High Behavioral Reactivity to Novelty as a Susceptibility Factor for Memory and Anxiety Disorders in Streptozotocin-Induced Neuroinflammation as a Rat Model of Alzheimer’s Disease
Abstract
1. Introduction
2. Results
2.1. Behavioural Results
2.1.1. Novelty Test
2.1.2. Fear/Anxiety, Spatial Memory, and Learning in the Elevated Plus-Maze Test (EPM)
2.1.3. Behavioral Activity and Fear/Anxiety in the White and Illuminated Open Field Test (OF)
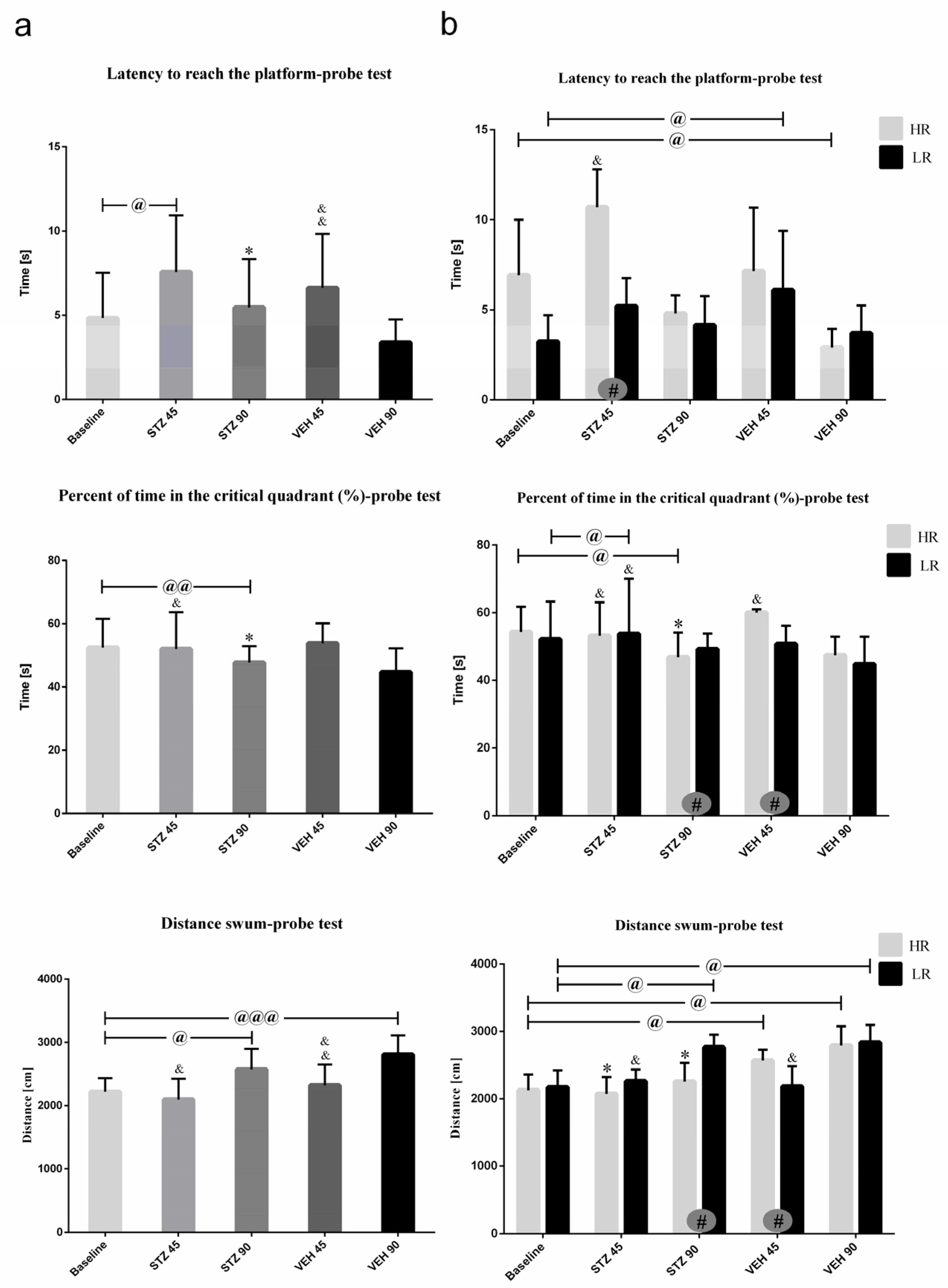
2.1.4. Behavioral Activity Associated with Memory Impairments in the Morris Water Maze Test (MWM)
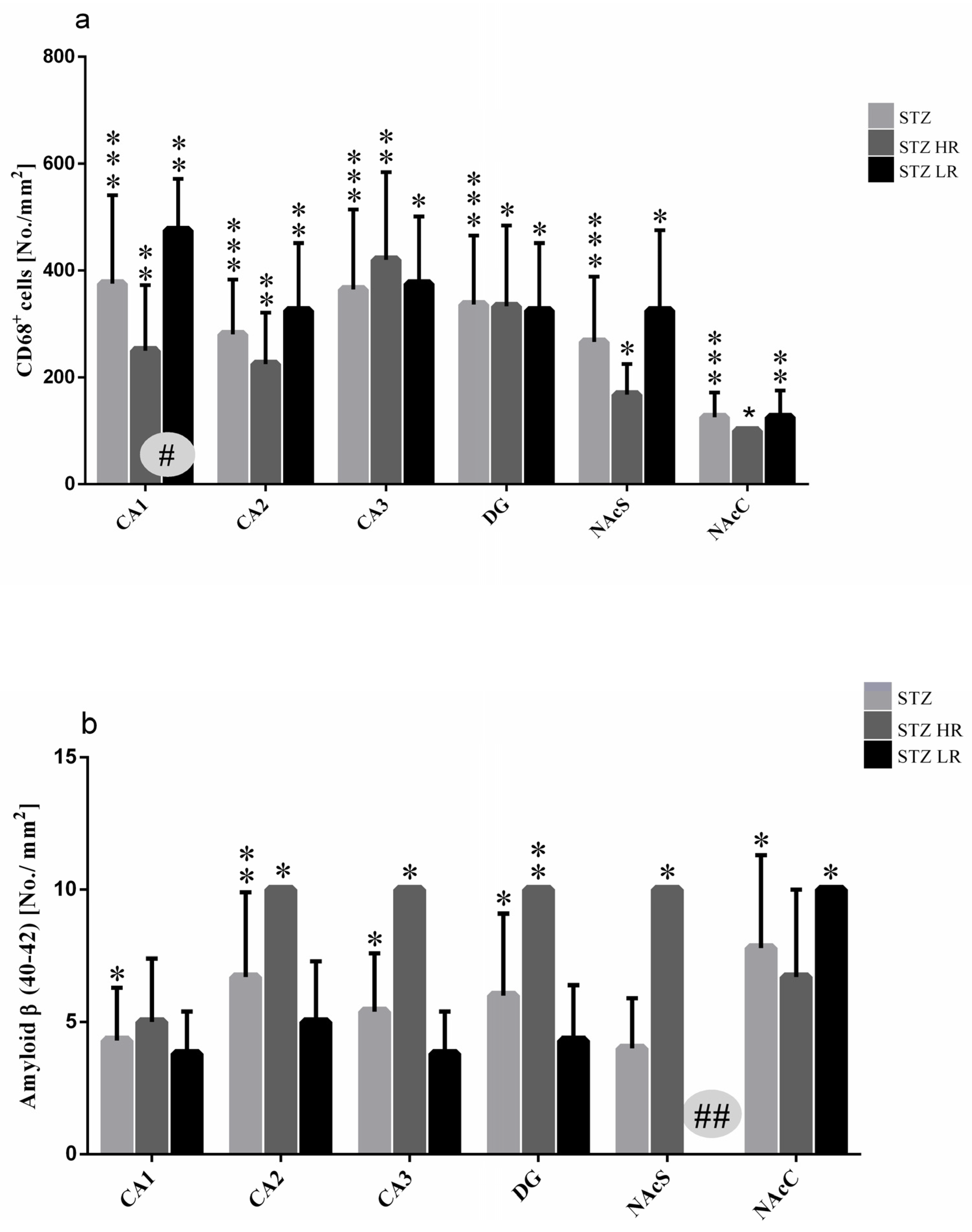
2.2. Microglia Activated Cells (CD68+ Cells) and Amyloid β (Aβ40–42) Deposits in the Hippocampal Areas (CA1, CA2, CA3, DG) and Nucleus Accumbens (NAcC and NAcS)
2.3. Peripheral Blood Leukocyte and Lymphocyte and Their Subpopulation Number
2.4. Relative Thymus and Spleen Weights
2.5. Plasma Interleukin-6 (IL-6)/Interleukin-10 (IL-10) and Corticosterone (CORT) Concentration
2.6. Hematological Parameters
3. Discussion
4. Materials and Methods
4.1. Animals
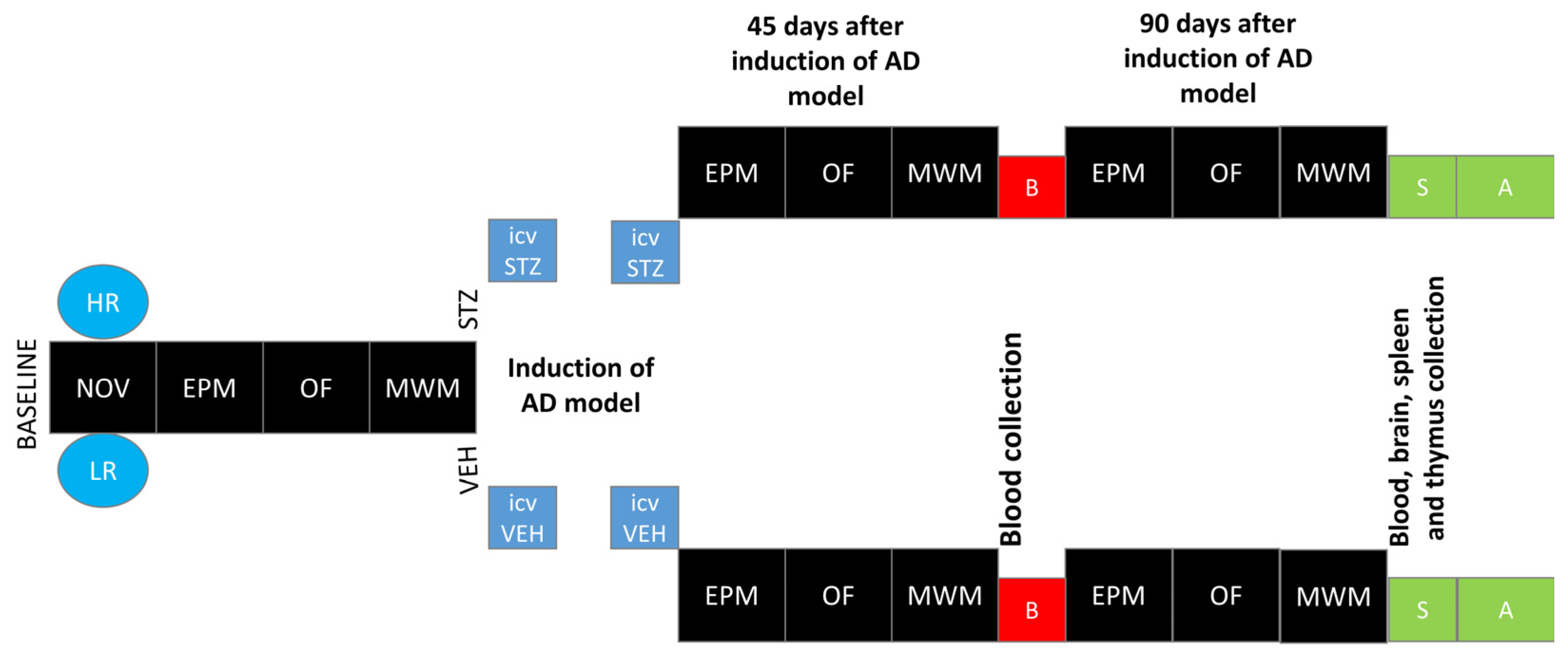
4.2. Experimental Procedure
4.2.1. Novelty Test (NOV)
4.2.2. Intracerebroventricular (ICV) Injections of Streptozotocin (STZ)—sAD Induction
4.2.3. Elevated Plus Maze Test (EPM)
4.2.4. White and Illuminated Open Field Test (OF)
4.2.5. Test of Spatial Memory in Morris Water Maze (MWM)
4.2.6. Immunohistochemistry
4.2.7. Quantification of Labelled Cells
4.2.8. Peripheral Blood Total Leukocyte and Leukocyte Subset Number
4.2.9. Determination of Pro-Inflammatory Interleukin (IL)-6 and Anti-Inflammatory Interleukin (IL)-10
4.2.10. Determination of Plasma Corticosterone (CORT) Concentration
4.2.11. Peripheral Blood Hematological Parameters
4.2.12. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, A.; Daya, T.; Carlton, K.; Yan, J.H.; Schmid, S. Differential effect of clomipramine on habituation and pre pulse inhibition in dominant versus subordinate rats. Eur. Neuropsychopharmacol. 2016, 26, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Selkoe, D.J. A beta oligomers—A decade of discovery. J. Neurochem. 2007, 101, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Reddy, P.H. Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer’s disease: Implications for early intervention and therapeutics. Biochim. Biophys. Acta 2011, 1812, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, X. Antioxidant Therapies for Alzheimer’s Disease. Oxidative Med. Cellalar Longev. 2012, 2012, 472932. [Google Scholar] [CrossRef] [PubMed]
- Esiri, M. The interplay between inflammation and neurodegeneration in cognitive impairment and mild Alzheimer’s disease. J. Neuroimmunol. 2007, 184, 4–16. [Google Scholar] [CrossRef]
- Naseri, N.N.; Wang, H.; Guo, J.; Sharma, M.; Luo, W. The complexity of tau in Alzheimer’s disease. Neurosci. Lett. 2019, 705, 183–194. [Google Scholar] [CrossRef]
- Lorenzi, E.; Franceschini, D.; Contardi, C.; Martino, R.; Seghetti, F.; Serra, M.; Bisceglia, F.; Pagetta, A.; Zusso, M.; Belluti, F. Modulation of Amyloid β-Induced Microglia Activation and Neuronal Cell Death by Curcumin and Analogues. Int. J. Mol. Sci. 2022, 23, 4381. [Google Scholar] [CrossRef]
