Double Heterozygous Pathogenic Variants in TP53 and CHEK2 in Boy with Undifferentiated Embryonal Sarcoma of the Liver
Abstract
1. Introduction
2. Results
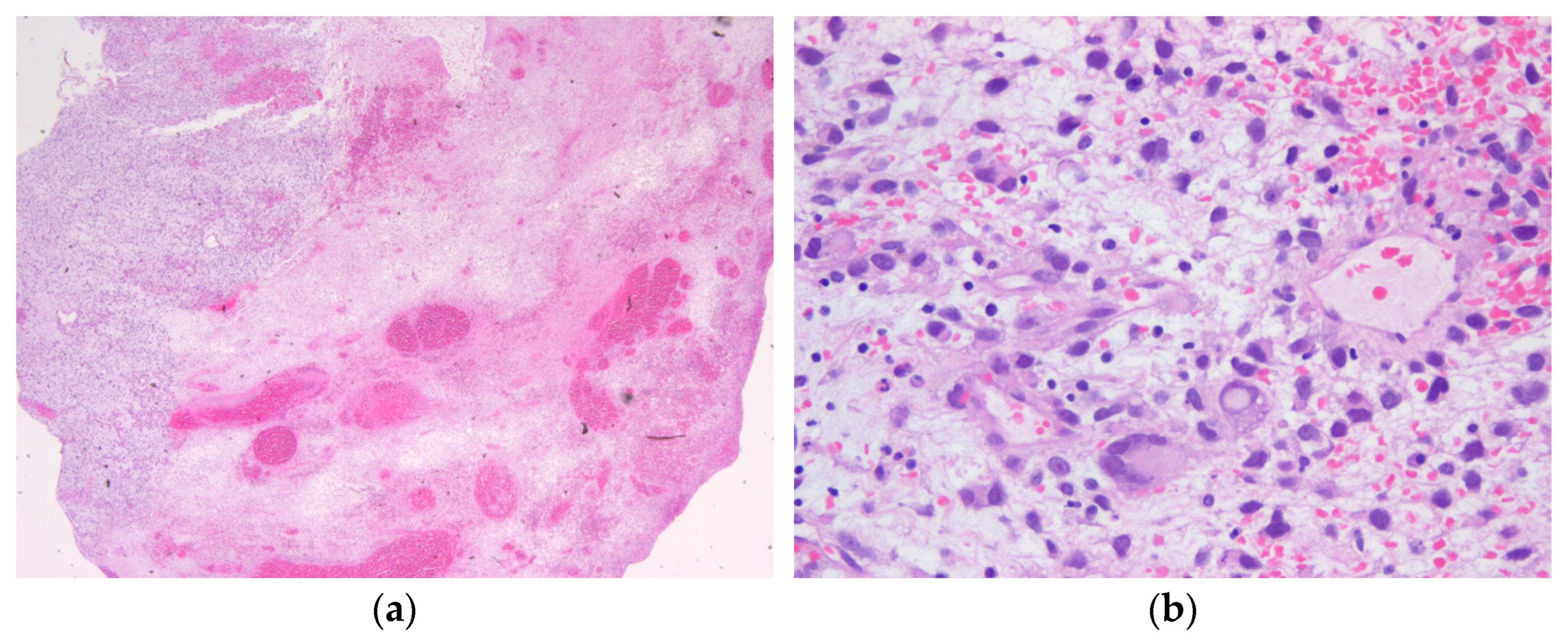
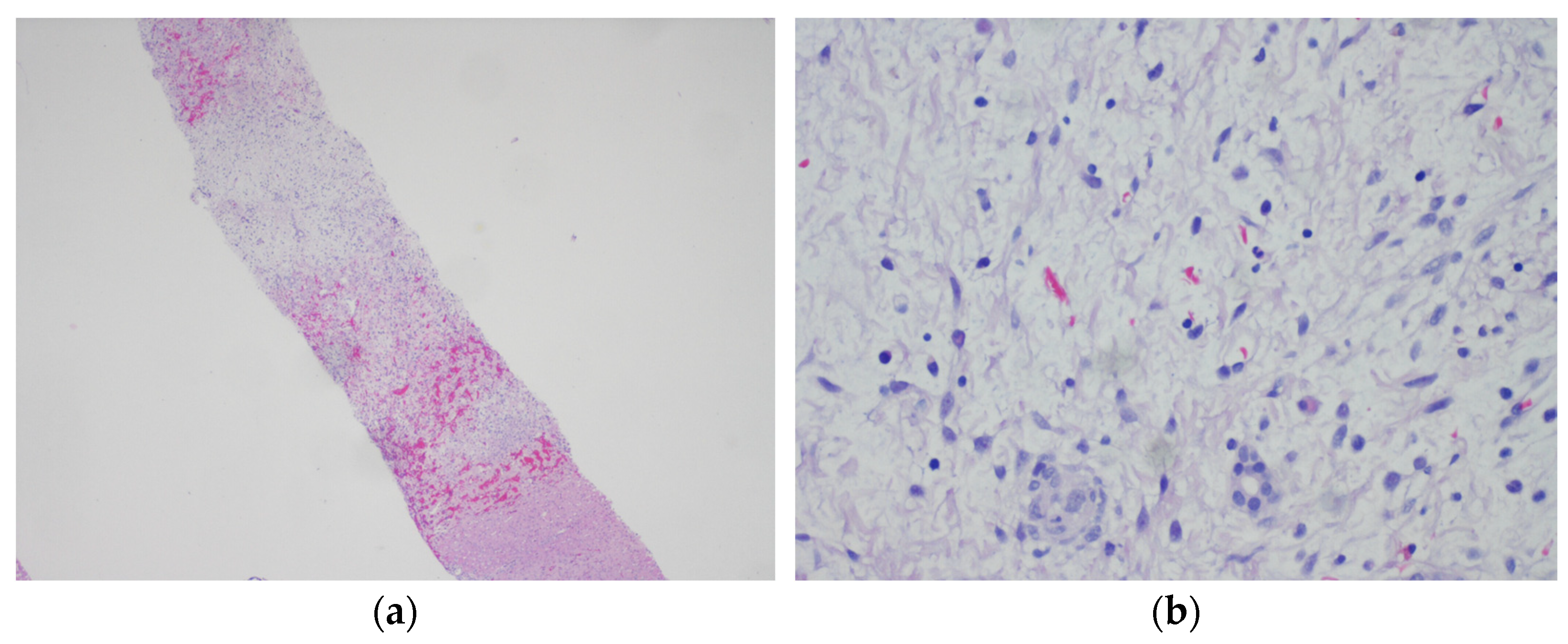
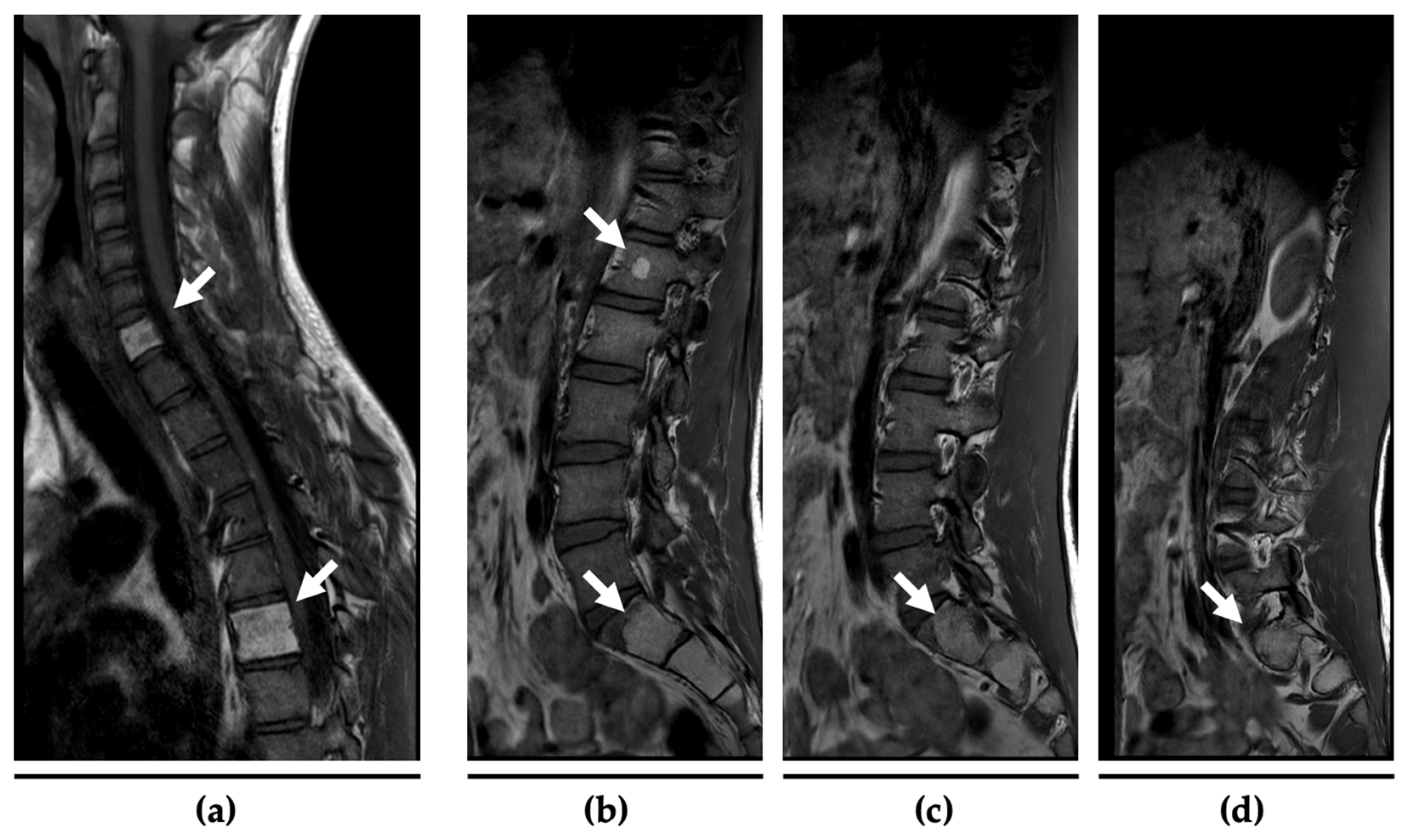
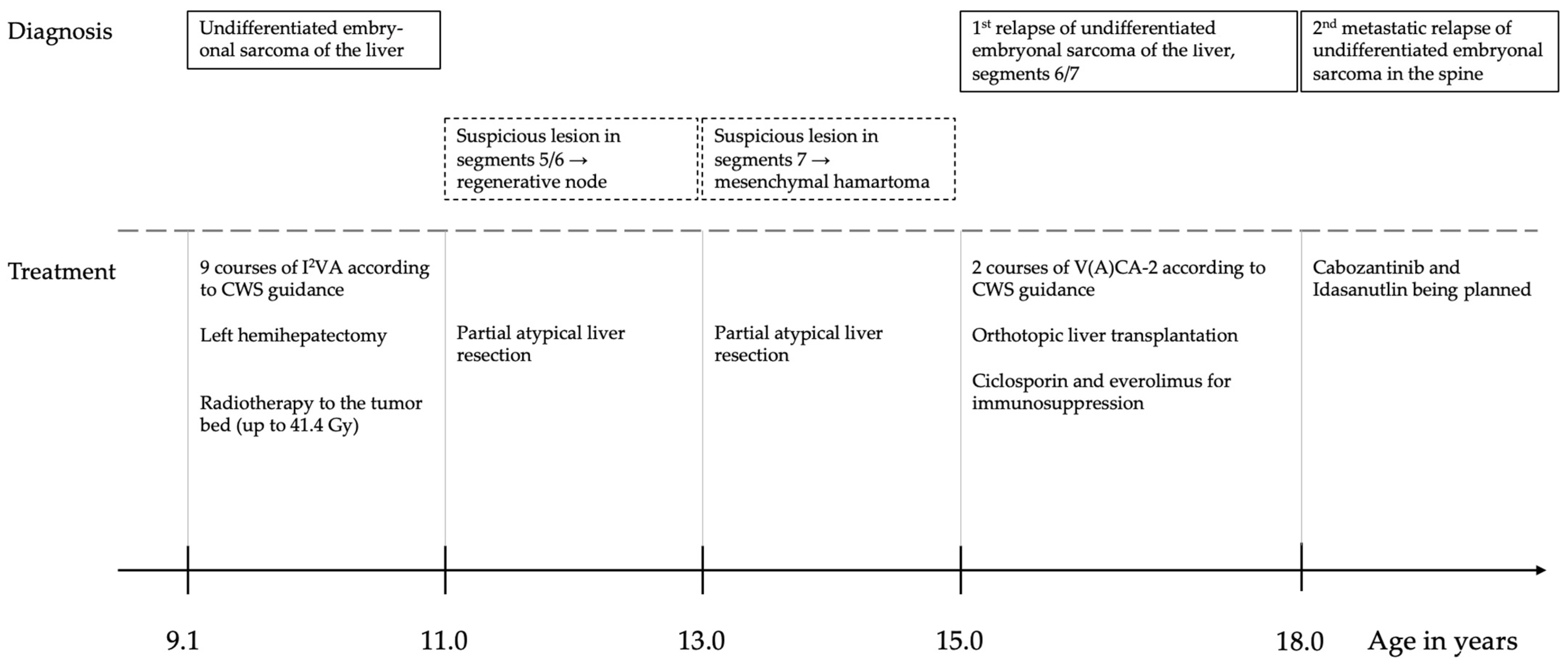
2.1. Case History
2.2. Comprehensive Genetic Tumor Analyses
2.3. Molecular Genetic Testing of TP53 and CHEK2 Genes
3. Discussion
4. Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grobner, S.N.; Worst, B.C.; Weischenfeldt, J.; Buchhalter, I.; Kleinheinz, K.; Rudneva, V.A.; Johann, P.D.; Balasubramanian, G.P.; Segura-Wang, M.; Brabetz, S.; et al. The landscape of genomic alterations across childhood cancers. Nature 2018, 555, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Walsh, M.F.; Wu, G.; Edmonson, M.N.; Gruber, T.A.; Easton, J.; Hedges, D.; Ma, X.; Zhou, X.; Yergeau, D.A.; et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. N. Engl. J. Med. 2015, 373, 2336–2346. [Google Scholar] [CrossRef] [PubMed]
- Goudie, C.; Cullinan, N.; Villani, A.; Mathews, N.; van Engelen, K.; Malkin, D.; Irwin, M.S.; Foulkes, W.D. Retrospective evaluation of a decision-support algorithm (MIPOGG) for genetic referrals for children with neuroblastic tumors. Pediatr. Blood Cancer 2018, 65, e27390. [Google Scholar] [CrossRef] [PubMed]
- Jongmans, M.C.; Loeffen, J.L.; Waanders, E.; Hoogerbrugge, P.M.; Ligtenberg, M.J.; Kuiper, R.P.; Hoogerbrugge, N. Recognition of genetic predisposition in pediatric cancer patients: An easy-to-use selection tool. Eur. J. Med. Genet. 2016, 59, 116–125. [Google Scholar] [CrossRef]
