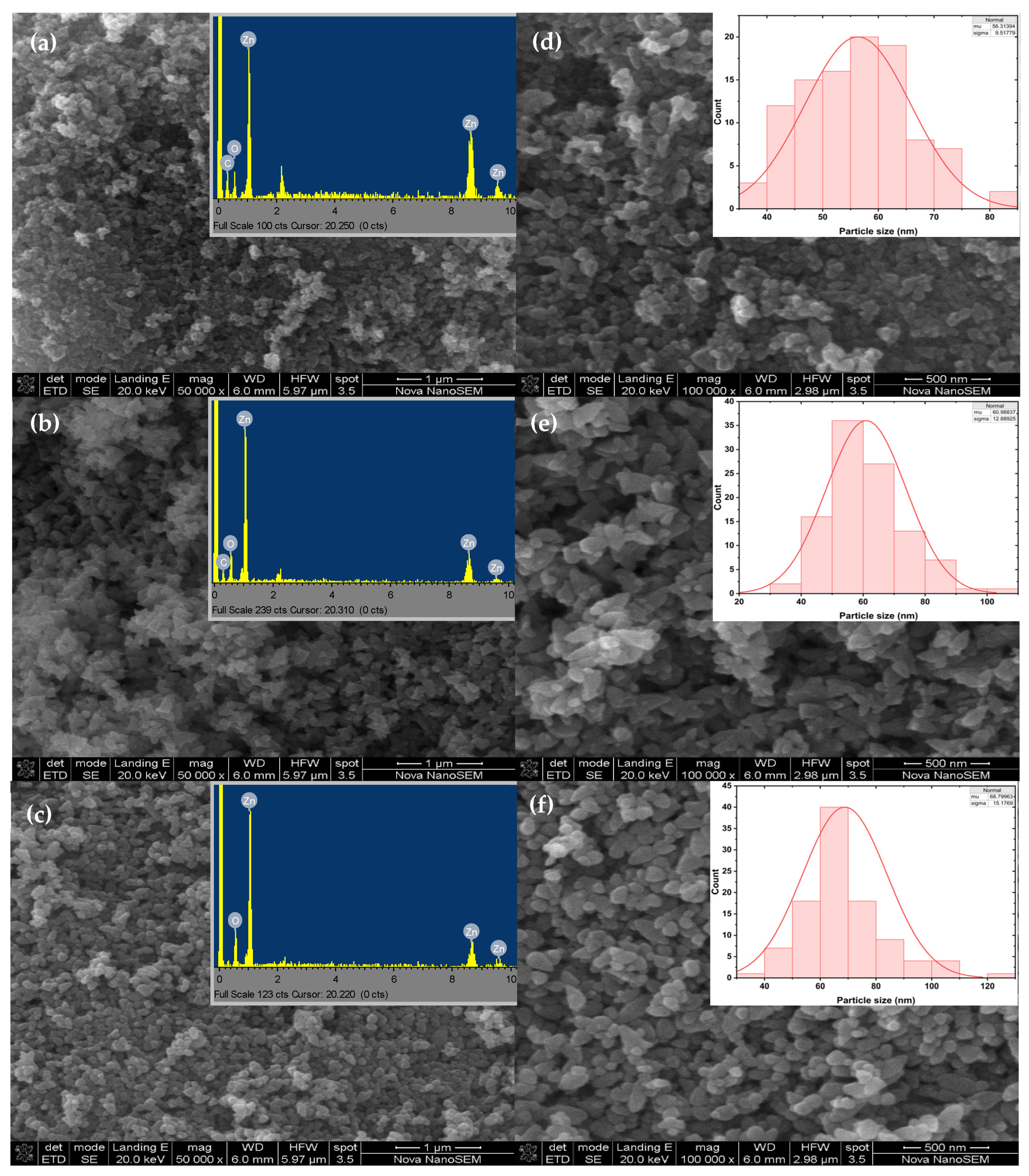
2.6. Morphological and Elemental Analysis
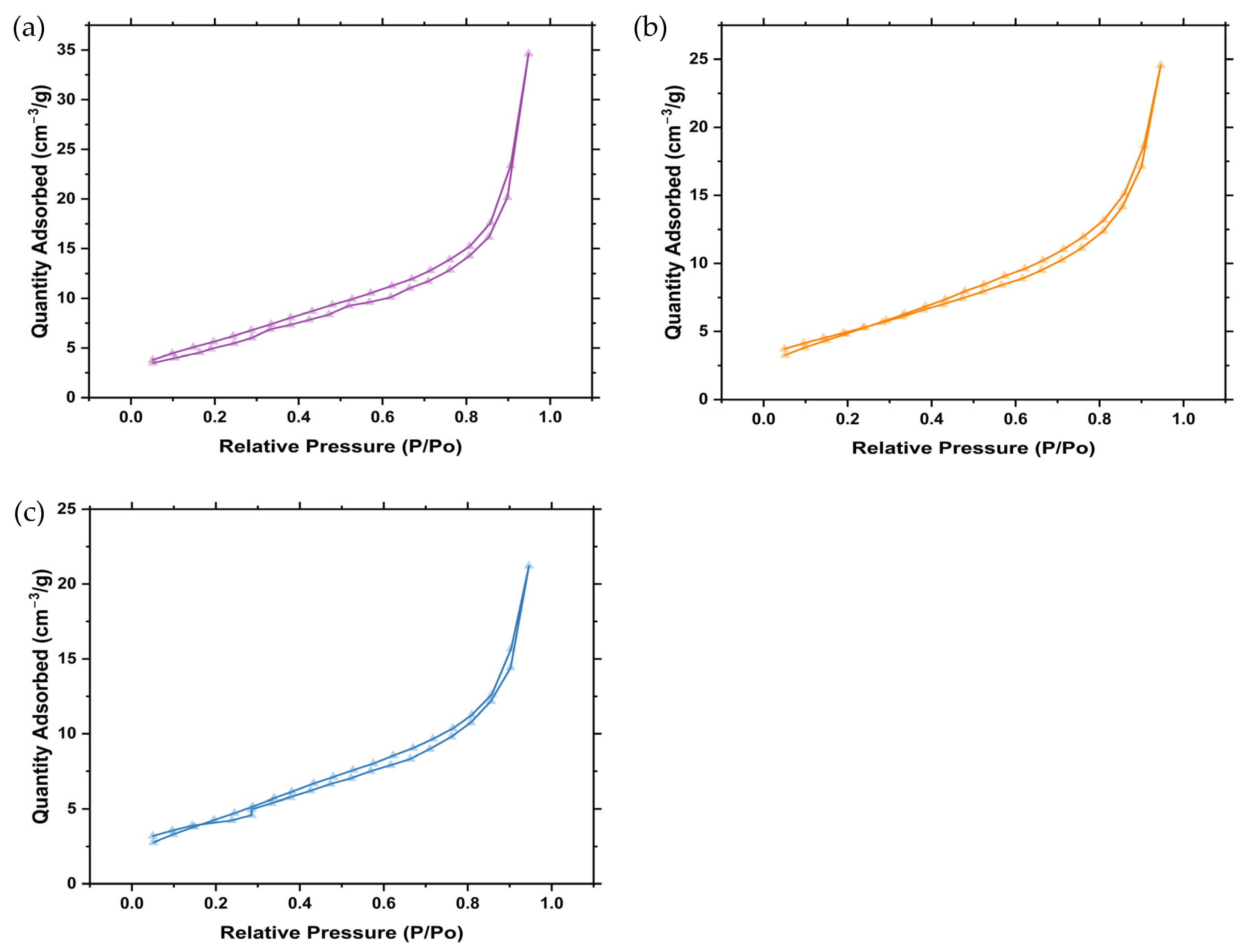
2.7. Brunauer, Emmett and Teller (BET) Analysis
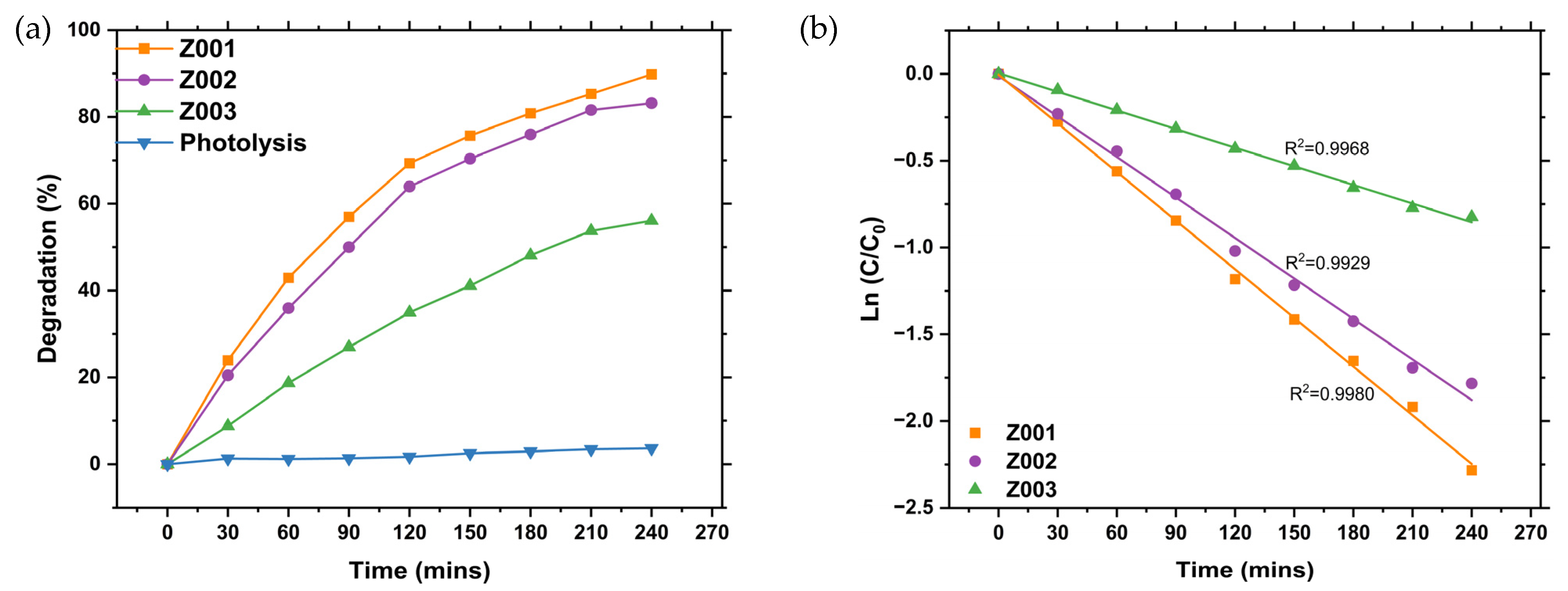
2.8. Photocatalytic Activity of the Synthesised ZnO NPs
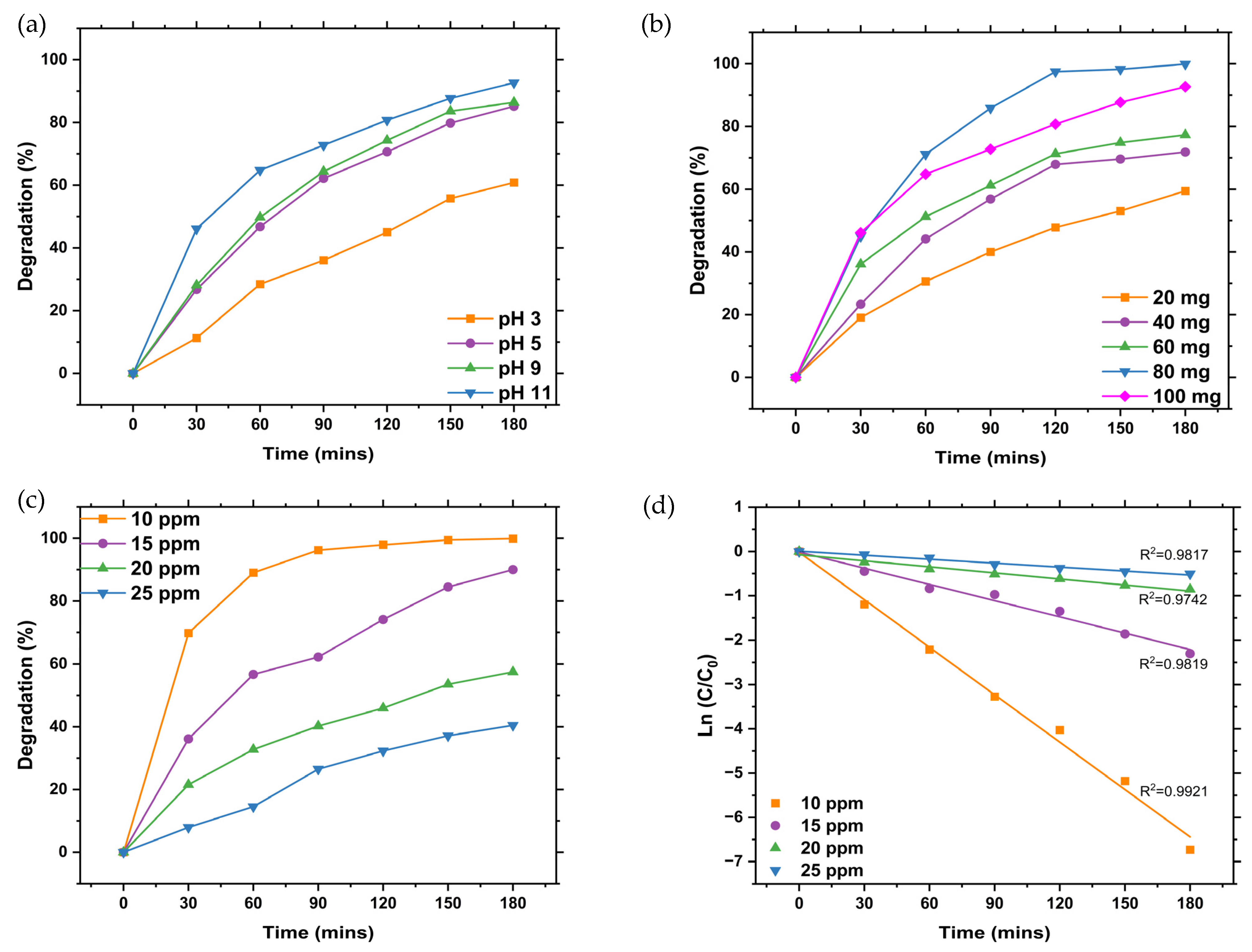
2.8.1. Optimisation of the Photodegradation of MB Dye Using L.O-Mediated ZnO NPs
2.8.2. Reusability
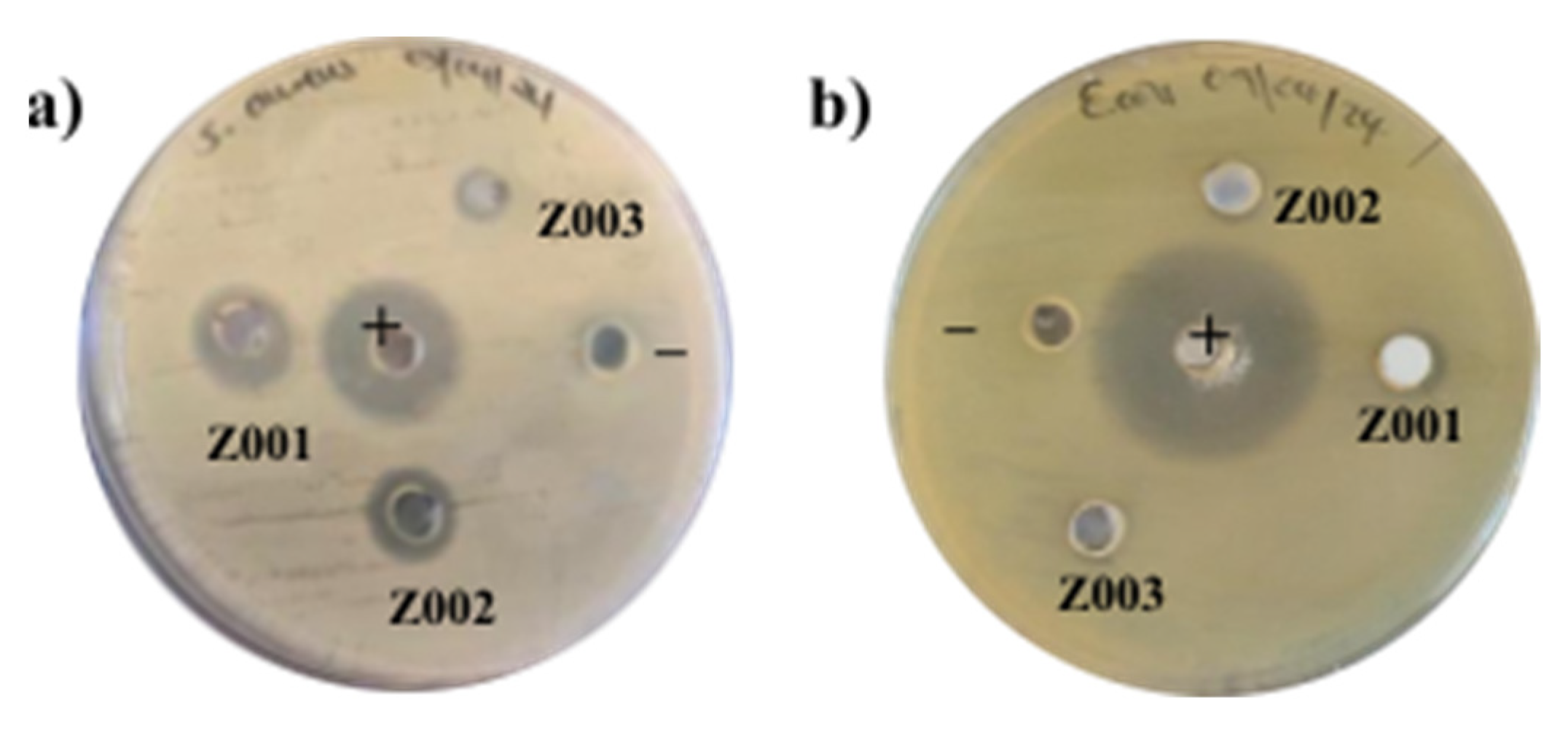
2.9. Antibacterial Activity
3. Materials and Methods
3.1. Preparation of Extract
3.2. Biomolecules Analysis
3.3. Synthesis of ZnO NPs
3.4. Characterisation Techniques
3.5. Photocatalytic Degradation Studies
3.6. Antibacterial Activity Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raha, S.; Ahmaruzzaman, M. ZnO Nanostructured Materials and Their Potential Applications: Progress, Challenges and Perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakkani, M.F. Biogenic Copper Nanoparticles and Their Applications: A Review. SN Appl. Sci. 2020, 2, 505. [Google Scholar] [CrossRef]
- Uzzaman, S.; Mashrai, A.; Khanam, H.; Aljawfi, R.N. Biological Synthesis of ZnO Nanoparticles Using C. albicans and Studying Their Catalytic Performance in the Synthesis of Steroidal Pyrazolines. Arab. J. Chem. 2017, 10, S1530–S1536. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, X.; Wu, F.; Ji, Y. High Adsorption Capability and Selectivity of ZnO Nanoparticles for Dye Removal. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 474–483. [Google Scholar] [CrossRef]
- Ghaffari, H.; Tavakoli, A.; Moradi, A.; Tabarraei, A.; Bokharaei-Salim, F.; Zahmatkeshan, M.; Farahmand, M.; Javanmard, D.; Kiani, S.J.; Esghaei, M.; et al. Inhibition of H1N1 Influenza Virus Infection by Zinc Oxide Nanoparticles: Another Emerging Application of Nanomedicine. J. Biomed. Sci. 2019, 26, 70. [Google Scholar] [CrossRef]
- Kamarajan, G.; Benny Anburaj, D.; Porkalai, V.; Muthuvel, A.; Nedunchezhian, G.; Mahendran, N. Green Synthesis of ZnO Nanoparticles and Their Photocatalyst Degradation and Antibacterial Activity. J. Water Environ. Nanotechnol. 2022, 7, 180–193. [Google Scholar] [CrossRef]