- Bomboi, G.; Castello, L.; Cosentino, F.; Giubilei, F.; Orzi, F.; Volpe, M. Alzheimer’s disease and endothelial dysfunction. Neurol. Sci. 2010, 31, 1–8. [Google Scholar] [CrossRef]
- Vlassara, H.; Cai, W.; Chen, X.; Serrano, E.J.; Shobha, M.S.; Uribarri, J.; Woodward, M.; Striker, G.E. Managing chronic inflammation in the aging diabetic patient with CKD by diet or sevelamer carbonate: A modern paradigm shift. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1410–1416. [Google Scholar] [CrossRef][Green Version]
- Puca, A.A.; Carrizzo, A.; Villa, F.; Ferrario, A.; Casaburo, M.; Maciag, A.; Vecchione, C. Vascular aging: The role of oxidative stress. Int. J. Biochem. Cell Biol. 2013, 45, 556–559. [Google Scholar] [CrossRef]
- Claessens, S.E.; Daskalakis, N.P.; van der Veen, R.; Oitzl, M.S.; de Kloet, E.; Champagne, D.L. Development of individual differences in stress responsiveness: An overview of factors mediating the outcome of early life experiences. Psychopharmacology 2011, 214, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Ebner, K.; Singewald, N. Individual differences in stress susceptibility and stress inhibitory mechanisms. Curr. Opin. Behav. Sci. 2017, 14, 54–64. [Google Scholar] [CrossRef]
- Piazza, P.V.; Deminiere, J.M.; Le Moal, M.; Simon, H. Factors that predict individual vulnerability to amphetamine self-administration. Science 1989, 245, 1511–1513. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.K.; Corfman, T.P. An overview of neurobiological comparisons in mouse strains. Neurosci. Biobehav. Rev. 1980, 4, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Piazza, P.V.; Maccari, S.; Deminiere, J.M.; Le Moal, M.; Mormede, P.; Simon, H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc. Natl. Acad. Sci. USA 1991, 88, 2088–2092. [Google Scholar] [CrossRef]
- Deroche, V.; Piazza, P.V.; Casolini, P.; Maccari, S.; Le Moal, M.; Simon, H. Stress-induced sensitization to amphetamine and morphine psychomotor effects depend on stress-induced corticosterone secretion. Brain Res. 1992, 598, 343–348. [Google Scholar] [CrossRef]
- Lecanu, L.; Papadopoulos, V. Modeling Alzheimer’s disease with non-transgenic rat models. Alzheimers Res. Ther. 2013, 5, 17. [Google Scholar] [CrossRef]
- Moreira-Silva, D.; Carrettiero, D.; Oliveira, A.; Rodrigues, S.; Santos-Lopes, J.; Canas, P.; Cunha, R.; Almeida, M.; Ferreira, T. Anandamide effects in a streptozotocin-induced Alzheimer’s Disease-Like Sporadic Dementia in Rats. Front. Neurosci. 2018, 12, 653. [Google Scholar] [CrossRef]
- Salkovic-Petrisic, M.; Hoyer, S. Central insulin resistance as a trigger for sporadic Alzheimer-like pathology: An experimental approach. J. Neural. Transm. Suppl. 2007, 72, 217–233. [Google Scholar] [CrossRef]
- Mayer, G.; Nitsch, R.; Hoyer, S. Impairments in passive avoidance learning after cerebral inhibition of cerebral glucose metabolism. J. Neural. Transm. 1989, 1, 103–104. [Google Scholar] [CrossRef]
- Grünblatt, E.; Salkovic-Petrisic, M.; Osmanovic, J.; Riederer, P.; Hoyer, S. Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J. Neurochem. 2007, 101, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, J.; Rocha, A.; Nunes, F.; Costa, M.S.; Schein, V.; Kazlauckas, V.; Kalinine, E.; Souza, D.O.; Cunha, R.A.; Porciúncula, L.O. Caffeine consumption prevents memory impairment, neuronal damage, and adenosine A2A receptors upregulation in the hippocampus of a rat model of sporadic dementia. J. Alzheimers Dis. 2013, 34, 509–518. [Google Scholar] [CrossRef]