- Ripperger, T.; Bielack, S.S.; Borkhardt, A.; Brecht, I.B.; Burkhardt, B.; Calaminus, G.; Debatin, K.M.; Deubzer, H.; Dirksen, U.; Eckert, C.; et al. Childhood cancer predisposition syndromes-A concise review and recommendations by the Cancer Predisposition Working Group of the Society for Pediatric Oncology and Hematology. Am. J. Med. Genet. A 2017, 173, 1017–1037. [Google Scholar] [CrossRef]
- Wurtemberger, J.; Ripperger, T.; Vokuhl, C.; Bauer, S.; Teichert-von Luttichau, I.; Wardelmann, E.; Niemeyer, C.M.; Kratz, C.P.; Schlegelberger, B.; Hettmer, S. Genetic susceptibility in children, adolescents, and young adults diagnosed with soft-tissue sarcomas. Eur. J. Med. Genet. 2023, 66, 104718. [Google Scholar] [CrossRef]
- King, M.C.; Marks, J.H.; Mandell, J.B.; New York Breast Cancer Study, G. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 2003, 302, 643–646. [Google Scholar] [CrossRef]
- McCabe, N.; Turner, N.C.; Lord, C.J.; Kluzek, K.; Bialkowska, A.; Swift, S.; Giavara, S.; O’Connor, M.J.; Tutt, A.N.; Zdzienicka, M.Z.; et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006, 66, 8109–8115. [Google Scholar] [CrossRef]
- Byrjalsen, A.; Hansen, T.V.O.; Stoltze, U.K.; Mehrjouy, M.M.; Barnkob, N.M.; Hjalgrim, L.L.; Mathiasen, R.; Lautrup, C.K.; Gregersen, P.A.; Hasle, H.; et al. Nationwide germline whole genome sequencing of 198 consecutive pediatric cancer patients reveals a high incidence of cancer prone syndromes. PLoS Genet. 2020, 16, e1009231. [Google Scholar] [CrossRef]
