The Basic Principles of Pathophysiology of Venous Thrombosis
Abstract
1. Introduction
2. The Basic Principles of the Pathophysiology of Venous Thrombosis
2.1. Venous Stasis
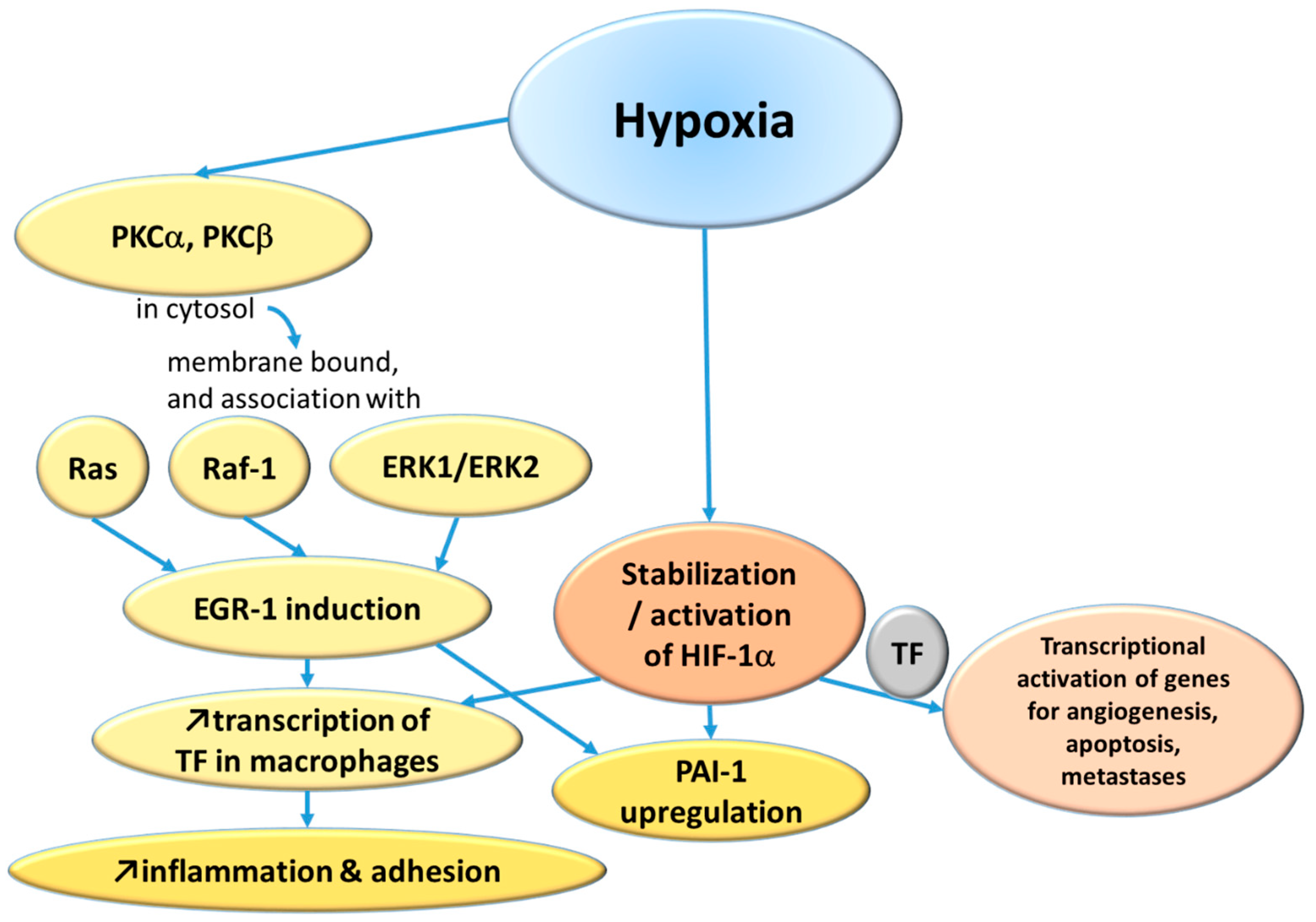
2.1.1. Hypoxemia
2.1.2. Reduced Clearance in Valve Pockets
2.1.3. A Genetic Component
2.2. Venous Flow Pattern
2.3. Interplay Between Blood Components and Endothelium
2.4. Inflammation
2.4.1. Interleukins and Their Possible Role in Air-Pollution-Evoked Venous Thromboembolism
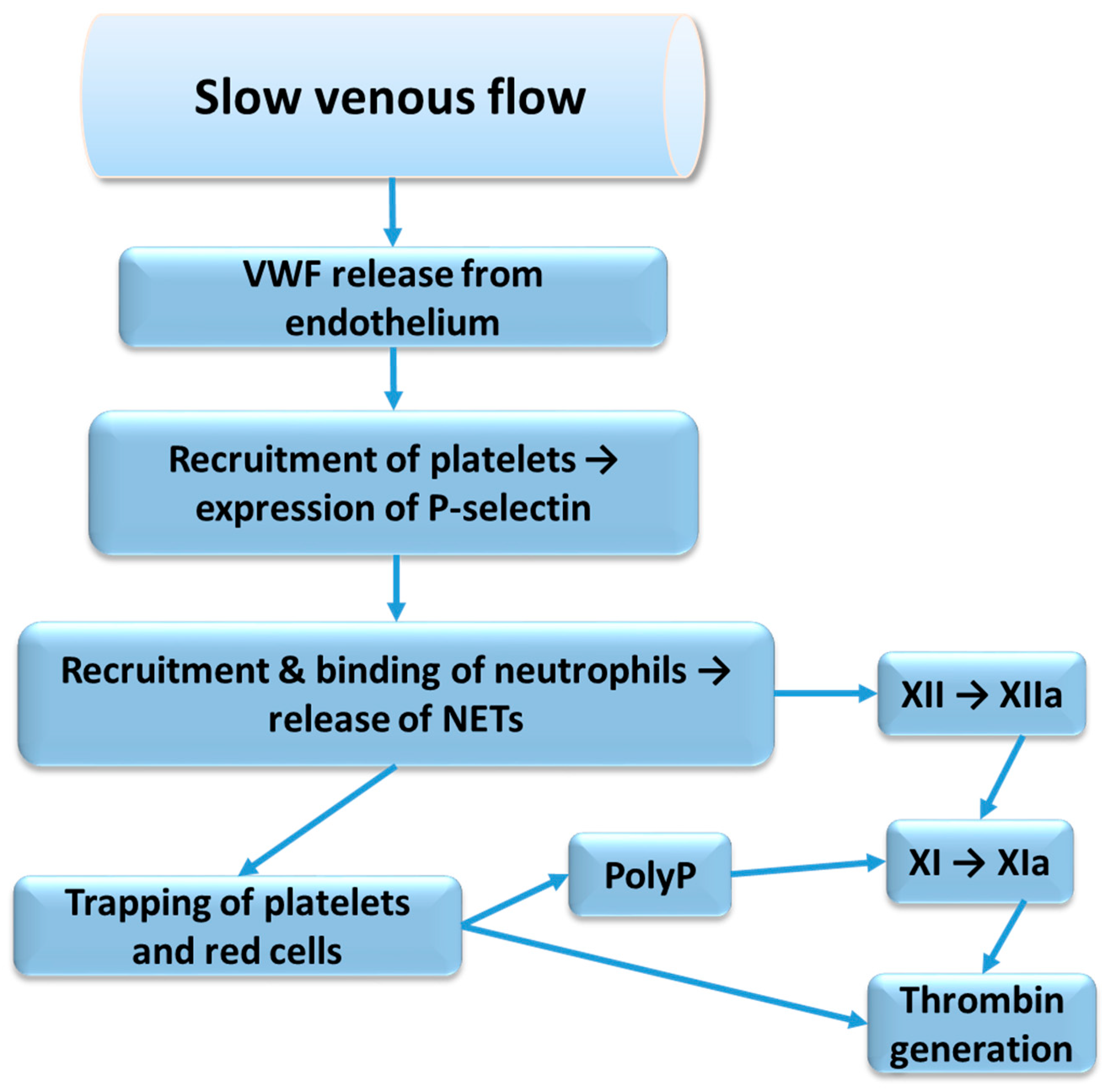
2.4.2. Neutrophil Extracellular Traps
2.4.3. Complement System
2.4.4. Genetic Susceptibility
2.5. Cancer and Thrombosis
2.5.1. Risk Factors of Cancer-Associated Thrombosis
