The Variability of the Salivary Antimicrobial Peptide Profile: Impact of Lifestyle
Abstract
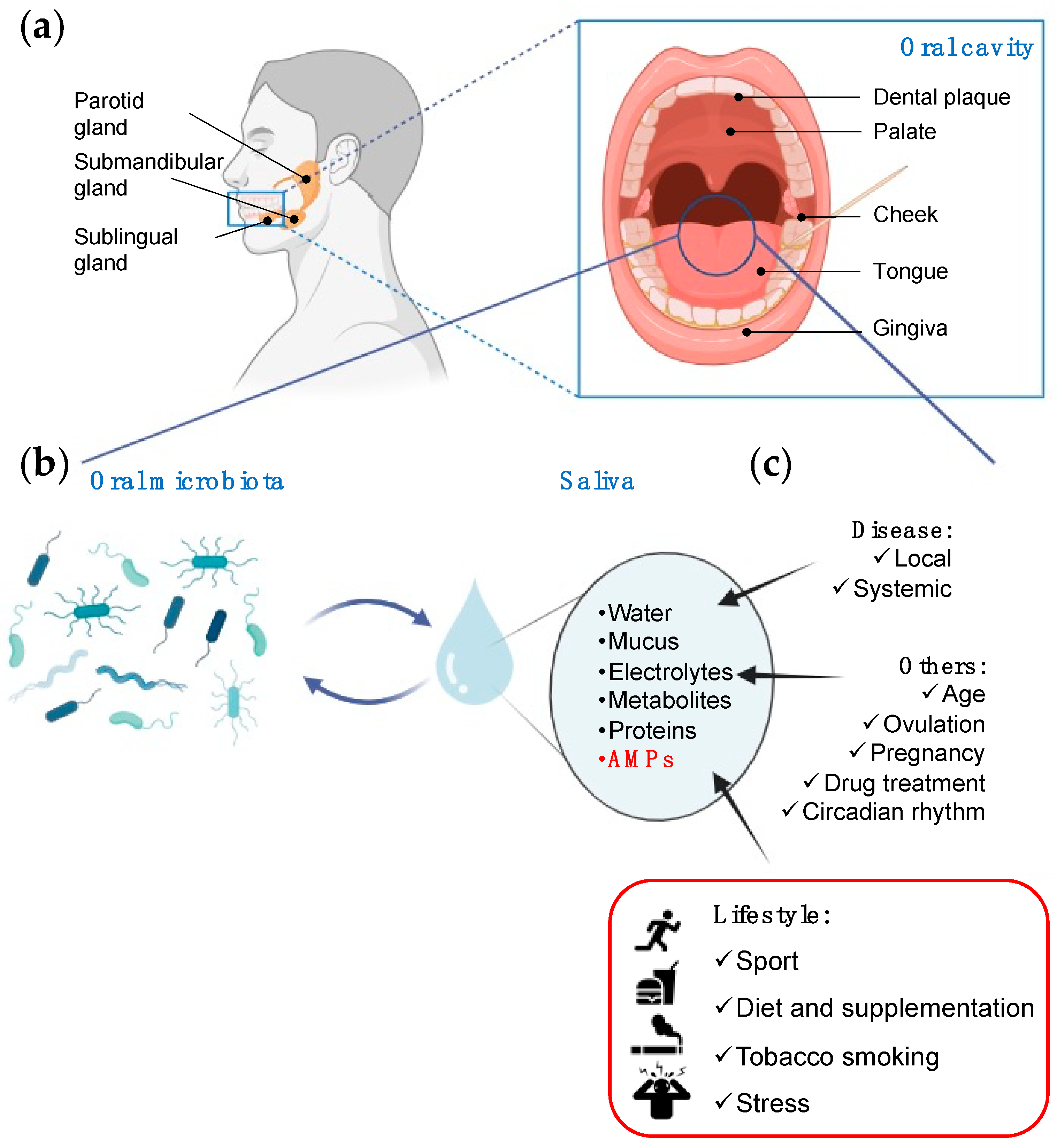
1. Introduction
2. The Interplay Between Saliva and Microbiota
3. Salivary AMPs
4. Salivary AMPs as Potential Biomarkers
5. The Effect of Lifestyle on AMP Expression Profiles
5.1. Physical Activity
5.2. Diet Supplementation
5.2.1. Vitamin D
5.2.2. Bovine Colostrum, Fermented Milk, and Probiotics
5.2.3. Carbohydrates and Proteins
5.2.4. Vitamin A
5.3. Tobacco Smoking
5.4. Psychological Stress
6. Discussion
7. Concluding Remarks
- Physical activity: Acute physical exercise tends to temporarily increase salivary AMP levels, while prolonged physical activity seems to be associated with a reduction of them. This may be associated with the increased incidence of upper respiratory tract symptoms in athletes.
- Tobacco smoking: It induces a decrease in LL-37. This decrease could mask the increase in LL-37 correlated with periodontal diseases in smokers.
- Diet supplementation: Different supplements are associated with variations in specific salivary AMPs. For instance, vitamin D provokes an increase in LL-37 and vitamin A and a decrease in hβD-2; fermented milk decreases hβD-3 and increases hNPs (as in carbohydrate-supplemented diets).
- Psychological stress: it seems to increase some AMPs in saliva, though more studies are needed to confirm this trend.