- McGee, R.B.; Oak, N.; Harrison, L.; Xu, K.; Nuccio, R.; Blake, A.K.; Mostafavi, R.; Lewis, S.; Taylor, L.M.; Kubal, M.; et al. Pathogenic Variants in Adult-Onset Cancer Predisposition Genes in Pediatric Cancer: Prevalence and Impact on Tumor Molecular Features and Clinical Management. Clin. Cancer Res. 2023, 29, 1243–1251. [Google Scholar] [CrossRef]
- Wagener, R.; Taeubner, J.; Walter, C.; Yasin, L.; Alzoubi, D.; Bartenhagen, C.; Attarbaschi, A.; Classen, C.F.; Kontny, U.; Hauer, J.; et al. Comprehensive germline-genomic and clinical profiling in 160 unselected children and adolescents with cancer. Eur. J. Hum. Genet. 2021, 29, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Kuhlen, M.; Hofmann, T.G.; Golas, M.M. Puzzling phenomenon: Adult-onset cancer predisposition and pediatric cancer. Trends Cancer 2024, 10, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Yin, L.; Liu, X.C.; Ying, R.C.; Kong, W.C. Adult versus paediatric undifferentiated embryonal sarcoma of the liver: A SEER database analysis. ANZ J. Surg. 2021, 91, 2690–2694. [Google Scholar] [CrossRef] [PubMed]
- Funato, M.; Tsunematsu, Y.; Yamazaki, F.; Tamura, C.; Kumamoto, T.; Takagi, M.; Kato, S.; Sugimura, H.; Tamura, K. Characteristics of Li-Fraumeni Syndrome in Japan; A Review Study by the Special Committee of JSHT. Cancer Sci. 2021, 112, 2821–2834. [Google Scholar] [CrossRef]
- Lack, E.E.; Schloo, B.L.; Azumi, N.; Travis, W.D.; Grier, H.E.; Kozakewich, H.P. Undifferentiated (embryonal) sarcoma of the liver. Clinical and pathologic study of 16 cases with emphasis on immunohistochemical features. Am. J. Surg. Pathol. 1991, 15, 1–16. [Google Scholar] [CrossRef]