- Santos, T.O.; Mazucanti, C.H.; Xavier, G.F.; Torrão, A.S. Early and late neurodegeneration and memory disruption after intracerebroventricular streptozotocin. Physiol. Behav. 2012, 107, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Pan, X.; Cui, J.; Ren, Y.; Zhang, J. Hyperphosphorylation of tau protein in hippocampus of central insulin-resistant rats is associated with cognitive impairment. Cell Physiol. Biochem. 2013, 32, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ma, J.; Liu, Z.; Lu, Y.; Hu, B.; Yu, H. Effect of naringenin on brain insulin signaling and cognitive functions in ICV-STZ induced dementia model of rats. Neurol. Sci. 2014, 35, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Deminiere, J.M.; Piazza, P.V.; Le Moal, M.; Simon, H. Experimental approach to individual vulnerability to psychostimulant addiction. Neurosci. Behav. Rev. 1989, 13, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Bradberry, C.W.; Gruen, R.J.; Berridge, C.W.; Roth, R.H. Individual differences in behavioral measures: Correlations with nucleus accumbens dopamine measured by microdialysis. Pharmacol. Biochem. Behav. 1991, 39, 877–882. [Google Scholar] [CrossRef]
- Hooks, M.S.; Jones, G.H.; Smith, A.D.; Neill, D.B.; Justice, J.B., Jr. Individual differences in locomotor activity and sensitization. Pharmacol. Biochem. Behav. 1991, 38, 467–470. [Google Scholar] [CrossRef]
- Piazza, P.V.; Rougé-Pont, F.; Deminière, J.M.; Kharoubi, M.; Le Moal, M.; Simon, H. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Res. 1991, 567, 169–174. [Google Scholar] [CrossRef]
- Cools, A.R.; Gingras, M.A. Nijmegen high and low responders to novelty: A new tool in the search after the neurobiology of drug abuse liability. Pharmacol. Biochem. Behav. 1998, 60, 151–159. [Google Scholar] [CrossRef]
- Basu, S.; Dasgupta, P.S. Dopamine, a neurotransmitter, influences the immune system. J. Neuroimmunol. 2000, 102, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Wrona, D.; Jurkowski, M.; Luszawska, D.; Tokarski, J.; Trojniar, W. The effects of lateral hypothalamic lesions on peripheral blood natural killer cell cytotoxicity in rats hyper- and hyporesponsive to novelty. Brain Behav. Immun. 2003, 17, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Wrona, D.; Jurkowski, M.K.; Tokarski, J. Blood and spleen natural killer cell cytotoxicity after exposure to open field stress in rats: Effect of spontaneous locomotor activity. J. Neuroimmunol. 2004, 150, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Wrona, D.; Sukiennik, L.; Jurkowski, M.K.; Jurkowlaniec, E.; Glac, W.; Tokarski, J. Effects of amphetamine on NK-related cytotoxicity in rats differing in locomotor reactivity and social position. Brain Behav. Immun. 2005, 19, 69–77. [Google Scholar] [CrossRef]
- Wrona, D.; Listowska, M.; Kubera, M.; Glac, W.; Grembecka, B.; Plucińska, K.; Majkutewicz, I.; Podlacha, M. Effects of chronic desipramine pretreatment on open field-induced suppression of blood natural killer cell activity and cytokine response depend on the rat’s behavioral characteristics. J. Neuroimmunol. 2014, 268, 13–24. [Google Scholar] [CrossRef]