Author Contributions
Funding
Conflicts of Interest
References
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Fábián, T.K.; Hermann, P.; Beck, A.; Fejérdy, P.; Fábián, G. Salivary Defense Proteins: Their Network and Role in Innate and Acquired Oral Immunity. Int. J. Mol. Sci. 2012, 13, 4295–4320. [Google Scholar] [CrossRef] [PubMed]
- Greabu, M.; Battino, M.; Mohora, M.; Totan, A.; Didilescu, A.; Spinu, T.; Totan, C.; Miricescu, D.; Radulescu, R. Saliva—A diagnostic window to the body, both in health and in disease. J. Med. Life 2009, 2, 124–132. [Google Scholar] [PubMed]
- Ferrari, E.; Gallo, M.; Spisni, A.; Antonelli, R.; Meleti, M.; Pertinhez, T.A. Human Serum and Salivary Metabolomes: Diversity and Closeness. Int. J. Mol. Sci. 2023, 24, 16603. [Google Scholar] [CrossRef]
- Zhang, C.-Z.; Cheng, X.-Q.; Li, J.-Y.; Zhang, P.; Yi, P.; Xu, X.; Zhou, X.-D. Saliva in the diagnosis of diseases. Int. J. Oral Sci. 2016, 8, 133–137. [Google Scholar] [CrossRef]
- Roi, A.; Rusu, L.C.; Roi, C.I.; Luca, R.E.; Boia, S.; Munteanu, R.I. A New Approach for the Diagnosis of Systemic and Oral Diseases Based on Salivary Biomolecules. Dis. Markers 2019, 2019, 8761860. [Google Scholar] [CrossRef]
- Prescott, S.L. History of medicine: Origin of the term microbiome and why it matters. Hum. Microbiome J. 2017, 4, 24–25. [Google Scholar] [CrossRef]
- Pathak, J.L.; Yan, Y.; Zhang, Q.; Wang, L.; Ge, L. The role of oral microbiome in respiratory health and diseases. Respir. Med. 2021, 185, 106475. [Google Scholar] [CrossRef]
- Baker, J.L.; Mark Welch, J.L.; Kauffman, K.M.; McLean, J.S.; He, X. The oral microbiome: Diversity, biogeography and human health. Nat. Rev. Microbiol. 2024, 22, 89–104. [Google Scholar] [CrossRef]
- Lynge Pedersen, A.M.; Belstrøm, D. The role of natural salivary defences in maintaining a healthy oral microbiota. J. Dent. 2019, 80 (Suppl. 1), S3–S12. [Google Scholar] [CrossRef]
- Gorr, S.-U.; Abdolhosseini, M. Antimicrobial peptides and periodontal disease. J. Clin. Periodontol. 2011, 38, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Vila, T.; Rizk, A.M.; Sultan, A.S.; Jabra-Rizk, M.A. The power of saliva: Antimicrobial and beyond. PLoS Pathog. 2019, 15, e1008058. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Sun, J.; Zhou, M.; Zhou, J.; Lao, X.; Zheng, H.; Xu, H. DRAMP: A comprehensive data repository of antimicrobial peptides. Sci. Rep. 2016, 6, 24482. [Google Scholar] [CrossRef] [PubMed]
- Satchanska, G.; Davidova, S.; Gergova, A. Diversity and Mechanisms of Action of Plant, Animal, and Human Antimicrobial Peptides. Antibiotics 2024, 13, 202. [Google Scholar] [CrossRef]
- Boparai, J.K.; Sharma, P.K. Mini Review on Antimicrobial Peptides, Sources, Mechanism and Recent Applications. Protein Pept. Lett. 2020, 27, 4–16. [Google Scholar] [CrossRef]
- Ciociola, T.; Pertinhez, T.A.; De Simone, T.; Magliani, W.; Ferrari, E.; Belletti, S.; D’Adda, T.; Conti, S.; Giovati, L. In Vitro and In Vivo Anti-Candida Activity and Structural Analysis of Killer Peptide (KP)-Derivatives. J. Fungi 2021, 7, 129. [Google Scholar] [CrossRef]
- Bischetti, M.; Alaimo, N.; Nardelli, F.; Punzi, P.; Amariei, C.; Ingenito, R.; Musco, G.; Gallo, M.; Cicero, D.O. Structural insights on the selective interaction of the histidine-rich piscidin antimicrobial peptide Of-Pis1 with membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 2023, 1865, 184080. [Google Scholar] [CrossRef]
- Jönsson, D.; Nilsson, B.O. The antimicrobial peptide LL-37 is anti-inflammatory and proapoptotic in human periodontal ligament cells. J. Periodontal Res. 2012, 47, 330–335. [Google Scholar] [CrossRef]
- Säll, J.; Carlsson, M.; Gidlöf, O.; Holm, A.; Humlén, J.; Ohman, J.; Svensson, D.; Nilsson, B.-O.; Jönsson, D. The antimicrobial peptide LL-37 alters human osteoblast Ca2+ handling and induces Ca2+-independent apoptosis. J. Innate Immun. 2013, 5, 290–300. [Google Scholar] [CrossRef]
- Tao, R.; Jurevic, R.J.; Coulton, K.K.; Tsutsui, M.T.; Roberts, M.C.; Kimball, J.R.; Wells, N.; Berndt, J.; Dale, B.A. Salivary antimicrobial peptide expression and dental caries experience in children. Antimicrob. Agents Chemother. 2005, 49, 3883–3888. [Google Scholar] [CrossRef]
- Bishop, N.C.; Gleeson, M. Acute and chronic effects of exercise on markers of mucosal immunity. Front. Biosci. (Landmark Ed.) 2009, 14, 4444–4456. [Google Scholar] [CrossRef] [PubMed]
- Castro-Quintas, Á.; Palma-Gudiel, H.; San Martín-González, N.; Caso, J.R.; Leza, J.C.; Fañanás, L. Salivary secretory immunoglobulin A as a potential biomarker of psychosocial stress response during the first stages of life: A systematic review. Front. Neuroendocrinol. 2023, 71, 101083. [Google Scholar] [CrossRef] [PubMed]
- Papacosta, E.; Nassis, G.P. Saliva as a tool for monitoring steroid, peptide and immune markers in sport and exercise science. J. Sci. Med. Sport 2011, 14, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Hertel, S.; Hannig, C.; Sterzenbach, T. The abundance of lysozyme, lactoferrin and cystatin S in the enamel pellicle of children-Potential biomarkers for caries? Arch. Oral Biol. 2023, 146, 105598. [Google Scholar] [CrossRef]
- Grant, M.; Kilsgård, O.; Åkerman, S.; Klinge, B.; Demmer, R.T.; Malmström, J.; Jönsson, D. The Human Salivary Antimicrobial Peptide Profile according to the Oral Microbiota in Health, Periodontitis and Smoking. J. Innate Immun. 2019, 11, 432–444. [Google Scholar] [CrossRef]
- Gorr, S.U. Antimicrobial peptides in periodontal innate defense. Front. Oral Biol. 2012, 15, 84–98. [Google Scholar] [CrossRef]
- Saitoh, E.; Taniguchi, M.; Ochiai, A.; Kato, T.; Imai, A.; Isemura, S. Bioactive peptides hidden in human salivary proteins. J. Oral Biosci. 2017, 59, 71–79. [Google Scholar] [CrossRef]