- Ecker, J.; Selt, F.; Sturm, D.; Sill, M.; Korshunov, A.; Hirsch, S.; Capper, D.; Dikow, N.; Sutter, C.; Muller, C.; et al. Molecular diagnostics enables detection of actionable targets: The Pediatric Targeted Therapy 2.0 registry. Eur. J. Cancer 2023, 180, 71–84. [Google Scholar] [CrossRef]
- Telli, M.L.; Timms, K.M.; Reid, J.; Hennessy, B.; Mills, G.B.; Jensen, K.C.; Szallasi, Z.; Barry, W.T.; Winer, E.P.; Tung, N.M.; et al. Homologous Recombination Deficiency (HRD) Score Predicts Response to Platinum-Containing Neoadjuvant Chemotherapy in Patients with Triple-Negative Breast Cancer. Clin. Cancer Res. 2016, 22, 3764–3773. [Google Scholar] [CrossRef]
- Kratz, C.P.; Villani, A.; Nichols, K.E.; Schiffman, J.; Malkin, D. Cancer surveillance for individuals with Li-Fraumeni syndrome. Eur. J. Hum. Genet. 2020, 28, 1481–1482. [Google Scholar] [CrossRef]
- Sutcliffe, E.G.; Stettner, A.R.; Miller, S.A.; Solomon, S.R.; Marshall, M.L.; Roberts, M.E.; Susswein, L.R.; Arvai, K.J.; Klein, R.T.; Murphy, P.D.; et al. Differences in cancer prevalence among CHEK2 carriers identified via multi-gene panel testing. Cancer Genet. 2020, 246–247, 12–17. [Google Scholar] [CrossRef]
- Agaoglu, N.B.; Doganay, L. Concurrent pathogenic variations in patients with hereditary cancer syndromes. Eur. J. Med. Genet. 2021, 64, 104366. [Google Scholar] [CrossRef]