- Bannerman, D.M.; Rawlins, J.N.; McHugh, S.B.; Deacon, R.M.; Yee, B.K.; Bast, T.; Zhang, W.N.; Pothuizen, H.H.; Feldon, J. Regional dissociations within the hippocampus—Memory and anxiety. Neurosci. Biobehav. Rev. 2004, 28, 273–283. [Google Scholar] [CrossRef]
- Bannerman, D.M.; Sprengel, R.; Sanderson, D.J.; McHugh, S.B.; Rawlins, J.N.; Monyer, H.; Seeburg, P.H. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat. Rev. Neurosci. 2014, 15, 181–192. [Google Scholar] [CrossRef]
- Hong, I.; Kaang, B.K. The complexity of ventral CA1 and its multiple functionalities. Genes Brain Behav. 2022, 21, e12826. [Google Scholar] [CrossRef]
- Richmond, M.A.; Yee, B.K.; Pouzet, B.; Veenman, L.; Rawlins, J.N.; Feldon, J.; Bannerman, D.M. Dissociating context and space within the hippocampus: Effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behav. Neurosci. 1999, 113, 1189–1203. [Google Scholar] [CrossRef]
- Phillips, M.L.; Robinson, H.A.; Pozzo-Miller, L. Ventral hippocampal projections to the medial prefrontal cortex regulate social memory. eLife 2019, 8, e44182. [Google Scholar] [CrossRef]
- Jimenez, J.C.; Berry, J.E.; Lim, S.C.; Ong, S.K.; Kheirbek, M.A.; Hen, R. Contextual fear memory retrieval by correlated ensembles of ventral CA1neurons. Nat. Commun. 2020, 11, 3492. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000, 23, 155–184. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, K.; Yoshida, K.; Suzuki, T.; Kobayashi, K.; Yoshida, K.; Mimura, M.; Tanaka, K.F. Activation of ventral CA1 hippocampal neurons projecting to the lateral septum during feeding. Hippocampus 2021, 31, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Brog, J.S.; Salyapongse, A.; Deutch, A.Y.; Zahm, D.S. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: Immunohistochemical detection of retrogradely transported fluoro-gold. J. Comp. Neurol. 1993, 338, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Mercado, D.; Padilla-Coreano, N.; Quirk, G. Dissociable roles of prelimbic and infralimbic cortices, ventral Hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 2010, 36, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, M.; Piazza, P.V. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur. J. Neurosci. 2002, 16, 387–394. [Google Scholar] [CrossRef]
- Simon, H.; Taghzouti, K.; Gozlan, H.; Studler, J.M.; Louilot, A.; Herve, D.; Glowinski, J.; Tassin, J.P.; Le Moal, M. Lesion of dopaminergic terminals in the amygdala produces enhanced locomotor response to Damphetamine and apposite changes in dopaminergic activity in prefrontal cortex and nucleus accumbens. Brain Res. 1988, 447, 335–340. [Google Scholar] [CrossRef]
- Meyer, M.E.; Hartesveldt, C.; Potter, T.J. Locomotor activity following intra-accumbens microinjections of dopamine D1 agonist SK and F 38393 in rats. Synapse 1993, 13, 310–314. [Google Scholar] [CrossRef]
- Iordanova, M.D.; Westbrook, R.F.; Killcross, A.S. Dopamine activity in the nucleus accumbens modulates blocking in fear conditioning. Eur. J. Neurosci. 2006, 24, 3265–3270. [Google Scholar] [CrossRef]
- Brauer, K.; Hausser, M.; Hartig, W.; Arendt, T. The core-shell dichotomy of nucleus accumbens in the rhesus monkey as revealed by double-immunofluorescence and morphology of cholinergic interneurons. Brain Res. 2000, 858, 151–162. [Google Scholar] [CrossRef]