- Mori, M.; Takeuchi, H.; Sato, M.; Sumitomo, S. Antimicrobial Peptides in Saliva and Salivary Glands: Their Roles in the Oral Defense System. Oral Med. Pathol. 2006, 11, 1–17. [Google Scholar] [CrossRef]
- Khurshid, Z.; Naseem, M.; Sheikh, Z.; Najeeb, S.; Shahab, S.; Zafar, M.S. Oral antimicrobial peptides: Types and role in the oral cavity. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2016, 24, 515–524. [Google Scholar] [CrossRef]
- Demir, B.; Çiçek, D.; Erden, İ.; Aydın, S.; Üçer, Ö.; Kuloğlu, T.; Kalaycı, M.; Yardım, M.; Yüksel, E. Investigation of serum and saliva dermcidin levels in patients with recurrent aphthous stomatitis and dermcidin analysis in salivary gland. Mucosa 2021, 4, 10–16. [Google Scholar] [CrossRef]
- Jenssen, H.; Hamill, P.; Hancock, R.E. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Güncü, G.N.; Yilmaz, D.; Könönen, E.; Gürsoy, U.K. Salivary Antimicrobial Peptides in Early Detection of Periodontitis. Front. Cell. Infect. Microbiol. 2015, 5, 99. [Google Scholar] [CrossRef] [PubMed]
- West, N.P.; Pyne, D.B.; Kyd, J.M.; Renshaw, G.M.; Fricker, P.A.; Cripps, A.W. The effect of exercise on innate mucosal immunity. Br. J. Sport. Med. 2010, 44, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Prodan, A.; Brand, H.; Imangaliyev, S.; Tsivtsivadze, E.; van der Weijden, F.; de Jong, A.; Paauw, A.; Crielaard, W.; Keijser, B.; Veerman, E. A Study of the Variation in the Salivary Peptide Profiles of Young Healthy Adults Acquired Using MALDI-TOF MS. PLoS ONE 2016, 11, e0156707. [Google Scholar] [CrossRef]
- Sant’Anna, M.L.; Oliveira, L.T.; Gomes, D.V.; Marques, S.T.F.; Provance, D.W., Jr.; Sorenson, M.M.; Salerno, V.P. Physical exercise stimulates salivary secretion of cystatins. PLoS ONE 2019, 14, e0224147. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Nagasawa, T.; Katagiri, S.; Kitagawara, S.; Kobayashi, H.; Koyanagi, T.; Izumi, Y. Salivary levels of antibacterial peptide (LL-37/hCAP-18) and cotinine in patients with chronic periodontitis. J. Periodontol. 2012, 83, 766–772. [Google Scholar] [CrossRef]
- Pereira, A.L.; Franco, G.C.; Cortelli, S.C.; Aquino, D.R.; Costa, F.O.; Raslan, S.A.; Cortelli, J.R. Influence of periodontal status and periodontopathogens on levels of oral human β-defensin-2 in saliva. J. Periodontol. 2013, 84, 1445–1453. [Google Scholar] [CrossRef]
- Salazar, M.G.; Jehmlich, N.; Murr, A.; Dhople, V.M.; Holtfreter, B.; Hammer, E.; Völker, U.; Kocher, T. Identification of periodontitis associated changes in the proteome of whole human saliva by mass spectrometric analysis. J. Clin. Periodontol. 2013, 40, 825–832. [Google Scholar] [CrossRef]
- Yilmaz, D.; Topcu, A.O.; Akcay, E.U.; Altındis, M.; Gursoy, U.K. Salivary human beta-defensins and cathelicidin levels in relation to periodontitis and type 2 diabetes mellitus. Acta Odontol. Scand. 2020, 78, 327–331. [Google Scholar] [CrossRef]
- Kzar, W.A.; Abbas, R.F.; Hussein, H.M. The Antimicrobial Peptide LL-37 as a Predictor Biomarker for Periodontitis with the Presence and Absence of Smoking: A Case-Control Study. BioMed Res. Int. 2023, 2023, 5581267. [Google Scholar] [CrossRef]
- Rudney, J.D.; Staikov, R.K.; Johnson, J.D. Potential biomarkers of human salivary function: A modified proteomic approach. Arch. Oral Biol. 2009, 54, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Tanida, T.; Okamoto, T.; Okamoto, A.; Wang, H.; Hamada, T.; Ueta, E.; Osaki, T. Decreased excretion of antimicrobial proteins and peptides in saliva of patients with oral candidiasis. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2003, 32, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Davidopoulou, S.; Diza, E.; Menexes, G.; Kalfas, S. Salivary concentration of the antimicrobial peptide LL-37 in children. Arch. Oral Biol. 2012, 57, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.R.; Dria, K.J.; de Carvalho, C.B.M.; Monteiro, A.J.; Fonteles, M.C.; de Moraes Carvalho, K.; Fonteles, C.S.R. Salivary peptide profile and its association with early childhood caries. Int. J. Paediatr. Dent. 2013, 23, 225–234. [Google Scholar] [CrossRef]
- Colombo, N.H.; Ribas, L.F.; Pereira, J.A.; Kreling, P.F.; Kressirer, C.A.; Tanner, A.C.; Duque, C. Antimicrobial peptides in saliva of children with severe early childhood caries. Arch. Oral Biol. 2016, 69, 40–46. [Google Scholar] [CrossRef]
- Wongpanuwich, W.; Yodsanga, S.; Chaisuparat, R.; Amornphimoltham, P. Association Between PD-L1 and Histatin1, 3 Expression in Advanced Head and Neck Squamous Cell Carcinoma. Anticancer Res. 2022, 42, 2689–2699. [Google Scholar] [CrossRef]
- Contini, C.; Olianas, A.; Serrao, S.; Deriu, C.; Iavarone, F.; Boroumand, M.; Bizzarro, A.; Lauria, A.; Faa, G.; Castagnola, M.; et al. Corrigendum: Top-Down Proteomics of Human Saliva Highlights Anti-inflammatory, Antioxidant, and Antimicrobial Defense Responses in Alzheimer Disease. Front. Neurosci. 2021, 15, 743596. [Google Scholar] [CrossRef]
- Kaneda, Y.; Yamaai, T.; Mizukawa, N.; Nagatsuka, H.; Yamachika, E.; Gunduz, M.; Sawaki, K.; Yamanishi, Y.; Matsubara, M.; Katase, N.; et al. Localization of antimicrobial peptides human β-defensins in minor salivary glands with Sjögren’s syndrome. Eur. J. Oral Sci. 2009, 117, 506–510. [Google Scholar] [CrossRef]
- Coker, M.O.; Cairo, C.; Garzino-Demo, A. HIV-Associated Interactions Between Oral Microbiota and Mucosal Immune Cells: Knowledge Gaps and Future Directions. Front. Immunol. 2021, 12, 676669. [Google Scholar] [CrossRef]
- Granger, J.; Cho, E.; Lindsey, K.; Lemoine, N.; Calvert, D.; Marucci, J.; Mullenix, S.; O’Neal, H.; Irving, B.A.; Johannsen, N.; et al. Salivary immunity of elite collegiate American football players infected with SARS-CoV-2 normalizes following isolation. Sci. Rep. 2022, 12, 9090. [Google Scholar] [CrossRef]
- Theotonio dos Santos, L.F.; Barbeiro, H.V.; Barbeiro, D.F.; de Souza, H.P.; Pinheiro da Silva, F. Antimicrobial peptides and other potential biomarkers of critical illness in SARS-CoV-2 patients with acute kidney injury. AMPAKI-CoV study. Physiol. Rep. 2024, 12, e15945. [Google Scholar] [CrossRef] [PubMed]
