Mitochondrial Dysfunction in Parkinson’s Disease: A Contribution to Cognitive Impairment?
Abstract
1. Introduction
2. Cognitive Impairment in PD
2.1. Clinical Symptoms of Cognitive Dysfunctions
2.2. Limits in Clinical Studies Dealing with Cognitive Impairment in PD
2.3. Cognitive Impairment in Animal Models of PD
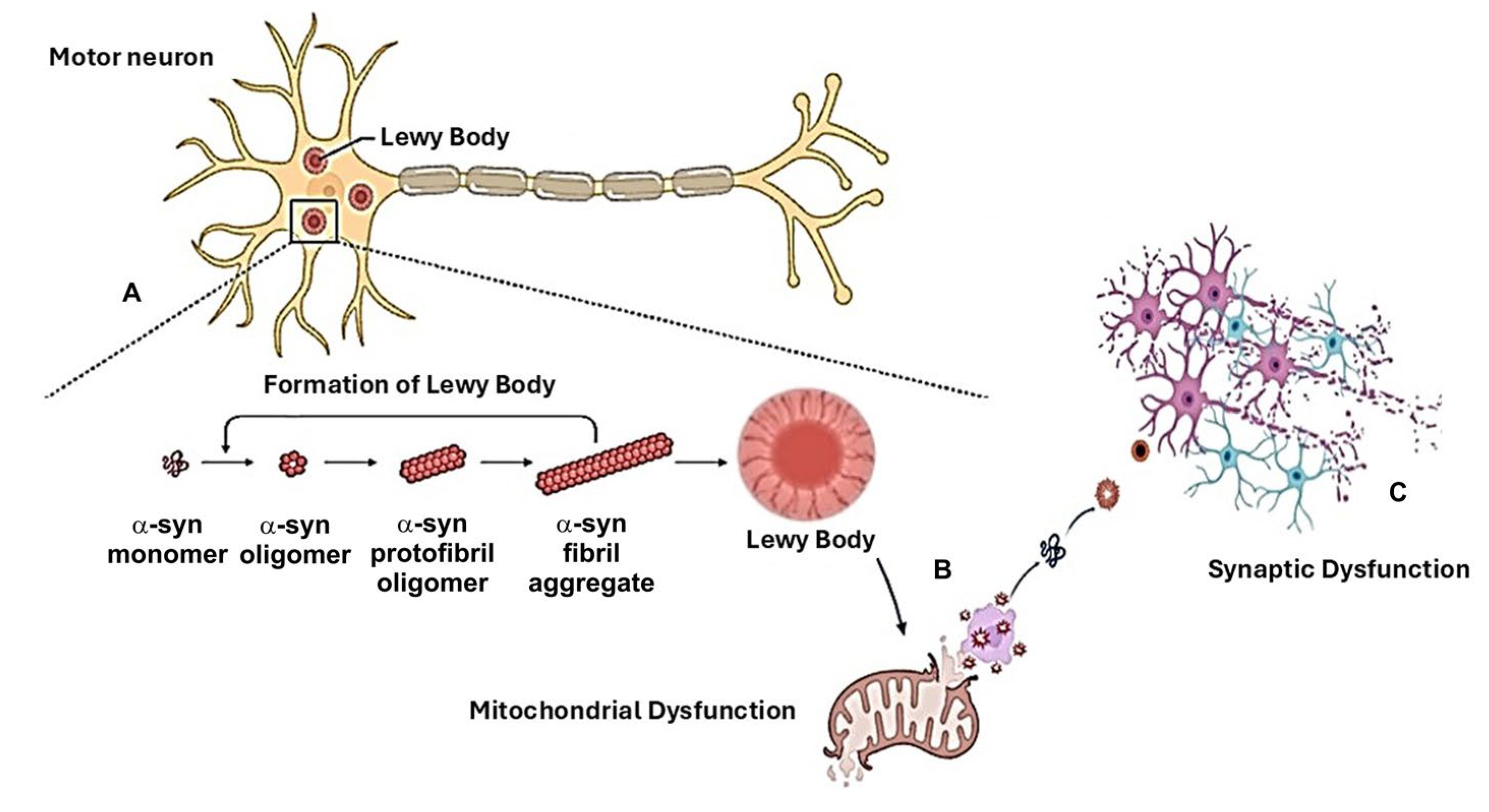
3. α-Synuclein and Mitochondrial Involvement in the Pathogenetic Mechanisms Related to Cognitive Decline in PD
3.1. Role of α-Syn
3.2. Mitochondrial Dysfunction
4. Treatment Strategies in PD: Unsatisfied Medical Needs
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloemd, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Parkinsons. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Obeso, J.A.; Stamelou, M.; Goetz, C.G.; Poewe, W.; Lang, A.E.; Weintraub, D.; Burn, D.; Halliday, G.M.; Bezard, E.; Przedborski, S.; et al. Past, Present, and Future of Parkinson’s Disease: A Special Essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 2017, 32, 1264–1310. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. α-Synuclein in Lewy Bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; De Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of Brain Pathology Related to Sporadic Parkinson’s Disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Ghebremedhin, E.; Rüb, U.; Bratzke, H.; Del Tredici, K. Stages in the Development of Parkinson’s Disease-Related Pathology. Cell Tissue Res. 2004, 318, 121–134. [Google Scholar] [CrossRef]
- Del Rey, N.L.G.; Quiroga-Varela, A.; Garbayo, E.; Carballo-Carbajal, I.; Fernández-Santiago, R.; Monje, M.H.G.; Trigo-Damas, I.; Blanco-Prieto, M.J.; Blesa, J. Advances in Parkinson’s Disease: 200 Years Later. Front. Neuroanat. 2018, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, E.; Tu, R. Genetics of Parkinson Disease. AJNR. Am. J. Neuroradiol. 2015, 36, 445–447. [Google Scholar] [CrossRef]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef]
- Briston, T.; Hicks, A.R. Mitochondrial Dysfunction and Neurodegenerative Proteinopathies: Mechanisms and Prospects for Therapeutic Intervention. Biochem. Soc. Trans. 2018, 46, 829–842. [Google Scholar] [CrossRef]
- Silver, I.; Erecińska, M. Oxygen and Ion Concentrations in Normoxic and Hypoxic Brain Cells. In Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1998; Volume 454. [Google Scholar] [CrossRef]
- Goyal, S.; Chaturvedi, R.K. Mitochondrial Protein Import Dysfunction in Pathogenesis of Neurodegenerative Diseases. Mol. Neurobiol. 2021, 58, 1418–1437. [Google Scholar] [CrossRef]
- Gonzalez-Latapi, P.; Bayram, E.; Litvan, I.; Marras, C. Cognitive Impairment in Parkinson’s Disease: Epidemiology, Clinical Profile, Protective and Risk Factors. Behav. Sci. 2021, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Buneeva, O.; Fedchenko, V.; Kopylov, A.; Medvedev, A. Mitochondrial Dysfunction in Parkinson’s Disease: Focus on Mitochondrial DNA. Biomedicines 2020, 8, 591. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Ikebe, S.-i.; Hattori, N.; Nakagawa-Hattori, Y.; Mochizuki, H.; Tanaka, M.; Ozawa, T. Role of Mitochondria in the Etiology and Pathogenesis of Parkinson’s Disease. BBA-Mol. Basis Dis. 1995, 1271, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Henrich, M.T.; Oertel, W.H.; Surmeier, D.J.; Geibl, F.F. Mitochondrial Dysfunction in Parkinson’s Disease—A Key Disease Hallmark with Therapeutic Potential. Mol. Neurodegener. 2023, 18, 83. [Google Scholar] [CrossRef]
- Qi, R.; Sammler, E.; Gonzalez-Hunt, C.P.; Barraza, I.; Pena, N.; Rouanet, J.P.; Naaldijk, Y.; Goodson, S.; Fuzzati, M.; Blandini, F.; et al. A Blood-Based Marker of Mitochondrial DNA Damage in Parkinson’s Disease. Sci. Transl. Med. 2023, 15, eabo1557. [Google Scholar] [CrossRef]
- Wang, R.; Sun, H.; Ren, H.; Wang, G. α-Synuclein Aggregation and Transmission in Parkinson’s Disease: A Link to Mitochondria and Lysosome. Sci. China Life Sci. 2020, 63, 1850–1859. [Google Scholar] [CrossRef]
- Gureev, A.P.; Khorolskaya, V.G.; Sadovnikova, I.S.; Shaforostova, E.A.; Cherednichenko, V.R.; Burakova, I.Y.; Plotnikov, E.Y.; Popov, V.N. Age-Related Decline in Nrf2/ARE Signaling Is Associated with the Mitochondrial DNA Damage and Cognitive Impairments. Int. J. Mol. Sci. 2022, 23, 15197. [Google Scholar] [CrossRef]
- Flønes, I.H.; Fernandez-Vizarra, E.; Lykouri, M.; Brakedal, B.; Skeie, G.O.; Miletic, H.; Lilleng, P.K.; Alves, G.; Tysnes, O.B.; Haugarvoll, K.; et al. Neuronal Complex I Deficiency Occurs throughout the Parkinson’s Disease Brain, but Is Not Associated with Neurodegeneration or Mitochondrial DNA Damage. Acta Neuropathol. 2018, 135, 409–425. [Google Scholar] [CrossRef]
- Czernecki, V.; Benchetrit, E.; Houot, M.; Pineau, F.; Mangone, G.; Corvol, J.C.; Vidailhet, M.; Levy, R. Social Cognitive Impairment in Early Parkinson’s Disease: A Novel “Mild Impairment”? Park. Relat. Disord. 2021, 85, 117–121. [Google Scholar] [CrossRef]
- Liepelt-Scarfone, I.; Ophey, A.; Kalbe, E. Cognition in Prodromal Parkinson’s Disease. Prog. Brain Res. 2022, 269, 93–111. [Google Scholar] [CrossRef]
- Adolphs, R. The Social Brain: Neural Basis of Social Knowledge. Annu. Rev. Psychol. 2009, 60, 693–716. [Google Scholar] [CrossRef] [PubMed]
- Christidi, F.; Migliaccio, R.; Santamaría-García, H.; Santangelo, G.; Trojsi, F. Social Cognition Dysfunctions in Neurodegenerative Diseases: Neuroanatomical Correlates and Clinical Implications. Behav. Neurol. 2018, 2018, 1849794. [Google Scholar] [CrossRef] [PubMed]
- Bora, E.; Walterfang, M.; Velakoulis, D. Theory of Mind in Parkinson’s Disease: A Meta-Analysis. Behav. Brain Res. 2015, 292, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Bodden, M.E.; Mollenhauer, B.; Trenkwalder, C.; Cabanel, N.; Eggert, K.M.; Unger, M.M.; Oertel, W.H.; Kessler, J.; Dodel, R.; Kalbe, E. Affective and Cognitive Theory of Mind in Patients with Parkinson’s Disease. Park. Relat. Disord. 2010, 16, 466–470. [Google Scholar] [CrossRef]