- Jensen, J.; McIntosh, A.R.; Crawley, A.; Mikulis, D.J.; Remington, G.; Kapur, S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron 2003, 40, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Levita, L.; Dalley, J.W.; Robbins, T.W. Nucleus accumbens dopamine and learned fear revisited: A review and some new findings. Behav. Brain Res. 2002, 137, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Beaver, J.; Halcomb, C.; Jasnow, A. Dissociable roles of the nucleus accumbens core and shell subregions in the expression and extinction of conditioned fear. Neurobiol. Stress 2021, 15, 100365. [Google Scholar] [CrossRef] [PubMed]
- Sandi, C. Glucocorticoids act on glutamatergic pathways to affect memory processes. Trends Neurosci. 2011, 34, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Piazza, P.V.; Rouge-Pont, F.; Deroche, V.; Maccari, S.H.; LeMoal, M. Glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proc. Natl Acad. Sci. USA 1996, 93, 8716–8720. [Google Scholar] [CrossRef]
- Piazza, P.V.; Le Moal, M. Glucocorticoids as a biological substrate of reward: Physiological and pathophysiological implications. Brain Res. Rev. 1997, 25, 359–372. [Google Scholar] [CrossRef]
- Rohleder, N.; Wolf, J.M.; Kirschbaum, C.; Wolf, O.T. Effects of cortisol on emotional but not on neutral memory are correlated with peripheral glucocorticoid sensitivity of inflammatory cytokine production. Int. J. Psychophysiol. 2009, 72, 74–80. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kiko, T.; Kuriwada, S.; Miyazawa, T.; Kimura, F.; Miyazawa, T. Amyloid β induces adhesion of erythrocytes to endothelial cells and affects endothelial viability and functionality. Biosci. Biotechnol. Biochem. 2011, 75, 2030–2033. [Google Scholar] [CrossRef]
- Pitychoutis, P.M.; Sanoudou, D.; Papandreou, M.; Nasias, D.; Kouskou, M.; Tomlinson, C.R.; Tsonis, P.A.; Papadopoulou-Daifoti, Z. Forced swim test induces divergent global transcriptomic alterations in the hippocampus of high versus low novelty-seeker rats. Hum. Genom. 2014, 8, 4. [Google Scholar] [CrossRef]
- Luo, J.; Thomassen, J.Q.; Bellenguez, C.; Grenier-Boley, B.; de Rojas, I.; Castillo, A.; Parveen, K.; Küçükali, F.; Nicolas, A.; Peters, O.; et al. Genetic associations between modifiable risk factors and Alzheimer disease. JAMA Netw. Open 2023, 6, e2313734. [Google Scholar] [CrossRef]
- Wrona, D.; Majkutewicz, I.; Świątek, G.; Dunacka, J.; Grembecka, B.; Glac, W. Dimethyl fumarate as the peripheral blood inflammatory mediators inhibitor in prevention of streptozotocin-induced neuroinflammation in aged rats. J. Neuroinflamm. Res. 2022, 15, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: San Diego, CA, USA, 2007; ISBN 978-0-0804-7515-8. [Google Scholar]
- Salkovic-Petrisic, M.; Osmanovic-Barilar, J.; Bruckner, M.K.; Hoyer, S.; Arendt, T.; Riederer, P. Cerebral amyloid angiopathy in streptozotocin rat model of sporadic Alzheimer’s disease: A long-term follow up study. J. Neural. Transm. 