- Alfaifi, A.; Sultan, A.S.; Montelongo-Jauregui, D.; Meiller, T.F.; Jabra-Rizk, M.A. Long-Term Post-COVID-19 Associated Oral Inflammatory Sequelae. Front. Cell. Infect. Microbiol. 2022, 12, 831744. [Google Scholar] [CrossRef] [PubMed]
- Olianas, A.; Guadalupi, G.; Cabras, T.; Contini, C.; Serrao, S.; Iavarone, F.; Castagnola, M.; Messana, I.; Onali, S.; Chessa, L.; et al. Top-Down Proteomics Detection of Potential Salivary Biomarkers for Autoimmune Liver Diseases Classification. Int. J. Mol. Sci. 2023, 24, 959. [Google Scholar] [CrossRef] [PubMed]
- He, C.S.; Bishop, N.C.; Handzlik, M.K.; Muhamad, A.S.; Gleeson, M. Sex differences in upper respiratory symptoms prevalence and oral-respiratory mucosal immunity in endurance athletes. Exerc. Immunol. Rev. 2014, 20, 8–22. [Google Scholar] [PubMed]
- Malcolm, J.; Sherriff, A.; Lappin, D.F.; Ramage, G.; Conway, D.I.; Macpherson, L.M.; Culshaw, S. Salivary antimicrobial proteins associate with age-related changes in streptococcal composition in dental plaque. Mol. Oral Microbiol. 2014, 29, 284–293. [Google Scholar] [CrossRef]
- Born, D.P.; Faiss, R.; Willis, S.J.; Strahler, J.; Millet, G.P.; Holmberg, H.C.; Sperlich, B. Circadian variation of salivary immunoglobin A, alpha-amylase activity and mood in response to repeated double-poling sprints in hypoxia. Eur. J. Appl. Physiol. 2016, 116, 1–10. [Google Scholar] [CrossRef]
- Saibaba, G.; Archunan, G. Does salivary protein(s) act an ovulation indicator for women? A hypothesis. Med. Hypotheses 2015, 84, 104–106. [Google Scholar] [CrossRef]
- Gürsoy, M.; Gürsoy, U.K.; Liukkonen, A.; Kauko, T.; Penkkala, S.; Könönen, E. Salivary antimicrobial defensins in pregnancy. J. Clin. Periodontol. 2016, 43, 807–815. [Google Scholar] [CrossRef]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative biology of exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef]
- Ntovas, P.; Loumprinis, N.; Maniatakos, P.; Margaritidi, L.; Rahiotis, C. The Effects of Physical Exercise on Saliva Composition: A Comprehensive Review. Dent. J. 2022, 10, 7. [Google Scholar] [CrossRef]
- Nakayasu, E.S.; Gritsenko, M.A.; Kim, Y.-M.; Kyle, J.E.; Stratton, K.G.; Nicora, C.D.; Munoz, N.; Navarro, K.M.; Claborne, D.; Gao, Y.; et al. Elucidating regulatory processes of intense physical activity by multi-omics analysis. Mil. Med. Res. 2023, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C. Risk of upper respiratory tract infection in athletes: An epidemiologic and immunologic perspective. J. Athl. Train. 1997, 32, 344–349. [Google Scholar] [PubMed]
- Kurowski, M.; Seys, S.; Bonini, M.; Del Giacco, S.; Delgado, L.; Diamant, Z.; Kowalski, M.L.; Moreira, A.; Rukhadze, M.; Couto, M. Physical exercise, immune response, and susceptibility to infections—Current knowledge and growing research areas. Allergy 2022, 77, 2653–2664. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.; Oliveira, M.; McCauley, T.; Tauler, P.; Muhamad, A.S. Respiratory infection risk in athletes: Association with antigen-stimulated IL-10 production and salivary IgA secretion. Scand. J. Med. Sci. Sport. 2012, 22, 410–417. [Google Scholar] [CrossRef]
- West, N.P.; Pyne, D.B.; Renshaw, G.; Cripps, A.W. Antimicrobial peptides and proteins, exercise and innate mucosal immunity. FEMS Immunol. Med. Microbiol. 2006, 48, 293–304. [Google Scholar] [CrossRef]
- Duarte-Mata, D.I.; Salinas-Carmona, M.C. Antimicrobial peptides’ immune modulation role in intracellular bacterial infection. Front. Immunol. 2023, 14, 1119574. [Google Scholar] [CrossRef]
- Davison, G.; Allgrove, J.; Gleeson, M. Salivary antimicrobial peptides (LL-37 and alpha-defensins HNP1-3), antimicrobial and IgA responses to prolonged exercise. Eur. J. Appl. Physiol. 2009, 106, 277–284. [Google Scholar] [CrossRef]
- Usui, T.; Yoshikawa, T.; Orita, K.; Ueda, S.-y.; Katsura, Y.; Fujimoto, S.; Yoshimura, M. Changes in salivary antimicrobial peptides, immunoglobulin A and cortisol after prolonged strenuous exercise. Eur. J. Appl. Physiol. 2011, 111, 2005–2014. [Google Scholar] [CrossRef]
- Gillum, T.L.; Kuennen, M.R.; Castillo, M.N.; Williams, N.L.; Jordan-Patterson, A.T. Exercise, But Not Acute Sleep Loss, Increases Salivary Antimicrobial Protein Secretion. J. Strength Cond. Res. 2015, 29, 1359–1366. [Google Scholar] [CrossRef]
- Kunz, H.; Bishop, N.C.; Spielmann, G.; Pistillo, M.; Reed, J.; Ograjsek, T.; Park, Y.; Mehta, S.K.; Pierson, D.L.; Simpson, R.J. Fitness level impacts salivary antimicrobial protein responses to a single bout of cycling exercise. Eur. J. Appl. Physiol. 2015, 115, 1015–1027. [Google Scholar] [CrossRef]
- Usui, T.; Yoshikawa, T.; Orita, K.; Ueda, S.-y.; Katsura, Y.; Fujimoto, S. Comparison of salivary antimicrobial peptides and upper respiratory tract infections in elite marathon runners and sedentary subjects. J. Phys. Fit. Sport. Med. 2012, 1, 175–181. [Google Scholar] [CrossRef]
- He, C.-S.; Tsai, M.-L.; Ko, M.-H.; Chang, C.-K.; Fang, S.-H. Relationships among salivary immunoglobulin A, lactoferrin and cortisol in basketball players during a basketball season. Eur. J. Appl. Physiol. 2010, 110, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.L.; Gleeson, M.; Pyne, D.B.; Callister, R.; Clancy, R.L. Variation of salivary immunoglobulins in exercising and sedentary populations. Med. Sci. Sport. Exerc. 2005, 37, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Arsati, F.; Cury, P.R.; Franciscon, C.; Simões, A.C.; De Oliveira, P.R.; De Araújo, V.C. The impact of a 17-day training period for an international championship on mucosal immune parameters in top-level basketball players and staff members. Eur. J. Oral Sci. 2008, 116, 431–437. [Google Scholar] [CrossRef]