- Santhoshkumar, J.; Kumar, S.V.; Rajeshkumar, S. Synthesis of Zinc Oxide Nanoparticles Using Plant Leaf Extract against Urinary Tract Infection Pathogen. Resour.-Effic. Technol. 2017, 3, 459–465. [Google Scholar] [CrossRef]
- Thema, F.T.; Manikandan, E.; Dhlamini, M.S.; Maaza, M. Green Synthesis of ZnO Nanoparticles via Agathosma betulina Natural Extract. Mater. Lett. 2015, 161, 124–127. [Google Scholar] [CrossRef]
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green Synthesis of Zinc Oxide Nanoparticles Using Hibiscus subdariffa Leaf Extract: Effect of Temperature on Synthesis, Anti-Bacterial Activity and Anti-Diabetic Activity. RSC Adv. 2015, 5, 4993–5003. [Google Scholar] [CrossRef]
- Shashanka, R.; Esgin, H.; Yilmaz, V.M.; Caglar, Y. Fabrication and Characterization of Green Synthesized ZnO Nanoparticle Based Dye-Sensitized Solar Cells. J. Sci. Adv. Mater. Devices 2020, 5, 185–191. [Google Scholar] [CrossRef]
- Vidya, C.; Prabha, M.N.C.; Raj, M.A.L.A. Green Mediated Synthesis of Zinc Oxide Nanoparticles for the Photocatalytic Degradation of Rose Bengal Dye. Env. Nanotechnol. Monit. Manag. 2016, 6, 134–138. [Google Scholar] [CrossRef]
- Nagar, A.; Kumar, A.; Tyagi, U.; Dhasmana, H.; Khan, M.A.M.; Husain, S.; Verma, A.; Jain, V.K. Ultrafast, Trace-Level Detection of NH3 Gas at Room Temperature Using Hexagonal-Shaped ZnO Nanoparticles Grown by Novel Green Synthesis Technique. Phys. B Condens. Matter 2022, 626, 413595. [Google Scholar] [CrossRef]
- Gawade, V.V.; Gavade, N.L.; Shinde, H.M.; Babar, S.B.; Kadam, A.N.; Garadkar, K.M. Green Synthesis of ZnO Nanoparticles by Using Calotropis procera Leaves for the Photodegradation of Methyl Orange. J. Mater. Sci. Mater. Electron. 2017, 28, 14033–14039. [Google Scholar] [CrossRef]
- Wall, K. Leonotis Ocymifolia. Available online: https://pza.sanbi.org/leonotis-ocymifolia (accessed on 11 September 2024).
- Alemu, A.; Tamiru, W.; Nedi, T.; Shibeshi, W. Analgesic and Anti-Inflammatory Effects of 80% Methanol Extract of Leonotis ocymifolia (Burm.f.) Iwarsson Leaves in Rodent Models. Evid.-Based Complement. Altern. Med. 2018, 2018, 1614793. [Google Scholar] [CrossRef]