2011, 118, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Bloch, K.; Gil-Ad, I.; Vanichkin, A.; Hornfeld, S.; Koroukhov, N.; Taler, M.; Vardi, P.; Weizman, A. Intracerebroventricular streptozotocin induces obesity and dementia in Luis rats. J Alzheimer’s Dis. 2017, 60, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Podlacha, M.; Glac, W.; Listowska, M.; Grembecka, B.; Majkutewicz, I.; Myślińska, D.; Plucińska, K.; Jerzemowska, G.; Grzybowska, M.; Wrona, D. Medial septal NMDA glutamate receptors are involved in modulation of blood natural killer cell activity in rats. J. Neuroimmune Pharmacol. 2016, 11, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Clamage, D.; Sanford, C.; Vander, A.; Mouw, D. Effects of psychosocial stimuli on plasma renin in rats. Am. J. Physiol. 1976, 231, 1290–1294. [Google Scholar] [CrossRef]
- Majkutewicz, I.; Kurowska, E.; Podlacha, M.; Myślińska, D.; Grembecka, B.; Ruciński, J.; Plucińska, K.; Jerzemowska, G.; Wrona, D. Dimethyl fumarate attenuates intracerebroventricular streptozotocin-induced spatial memory impairment and hippocampal neurodegeneration in rats. Behav. Brain Res. 2016, 308, 24–37. [Google Scholar] [CrossRef]
- Slepko, N.; Levi, G. Progressive activation of adult microglia cells in vitro. Glia 1996, 16, 241–246. [Google Scholar] [CrossRef]
- Schnieder, T.P.; Trenceska, I.; Rosoklia, G.; Stankov, A.; Mann, J.J.; Smiley, J.; Dwork, A.J. Microglia of prefrontal white matter in suicide. J. Neuropathol. Exp. 2014, 73, 880–890. [Google Scholar] [CrossRef]
- Listowska, M.; Glac, W.; Grembecka, B.; Grzybowska, M.; Wrona, D. Change in blood CD4+T and CD8+T lymphocytes in stressed rats pretreated chronically with desipramine are more pronounced after chronic open field stress challenge. J. Neuroimmunol. 2015, 282, 54–62. [Google Scholar] [CrossRef]
- Ulrich-LAI, Y.; Herman, J. Neural regulation of endocrine and autonomic stress response. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef]
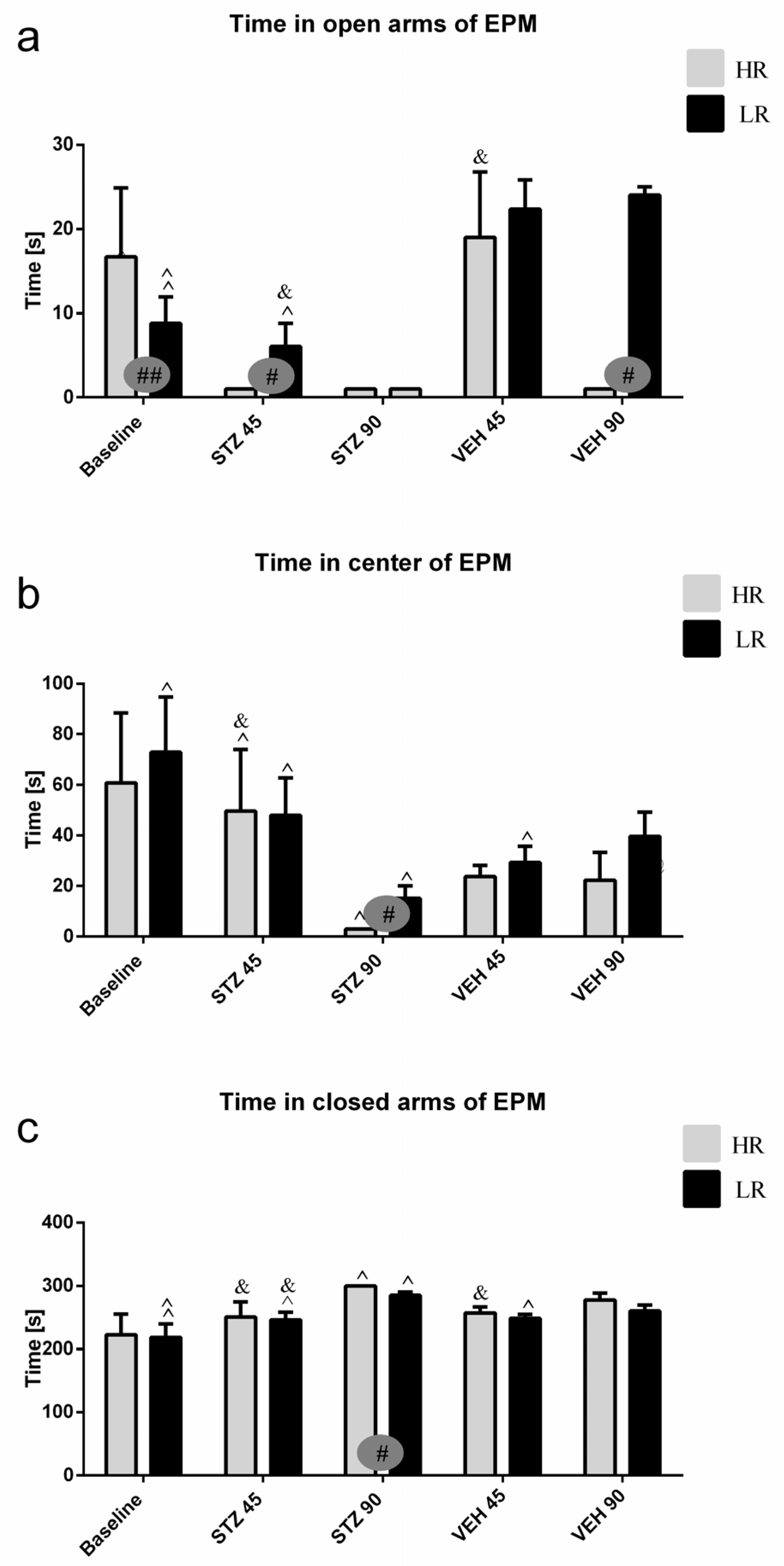



| Parameter/Group | Rearing (No.) | Grooming (No.) | Time at Periphery (s) | Time at the Center (s) | Center Entries (No.) | Miction (No.) | Defecation (No.) | |
|---|---|---|---|---|---|---|---|---|
| Baseline Mean ± SD | Non-divided | 14.39 ± 6.66 | 2.70 ± 1.11 | 1791.7 ± 7.70 | 8.3 ± 7.70 | 4.67 ± 4.32 | 5.69 ± 2.55 | 4.75 ± 2.18 ## |
| HR | 14.71 ± 6.47 | 2.71 ± 0.76 | 1798.67 ± 0.58 # | 1.33 ± 0.58 # | 1.33 ± 0.58 # | 4.14 ± 1.68 | 3.25 ± 0.5 | |
| LR | 15.91 ± 7.22 | 2.82 ± 1.08 | 1784.73 ± 1.55 | 15.27 ± 1.55 | 8. ± 3.61 | 6.36 ± 3.04 | 5.67 ± 2.35 | |
| STZ 45 Mean ± SD | Non-divided | 10.25 ± 3.23 **&&& | 2.43 ± 0.79 | 1800 ± 0 @@ | 0 ± 0 @@ | 0 ± 0 @@ | 1.63 ± 0.52 *@@@ | 3.63 ± 1.60 * |
| HR | 10 ± 1 $& | 3 ± 0 $#& | 1800 ± 0 @ | 0 ± 0 @ | 0 ± 0 @ | 1.66 ± 0.58 @ | 4.33 ± 1.15 $ | |
| LR | 10.75 ± 4.92 & | 1.67 ± 0.58 $ | 1800 ± 0 @ | 0 ± 0 @ | 0 ± 0 @ | 1.75 ± 0.5 $;@ | 3.75 ± 1.5 | |
| STZ 90 Mean ± SD | Non-divided | 3 ± 1.41 @@@ | 1.71 ± 0.49 @ | 1800 ± 0 @@ | 0 ± 0 @@ | 0 ± 0 @@ | 1.17 ± 0.41 @@@ | 4 ± 1.26 * |
| HR | 3 ± 1.41 @ | 1.67 ± 0.58 | 1800 ± 0 @ | 0 ± 0 @ | 0 ± 0 @ | 1.33 ± 0.58 @ | 3 ± 0 # | |
| LR | 3 ± 1.41 @@ | 1.75 ± 0.5 | 1800 ± 0 @ | 0 ± 0 @ | 0 ± 0 @ | 1 ± 0 $@@ | 5 ± 1 | |
| VEH 45 Mean ± SD | Non-divided | 6.2 ± 1.87 &&&@@@ | 3.13 ± 2.36 | 1800 ± 0 @@@ | 0 ± 0 @@@ | 0 ± 0 @@@ | 1.08 ± 0.29 @@@ | 2.13 ± 1.55 @@ |
| HR | 4.5 ± 0.58 #&@@ | 1 ± 0 #;@ | 1800 ± 0 @ | 0 ± 0 @ | 0 ± 0 @ | 1.33 ± 0.58 @ | 1 ± 0 #;@ | |
| LR | 8 ± 1.73 &@ | 4.4 ± 2.07 & | 1800 ± 0 @ | 0 ± 0 @ | 0 ± 0 @ | 1 ± 0 &&;@@ | 3.25 ± 1.5 | |