- Robinson, K.; Ma, X.; Liu, Y.; Qiao, S.; Hou, Y.; Zhang, G. Dietary modulation of endogenous host defense peptide synthesis as an alternative approach to in-feed antibiotics. Anim. Nutr. (Zhongguo Xu Mu Shou Yi Xue Hui) 2018, 4, 160–169. [Google Scholar] [CrossRef]
- Morio, K.A.; Sternowski, R.H.; Brogden, K.A. Induction of Endogenous Antimicrobial Peptides to Prevent or Treat Oral Infection and Inflammation. Antibiotics 2023, 12, 361. [Google Scholar] [CrossRef]
- Williams, N.C.; Killer, S.C.; Svendsen, I.S.; Jones, A.W. Immune nutrition and exercise: Narrative review and practical recommendations. Eur. J. Sport Sci. 2019, 19, 49–61. [Google Scholar] [CrossRef]
- He, C.S.; Fraser, W.D.; Tang, J.; Brown, K.; Renwick, S.; Rudland-Thomas, J.; Teah, J.; Tanqueray, E.; Gleeson, M. The effect of 14 weeks of vitamin D3 supplementation on antimicrobial peptides and proteins in athletes. J. Sport. Sci. 2016, 34, 67–74. [Google Scholar] [CrossRef]
- Scott, J.M.; Kazman, J.B.; Palmer, J.; McClung, J.P.; Gaffney-Stomberg, E.; Gasier, H.G. Effects of vitamin D supplementation on salivary immune responses during Marine Corps basic training. Scand. J. Med. Sci. Sport. 2019, 29, 1322–1330. [Google Scholar] [CrossRef]
- Alhelfi, N.M.; Hobi, N.M. Influence of Vitamin D Deficiency on the Level of Salivary cathelicidin LL-37 in relation to Dental Caries Experience: A Case-Control Study. AL-Kindy Coll. Med. J. 2023, 19, 86–89. [Google Scholar] [CrossRef]
- Nireeksha; Hegde, M.N.; Kumari, N.S. Potential role of salivary vitamin D antimicrobial peptide LL-37 and interleukins in severity of dental caries: An exvivo study. BMC Oral Health 2024, 24, 79. [Google Scholar] [CrossRef] [PubMed]
- Vaisberg, M.; Paixão, V.; Almeida, E.B.; Santos, J.M.B.; Foster, R.; Rossi, M.; Pithon-Curi, T.C.; Gorjão, R.; Momesso, C.M.; Andrade, M.S.; et al. Daily Intake of Fermented Milk Containing Lactobacillus casei Shirota (Lcs) Modulates Systemic and Upper Airways Immune/Inflammatory Responses in Marathon Runners. Nutrients 2019, 11, 1678. [Google Scholar] [CrossRef] [PubMed]
- Wattanarat, O.; Makeudom, A.; Sastraruji, T.; Piwat, S.; Tianviwat, S.; Teanpaisan, R.; Krisanaprakornkit, S. Enhancement of salivary human neutrophil peptide 1–3 levels by probiotic supplementation. BMC Oral Health 2015, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Wattanarat, O.; Nirunsittirat, A.; Piwat, S.; Manmontri, C.; Teanpaisan, R.; Pahumunto, N.; Makeudom, A.; Sastraruji, T.; Krisanaprakornkit, S. Significant elevation of salivary human neutrophil peptides 1-3 levels by probiotic milk in preschool children with severe early childhood caries: A randomized controlled trial. Clin. Oral Investig. 2021, 25, 2891–2903. [Google Scholar] [CrossRef]
- Sandoval, F.; Faleiros, S.; Cabello, R.; Díaz-Dosque, M.; Rodríguez, G.; Escobar, A. The consumption of milk supplemented with probiotics decreases the occurrence of caries and the salivary concentration of hβD-3 in children. Clin. Oral Investig. 2021, 25, 3823–3830. [Google Scholar] [CrossRef]
- Naclerio, F.; Larumbe-Zabala, E.; Seijo, M.; Ashrafi, N.; Nielsen, B.V.; Earnest, C.P. Effects of Protein Versus Carbohydrate Supplementation on Markers of Immune Response in Master Triathletes: A Randomized Controlled Trial. J. Am. Coll. Nutr. 2019, 38, 395–404. [Google Scholar] [CrossRef]
- Naclerio, F.; Larumbe-Zabala, E.; Ashrafi, N.; Seijo, M.; Nielsen, B.; Allgrove, J.; Earnest, C.P. Effects of protein–carbohydrate supplementation on immunity and resistance training outcomes: A double-blind, randomized, controlled clinical trial. Eur. J. Appl. Physiol. 2017, 117, 267–277. [Google Scholar] [CrossRef]
- Atalay, N.; Balci, N.; Toygar, H.U.; Yardimci, G.; Gürsoy, U.K. Serum, saliva, and gingival tissue human β-defensin levels in relation to retinoic acid use. J. Periodontol. 2023, 94, 597–605. [Google Scholar] [CrossRef]
- Gombart, A.F. The Vitamin D–Antimicrobial Peptide Pathway and its Role in Protection Against Infection. Future Microbiol. 2009, 4, 1151–1165. [Google Scholar] [CrossRef]
- He, C.S.; Handzlik, M.; Fraser, W.D.; Muhamad, A.; Preston, H.; Richardson, A.; Gleeson, M. Influence of vitamin D status on respiratory infection incidence and immune function during 4 months of winter training in endurance sport athletes. Exerc. Immunol. Rev. 2013, 19, 86–101. [Google Scholar]
- Mahmood, M.K.; Tassery, H.; Tardivo, D.; Lan, R. Association between Vitamin D Levels and Dental Caries: A Systematic Review and Dose-Response Meta-Analysis of Cross-Sectional Studies. Appl. Sci. 2023, 13, 9883. [Google Scholar] [CrossRef]
- Ghosh, S.; Iacucci, M. Diverse Immune Effects of Bovine Colostrum and Benefits in Human Health and Disease. Nutrients 2021, 13, 3798. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.W.; March, D.S.; Curtis, F.; Bridle, C. Bovine colostrum supplementation and upper respiratory symptoms during exercise training: A systematic review and meta-analysis of randomised controlled trials. BMC Sport. Sci. Med. Rehabil. 2016, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.J.; Pyne, D.B.; Saunders, P.U.; Fricker, P.A. Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. Br. J. Sport. Med. 2010, 44, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Oliveira, M.; Tauler, P. Daily probiotic’s (Lactobacillus casei Shirota) reduction of infection incidence in athletes. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 55–64. [Google Scholar] [CrossRef]
- Patil, S.; Zamwar, U.M.; Mudey, A. Etiology, Epidemiology, Pathophysiology, Signs and Symptoms, Evaluation, and Treatment of Vitamin A (Retinol) Deficiency. Cureus 2023, 15, e49011. [Google Scholar] [CrossRef]
- Borovaya, A.; Dombrowski, Y.; Zwicker, S.; Olisova, O.; Ruzicka, T.; Wolf, R.; Schauber, J.; Sárdy, M. Isotretinoin therapy changes the expression of antimicrobial peptides in acne vulgaris. Arch. Dermatol. Res. 2014, 306, 689–700. [Google Scholar] [CrossRef]