- Mutukwa, D.; Taziwa, R.; Khotseng, L.E. A Review of the Green Synthesis of ZnO Nanoparticles Utilising Southern African Indigenous Medicinal Plants. Nanomaterials 2022, 12, 3456. [Google Scholar] [CrossRef]
- Soto-Robles, C.A.; Luque, P.A.; Gómez-Gutiérrez, C.M.; Nava, O.; Vilchis-Nestor, A.R.; Lugo-Medina, E.; Ranjithkumar, R.; Castro-Beltrán, A. Study on the Effect of the Concentration of Hibiscus sabdariffa Extract on the Green Synthesis of ZnO Nanoparticles. Results Phys. 2019, 15, 102807. [Google Scholar] [CrossRef]
- Zewde, D.; Geremew, B. Biosynthesis of ZnO Nanoparticles Using Hagenia abyssinica Leaf Extracts; Their Photocatalytic and Antibacterial Activities. Environ. Pollut. Bioavailab. 2022, 34, 224–235. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, S.; Kaur, G.; Basu, S.; Rawat, M. Biogenic ZnO Nanoparticles: A Study of Blueshift of Optical Band Gap and Photocatalytic Degradation of Reactive Yellow 186 Dye under Direct Sunlight. Green Process. Synth. 2019, 8, 272–280. [Google Scholar] [CrossRef]
- Alshehri, A.A.; Malik, M.A. Biogenic Fabrication of ZnO Nanoparticles Using Trigonella Foenum-Graecum (Fenugreek) for Proficient Photocatalytic Degradation of Methylene Blue under UV Irradiation. J. Mater. Sci. Mater. Electron. 2019, 30, 16156–16173. [Google Scholar] [CrossRef]
- Akhter, S.M.H.; Mahmood, Z.; Ahmad, S.; Mohammad, F. Plant-Mediated Green Synthesis of Zinc Oxide Nanoparticles Using Swertia Chirayita Leaf Extract, Characterization and Its Antibacterial Efficacy Against Some Common Pathogenic Bacteria. Bionanoscience 2018, 8, 811–817. [Google Scholar] [CrossRef]
- Haiouani, K.; Hegazy, S.; Alsaeedi, H.; Bechelany, M.; Barhoum, A. Green Synthesis of Hexagonal-like ZnO Nanoparticles Modified with Phytochemicals of Clove (Syzygium aromaticum) and Thymus Capitatus Extracts: Enhanced Antibacterial, Antifungal, and Antioxidant Activities. Materials 2024, 17, 4340. [Google Scholar] [CrossRef] [PubMed]