| VEH 90 Mean ± SD | Non-divided | 2.71 ± 0.95 @@@ | 1.7 ± 0.67 @ | 1800 ± 0 @@@ | 0 ± 0 @@@ | 0 ± 0 @@@ | 0.67 ± 0.65 @@@ | 2.55 ± 1.04 @@ |
| HR | 3 ± 0 @ | 1.33 ± 0.58 @ | 1800 ± 0 @ | 0 ± 0 @ | 0 ± 0 @ | 1.25 ± 0.5 ##@@ | 2.25 ± 1.26 | |
| LR | 2 ± 1 @@ | 1.8 ± 0.84 | 1800 ± 0 @ | 0 ± 0 @ | 0 ± 0 @ | 0 ± 0 @@ | 3 ± 1.15 @ | |
| (a) | Latency to Reach the Platform (s) | ||||
| Group | Trial 1 | Trial 2 | Trial 3 | Trial 4 | |
| Baseline | 92.02 ± 30.26 | 7.51 ± 3.9 ^^^ | 5.28 ± 2.6 %% | 4.78 ± 2.1 | |
| STZ 45 | 100.43 ± 19.78 ***&&& | 86.08 ± 27.12 ***&&&@@^ | 78.09 ± 35.93 ***&&&@@@ | 82.98 ± 29.89 ***&&&@@@ | |
| STZ 90 | 25.43 ± 9.84 ***@@@ | 19.73 ± 10.68 ***@@@^ | 22.03 ± 14.32 ***@@@ | 20.18 ± 13.75 ***@@@ | |
| VEH 45 | 36.75 ± 11.08 &&&@@@ | 6.68 ± 3.37 &&&^^^ | 3.41 ± 1.37 @@%%% | 2.88 ± 1.26 @@@! | |
| VEH 90 | 10.89 ± 6.41 @@@ | 3.73 ± 1.43 @@@^^^ | 3.28 ± 1.35 @@@ | 2.83 ± 1.05 @@@ | |
| Baseline HR | 90.68 ± 28.26 | 6.08 ± 2.58 #^^^ | 4.95 ± 2.38 % | 5.08 ± 2.41 | |
| Baseline LR | 93.37 ± 32.68 | 8.95 ± 4.50 ^^^ | 5.6 ± 2.8 %% | 4.48 ± 1.73 | |
| STZ 45 HR | 99.63 ± 20.62 $$$&&& | 90.38 ± 27.43 $$$&&&@@@ | 81.57 ± 37.53 $$$&&&@@@ | 87.4 ± 25.53 $$$&&&@@@ | |
| STZ 45 LR | 101.22 ± 19.77 $$$&&& | 81.78 ± 27.30 $$$&&&@@@^ | 74.62 ± 35.56 $$$&&@@@ | 78.55 ± 34.25 $$$&&@@@ | |
| STZ 90 HR | 22.35 ± 8.87 @@@ | 12.33 ± 5.4 $$$###@@@^^ | 10.95 ± 5.09 $$$###@@@ | 7.9 ± 2.79 $$$#@@ | |
| STZ 90 LR | 28.52 ± 10.15 $$$@@@ | 27.42 ± 9.06 $$$@@@ | 33.5 ± 10.77 $$$@@@ | 32.47 ± 7.66 @@@ | |
| VEH 45 HR | 39.88 ± 10.89 &&&@@@ | 8.45 ± 3.7 &&#@^^^ | 3.64 ± 1.76 %% | 3.32 ± 1.57 @@@ | |
| VEH 45 LR | 33.60 ± 10.80 &&&@@@ | 4.9 ± 1.8 @@^^^ | 3.20 ± 0.87 @@% | 2.45 ± 0.68 @@@! | |
| VEH 90 HR | 16.15 ± 4.35 ###@@@ | 3.87 ± 1.65 @@^^ | 3.37 ± 1.24 @ | 2.53 ± 0.81 @ | |
| VEH 90 LR | 5.62 ± 2.56 @@@ | 3.6 ± 1.22 @@@^ | 3.2 ± 1.49 @@ | 3.12 ± 1.22 @ | |
| (b) | Latency to Reach the Platform (s) | Total Distance Swum (cm) | |||
| Group | Day 1 | Day 2 | Day 3 | Day 1 | |
| Baseline | 97.32 ± 44.71 | 26.58 ± 12.90 ^^^ | 20.39 ± 12.35 ^^^% | 625.23 ± 293.05 | |
| STZ 45 | 42.23 ± 17.02 ***&@@ | 10.97 ± 3.38 *@@@^^ | 11.09 ± 7.93 **@^^ | 715.74 ± 324.31 ***& | |
| STZ 90 | 23.79 ± 8.89 ***@@@ | 17.53 ± 8.56 ***@ | 13.16 ± 9.60 **^ | 428.65 ± 114.29 ***@ | |
| VEH 45 | 8.52 ± 1.81 &@@@ | 6.54 ± 4.83 @@@^ | 4.64 ± 2.32 @@@^^ | 255.83 ± 141.97 @@@ | |
| VEH 90 | 5.75 ± 2.28 @@@ | 4.36 ± 1.99 @@@ | 3.69 ± 1.24 @@@^^ | 148.40 ± 41.49 @@@ | |
| Baseline HR | 100.98 ± 44.66 | 22.7 ± 9.5 ^^ | 17.15 ± 5.97 ^^^ | 643.96 ± 280.13 | |
| Baseline LR | 86.38 ± 27.86 | 26.85 ± 10.63 ^^^ | 17.2 ± 8.27 ^^^% | 578.88 ± 280.76 | |
| STZ 45 HR | 28.87 ± 10.25 $#@ | 13.77 ± 3.7 $#^ | 17.92 ± 7.98 $# | 426.02 ± 158.81 # | |
| STZ 45 LR | 51.2 ± 16.99 $& | 8.9 ± 0.35 @@^ | 6.02 ± 1.52 &@^% | 993.47 ± 251.72 $&@ | |
| STZ 90 HR | 30.62 ± 0.08 $#@ | 18.38 ± 10.13 $^ | 13.63 ± 6 $#^ | 504.45 ± 66.46 $ | |
| STZ 90 LR | 13.2 ± 2.85 $@@ | 10.72 ± 2.33 $@@ | 3.97 ± 0.21 @@^% | 374.04 ± 124.45 $ | |
| VEH 45 HR | 9.66 ± 0.65 #&&@@ | 4.28 ± 2.04 @@^ | 3.85 ± 2.23 @@^ | 339.36 ± 157.47 &##@ | |
| VEH 45 LR | 7.75 ± 1.96 @@@ | 8.05 ± 5.72 @@ | 5.17 ± 2.43 @@@ | 158.81 ± 28.66 @@@ | |
| VEH 90 HR | 5.88 ± 2.15 @@ | 4.53 ± 2.17 @@ | 3.59 ± 1.31 @@ | 152.67 ± 46.93 @@ | |