- Sokolenko, A.P.; Bogdanova, N.; Kluzniak, W.; Preobrazhenskaya, E.V.; Kuligina, E.S.; Iyevleva, A.G.; Aleksakhina, S.N.; Mitiushkina, N.V.; Gorodnova, T.V.; Bessonov, A.A.; et al. Double heterozygotes among breast cancer patients analyzed for BRCA1, CHEK2, ATM, NBN/NBS1, and BLM germ-line mutations. Breast Cancer Res. Treat. 2014, 145, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Megid, T.B.C.; Barros-Filho, M.C.; Pisani, J.P.; Achatz, M.I. Double heterozygous pathogenic variants prevalence in a cohort of patients with hereditary breast cancer. Front. Oncol. 2022, 12, 873395. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.L.; Michailidou, K.; Parsons, M.T.; Fortuno, C.; Bolla, M.K.; Wang, Q.; Dennis, J.; Naven, M.; Abubakar, M.; Ahearn, T.U.; et al. Co-observation of germline pathogenic variants in breast cancer predisposition genes: Results from analysis of the BRIDGES sequencing dataset. Am. J. Hum. Genet. 2024, 111, 2059–2069. [Google Scholar] [CrossRef]
- Matt, S.; Hofmann, T.G. The DNA damage-induced cell death response: A roadmap to kill cancer cells. Cell. Mol. Life Sci. 2016, 73, 2829–2850. [Google Scholar] [CrossRef]
- Jack, M.T.; Woo, R.A.; Hirao, A.; Cheung, A.; Mak, T.W.; Lee, P.W. Chk2 is dispensable for p53-mediated G1 arrest but is required for a latent p53-mediated apoptotic response. Proc. Natl. Acad. Sci. USA 2002, 99, 9825–9829. [Google Scholar] [CrossRef]
- Hirao, A.; Kong, Y.Y.; Matsuoka, S.; Wakeham, A.; Ruland, J.; Yoshida, H.; Liu, D.; Elledge, S.J.; Mak, T.W. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science 2000, 287, 1824–1827. [Google Scholar] [CrossRef]
- Yu, Q.; Rose, J.H.; Zhang, H.; Pommier, Y. Antisense inhibition of Chk2/hCds1 expression attenuates DNA damage-induced S and G2 checkpoints and enhances apoptotic activity in HEK-293 cells. FEBS Lett. 2001, 505, 7–12. [Google Scholar] [CrossRef]