- Aldalbahi, A.; Alterary, S.; Ali Abdullrahman Almoghim, R.; Awad, M.A.; Aldosari, N.S.; Fahad Alghannam, S.; Nasser Alabdan, A.; Alharbi, S.; Ali Mohammed Alateeq, B.; Abdulrahman Al Mohsen, A.; et al. Greener Synthesis of Zinc Oxide Nanoparticles: Characterization and Multifaceted Applications. Molecules 2020, 25, 4198. [Google Scholar] [CrossRef]
- Aziz, A.; Memon, Z.; Bhutto, A. Efficient Photocatalytic Degradation of Industrial Wastewater Dye by Grewia asiatica Mediated Zinc Oxide Nanoparticles. Optik 2023, 272, 170352. [Google Scholar] [CrossRef]
- Tang, Q.; Xia, H.; Liang, W.; Huo, X.; Wei, X. Synthesis and Characterization of Zinc Oxide Nanoparticles from Morus nigra and Its Anticancer Activity of AGS Gastric Cancer Cells. J. Photochem. Photobiol. B 2020, 202, 111698. [Google Scholar] [CrossRef] [PubMed]
- Sepasgozar, S.M.E.; Mohseni, S.; Feizyzadeh, B.; Morsali, A. Green Synthesis of Zinc Oxide and Copper Oxide Nanoparticles Using Achillea Nobilis Extract and Evaluating Their antioxidant and Antibacterial Properties. Bull. Mater. Sci. 2021, 44, 1–13. [Google Scholar] [CrossRef]
- Ashraf, H.; Meer, B.; Iqbal, J.; Ali, J.S.; Andleeb, A.; Butt, H.; Zia, M.; Mehmood, A.; Nadeem, M.; Drouet, S.; et al. Comparative Evaluation of Chemically and Green Synthesized Zinc Oxide Nanoparticles: Their in Vitro Antioxidant, Antimicrobial, Cytotoxic and Anticancer Potential towards HepG2 Cell Line. J. Nanostruct. Chem. 2023, 13, 243–261. [Google Scholar] [CrossRef]
- Geetha, M.S.; Nagabhushana, H.; Shivananjaiah, H.N. Green Mediated Synthesis and Characterization of ZnO Nanoparticles Using Euphorbia Jatropa Latex as Reducing Agent. J. Sci. Adv. Mater. Devices 2016, 1, 301–310. [Google Scholar] [CrossRef]
- Loganathan, S.; Selvam, K.; Govindasamy, M.; Habila, M.A. Bioynthesis and Characterization of Zinc Oxide Nanoparticles (ZnO-NPs) Using Passiflora foetida Linn Leaves Extract and Their Biological Applications. Biomass Convers. Biorefin 2024. [Google Scholar] [CrossRef]
- Muhammad, W.; Ullah, N.; Haroon, M.; Abbasi, B.H. Optical, Morphological and Biological Analysis of Zinc Oxide Nanoparticles (ZnO NPs) Using: Papaver somniferum L. RSC Adv. 2019, 9, 29541–29548. [Google Scholar] [CrossRef]