- Poletti, M.; Vergallo, A.; Ulivi, M.; Sonnoli, A.; Bonuccelli, U. Affective Theory of Mind in Patients with Parkinson’s Disease. Psychiatry Clin. Neurosci. 2013, 67, 273–276. [Google Scholar] [CrossRef]
- Enrici, I.; Adenzato, M.; Ardito, R.B.; Mitkova, A.; Cavallo, M.; Zibetti, M.; Lopiano, L.; Castelli, L. Emotion Processing in Parkinson’s Disease: A Three-Level Study on Recognition, Representation, and Regulation. PLoS ONE 2015, 10, e0131470. [Google Scholar] [CrossRef]
- Gray, H.M.; Tickle-Degnen, L. A Meta-Analysis of Performance on Emotion Recognition Tasks in Parkinson’s Disease. Neuropsychology 2010, 24, 176. [Google Scholar] [CrossRef]
- Santangelo, G.; Vitale, C.; Trojano, L.; Errico, D.; Amboni, M.; Barbarulo, A.M.; Grossi, D.; Barone, P. Neuropsychological Correlates of Theory of Mind in Patients with Early Parkinson’s Disease. Mov. Disord. 2012, 27, 98–105. [Google Scholar] [CrossRef]
- Roca, M.; Torralva, T.; Gleichgerrcht, E.; Chade, A.; Arévalo, G.G.; Gershanik, O.; Manes, F. Impairments in Social Cognition in Early Medicated and Unmedicated Parkinson Disease. Cogn. Behav. Neurol. 2010, 23, 152–158. [Google Scholar] [CrossRef]
- Péron, J.; Vicente, S.; Leray, E.; Drapier, S.; Drapier, D.; Cohen, R.; Biseul, I.; Rouaud, T.; Le Jeune, F.; Sauleau, P.; et al. Are Dopaminergic Pathways Involved in Theory of Mind? A Study in Parkinson’s Disease. Neuropsychologia 2009, 47, 406–414. [Google Scholar] [CrossRef]
- Thaler, A.; Posen, J.; Giladi, N.; Manor, Y.; Mayanz, C.; Mirelman, A.; Gurevich, T. Appreciation of Humor Is Decreased among Patients with Parkinson’s Disease. Park. Relat. Disord. 2012, 18, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Boller, F.; Passafiume, D.; Keefe, N.C.; Rogers, K.; Morrow, L.; Kim, Y. Visuospatial Impairment in Parkinson’s Disease. Role of Perceptual and Motor Factors. Arch. Neurol. 1984, 41, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Emre, M. Dementia Associated with Parkinson’s Disease. Lancet Neurol. 2003, 2, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yuan, X.; Chen, L.; Hu, B.; Jiang, L.; Shi, T.; Wang, H.; Huang, W. Subjective Cognitive Decline in Patients with Parkinson’s Disease: An Updated Review. Front. Aging Neurosci. 2023, 15, 1117068. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.F. Non-Motor Symptoms in Parkinson’s Disease. Park. Relat. Disord. 2016, 22, S119–S122. [Google Scholar] [CrossRef]
- Burton, E.J.; McKeith, I.G.; Burn, D.J.; Williams, E.D.; O’Brien, J.T. Cerebral Atrophy in Parkinson’s Disease with and without Dementia: A Comparison with Alzheimer’s Disease, Dementia with Lewy Bodies and Controls. Brain 2004, 127 Pt 4, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-Motor Features of Parkinson Disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Shiba, M.; Bower, J.H.; Maraganore, D.M.; McDonnell, S.K.; Peterson, B.J.; Ahlskog, J.E.; Schaid, D.J.; Rocca, W.A. Anxiety Disorders and Depressive Disorders Preceding Parkinson’s Disease: A Case-Control Study. Mov. Disord. 2000, 15, 669–677. [Google Scholar] [CrossRef]
- Dissanayaka, N.N.W.; Sellbach, A.; Silburn, P.A.; O’Sullivan, J.D.; Marsh, R.; Mellick, G.D. Factors Associated with Depression in Parkinson’s Disease. J. Affect. Disord. 2011, 132, 82–88. [Google Scholar] [CrossRef]
- Santangelo, G.; Vitale, C.; Trojano, L.; Angrisano, M.G.; Picillo, M.; Errico, D.; Agosti, V.; Grossi, D.; Barone, P. Subthreshold Depression and Subjective Cognitive Complaints in Parkinson’s Disease. Eur. J. Neurol. 2014, 21, 541–544. [Google Scholar] [CrossRef]
- Even, C.; Weintraub, D. Is Depression in Parkinson’s Disease (PD) a Specific Entity? J. Affect. Disord. 2012, 139, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Seppi, K.; Weintraub, D.; Coelho, M.; Perez-Lloret, S.; Fox, S.H.; Katzenschlager, R.; Hametner, E.M.; Poewe, W.; Rascol, O.; Goetz, C.G.; et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the Non-Motor Symptoms of Parkinson’s Disease. Mov. Disord. 2011, 26 (Suppl. S3), S42–S80. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.P.; McKeith, I.G.; Burn, D.J.; Boeve, B.F.; Weintraub, D.; Bamford, C.; Allan, L.M.; Thomas, A.J.; O’Brien, J.T. New Evidence on the Management of Lewy Body Dementia. Lancet Neurol. 2020, 19, 157–169. [Google Scholar] [CrossRef]
- Prasad, S.; Katta, M.R.; Abhishek, S.; Sridhar, R.; Valisekka, S.S.; Hameed, M.; Kaur, J.; Walia, N. Recent Advances in Lewy Body Dementia: A Comprehensive Review. Dis.-Mon. 2023, 69, 101441. [Google Scholar] [CrossRef]
- Sezgin, M.; Bilgic, B.; Tinaz, S.; Emre, M. Parkinson’s Disease Dementia and Lewy Body Disease. Semin. Neurol. 2019, 39, 274–282. [Google Scholar] [CrossRef]
- Calabresi, P.; Di Lazzaro, G.; Marino, G.; Campanelli, F.; Ghiglieri, V. Advances in Understanding the Function of Alpha-Synuclein: Implications for Parkinson’s Disease. Brain 2023, 146, 3587–3597. [Google Scholar] [CrossRef]
- Pastukhov, R.; Korshunov, A.; Turdakov, D.; Kuznetsov, S. Improving Quality of Graph Partitioning Using Multilevel Optimization. Proc. Inst. Syst. Program. RAS 2014, 26, 302–306. [Google Scholar] [CrossRef]
- Biundo, R.; Weis, L.; Facchini, S.; Formento-Dojot, P.; Vallelunga, A.; Pilleri, M.; Antonini, A. Cognitive Profiling of Parkinson Disease Patients with Mild Cognitive Impairment and Dementia. Park. Relat. Disord. 2014, 20, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Hobson, P.; Meara, J. Mild Cognitive Impairment in Parkinson’s Disease and Its Progression onto Dementia: A 16-Year Outcome Evaluation of the Denbighshire Cohort. Int. J. Geriatr. Psychiatry 2015, 30, 1048–1055. [Google Scholar] [CrossRef]
- Kehagia, A.A.; Barker, R.A.; Robbins, T.W. Cognitive Impairment in Parkinson’s Disease: The Dual Syndrome Hypothesis. Neurodegener. Dis. 2012, 11, 79–92. [Google Scholar] [CrossRef]