- Madi, M.; Smith, S.; Alshehri, S.; Zakaria, O.; Almas, K. Influence of Smoking on Periodontal and Implant Therapy: A Narrative Review. Int. J. Environ. Res. Public Health 2023, 20, 5368. [Google Scholar] [CrossRef]
- Karsiyaka Hendek, M.; Erkmen Almaz, M.; Olgun, E.; Kisa, U. Salivary LL-37 and periodontal health in children exposed to passive smoking. Int. J. Paediatr. Dent. 2019, 29, 369–374. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; He, B.; Huang, R.; Li, M. Effect of tobacco on periodontal disease and oral cancer. Tob. Induc. Dis. 2019, 17, 40. [Google Scholar] [CrossRef] [PubMed]
- Persson, L.; Bergström, J.; Ito, H.; Gustafsson, A. Tobacco smoking and neutrophil activity in patients with periodontal disease. J. Periodontol. 2001, 72, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Štěpánek, L.; Ševčíková, J.; Horáková, D.; Patel, M.S.; Durďáková, R. Public Health Burden of Secondhand Smoking: Case Reports of Lung Cancer and a Literature Review. Int J Env. Res Public Health 2022, 19, 13152. [Google Scholar] [CrossRef] [PubMed]
- Kopa-Stojak, P.N.; Pawliczak, R. Comparison of the effects of active and passive smoking of tobacco cigarettes, electronic nicotine delivery systems and tobacco heating products on the expression and secretion of oxidative stress and inflammatory response markers. A systematic review. Inhal. Toxicol. 2024, 36, 75–89. [Google Scholar] [CrossRef]
- Palmer, R.M.; Scott, D.A.; Meekin, T.N.; Poston, R.N.; Odell, E.W.; Wilson, R.F. Potential mechanisms of susceptibility to periodontitis in tobacco smokers. J. Periodontal Res. 1999, 34, 363–369. [Google Scholar] [CrossRef]
- Yang, E.V.; Glaser, R. Stress-induced immunomodulation and the implications for health. Int. Immunopharmacol. 2002, 2, 315–324. [Google Scholar] [CrossRef]
- Sabbah, W.; Gomaa, N.; Gireesh, A. Stress, allostatic load, and periodontal diseases. Periodontol. 2000 2018, 78, 154–161. [Google Scholar] [CrossRef]
- Forte, L.F.; Cortelli, S.C.; Cortelli, J.R.; Aquino, D.R.; de Campos, M.V.; Cogo, K.; Costa, F.O.; Franco, G.C. Psychological stress has no association with salivary levels of β-defensin 2 and β-defensin 3. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2010, 39, 765–769. [Google Scholar] [CrossRef]
- Eda, N.; Shimizu, K.; Suzuki, S.; Tanabe, Y.; Lee, E.; Akama, T. Effects of yoga exercise on salivary beta-defensin 2. Eur. J. Appl. Physiol. 2013, 113, 2621–2627. [Google Scholar] [CrossRef]
- Agha, N.H.; Baker, F.L.; Kunz, H.E.; Spielmann, G.; Mylabathula, P.L.; Rooney, B.V.; Mehta, S.K.; Pierson, D.L.; Laughlin, M.S.; Markofski, M.M.; et al. Salivary antimicrobial proteins and stress biomarkers are elevated during a 6-month mission to the International Space Station. J. Appl. Physiol. 2020, 128, 264–275. [Google Scholar] [CrossRef]
- Şemsi, R.; Ergünol, E.; Er, E.K.; Sepici Dinçel, A. Academic examination stress: Effects on salivary cortisol, neuropeptide Y and interleukin-1β. Asp. Mol. Med. 2023, 2, 100030. [Google Scholar] [CrossRef]
- Aberg, K.M.; Radek, K.A.; Choi, E.-H.; Kim, D.-K.; Demerjian, M.; Hupe, M.; Kerbleski, J.; Gallo, R.L.; Ganz, T.; Mauro, T.; et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J. Clin. Investig. 2007, 117, 3339–3349. [Google Scholar] [CrossRef] [PubMed]
- Crucian, B.; Stowe, R.; Mehta, S.; Uchakin, P.; Quiriarte, H.; Pierson, D.; Sams, C. Immune system dysregulation occurs during short duration spaceflight on board the space shuttle. J. Clin. Immunol. 2013, 33, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Laudenslager, M.L.; Stowe, R.P.; Crucian, B.E.; Feiveson, A.H.; Sams, C.F.; Pierson, D.L. Latent virus reactivation in astronauts on the international space station. NPJ Microgravity 2017, 3, 11. [Google Scholar] [CrossRef]
- Nollet, M.; Wisden, W.; Franks, N.P. Sleep deprivation and stress: A reciprocal relationship. Interface Focus 2020, 10, 20190092. [Google Scholar] [CrossRef]
- Reichmann, F.; Holzer, P. Neuropeptide Y: A stressful review. Neuropeptides 2016, 55, 99–109. [Google Scholar] [CrossRef]
- Walsh, N.P. Nutrition and Athlete Immune Health: New Perspectives on an Old Paradigm. Sport. Med. 2019, 49, 153–168. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Y.; Shui, Y.; Zhou, X.; Cheng, L.; Ren, B.; Chen, Z.; Li, M. Interactions Between Neutrophils and Periodontal Pathogens in Late-Onset Periodontitis. Front. Cell. Infect. Microbiol. 2021, 11, 627328. [Google Scholar] [CrossRef]
- Tokajuk, J.; Deptuła, P.; Piktel, E.; Daniluk, T.; Chmielewska, S.; Wollny, T.; Wolak, P.; Fiedoruk, K.; Bucki, R. Cathelicidin LL-37 in Health and Diseases of the Oral Cavity. Biomedicines 2022, 10, 1086. [Google Scholar] [CrossRef]

| AMP | Structural Characteristics | Produced by | Biological Activity | Average Concentration (μg/mL) 1 | References |
|---|---|---|---|---|---|
| Histatins (Histatin 1, 3, 5) and proteolytic fragments | 7–38 AA, cationic, histidine-rich | Salivary gland and ductus |
| Histatin 1: 10.1 (parotid), 34.7 (SM/SL) Histatin 3: 7.3 (parotid), 10.2 (SM/SL) | [2,11,28,29] |
| α-Defensins, human neutrophil peptides (hNPs 1–4) | 29–35 AA, cationic, cysteine-rich, three intramolecular S-S bonds (I-VI, II-IV, III-V), β-sheet fold | Neutrophils Gingival sulcus Inflammation site Salivary gland cells |
| hNP-1: 8.6 hNP-2: 5.6 hNP-3: 0–2.7 | [2,11,28,29] |
| β-Defensins (hβD-1, hβD-2, and hβD-3) | 38–42 AA, cationic, cysteine-rich, three intramolecular S-S bonds (I-V, II-IV, III-VI), β-sheet fold | Epithelial cells Salivary duct |
| hβD-1: 0.15 hβD-2: 0.15 hβD-3: 0.31 | [2,11,28,29] |
| Cathelicidins (LL-37) | C-terminal region of the hCAP-18, 37 AA, cationic, no cysteine, α-helical conformation | Neutrophils, monocytes, T cells Gingival sulcus Salivary gland and ducts |
| 1.6 | [2,11,29] |