- Thirunavoukkarasu, M.; Balaji, U.; Behera, S.; Panda, P.K.; Mishra, B.K. Biosynthesis of Silver Nanoparticle from Leaf Extract of Desmodium gangeticum (L.) DC. and Its Biomedical Potential. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 116, 424–427. [Google Scholar] [CrossRef]
- Albeladi, S.S.R.; Malik, M.A.; Al-Thabaiti, S.A. Facile Biofabrication of Silver Nanoparticles Using Salvia officinalis Leaf Extract and Its Catalytic Activity towards Congo Red Dye Degradation. J. Mater. Res. Technol. 2020, 9, 10031–10044. [Google Scholar] [CrossRef]
- Thongam, D.D.; Gupta, J.; Sahu, N.K. Effect of Induced Defects on the Properties of ZnO Nanocrystals: Surfactant Role and Spectroscopic Analysis. SN Appl. Sci. 2019, 1, 1030. [Google Scholar] [CrossRef]
- Gatou, M.A.; Lagopati, N.; Vagena, I.A.; Gazouli, M.; Pavlatou, E.A. ZnO Nanoparticles from Different Precursors and Their Photocatalytic Potential for Biomedical Use. Nanomaterials 2023, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Sekar, A.; Murugan, P.J.; Paularokiadoss, F. Biological Synthesis and Characterization of Zinc Oxide Nanoparticles (ZnONPs) from Anisomeles malabarica. Vietnam J. Chem. 2022, 60, 459–471. [Google Scholar] [CrossRef]
- Muthuvel, A.; Jothibas, M.; Manoharan, C. Effect of Chemically Synthesis Compared to Biosynthesized ZnO-NPs Using Solanum nigrum Leaf Extract and Their Photocatalytic, Antibacterial and in-Vitro Antioxidant Activity. J. Env. Chem. Eng. 2020, 8, 103705. [Google Scholar] [CrossRef]
- Lal, R.; Gour, T.; Dave, N.; Singh, N.; Yadav, J.; Khan, A.; Jain, A.; Agarwal, L.K.; Sharma, Y.K.; Sharma, K. Green Route to Fabrication of Semal-ZnO Nanoparticles for Efficient Solar-Driven Catalysis of Noxious Dyes in Diverse Aquatic Environments. Front. Chem. 2024, 12, 1370667. [Google Scholar] [CrossRef]
- Maheswaran, H.; Djearamane, S.; Tanislaus Antony Dhanapal, A.C.; Wong, L.S. Cytotoxicity of Green Synthesized Zinc Oxide Nanoparticles Using Musa acuminata on Vero Cells. Heliyon 2024, 10, e31316. [Google Scholar] [CrossRef]