- Biundo, R.; Weis, L.; Antonini, A. Cognitive Decline in Parkinson’s Disease: The Complex Picture. NPJ Park. Dis. 2016, 2, 16018. [Google Scholar] [CrossRef] [PubMed]
- Kotzbauer, P.T.; Cairns, N.J.; Campbell, M.C.; Willis, A.W.; Racette, B.A.; Tabbal, S.D.; Perlmutter, J.S. Pathologic Accumulation of α-Synuclein and Aβ in Parkinson Disease Patients with Dementia. Arch. Neurol. 2012, 69, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Petrou, M.; Dwamena, B.A.; Foerster, B.R.; Maceachern, M.P.; Bohnen, N.I.; Müller, M.L.; Albin, R.L.; Frey, K.A. Amyloid Deposition in Parkinson’s Disease and Cognitive Impairment: A Systematic Review. Mov. Disord. 2015, 30, 928–935. [Google Scholar] [CrossRef]
- Theilmann, R.J.; Reed, J.D.; Song, D.D.; Huang, M.X.; Lee, R.R.; Litvan, I.; Harrington, D.L. White-Matter Changes Correlate with Cognitive Functioning in Parkinson’s Disease. Front. Neurol. 2013, 4, 37. [Google Scholar] [CrossRef]
- Zheng, Z.; Shemmassian, S.; Wijekoon, C.; Kim, W.; Bookheimer, S.Y.; Pouratian, N. DTI Correlates of Distinct Cognitive Impairments in Parkinson’s Disease. Hum. Brain Mapp. 2014, 35, 1325–1333. [Google Scholar] [CrossRef]
- Picconi, B.; Piccoli, G.; Calabresi, P. Synaptic Dysfunction in Parkinson’s Disease. Adv. Exp. Med. Biol. 2012, 970, 553–572. [Google Scholar] [CrossRef]
- Williams-Gray, C.H.; Foltynie, T.; Lewis, S.J.G.; Barker, R.A. Cognitive Deficits and Psychosis in Parkinson’s Disease: A Review of Pathophysiology and Therapeutic Options. CNS Drugs. 2006, 20, 477–505. [Google Scholar] [CrossRef] [PubMed]
- Le Govic, Y.; Demey, B.; Cassereau, J.; Bahn, Y.-S.; Papon, N. Pathogens Infecting the Central Nervous System. PLoS Pathog. 2022, 18, e1010234. [Google Scholar] [CrossRef]
- Peterson, C.T. Dysfunction of the Microbiota-Gut-Brain Axis in Neurodegenerative Disease: The Promise of Therapeutic Modulation with Prebiotics, Medicinal Herbs, Probiotics, and Synbiotics. J. Evid.-Based Integr. Med. 2020, 25, 2515690X20957225. [Google Scholar] [CrossRef]
- Menezes-Rodrigues, F.S.; Scorza, C.S.; Fiorini, A.C.; Caricati-Neto, A.; Scorza, C.A.; Finsterer, J.; Scorza, F.A. Sudden Unexpected Death in Parkinson’s Disease: Why Is Drinking Water Important? Neurodegener. Dis. Manag. 2019, 9, 241–246. [Google Scholar] [CrossRef]
- Jankovic, J.; Chen, S.; Le, W.D. The Role of Nurr1 in the Development of Dopaminergic Neurons and Parkinson’s Disease. Prog. Neurobiol. 2005, 77, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Kim, S.M.; Kim, C.H.; Pagire, S.H.; Pagire, H.S.; Chung, H.Y.; Ahn, J.H.; Park, C.H. Dopamine Neuron Induction and the Neuroprotective Effects of Thyroid Hormone Derivatives. Sci. Rep. 2019, 9, 13659. [Google Scholar] [CrossRef] [PubMed]
- Venditti, P.; Di Meo, S. Thyroid Hormone-Induced Oxidative Stress. Cell. Mol. Life Sci. 2006, 63, 414–434. [Google Scholar] [CrossRef]
- Villanueva, I.; Alva-Sánchez, C.; Pacheco-Rosado, J. The Role of Thyroid Hormones as Inductors of Oxidative Stress and Neurodegeneration. Oxidative Med. Cell. Longev. 2013, 2013, 218145. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Dolatshahi, M.; Rahmani, F. Shedding Light on Thyroid Hormone Disorders and Parkinson Disease Pathology: Mechanisms and Risk Factors. J. Endocrinol. Investig. 2021, 44, 1–13. [Google Scholar] [CrossRef]
- Tenison, E.; James, A.; Ebenezer, L.; Henderson, E.J. A Narrative Review of Specialist Parkinson’s Nurses: Evolution, Evidence and Expectation. Geriatrics 2022, 7, 46. [Google Scholar] [CrossRef]
- Rojo-Sebastián, A.; González-Robles, C.; Garciá De Yébenes, J. Vitamin B6 Deficiency in Patients with Parkinson Disease Treated with Levodopa/Carbidopa. Clin. Neuropharmacol. 2020, 43, 151–157. [Google Scholar] [CrossRef]
- Planas-Ballvé, A.; Vilas, D. Cognitive Impairment in Genetic Parkinson’s Disease. Parkinsons. Dis. 2021, 2021, 8610285. [Google Scholar] [CrossRef]
- Degirmenci, Y.; Angelopoulou, E.; Georgakopoulou, V.E.; Bougea, A. Cognitive Impairment in Parkinson’s Disease: An Updated Overview Focusing on Emerging Pharmaceutical Treatment Approaches. Medicina 2023, 59, 1756. [Google Scholar] [CrossRef]
- Lee, E.Y. Memory Deficits in Parkinson’s Disease Are Associated with Impaired Attentional Filtering and Memory Consolidation Processes. J. Clin. Med. 2023, 12, 4594. [Google Scholar] [CrossRef]
- Aarsland, D.; Batzu, L.; Halliday, G.M.; Geurtsen, G.J.; Ballard, C.; Ray Chaudhuri, K.; Weintraub, D. Parkinson Disease-Associated Cognitive Impairment. Nat. Rev. Dis. Prim. 2021, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Ohtsuka, H.; Kamata, N.; Yamamoto, S.; Sawada, M.; Nakamura, J.; Okamoto, M.; Narita, M.; Nikaido, Y.; Urakami, H.; et al. Effectiveness of Long-Term Physiotherapy in Parkinson’s Disease: A Systematic Review and Meta-Analysis. J. Park. Dis. 2021, 11, 1619–1630. [Google Scholar] [CrossRef]
- Orgeta, V.; McDonald, K.R.; Poliakoff, E.; Hindle, J.V.; Clare, L.; Leroi, I. Cognitive Training Interventions for Dementia and Mild Cognitive Impairment in Parkinson’s Disease. Cochrane Database Syst. Rev. 2020, 2, CD011961. [Google Scholar] [CrossRef]
- Costa, C.; Sgobio, C.; Siliquini, S.; Tozzi, A.; Tantucci, M.; Ghiglieri, V.; Di Filippo, M.; Pendolino, V.; De Iure, A.; Marti, M.; et al. Mechanisms Underlying the Impairment of Hippocampal Long-Term Potentiation and Memory in Experimental Parkinson’s Disease. Brain 2012, 135 Pt 6, 1884–1899. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive Decline in Parkinson Disease. Nat. Rev. Neurol. 2017, 13, 217–231. [Google Scholar] [CrossRef]
- Goldman, J.G.; Sieg, E. Cognitive Impairment and Dementia in Parkinson Disease. Clin. Geriatr. Med. 2020, 36, 365–377. [Google Scholar] [CrossRef]
- Novikov, N.I.; Brazhnik, E.S.; Kitchigina, V.F. Pathological Correlates of Cognitive Decline in Parkinson’s Disease: From Molecules to Neural Networks. Biochemistry 2023, 88, 1890–1904. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.J.; Tan, E.K.; Chao, Y.X. Historical Perspective: Models of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 2464. [Google Scholar] [CrossRef]