- Harvey, M.; Vogel, H.; Morris, D.; Bradley, A.; Bernstein, A.; Donehower, L.A. A mutant p53 transgene accelerates tumour development in heterozygous but not nullizygous p53-deficient mice. Nat. Genet. 1995, 9, 305–311. [Google Scholar] [CrossRef]
- Knudson, A.G., Jr. Mutation and cancer: Statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA 1971, 68, 820–823. [Google Scholar] [CrossRef]
- Ciani, Y.; Fedrizzi, T.; Prandi, D.; Lorenzin, F.; Locallo, A.; Gasperini, P.; Franceschini, G.M.; Benelli, M.; Elemento, O.; Fava, L.L.; et al. Allele-specific genomic data elucidate the role of somatic gain and copy-number neutral loss of heterozygosity in cancer. Cell Syst. 2022, 13, 183–193.e7. [Google Scholar] [CrossRef]
- Dobzhansky, T. Genetics of natural populations; recombination and variability in populations of Drosophila pseudoobscura. Genetics 1946, 31, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Topatana, W.; Juengpanich, S.; Cao, J.; Hu, J.; Zhang, B.; Ma, D.; Cai, X.; Chen, M. Development of synthetic lethality in cancer: Molecular and cellular classification. Signal Transduct. Target. Ther. 2020, 5, 241. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Simon, R. Identification of potential synthetic lethal genes to p53 using a computational biology approach. BMC Med. Genomics 2013, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- den Dunnen, J.T.; Dalgleish, R.; Maglott, D.R.; Hart, R.K.; Greenblatt, M.S.; McGowan-Jordan, J.; Roux, A.F.; Smith, T.; Antonarakis, S.E.; Taschner, P.E. HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum. Mutat. 