- Kahsay, M.H.; Tadesse, A.; Ramadevi, D.; Belachew, N.; Basavaiah, K. Green Synthesis of Zinc Oxide Nanostructures and Investigation of Their Photocatalytic and Bactericidal Applications. RSC Adv. 2019, 9, 36967–36981. [Google Scholar] [CrossRef]
- Jayappa, M.D.; Ramaiah, C.K.; Kumar, M.A.P.; Suresh, D.; Prabhu, A.; Devasya, R.P.; Sheikh, S. Green Synthesis of Zinc Oxide Nanoparticles from the Leaf, Stem and in Vitro Grown Callus of Mussaenda frondosa L.: Characterization and Their Applications. Appl. Nanosci. 2020, 10, 3057–3074. [Google Scholar] [CrossRef]
- Anil Kumar, M.R.; Ravikumar, C.R.; Nagaswarupa, H.P.; Purshotam, B.; Gonfa, B.A.; Murthy, H.C.A.; Sabir, F.K.; Tadesse, S. Evaluation of Bi-Functional Applications of ZnO Nanoparticles Prepared by Green and Chemical Methods. J. Env. Chem. Eng. 2019, 7, 103468. [Google Scholar] [CrossRef]
- Luque, P.A.; Garrafa-Gálvez, H.E.; García-Maro, C.A.; Soto-Robles, C.A. Study of the Optical Properties of ZnO Semiconductor Nanoparticles Using Origanum vulgare and Its Effect in Rhodamine B Degradation. Optik 2022, 258, 168937. [Google Scholar] [CrossRef]
- Kazeminezhad, I.; Sadollahkhani, A. Influence of PH on the Photocatalytic Activity of ZnO Nanoparticles. J. Mater. Sci. Mater. Electron. 2016, 27, 4206–4215. [Google Scholar] [CrossRef]
- Ali, T.T.; Narasimharao, K.; Parkin, I.P.; Carmalt, C.J.; Sathasivam, S.; Basahel, S.N.; Bawaked, S.M.; Al-Thabaiti, S.A. Effect of Pretreatment Temperature on the Photocatalytic Activity of Microwave Irradiated Porous Nanocrystalline ZnO. New J. Chem. 2015, 39, 321–332. [Google Scholar] [CrossRef]
- Etay, H.; Kumar, A.; Yadav, O. Kinetics of Photocatalytic Degradation of Methylene Blue Dye in Aqueous Medium Using ZnO Nanoparticles under UV Radiation. J. Anal. Pharm. Res. 2023, 12, 32–37. [Google Scholar] [CrossRef]
- Kadhim, M.J.; Mahdi, M.A.; Selman, A.M.; Al-Ani, S.K.J.; Hassan, J.J.; Ahmed, N.M. The Most Important Parameters That Affect the Photocatalytic Activity of ZnO Nanostructures against Organic Dyes: A Review. Iran. J. Catal. 2023, 13, 1–21. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An Overview of Photocatalytic Degradation: Photocatalysts, Mechanisms, and Development of Photocatalytic Membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef]