- Dovonou, A.; Bolduc, C.; Soto Linan, V.; Gora, C.; Peralta, M.R.; Lévesque, M. Animal Models of Parkinson’s Disease: Bridging the Gap between Disease Hallmarks and Research Questions. Transl. Neurodegener. 2023, 12, 36. [Google Scholar] [CrossRef]
- Luk, K.C.; Kehm, V.; Carroll, J.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M.Y. Pathological α-Synuclein Transmission Initiates Parkinson-like Neurodegeneration in Nontransgenic Mice. Science 2012, 338, 949–953. [Google Scholar] [CrossRef]
- Przedborski, S.; Vila, M. The 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Mouse Model: A Tool to Explore the Pathogenesis of Parkinson’s Disease. In Annals of the New York Academy of Sciences; The New York Academy of Sciences: New York, NY, USA, 2003; Volume 991. [Google Scholar] [CrossRef]
- Blandini, F.; Armentero, M.T.; Martignoni, E. The 6-Hydroxydopamine Model: News from the Past. Park. Relat. Disord. 2008, 14 (Suppl. S2), S124–S129. [Google Scholar] [CrossRef] [PubMed]
- Giasson, B.I.; Duda, J.E.; Quinn, S.M.; Zhang, B.; Trojanowski, J.Q.; Lee, V.M.Y. Neuronal α-Synucleinopathy with Severe Movement Disorder in Mice Expressing A53T Human α-Synuclein. Neuron 2002, 34, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Sisalli, M.J.; Simola, N.; Della Notte, S.; Casu, M.A.; Serra, M.; Pinna, A.; Feliciello, A.; Annunziato, L.; Scorziello, A.; et al. Gender Differences in Neurodegeneration, Neuroinflammation and Na+-Ca2+ Exchangers in the Female A53T Transgenic Mouse Model of Parkinson’s Disease. Front. Aging Neurosci. 2020, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Jerčić, K.G.; Blažeković, A.; Borovečki, S.; Borovečki, F. Non-Motor Symptoms of Parkinson`s Disease-Insights from Genetics. J. Neural Transm. 2024. [Google Scholar] [CrossRef]
- Takahashi, R.N.; Tadaiesky, M.T.; Dombrowski, P.A.; Da Cunha, C. Effects of SR141716A on Cognitive and Depression-Related Behavior in an Animal Model of Premotor Parkinson’s Disease. Parkinsons. Dis. 2010, 2010, 238491. [Google Scholar] [CrossRef]
- Ferro, M.M.; Angelucci, M.E.M.; Anselmo-Franci, J.A.; Canteras, N.S.; Da Cunha, C. Neuroprotective Effect of Ketamine/Xylazine on Two Rat Models of Parkinson’s Disease. Braz. J. Med. Biol. Res. 2007, 40, 89–96. [Google Scholar] [CrossRef]
- Masato, A.; Plotegher, N.; Terrin, F.; Sandre, M.; Faustini, G.; Thor, A.; Adams, S.; Berti, G.; Cogo, S.; De Lazzari, F.; et al. DOPAL Initiates ASynuclein-Dependent Impaired Proteostasis and Degeneration of Neuronal Projections in Parkinson’s Disease. npj Park. Dis. 2023, 9, 42. [Google Scholar] [CrossRef]
- Giordano, N.; Iemolo, A.; Mancini, M.; Cacace, F.; Risi, M.D.; Latagliata, E.C.; Ghiglieri, V.; Bellenchi, G.C.; Puglisi-Allegra, S.; Calabresi, P.; et al. Motor Learning and Metaplasticity in Striatal Neurons: Relevance for Parkinson’s Disease. Brain 2018, 141, 505–520. [Google Scholar] [CrossRef]
- Marino, G.; Calabresi, P.; Ghiglieri, V. Alpha-Synuclein and Cortico-Striatal Plasticity in Animal Models of Parkinson Disease. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2022; Volume 184. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Burns, M.R.; Brundin, P.; Wesson, D.W. Linking α-Synuclein-Induced Synaptopathy and Neural Network Dysfunction in Early Parkinson’s Disease. Brain Commun. 2022, 4, fcac165. [Google Scholar] [CrossRef]
- Hong, J.Y.; Lee, P.H. Subjective Cognitive Complaints in Cognitively Normal Patients with Parkinson’s Disease: A Systematic Review. J. Mov. Disord. 2023, 16, 1. [Google Scholar] [CrossRef]
- Montenegro, M.V.B.; de Melo Amaral, C.E.; da Silva, L.C.S. Genetic Risk Factors and Lysosomal Function in Parkinson Disease. In Methods in Molecular Medicine; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Castro, A.A.; Ghisoni, K.; Latini, A.; Quevedo, J.; Tasca, C.I.; Prediger, R.D.S. Lithium and Valproate Prevent Olfactory Discrimination and Short-Term Memory Impairments in the Intranasal 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP) Rat Model of Parkinson’s Disease. Behav. Brain Res. 2012, 229, 208–215. [Google Scholar] [CrossRef]
- Moreira, E.L.G.; Rial, D.; Aguiar, A.S.; Figueiredo, C.P.; Siqueira, J.M.; Dalbó, S.; Horst, H.; De Oliveira, J.; Mancini, G.; Dos Santos, T.S.; et al. Proanthocyanidin-Rich Fraction from Croton Celtidifolius Baill Confers Neuroprotection in the Intranasal 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Rat Model of Parkinson’s Disease. J. Neural Transm. 2010, 117, 1337–1351. [Google Scholar] [CrossRef]
- Das, D.S.; Ray, A.; Das, A.; Song, Y.; Tian, Z.; Oronsky, B.; Richardson, P.; Scicinski, J.; Chauhan, D.; Anderson, K.C. A Novel Hypoxia-Selective Epigenetic Agent RRx-001 Triggers Apoptosis and Overcomes Drug Resistance in Multiple Myeloma Cells. Leukemia 2016, 30, 2187–2197. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nemani, V.M.; Wallender, E.K.; Kaehlcke, K.; Ott, M.; Edwards, R.H. Optical Reporters for the Conformation of α-Synuclein Reveal a Specific Interaction with Mitochondria. J. Neurosci. 2008, 28, 12305–12317. [Google Scholar] [CrossRef] [PubMed]
- Vargas, K.J.; Schrod, N.; Davis, T.; Fernandez-Busnadiego, R.; Taguchi, Y.V.; Laugks, U.; Lucic, V.; Chandra, S.S. Synucleins Have Multiple Effects on Presynaptic Architecture. Cell Rep. 2017, 18, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Iwai, A.; Masliah, E.; Yoshimoto, M.; Ge, N.; Flanagan, L.; Rohan de Silva, H.A.; Kittel, A.; Saitoh, T. The Precursor Protein of Non-A Beta Component of Alzheimer’s Disease Amyloid Is a Presynaptic Protein of the Central Nervous System. Neuron 1995, 14, 467–475. [Google Scholar] [CrossRef]