| Statherin | Phosphoprotein, 43 AA | Salivary gland cells |
| 26.5 | [2,11,28,29] |
| Adrenomedullin | 52 AA, cationic, one S-S bond | Epithelia |
| 0.06 | [2,11,29] |
| Neuropeptides (substance P, neurokinin A, calcitonin gene-related peptide, neuropeptide Y, and vasoactive intestinal polypeptide) | 10–37 AA, cationic | Salivary gland cells |
| substance P: 7.5 × 10−6 neuropeptide Y: 41.4 × 10−6 calcitonin gene-related peptide: 23.5 × 10−6 vasoactive intestinal polypeptide: 39.9 × 10−6 | [11,29] |
| Fragments from PRPs (P-B1, P-B, and BPLP) | Different fragments derived from PRPs | Parotid and submandibular glands |
| - | [2,11] |
| Dermcidin | Protein, 110 AA, processed to generate a 48 AA peptide with partial helical conformation | Striated cells in salivary glands |
| 0.45 | [30] |
| Pathology | Changes in Salivary AMP Levels 1 | References | |
|---|---|---|---|
| Oral | Periodontitis | hβD-1, hβD-2, hβD-3 (↑) hNP 1 (↑) LL-37 (↑) | [25,36,37,38,39,40] |
| Infections | Statherin (↑) | [41] | |
| Candidiasis | hβD-1, hβD-2 (↓) | [42] | |
| Caries | hNP 1–3 (↓) hβD-3 (↓) Histatin 5 (↓) LL-37 (↓) PPR IB-4 (↑) Statherin (↑) | [20,41,43,44,45] | |
| Head and neck squamous cell carcinoma | Histatin 1 (↑) | [46] | |
| Systemic | Alzheimer disease | hNPs 1–4 (↑) Statherin (↑) Histatin 1 (↑) | [47] |
| Sjögren syndrome | hβD-1, hβD-2 (↑) | [48] | |
| Type 2 diabetes mellitus | hβD-1 (↑) LL-37 (↑) | [39] | |
| HIV infection | hβD-1, hβD-2 (↑) | [49,50] | |
| COVID-19 | hNP 1, hNP 3 (↑) hβD-3 (↑) | [51] | |
| Long COVID-19 | Histatin 5 (↓) | [52] | |
| Autoimmune hepatitis | Histatins 3, 5, 6 (↑) Statherin (↑) | [53] | |
| Participants and Study Design | Observed Changes in Salivary AMP Levels 1 | References |
|---|---|---|
| 12 M (24 ± 8 y), 2.5 h of exercise on a cycle ergometer at 60% VO2max. Saliva sampling: immediately before and after exercise | Post- vs. pre-exercise: sIgA (=) hNP 1–3 (↑) LL-37 (↑) | Davison et al., Eur. J. Appl. Physiol. 2009 [67] |
| 10 M (23 ± 3 y), 60 min of exercise on a cycle ergometer at 75% VO2max. Saliva sampling: during exercise and resting sessions at t0, 60, 120, and 180 min | During and post- vs. pre-exercise: LL-37 (↑) hβD-2 (↑) sIgA (↓) | Usui et al., Eur. J. Appl. Physiol. 2011 [68] |
| 4 M and 4 F (23 ± 2 y) completed 2 exercise trials (45 min of running at 75% VO2max). Saliva sampling: before, immediately after, and 1 h after exercise | Post (1 h)- vs. pre-exercise: hNP 1–3 (↑) sIgA (↑) Lysozyme (↑) Lactoferrin (↑) LL-37 (↑) | Gillum et al., J. Strength Cond. Res. 2015 [69] |
| 11 M (25 ± 3 y) wildland firefighters. Saliva sampling: before and immediately after 45 min of intense exercise regimen | Post- vs. pre-exercise: Lysozyme (=) Dermcidin (↑) Cystatins: S, SN, SA, C, and D (↑) hβD-1 (↑) Histatin 1 (↑) | Nakayasu et al., Mil. Med. Res. 2023 [61] |
| 17 experienced cyclists (31 ± 5 y): 9 high-fit (6 M, 3 F) and 8 low-fit (7 M, 1F) completed three × 30 min exercise trials at varying workloads. Saliva sampling: before and immediately after exercise | Post- vs. pre-exercise: hNP 1–3 (↑) sIgA (↑) Lysozyme (↑) Lactoferrin (↑) LL-37 (↑) (increases were higher for high- vs. low-fit) High- vs. low-fit cyclists: hNP 1–3 (↓) Lactoferrin (↓) Lysozyme (=) LL-37 (=) sIgA (=) | Kunz et al., Eur. J. Appl. Physiol. 2015 [70] |
| 20 marathon runners (M, 21 ± 2 y), 20 sedentary controls (M, 20 ± 5 y) | Marathon runners vs. sedentary individuals: LL-37 (↓) hβD-2 (↓) | Usui et al., J. Sports Med. Phys. Fitness 2012 [71] |
| Supplement | Participants and Study Design | Observed Changes in Salivary AMPs Levels 1 | References |
|---|---|---|---|
| Vitamin D | 39 athletes (M, 20 ± 2 y) were daily supplemented with vitamin D3 (5000 IU, n = 20) or placebo (n = 19) for 14 wk during the winter training period. Saliva sampling: at t0, 7, and 14 wk | Supplemented vs. placebo: sIgA (=) lactoferrin (=) lysozyme (=) LL-37 (=) SR of sIgA (↑) LL-37 (↑) | He et al., J. Sports Sci. 2016 [78] |
| 149 subjects (75 M, 74 F, 19 ± 2 y) completed 12 wk of basic military training with supplementation of vitamin D3 (1000 IU) + 2000 mg calcium/d (n = 73) or placebo (n = 76). Saliva sampling: pre-, during (4 and 8 wk), and post-training (12 wk) | Supplemented vs. placebo: SR of sIgA (↑) LL-37 (↑) only in M | Scott et al., Scand. J. Med. Sci. Sports 2019 [79] | |
| 80 F (20–30 y) divided into two groups: low level (<0.4 IU/mL, n = 40) and high level of serum vitamin D (>1.2 IU/mL, n = 40) | High vs. low vit D levels: LL-37 (↑) | Alhelfi et al., Al-Kindy Col. Med. J. 2023 [80] | |
| Two groups of subjects (18–40 y): caries-free (n = 105, 38 M, 67 F) and caries-active (n = 272, 100 M, 172 F) | Caries-free vs. caries-active: vitamin D (↑) LL-37 (=) | Nireeksha et al., BMC Oral Health 2024 [81] | |
| Fermented milk and probiotics | 42 M marathonists ingested probiotic Lactobacillus fermentum (40 billion CFU/d, n = 20, 40 ± 9 y) or a placebo (n = 22, 40 ± 10 y) for 30 d pre-marathon. Saliva sampling: before and after supplementation, immediately, 72 h, and 14 d post-marathon | Supplemented vs. placebo: sIgA (↑) hNP 1 (↑) LL-37 (=) lactoferrin (=) lysozyme (=) | Vaisberg et al., Nutrients 2019 [82] |
| 60 children (26 M, 34 F, 13–15 y) were randomly assigned to intervention or control groups. Supplemented for 1 y with L. paracasei (6 billion CFU/d) or a placebo for 6 mth Saliva sampling: at baseline and every 3 mth for 1 y | Supplemented vs. placebo: hNP 1–3 (↑) | Wattanarat et al., BMC Oral Health 2015 [83] | |
| A cohort of children (1–5 y) without early childhood caries, with early childhood caries, and with severe early childhood caries were randomly assigned to three groups: (i) placebo (n = 86); (ii) daily probiotic (n = 89, 3 × 107 CFU of L. paracasei/d), and triweekly probiotic (n = 93, 3 × 107 CFU of L. paracasei). Saliva sampling: at baseline, 6 mth, and 12 mth | Daily and weekly vs. placebo: hNP 1–3 (↑) between baseline and month 12 only for children with severe early childhood caries | Wattanarat et al., Clin. Oral Investig. 2021 [84] | |