- Hossain, M.K.; Hossain, M.M.; Akhtar, S. Photodegradation and Reaction Kinetics for Eosin Yellow Using ZnO Nanoparticles as Catalysts. React. Kinet. Mech. Catal. 2022, 135, 2247–2263. [Google Scholar] [CrossRef]
- Fu, L.; Fu, Z. Plectranthus amboinicus Leaf Extract-Assisted Biosynthesis of ZnO Nanoparticles and Their Photocatalytic Activity. Ceram. Int. 2015, 41, 2492–2496. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the Improvement of the Photocatalytic and Antibacterial Activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Inamdar, H.K.; Sasikala, M.; Agsar, D.; Prasad, M.V.N.A. Facile Green Fabrication of ZnO Nanopowders: Their Antibacterial, Antifungal and Photoluminescent Properties. Mater. Today Proc. 2018, 5, 21263–21270. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Malaikozhundan, B.; Shanthi, S.; Vaseeharan, B.; Thajuddin, N. Control of Biofilm Forming Clinically Important Bacteria by Green Synthesized ZnO Nanoparticles and Its Ecotoxicity on Ceriodaphnia Cornuta. Microb. Pathog. 2017, 107, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Umamaheswari, A.; Prabu, S.L.; John, S.A.; Puratchikody, A. Green Synthesis of Zinc Oxide Nanoparticles Using Leaf Extracts of Raphanus sativus Var. Longipinnatus and Evaluation of Their Anticancer Property in A549 Cell Lines. Biotechnol. Rep. 2021, 29, e00595. [Google Scholar] [CrossRef] [PubMed]
- Mugumo, R.; Ichipi, E.O.; Tichapondwa, S.M.; Chirwa, E.M.N. Bandgap Tailoring of ZnO Using Metallic sulphides for Enhanced Visible-Light-Active Photocatalytic Water Treatment. Chem. Eng. Trans. 2023, 103, 829–834. [Google Scholar] [CrossRef]
- Selvanathan, V.; Aminuzzaman, M.; Tan, L.X.; Win, Y.F.; Guan Cheah, E.S.; Heng, M.H.; Tey, L.H.; Arullappan, S.; Algethami, N.; Alharthi, S.S.; et al. Synthesis, Characterization, and Preliminary in Vitro Antibacterial Evaluation of ZnO Nanoparticles Derived from Soursop (Annona muricata L.) Leaf Extract as a Green Reducing Agent. J. Mater. Res. Technol. 2022, 20, 2931–2941. [Google Scholar] [CrossRef]




| Phytochemical | Results |