- Nakajo, S.; Omata, K.; Aiuchi, T.; Shibayama, T.; Okahashi, I.; Ochiai, H.; Nakai, Y.; Nakaya, K.; Nakamura, Y. Purification and Characterization of a Novel Brain-Specific 14-kDa Protein. J. Neurochem. 1990, 55, 2031–2038. [Google Scholar] [CrossRef]
- Fortin, D.L.; Troyer, M.D.; Nakamura, K.; Kubo, S.I.; Anthony, M.D.; Edwards, R.H. Lipid Rafts Mediate the Synaptic Localization of α-Synuclein. J. Neurosci. 2004, 24, 6715–6723. [Google Scholar] [CrossRef]
- Petrosini, L.; De Bartolo, P.; Foti, F.; Gelfo, F.; Cutuli, D.; Leggio, M.G.; Mandolesi, L. On Whether the Environmental Enrichment May Provide Cognitive and Brain Reserves. Brain Res. Rev. 2009, 61, 221–239. [Google Scholar] [CrossRef]
- Rosenberg, A.; Ngandu, T.; Rusanen, M.; Antikainen, R.; Bäckman, L.; Havulinna, S.; Hänninen, T.; Laatikainen, T.; Lehtisalo, J.; Levälahti, E.; et al. Multidomain Lifestyle Intervention Benefits a Large Elderly Population at Risk for Cognitive Decline and Dementia Regardless of Baseline Characteristics: The FINGER Trial. Alzheimer’s Dement. 2018, 14, 263–270. [Google Scholar] [CrossRef]
- Pantiya, P.; Thonusin, C.; Chattipakorn, N.; Chattipakorn, S.C. Mitochondrial Abnormalities in Neurodegenerative Models and Possible Interventions: Focus on Alzheimer’s Disease, Parkinson’s Disease, Huntington’s Disease. Mitochondrion 2020, 55, 14–47. [Google Scholar] [CrossRef] [PubMed]
- Monzio Compagnoni, G.; Di Fonzo, A.; Corti, S.; Comi, G.P.; Bresolin, N.; Masliah, E. The Role of Mitochondria in Neurodegenerative Diseases: The Lesson from Alzheimer’s Disease and Parkinson’s Disease. Mol. Neurobiol. 2020, 57, 2959–2980. [Google Scholar] [CrossRef]
- Gatt, A.P.; Duncan, O.F.; Attems, J.; Francis, P.T.; Ballard, C.G.; Bateman, J.M. Dementia in Parkinson’s Disease Is Associated with Enhanced Mitochondrial Complex I Deficiency. Mov. Disord. 2016, 31, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Mercado-Ayon, E.; Mercado-Ayon, Y.; Dong, Y.N.; Halawani, S.; Ngaba, L.; Lynch, D.R. Mitochondrial Dysfunction in the Development and Progression of Neurodegenerative Diseases. Arch. Biochem. Biophys. 2021, 702, 108698. [Google Scholar] [CrossRef]
- Obergasteiger, J.; Castonguay, A.M.; Pizzi, S.; Magnabosco, S.; Frapporti, G.; Lobbestael, E.; Baekelandt, V.; Hicks, A.A.; Pramstaller, P.P.; Gravel, C.; et al. The Small GTPase Rit2 Modulates LRRK2 Kinase Activity, Is Required for Lysosomal Function and Protects against Alpha-Synuclein Neuropathology. npj Park. Dis. 2023, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Requejo-Aguilar, R.; Bolaños, J.P. Mitochondrial Control of Cell Bioenergetics in Parkinson’s Disease. Free. Radic. Biol. Med. 2016, 100, 123–137. [Google Scholar] [CrossRef]
- Lin, C.H.; Yang, S.Y.; Horng, H.E.; Yang, C.C.; Chieh, J.J.; Chen, H.H.; Liu, B.H.; Chiu, M.J. Plasma α-Synuclein Predicts Cognitive Decline in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2017, 88, 818–824. [Google Scholar] [CrossRef]
- Chinta, S.J.; Mallajosyula, J.K.; Rane, A.; Andersen, J.K. Mitochondrial α-Synuclein Accumulation Impairs Complex I Function in Dopaminergic Neurons and Results in Increased Mitophagy in Vivo. Neurosci. Lett. 2010, 486, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Bridi, J.C.; Hirth, F. Mechanisms of α-Synuclein Induced Synaptopathy in Parkinson’s Disease. Front. Neurosci. 2018, 12, 80. [Google Scholar] [CrossRef]
- Burré, J.; Sharma, M.; Tsetsenis, T.; Buchman, V.; Etherton, M.R.; Südhof, T.C. Alpha-Synuclein Promotes SNARE-Complex Assembly in Vivo and in Vitro. Science 2010, 329, 1663–1667. [Google Scholar] [CrossRef]
- Rocha, E.M.; De Miranda, B.; Sanders, L.H. Alpha-Synuclein: Pathology, Mitochondrial Dysfunction and Neuroinflammation in Parkinson’s Disease. Neurobiol. Dis. 2018, 109, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Emmanouilidou, E.; Melachroinou, K.; Roumeliotis, T.; Garbis, S.D.; Ntzouni, M.; Margaritis, L.H.; Stefanis, L.; Vekrellis, K. Cell-Produced Alpha-Synuclein Is Secreted in a Calcium-Dependent Manner by Exosomes and Impacts Neuronal Survival. J. Neurosci. 2010, 30, 6838–6851. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J.; Obeso, J.A.; Halliday, G.M. Selective Neuronal Vulnerability in Parkinson Disease. Nat. Rev. Neurosci. 2017, 18, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Ghiglieri, V.; Calabrese, V.; Calabresi, P. Alpha-Synuclein: From Early Synaptic Dysfunction to Neurodegeneration. Front. Neurol. 2018, 9, 295. [Google Scholar] [CrossRef]
- Ferrari, E.; Scheggia, D.; Zianni, E.; Italia, M.; Brumana, M.; Palazzolo, L.; Parravicini, C.; Pilotto, A.; Padovani, A.; Marcello, E.; et al. Rabphilin-3A as a Novel Target to Reverse α-Synuclein-Induced Synaptic Loss in Parkinson’s Disease. Pharmacol. Res. 2022, 183, 106375. [Google Scholar] [CrossRef]
- Franchini, L.; Stanic, J.; Barzasi, M.; Zianni, E.; Mauceri, D.; Diluca, M.; Gardoni, F. Rabphilin-3A Drives Structural Modifications of Dendritic Spines Induced by Long-Term Potentiation. Cells 2022, 11, 1616. [Google Scholar] [CrossRef]
- Italia, M.; Ferrari, E.; Diluca, M.; Gardoni, F. NMDA and AMPA Receptors at Synapses: Novel Targets for Tau and A-Synuclein Proteinopathies. Biomedicines 2022, 10, 1550. [Google Scholar] [CrossRef]
- Tozzi, A.; de Iure, A.; Bagetta, V.; Tantucci, M.; Durante, V.; Quiroga-Varela, A.; Costa, C.; Di Filippo, M.; Ghiglieri, V.; Latagliata, E.C.; et al. Alpha-Synuclein Produces Early Behavioral Alterations via Striatal Cholinergic Synaptic Dysfunction by Interacting with GluN2D N-Methyl-D-Aspartate Receptor Subunit. Biol. Psychiatry 2016, 79, 402–414. [Google Scholar] [CrossRef]
- Durante, V.; De Iure, A.; Loffredo, V.; Vaikath, N.; De Risi, M.; Paciotti, S.; Quiroga-Varela, A.; Chiasserini, D.; Mellone, M.; Mazzocchetti, P.; et al. Alpha-Synuclein Targets GluN2A NMDA Receptor Subunit Causing Striatal Synaptic Dysfunction and Visuospatial Memory Alteration. Brain 2019, 142, 1365–1385. [Google Scholar] [CrossRef]