| VEH 90 LR | 5.66 ± 2.54 @@@ | 4.24 ± 2.02 @@@ | 3.76 ± 1.28 @@@ | 145.96 ± 41.82 @@@ | |
| (a) | Microglia Cells (CD68+) No./mm2 | ||||||
| Group/Structure | STZ Mean ± SD | VEH Mean ± SD | STZ HR Mean ± SD | STZ LR Mean ± SD | VEH HR Mean ± SD | VEH LR Mean ± SD | |
| Prefrontal cortex | 117 ± 41 *** | 0 ± 0 | 133 ± 58 $ | 100 ± 0 $ | 0 ± 0 | 0 ± 0 | |
| Corpus callosum | 1018 ± 268 *** | 280 ± 131 | 1000 ± 322 $$ | 1000 ± 23 $$ | 300 ± 115 | 267 ± 170 | |
| Caudate putamen | 463 ± 207 ** | 100 ± 0 | 325 ± 50 $ | 533 ± 208 $ | 100 ± 0 | 100 ± 0 | |
| Medial septal nucleus | 473 ± 142 *** | 0 ± 0 | 400 ± 155 $$ | 550 ± 58 $$ | 0 ± 0 | 0 ± 0 | |
| Lateral preoptic area | 200 ± 87 *** | 0 ± 0 | 175 ± 50 $$ | 200 ± 115 $ | 0 ± 0 | 0 ± 0 | |
| Medial preoptic nucleus | 244 ± 124 *** | 0 ± 0 | 225 ± 150 $ | 250 ± 129 $ | 0 ± 0 | 0 ± 0 | |
| Supraoptic nucleus | 189 ± 93 * | 100 ± 0 | 150 ± 58 | 200 ± 115 | 100 ± 0 | 100 ± 0 | |
| Arcuate hypothalamic nucleus | 464 ± 225 ** | 100 ± 65 | 767 ± 58 | 300 ± 0 | 0 ± 0 | 233 ± 115 | |
| Dorsomedial hypothalamic nucleus | 220 ± 103 *** | 0 ± 0 | 300 ± 71 #$$ | 125 ± 50 $ | 0 ± 0 | 0 ± 0 | |
| Lateral hypothalamus | 220 ± 79 *** | 0 ± 0 | 250 ± 58 $$ | 133 ± 58 $ | 0 ± 0 | 0 ± 0 | |
| Paraventricular hypothalamic nucleus | 163 ± 74 *** | 0 ± 0 | 160 ± 89 $$ | 167 ± 58 $ | 0 ± 0 | 0 ± 0 | |
| Ventromedial hypothalamic nucleus | 113 ± 35 *** | 0 ± 0 | 133 ± 58 $ | 100 ± 0 $ | 0 ± 0 | 0 ± 0 | |
| (b) | Group/Structure | Amyloid β (40–42)/No./mm2 | |||||
| STZ Mean ± SD | VEH Mean ± SD | STZ HR Mean ± SD | STZ LR Mean ± SD | VEH HR Mean ± SD | VEH LR Mean ± SD | ||
| Prefrontal cortex | 2.5 ± 2.25 | 0 ± 0 | 5 ± 2.75 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Lateral preoptic area | 8.9 ± 1.65 ** | 0 ± 0 | 8.3 ± 2.05 $ | 1 ± 0 $ | 0 ± 0 | 0 ± 0 | |
| Medial preoptic nucleus | 5 ± 2.6 | 0 ± 0 | 6.67 ± 2.6 | 3.33 ± 2.6 | 0 ± 0 | 0 ± 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunacka, J.; Świątek, G.; Wrona, D. High Behavioral Reactivity to Novelty as a Susceptibility Factor for Memory and Anxiety Disorders in Streptozotocin-Induced Neuroinflammation as a Rat Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 11562. https://doi.org/10.3390/ijms252111562
Dunacka J, Świątek G, Wrona D. High Behavioral Reactivity to Novelty as a Susceptibility Factor for Memory and Anxiety Disorders in Streptozotocin-Induced Neuroinflammation as a Rat Model of Alzheimer’s Disease. International Journal of Molecular Sciences. 2024; 25(21):11562. https://doi.org/10.3390/ijms252111562
Chicago/Turabian StyleDunacka, Joanna, Grzegorz Świątek, and Danuta Wrona. 2024. "High Behavioral Reactivity to Novelty as a Susceptibility Factor for Memory and Anxiety Disorders in Streptozotocin-Induced Neuroinflammation as a Rat Model of Alzheimer’s Disease" International Journal of Molecular Sciences 25, no. 21: 11562. https://doi.org/10.3390/ijms252111562
APA StyleDunacka, J., Świątek, G., & Wrona, D. (2024). High Behavioral Reactivity to Novelty as a Susceptibility Factor for Memory and Anxiety Disorders in Streptozotocin-Induced Neuroinflammation as a Rat Model of Alzheimer’s Disease. International Journal of Molecular Sciences, 25(21), 11562. https://doi.org/10.3390/ijms252111562