|---|---|
| Flavonoids | Positive |
| Alkaloids | Negative |
| Phenols | Positive |
| Tannins | Positive |
| Saponins | Positive |
| Terpenoids | Positive |
| Glycosides | Positive |
| Proteins | Negative |
| Sample | Absorption Edge (nm) | Bandgap (eV) |
|---|---|---|
| Z001 | 425.68 | 3.18 |
| Z002 | 439.48 | 3.13 |
| Z003 | 450.89 | 3.07 |
| Sample | BET Surface Area (m2g) | Pore Volume (cc/g) | Pore Diameter (nm) |
|---|---|---|---|
| Z001 | 20.3 | 0.0336 | 10.6 |
| Z002 | 18.0 | 0.0269 | 8.4 |
| Z003 | 15.7 | 0.0223 | 8.5 |
| Sample | ZOI (mm) |
|---|---|
| Z001 | 12 |
| Z002 | 10 |
| Z003 | 7 |
| Positive control | 19 |
| Negative control | Nil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutukwa, D.; Taziwa, R.T.; Tichapondwa, S.M.; Khotseng, L. Optimisation, Synthesis, and Characterisation of ZnO Nanoparticles Using Leonotis ocymifolia (L. ocymifolia) Leaf Extracts for Antibacterial and Photodegradation Applications. Int. J. Mol. Sci. 2024, 25, 11621. https://doi.org/10.3390/ijms252111621
Mutukwa D, Taziwa RT, Tichapondwa SM, Khotseng L. Optimisation, Synthesis, and Characterisation of ZnO Nanoparticles Using Leonotis ocymifolia (L. ocymifolia) Leaf Extracts for Antibacterial and Photodegradation Applications. International Journal of Molecular Sciences. 2024; 25(21):11621. https://doi.org/10.3390/ijms252111621
Chicago/Turabian StyleMutukwa, Dorcas, Raymond Tichaona Taziwa, Shepherd Masimba Tichapondwa, and Lindiwe Khotseng. 2024. "Optimisation, Synthesis, and Characterisation of ZnO Nanoparticles Using Leonotis ocymifolia (L. ocymifolia) Leaf Extracts for Antibacterial and Photodegradation Applications" International Journal of Molecular Sciences 25, no. 21: 11621. https://doi.org/10.3390/ijms252111621
APA StyleMutukwa, D., Taziwa, R. T., Tichapondwa, S. M., & Khotseng, L. (2024). Optimisation, Synthesis, and Characterisation of ZnO Nanoparticles Using Leonotis ocymifolia (L. ocymifolia) Leaf Extracts for Antibacterial and Photodegradation Applications. International Journal of Molecular Sciences, 25(21), 11621. https://doi.org/10.3390/ijms252111621