- Tozzi, A.; Sciaccaluga, M.; Loffredo, V.; Megaro, A.; Ledonne, A.; Cardinale, A.; Federici, M.; Bellingacci, L.; Paciotti, S.; Ferrari, E.; et al. Dopamine-Dependent Early Synaptic and Motor Dysfunctions Induced by a-Synuclein in the Nigrostriatal Circuit. Brain 2021, 144, 3477–3491. [Google Scholar] [CrossRef]
- Hallam, R.D.; Buchner-Duby, B.; Stykel, M.G.; Coackley, C.L.; Ryan, S.D. Intracellular Accumulation of A-Synuclein Aggregates Promotes S-Nitrosylation of MAP1A Leading to Decreased NMDAR-Evoked Calcium Influx and Loss of Mature Synaptic Spines. J. Neurosci. 2022, 42, 9473–9487. [Google Scholar] [CrossRef] [PubMed]
- Lautenschläger, J.; Stephens, A.D.; Fusco, G.; Ströhl, F.; Curry, N.; Zacharopoulou, M.; Michel, C.H.; Laine, R.; Nespovitaya, N.; Fantham, M.; et al. C-Terminal Calcium Binding of α-Synuclein Modulates Synaptic Vesicle Interaction. Nat. Commun. 2018, 9, 72. [Google Scholar] [CrossRef]
- Stephens, A.D.; Zacharopoulou, M.; Schierle, G.S.K. The Cellular Environment Affects Monomeric α-Synuclein Structure. Trends Biochem. Sci. 2019, 44, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Giasson, B.I.; Murray, I.V.J.; Trojanowski, J.Q.; Lee, V.M.Y. A Hydrophobic Stretch of 12 Amino Acid Residues in the Middle of α-Synuclein Is Essential for Filament Assembly. J. Biol. Chem. 2001, 276, 2380–2386. [Google Scholar] [CrossRef] [PubMed]
- Candelise, N.; Schmitz, M.; Thüne, K.; Cramm, M.; Rabano, A.; Zafar, S.; Stoops, E.; Vanderstichele, H.; Villar-Pique, A.; Llorens, F.; et al. Effect of the Micro-Environment on α-Synuclein Conversion and Implication in Seeded Conversion Assays. Transl. Neurodegener. 2020, 9, 5. [Google Scholar] [CrossRef]
- Kumari, M.; Bisht, K.S.; Ahuja, K.; Motiani, R.K.; Maiti, T.K. Glycation Produces Topologically Different α—Synuclein Oligomeric Strains and Modulates Microglia Response via the NLRP3- Inflammasome Pathway. ACS Chem. Neurosci. 2024, 15, 3640–3654. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.G.; Andrews, H.; Amara, A.; Naito, A.; Alcalay, R.N.; Shaw, L.M.; Taylor, P.; Xie, T.; Tuite, P.; Henchcliffe, C.; et al. Cerebrospinal Fluid, Plasma, and Saliva in the BioFIND Study: Relationships among Biomarkers and Parkinson’s Disease Features. Mov. Disord. 2018, 33, 282–288. [Google Scholar] [CrossRef]
- Marui, W.; Iseki, E.; Nakai, T.; Miura, S.; Kato, M.; Uéda, K.; Kosaka, K. Progression and Staging of Lewy Pathology in Brains from Patients with Dementia with Lewy Bodies. J. Neurol. Sci. 2002, 195, 153–159. [Google Scholar] [CrossRef]
- Griffiths, E.J.; Rutter, G.A. Mitochondrial Calcium as a Key Regulator of Mitochondrial ATP Production in Mammalian Cells. Biochim. Biophys. Acta—Bioenerg. 2009, 1787, 1324–1333. [Google Scholar] [CrossRef]
- Scorziello, A.; Savoia, C.; Secondo, A.; Boscia, F.; Sisalli, M.J.; Esposito, A.; Carlucci, A.; Molinaro, P.; Lignitto, L.; Di Renzo, G.; et al. New Insights in Mitochondrial Calcium Handling by Sodium/Calcium Exchanger. In Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2013; Volume 961. [Google Scholar] [CrossRef]
- McCormack, J.G.; Denton, R.M. A Comparative Study of the Regulation of Ca2+ of the Activities of the 2-Oxoglutarate Dehydrogenase Complex and NAD+-Isocitrate Dehydrogenase from a Variety of Sources. Biochem. J. 1981, 196, 619–624. [Google Scholar] [CrossRef]
- Berthold, C.H.; Fabricius, C.; Rydmark, M.; Andersén, B. Axoplasmic Organelles at Nodes of Ranvier. I. Occurrence and Distribution in Large Myelinated Spinal Root Axons of the Adult Cat. J. Neurocytol. 1993, 22, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Rowland, K.C.; Irby, N.K.; Spirou, G.A. Specialized Synapse-Associated Structures within the Calyx of Held. J. Neurosci. 2000, 20, 9135–9144. [Google Scholar] [CrossRef]
- Cheng, S.T.; Lam, L.C.W. Improving Diagnostic Communication in Dementia. Int. Psychogeriatr. 2018, 30, 1149–1152. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, C.; Giguère, N.; Bourque, M.J.; Lévesque, M.; Slack, R.S.; Trudeau, L.É. Elevated Mitochondrial Bioenergetics and Axonal Arborization Size Are Key Contributors to the Vulnerability of Dopamine Neurons. Curr. Biol. 2015, 25, 2349–2360. [Google Scholar] [CrossRef]
- Stanga, S.; Caretto, A.; Boido, M.; Vercelli, A. Mitochondrial Dysfunctions: A Red Thread across Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 3719. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.J.; Roy, D.S.; Pignatelli, M.; Arons, A.; Tonegawa, S. Engram Cells Retain Memory under Retrograde Amnesia. Science 2015, 348, 1007–1013. [Google Scholar] [CrossRef]
- Sirabella, R.; Sisalli, M.J.; Costa, G.; Omura, K.; Ianniello, G.; Pinna, A.; Morelli, M.; Di Renzo, G.M.; Annunziato, L.; Scorziello, A. NCX1 and NCX3 as Potential Factors Contributing to Neurodegeneration and Neuroinflammation in the A53T Transgenic Mouse Model of Parkinson’s Disease Article. Cell Death Dis. 2018, 9, 725. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, E.; Musich, P.R.; Lin, F. Mitochondrial Dysfunction in Neurodegenerative Diseases and the Potential Countermeasure. CNS Neurosci. Ther. 2019, 25, 816–824. [Google Scholar] [CrossRef]
- Tresse, E.; Marturia-Navarro, J.; Sew, W.Q.G.; Cisquella-Serra, M.; Jaberi, E.; Riera-Ponsati, L.; Fauerby, N.; Hu, E.; Kretz, O.; Aznar, S.; et al. Mitochondrial DNA Damage Triggers Spread of Parkinson’s Disease-like Pathology. Mol. Psychiatry 2023, 28, 4902–4914. [Google Scholar] [CrossRef]
- Flønes, I.H.; Toker, L.; Sandnes, D.A.; Castelli, M.; Mostafavi, S.; Lura, N.; Shadad, O.; Fernandez-Vizarra, E.; Painous, C.; Pérez-Soriano, A.; et al. Mitochondrial Complex I Deficiency Stratifies Idiopathic Parkinson’s Disease. Nat. Commun. 2024, 15, 3631. [Google Scholar] [CrossRef]