| 42 children were randomly assigned to 2 groups: (i) 11 M and 10 F (2.9 ± 0.3 y) were given a placebo, 11 M and 10 F (3.0 ± 0.3 y) were given L. rhamnosus supplemented milk (1.5 billion CFU/d). Saliva sampling: at baseline and end of the study (10 mth) | Supplemented vs. placebo: hβD-3 (↓) | Sandoval et al., Clin. Oral Investig. 2021 [85] | |
| Carbohydrates vs. proteins | Master-aged triathletes (n = 16, 35–60 y) were randomly assigned to ingest, during a 10 wk endurance training, either a hydrolyzed beef protein (n = 8) or a non-protein isoenergetic carbohydrate (n = 8). Saliva sampling: before and after performing an incremental endurance test to exhaustion, pre- and post-intervention | Baselines: hNP 1–3 (=) Only for the protein group, post- vs. before exercise: hNP1–3 (↓) | Naclerio et al., J. Am. Coll. Nutr. 2019 [86] |
| 27 recreationally physically active subjects (n = 9/treatment) were randomly assigned to 1 of 3 groups: (i) hydrolyzed beef protein (26 ± 5 y), (ii) whey protein (28 ± 5 y), (iii) non-protein isoenergetic carbohydrate (24 ± 7 y). Products were taken once a day for 8 wk during a resistance training program | Baselines: hNP 1–3 (=) Only for the beef protein group, post- vs. before exercise: hNP1–3 (↓) | Naclerio et al., Eur. J. Appl. Physiol. 2017 [87] | |
| Vitamin A (retinoic acid) | 69 subjects: 34 RA users (16 M, 18 F, 24 ± 4 y) and 35 controls (17 M, 18 F, 25 ± 3 y) | RA vs. controls: hβD-2 (↓) hβD-1 (=) hβD-3 (=) | Atalay et al., J. Periodontol. 2023 [88] |
| Participants and Study Design | Changes in Salivary AMP Levels 1 | References |
|---|---|---|
| 40 healthy non-smoker subjects (HNS, 31 M, 9 F, 43 ± 10 y), 40 healthy smokers (HS, 40 M, 0 F, 45 ± 10 y), 40 non-smokers with periodontal disease (PNS, 35 M, 5 F, 45 ± 9 y), 40 smokers with periodontal disease (PS, 40 M, 0 F, 45 ± 9 y) | HS vs. HNS and PS vs. PNS: LL-37 (↓) | Kzar et al., Biomed. Res. Int. 2023 [40] |
| 69 patients with chronic periodontitis (31 M, 38 F, 43 ± 10 y). Two groups were defined based on the salivary concentration of cotinine (marker of smoking): high (≥8 ng/mL, 14 patients) and low (<8 ng/mL, 55) | High vs. low cotinine: LL-37 (↓) | Takeuchi et al., J. Periodontol. 2012 [36] |
| 180 children: 90 passive smoking-exposed (PSE, 52 M, 38 F, 9.4 ± 1.6 y) and 90 passive smoking non-exposed (PSU, 47 M, 43 F, 9.0 ± 1.7 y) | PSE vs. PSU: LL-37 (↓) | Karsiyaka Hendek et al., Int. J. Paediatr. Dent. 2019 [99] |
| 41 individuals: HNS (5 M, 6 F, 53 ± 13 y); PNS (5 M, F 5, 51 ± 18 y); HS (5 M, F 5, 33 ± 11 y); PS (5 M, F 4, 51 ± 15 y) | HNS vs. HS: 63 AMPs and proteins were measured. AMPs: adrenomedullin (=), dermcidin (=), different hβDs (=), hNP 1 (=), LL-37 precursor (=), neuropeptide Y (=) PS vs. PNS: Adrenomedullin (↑) Eosinophil peroxidase (↑) Three histones (↑) Myeloperoxidase (↑) hNP 1 (↑) | Grant et al., J. Innate Immun. 2019 [25] |
| Participants and Study Design | Changes in Salivary AMP Levels 1 | References |
|---|---|---|
| 75 army students were classified as not stressed (22 M, 4 F, 24 ± 5 y) or stressed (14 M, 35 F, 22 ± 2 y), according to a stress system inventory | Stressed vs. non-stressed: hβD-2 (=), hβD-3 (=) | Forte et al., J. Oral Pathol. Med. 2010 [108] |
| 15 adults (60 ± 8 y). Saliva sampling: before and immediately after performing yoga or resting for 90 min | Pre- vs. post-yoga session: hβD-2 (↑) | Eda et al., Eur. J. Appl. Physiol. 2013 [109] |
| 4 veteran (4 M, 51–53 y) and 4 rookie (3 M, 1 F, 37–45 y) ISS crew members in a 6 mth mission to the ISS, 6 ground-based control subjects (5 M, 1 F, 27–42 y). Saliva sampling: at 180 and 60 d before launch, 10 and 90 d of flight, and 1 d before return | ISS crew vs. controls: sIgA (↑) Lysozyme (↑) LL-37 (↑) Lactoferrin (=) hNP 1–3 (=). Rookies vs. veterans: sIgA (↓) Lysozyme (↑) LL-37 (↑) Lactoferrin (=) hNP 1–3 (=) | Agha et al., J. Appl. Physiol. 2020 [110] |
| 4 M and 4 F (23 ± 2 y) completed 2 exercise trials (45 min of running at 75% VO2max) after a normal night of sleep (CON) and after a night without sleep (WS). Saliva sampling: before, immediately after, and 1 h after exercise | WS vs. CON: hNP 1–3 (=) sIgA (=) Lysozyme (=) Lactoferrin (=) LL-37 (=) | Gillum et al., J. Strength Cond. Res. 2015 [69] |
| 44 students (18–25 y). Saliva sampling: before and immediately after the examination | Post- vs. pre-examination: Neuropeptide Y(↑) | Semsi et al., Mol. Aspects Med. 2023 [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, M.; Ferrari, E.; Giovati, L.; Pertinhez, T.A.; Artesani, L.; Conti, S.; Ciociola, T. The Variability of the Salivary Antimicrobial Peptide Profile: Impact of Lifestyle. Int. J. Mol. Sci. 2024, 25, 11501. https://doi.org/10.3390/ijms252111501
Gallo M, Ferrari E, Giovati L, Pertinhez TA, Artesani L, Conti S, Ciociola T. The Variability of the Salivary Antimicrobial Peptide Profile: Impact of Lifestyle. International Journal of Molecular Sciences. 2024; 25(21):11501. https://doi.org/10.3390/ijms252111501
Chicago/Turabian StyleGallo, Mariana, Elena Ferrari, Laura Giovati, Thelma A. Pertinhez, Lorenza Artesani, Stefania Conti, and Tecla Ciociola. 2024. "The Variability of the Salivary Antimicrobial Peptide Profile: Impact of Lifestyle" International Journal of Molecular Sciences 25, no. 21: 11501. https://doi.org/10.3390/ijms252111501
APA StyleGallo, M., Ferrari, E., Giovati, L., Pertinhez, T. A., Artesani, L., Conti, S., & Ciociola, T. (2024). The Variability of the Salivary Antimicrobial Peptide Profile: Impact of Lifestyle. International Journal of Molecular Sciences, 25(21), 11501. https://doi.org/10.3390/ijms252111501