2016, 37, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuhlen, M.; Schaller, T.; Dintner, S.; Stadler, N.; Hofmann, T.G.; Schmutz, M.; Claus, R.; Frühwald, M.C.; Golas, M.M. Double Heterozygous Pathogenic Variants in TP53 and CHEK2 in Boy with Undifferentiated Embryonal Sarcoma of the Liver. Int. J. Mol. Sci. 2024, 25, 11489. https://doi.org/10.3390/ijms252111489
Kuhlen M, Schaller T, Dintner S, Stadler N, Hofmann TG, Schmutz M, Claus R, Frühwald MC, Golas MM. Double Heterozygous Pathogenic Variants in TP53 and CHEK2 in Boy with Undifferentiated Embryonal Sarcoma of the Liver. International Journal of Molecular Sciences. 2024; 25(21):11489. https://doi.org/10.3390/ijms252111489
Chicago/Turabian StyleKuhlen, Michaela, Tina Schaller, Sebastian Dintner, Nicole Stadler, Thomas G. Hofmann, Maximilian Schmutz, Rainer Claus, Michael C. Frühwald, and Monika M. Golas. 2024. "Double Heterozygous Pathogenic Variants in TP53 and CHEK2 in Boy with Undifferentiated Embryonal Sarcoma of the Liver" International Journal of Molecular Sciences 25, no. 21: 11489. https://doi.org/10.3390/ijms252111489
APA StyleKuhlen, M., Schaller, T., Dintner, S., Stadler, N., Hofmann, T. G., Schmutz, M., Claus, R., Frühwald, M. C., & Golas, M. M. (2024). Double Heterozygous Pathogenic Variants in TP53 and CHEK2 in Boy with Undifferentiated Embryonal Sarcoma of the Liver. International Journal of Molecular Sciences, 25(21), 11489. https://doi.org/10.3390/ijms252111489