- Parihar, M.S.; Parihar, A.; Fujita, M.; Hashimoto, M.; Ghafourifar, P. Alpha-Synuclein Overexpression and Aggregation Exacerbates Impairment of Mitochondrial Functions by Augmenting Oxidative Stress in Human Neuroblastoma Cells. Int. J. Biochem. Cell Biol. 2009, 41, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Guardia-Laguarta, C.; Area-Gomez, E.; Rüb, C.; Liu, Y.; Magrané, J.; Becker, D.; Voos, W.; Schon, E.A.; Przedborski, S. α-Synuclein Is Localized to Mitochondria-Associated ER Membranes. J. Neurosci. 2014, 34, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Robotta, M.; Gerding, H.R.; Vogel, A.; Hauser, K.; Schildknecht, S.; Karreman, C.; Leist, M.; Subramaniam, V.; Drescher, M. Cover Picture: Alpha-Synuclein Binds to the Inner Membrane of Mitochondria in an A-Helical Conformation (ChemBioChem 17/2014). ChemBioChem 2014, 15, 2473. [Google Scholar] [CrossRef][Green Version]
- Du, X.Y.; Xie, X.X.; Liu, R.T. The Role of α-Synuclein Oligomers in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8645. [Google Scholar] [CrossRef]
- Bartman, S.; Coppotelli, G.; Ross, J.M. Mitochondrial Dysfunction: A Key Player in Brain Aging and Diseases. Curr. Issues Mol. Biol. 2024, 46, 1987–2026. [Google Scholar] [CrossRef]
- Choi, M.L.; Chappard, A.; Singh, B.P.; Maclachlan, C.; Rodrigues, M.; Fedotova, E.I.; Berezhnov, A.V.; De, S.; Peddie, C.J.; Athauda, D.; et al. Pathological Structural Conversion of α-Synuclein at the Mitochondria Induces Neuronal Toxicity. Nat. Neurosci. 2022, 25, 1134–1148. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, A.; Kumar, K.R.; Sue, C.M. New Insights into the Complex Role of Mitochondria in Parkinson’s Disease. Prog. Neurobiol. 2019, 177, 73–93. [Google Scholar] [CrossRef]
- Ramesh, S.; Arachchige, A.S.P.M. Depletion of Dopamine in Parkinson’s Disease and Relevant Therapeutic Options: A Review of the Literature. AIMS Neurosci. 2023, 10, 200. [Google Scholar] [CrossRef]
- Sohrabi, T.; Mirzaei-Behbahani, B.; Zadali, R.; Pirhaghi, M.; Morozova-Roche, L.A.; Meratan, A.A. Common Mechanisms Underlying α-Synuclein-Induced Mitochondrial Dysfunction in Parkinson’s Disease. J. Mol. Biol. 2023, 435, 167992. [Google Scholar] [CrossRef]
- Yang, S.; Qin, C.; Hu, Z.W.; Zhou, L.Q.; Yu, H.H.; Chen, M.; Bosco, D.B.; Wang, W.; Wu, L.J.; Tian, D.S. Microglia Reprogram Metabolic Profiles for Phenotype and Function Changes in Central Nervous System. Neurobiol. Dis. 2021, 152, 105290. [Google Scholar] [CrossRef]
- Takeda, H.; Yamaguchi, T.; Yano, H.; Tanaka, J. Microglial Metabolic Disturbances and Neuroinflammation in Cerebral Infarction. J. Pharmacol. Sci. 2021, 145, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Martínez, L.; Sierra-Filardi, E.; Boya, P. Mitophagy, Metabolism, and Cell Fate. Mol. Cell. Oncol. 2017, 4, e1353854. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Ma, Z.; Ma, H.; Li, Q.; Zhai, Q.; Jiang, T.; Zhang, Z.; Wang, Q. Mitochondrial Transplantation Attenuates Brain Dysfunction in Sepsis by Driving Microglial M2 Polarization. Mol. Neurobiol. 2020, 57, 3875–3890. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Niu, W.; Lv, J.; Jia, J.; Zhu, M.; Yang, S. PGC-1α-Mediated Mitochondrial Biogenesis Is Involved in Cannabinoid Receptor 2 Agonist AM1241-Induced Microglial Phenotype Amelioration. Cell. Mol. Neurobiol. 2018, 38, 1529–1537. [Google Scholar] [CrossRef]
- Diaz-Galvan, P.; Miyagawa, T.; Przybelski, S.A.; Lesnick, T.G.; Senjem, M.L.; Jack, C.R.; Forsberg, L.K.; Min, H.K.; St. Louis, E.K.; Savica, R.; et al. Brain Glucose Metabolism and Nigrostriatal Degeneration in Isolated Rapid Eye Movement Sleep Behaviour Disorder. Brain Commun. 2023, 5, fcad021. [Google Scholar] [CrossRef]
- Palmas, M.F.; Etzi, M.; Pisanu, A.; Camoglio, C.; Sagheddu, C.; Santoni, M.; Manchinu, M.F.; Pala, M.; Fusco, G.; De Simone, A.; et al. The Intranigral Infusion of Human-Alpha Synuclein Oligomers Induces a Cognitive Impairment in Rats Associated with Changes in Neuronal Firing and Neuroinflammation in the Anterior Cingulate Cortex. Cells 2022, 11, 2628. [Google Scholar] [CrossRef]
- Rehman, K.; Irshad, K.; Kamal, S.; Imran, I.; Akash, M.S.H. Exposure of Environmental Contaminants and Development of Neurological Disorders. Crit. Rev. Eukaryot. Gene Expr. 2021, 31, 35–53. [Google Scholar] [CrossRef]
- Mansour, R.M.; Ahmed, M.A.E.; El-Sahar, A.E.; El Sayed, N.S. Montelukast Attenuates Rotenone-Induced Microglial Activation/P38 MAPK Expression in Rats: Possible Role of Its Antioxidant, Anti-Inflammatory and Antiapoptotic Effects. Toxicol. Appl. Pharmacol. 2018, 358, 76–85. [Google Scholar] [CrossRef]
- Yan, X.; Liu, D.F.; Zhang, X.Y.; Liu, D.; Xu, S.Y.; Chen, G.X.; Huang, B.X.; Ren, W.Z.; Wang, W.; Fu, S.P.; et al. Vanillin Protects Dopaminergic Neurons against Inflammation-Mediated Cell Death by Inhibiting ERK1/2, P38 and the NF-ΚB Signaling Pathway. Int. J. Mol. Sci. 2017, 18, 389. [Google Scholar] [CrossRef]
- Yan, A.; Liu, Z.; Song, L.; Wang, X.; Zhang, Y.; Wu, N.; Lin, J.; Liu, Y.; Liu, Z. Idebenone Alleviates Neuroinflammation and Modulates Microglial Polarization in LPS-Stimulated BV2 Cells and MPTP-Induced Parkinson’s Disease Mice. Front. Cell. Neurosci. 2019, 12, 259. [Google Scholar] [CrossRef]
- Pisanu, A.; Lecca, D.; Mulas, G.; Wardas, J.; Simbula, G.; Spiga, S.; Carta, A.R. Dynamic Changes in Pro-and Anti-Inflammatory Cytokines in Microglia after PPAR-γ Agonist Neuroprotective Treatment in the MPTPp Mouse Model of Progressive Parkinson’s Disease. Neurobiol. Dis. 2014, 71, 280–291. [Google Scholar] [CrossRef]
- Machado, M.M.F.; Bassani, T.B.; Cóppola-Segovia, V.; Moura, E.L.R.; Zanata, S.M.; Andreatini, R.; Vital, M.A.B.F. PPAR-γ Agonist Pioglitazone Reduces Microglial Proliferation and NF-ΚB Activation in the Substantia Nigra in the 6-Hydroxydopamine Model of Parkinson’s Disease. Pharmacol. Rep. 2019, 71, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Xue, T.F.; Guo, X.D.; Yang, J.; Guo, R.B.; Wang, J.; Huang, J.Y.; Zhao, X.J.; Sun, X.L. Antagonizing Peroxisome Proliferator-Activated Receptor γ Facilitates M1-to-M2 Shift of Microglia by Enhancing Autophagy via the LKB1–AMPK Signaling Pathway. Aging Cell 2018, 17, e12774. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, A.I.; Sucunza, D.; Pedrosa, M.A.; Garrido-Gil, P.; Kulisevsky, J.; Lanciego, J.L.; Labandeira-Garcia, J.L. Angiotensin Type 1 Receptor Antagonists Protect Against Alpha-Synuclein-Induced Neuroinflammation and Dopaminergic Neuron Death. Neurotherapeutics 2018, 15, 1063–1081. [Google Scholar] [CrossRef]
- Liu, G.; Ni, C.; Zhan, J.; Li, W.; Luo, J.; Liao, Z.; Locascio, J.J.; Xian, W.; Chen, L.; Pei, Z.; et al. Mitochondrial Haplogroups and Cognitive Progression in Parkinson’s Disease. Brain 2023, 146, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Trinh, D.; Israwi, A.R.; Arathoon, L.R.; Gleave, J.A.; Nash, J.E. The Multi-Faceted Role of Mitochondria in the Pathology of Parkinson’s Disease. J. Neurochem. 2021, 156, 715–752. [Google Scholar] [CrossRef]
- Gao, X.-Y.; Yang, T.; Gu, Y.; Sun, X.-H. Mitochondrial Dysfunction in Parkinson’s Disease: From Mechanistic Insights to Therapy. Front. Aging Neurosci. 2022, 14, 885500. [Google Scholar] [CrossRef]
- Karimian, A.; Gorjizadeh, N.; Alemi, F.; Asemi, Z.; Azizian, K.; Soleimanpour, J.; Malakouti, F.; Targhazeh, N.; Majidinia, M.; Yousefi, B. CRISPR/Cas9 Novel Therapeutic Road for the Treatment of Neurodegenerative Diseases. Life Sci. 2020, 259, 118165. [Google Scholar] [CrossRef]
- Mishra, A.; Singh, S.; Tiwari, V.; Bano, S.; Shukla, S. Dopamine D1 Receptor Agonism Induces Dynamin Related Protein-1 Inhibition to Improve Mitochondrial Biogenesis and Dopaminergic Neurogenesis in Rat Model of Parkinson’s Disease. Behav. Brain Res. 2020, 378, 112304. [Google Scholar] [CrossRef]
- Aman, Y.; Ryan, B.; Torsetnes, S.B.; Knapskog, A.B.; Watne, L.O.; McEwan, W.A.; Fang, E.F. Enhancing Mitophagy as a Therapeutic Approach for Neurodegenerative Diseases. Int. Rev. Neurobiol. 2020, 155, 169–202. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Ishikawa, K.-i.; Inoshita, T.; Shiba-Fukushima, K.; Saiki, S.; Hatano, T.; Mori, A.; Oji, Y.; Okuzumi, A.; Li, Y.; et al. Identifying Therapeutic Agents for Amelioration of Mitochondrial Clearance Disorder in Neurons of Familial Parkinson Disease. Stem Cell Rep. 2020, 14, 1060–1075. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.L.; Champarini, L.G.; Basmadjian, O.M.; Bellini, M.J.; Hereñú, C.B. IGF-1 Gene Therapy Prevents Spatial Memory Deficits and Modulates Dopaminergic Neurodegeneration and Inflammation in a Parkinsonism Model. Brain. Behav. Immun. 2024, 119, 851–866. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Lang, L.; Ye, Z.; Wu, J. Subthalamic Nucleus Stimulation Modulates Cognitive Theory of Mind in Parkinson’s Disease. Mov. Disord. 2024, 39, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, D.; Aarsland, D.; Chaudhuri, K.R.; Dobkin, R.D.; Leentjens, A.F.; Rodriguez-Violante, M.; Schrag, A. The Neuropsychiatry of Parkinson’s Disease: Advances and Challenges. Lancet Neurol. 2022, 21, 89–102. [Google Scholar] [CrossRef]
- Ye, H.; Robak, L.A.; Yu, M.; Cykowski, M.; Shulman, J.M. Genetics and Pathogenesis of Parkinson’s Syndrome. Annu. Rev. Pathol. Mech. Dis. 2023, 18, 95–121. [Google Scholar] [CrossRef]

| Mitochondrial Dysfunction | Brain Areas | Cognitive Impairment | Models | Ref. |
|---|---|---|---|---|
| Reduced ATP production | Hippocampus and prefrontal cortex | Memory deficits | Preclinical cellular models | [11] |
| Increased oxidative stress | Prefrontal cortex and basal ganglia | Executive dysfunction | Clinical models (observations in patients) | [12] |
| Impairment in mitochondrial dynamics (fission/fusion) | Parietal lobe and occipital lobe | Visuospatial impairments | Preclinical cellular models | [13] |
| Altered mitochondrial morphology | Cerebral cortex and basal ganglia | Slowed cognitive processing | Human post-mortem tissues | [14] |
| Impaired electron transport chain | Temporal lobe, frontal lobe | Language difficulties | Clinical models (patients) | [15] |
| Mutations in mitochondrial DNA | Prefrontal cortex and parietal lobe | Attention deficits | Preclinical animal models | [16] |
| Reduction in mitochondrial membrane potential | Frontal lobe | Impaired problem-solving abilities | Preclinical cellular and animal models | [17] |
| Accumulation of mitochondrial DNA damage | Limbic system and prefrontal cortex | Mood disorders (depression and anxiety) | [18] | |
| Complex I deficiency of the electron transport chain | Prefrontal cortex and basal ganglia | Difficulties in multitasking | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scorziello, A.; Sirabella, R.; Sisalli, M.J.; Tufano, M.; Giaccio, L.; D’Apolito, E.; Castellano, L.; Annunziato, L. Mitochondrial Dysfunction in Parkinson’s Disease: A Contribution to Cognitive Impairment? Int. J. Mol. Sci. 2024, 25, 11490. https://doi.org/10.3390/ijms252111490
Scorziello A, Sirabella R, Sisalli MJ, Tufano M, Giaccio L, D’Apolito E, Castellano L, Annunziato L. Mitochondrial Dysfunction in Parkinson’s Disease: A Contribution to Cognitive Impairment? International Journal of Molecular Sciences. 2024; 25(21):11490. https://doi.org/10.3390/ijms252111490
Chicago/Turabian StyleScorziello, Antonella, Rossana Sirabella, Maria Josè Sisalli, Michele Tufano, Lucia Giaccio, Elena D’Apolito, Lorenzo Castellano, and Lucio Annunziato. 2024. "Mitochondrial Dysfunction in Parkinson’s Disease: A Contribution to Cognitive Impairment?" International Journal of Molecular Sciences 25, no. 21: 11490. https://doi.org/10.3390/ijms252111490
APA StyleScorziello, A., Sirabella, R., Sisalli, M. J., Tufano, M., Giaccio, L., D’Apolito, E., Castellano, L., & Annunziato, L. (2024). Mitochondrial Dysfunction in Parkinson’s Disease: A Contribution to Cognitive Impairment? International Journal of Molecular Sciences, 25(21), 11490. https://doi.org/10.3390/ijms252111490