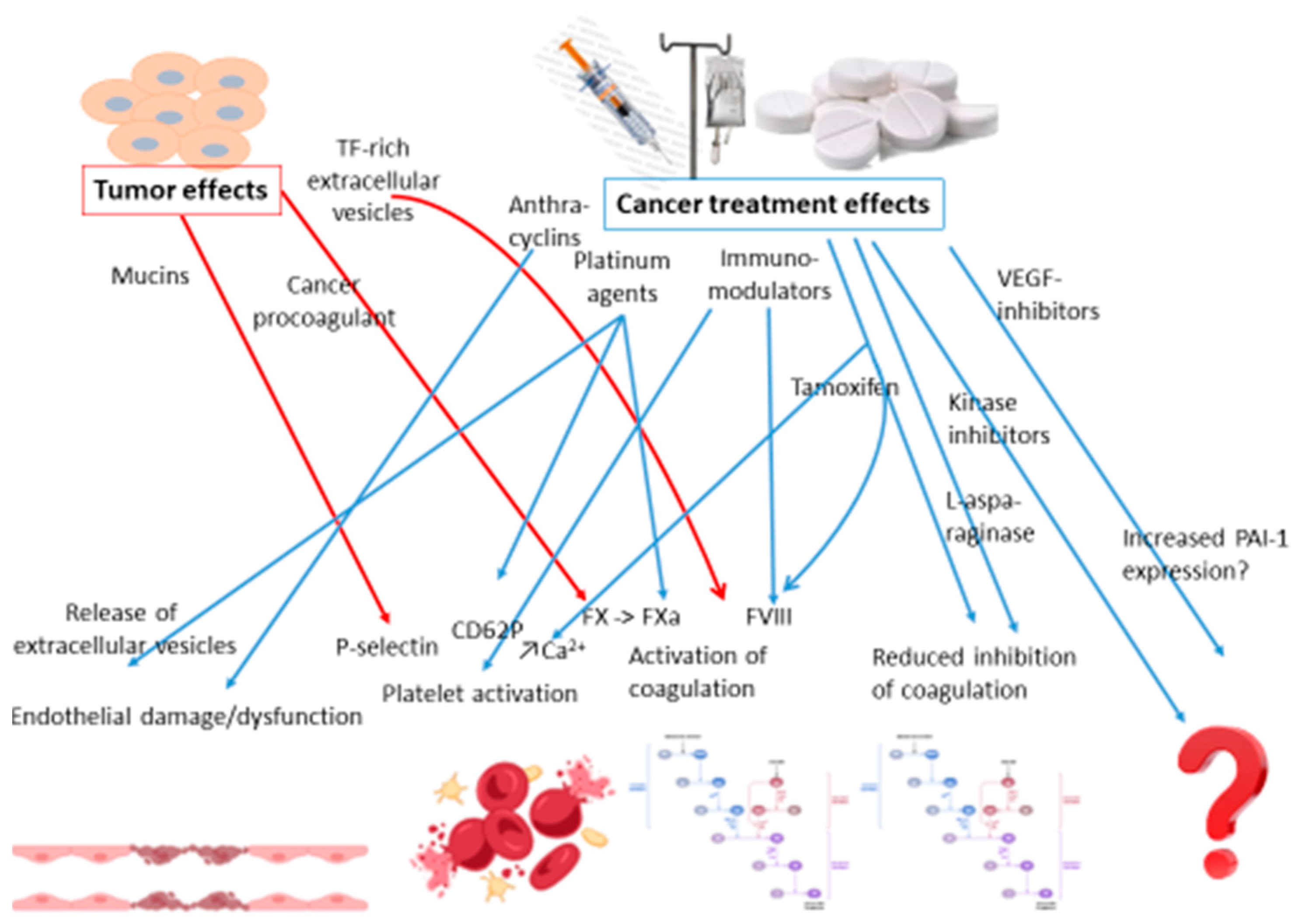
2.5.2. Pharmaceutical Treatments for Cancer and Risk of Thrombosis
2.5.3. Cancer Related Factors
2.6. Hereditary Thrombophilia
3. Future Research
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bagot, C.N.; Arya, R. Virchow and his triad: A question of attribution. Br. J. Haematol. 2008, 143, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Wessler, S. Thrombosis in the presence of vascular stasis. Am. J. Med. 1962, 33, 648–666. [Google Scholar] [CrossRef] [PubMed]
- Hamer, J.D.; Malone, P.C.; Silver, I.A. The PO2 in venous valve pockets: Its possible bearing on thrombogenesis. Br. J. Surg. 1981, 68, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.F.; Mackman, N.; Kisiel, W.; Stern, D.M.; Pinsky, D.J. Hypoxia/Hypoxemia-Induced activation of the procoagulant pathways and the pathogenesis of ischemia-associated thrombosis. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2029–2035. [Google Scholar] [CrossRef]
- Yan, S.F.; Zou, Y.S.; Gao, Y.; Zhai, C.; Mackman, N.; Lee, S.L.; Milbrandt, J.; Pinsky, D.; Kisiel, W.; Stern, D. Tissue factor transcription driven by Egr-1 is a critical mechanism of murine pulmonary fibrin deposition in hypoxia. Proc. Natl. Acad. Sci. USA 1998, 95, 8298–8303. [Google Scholar] [CrossRef]
- Yan, S.F.; Lu, J.; Zou, Y.S.; Kisiel, W.; Mackman, N.; Leitges, M.; Steinberg, S.; Pinsky, D.; Stern, D. Protein kinase C-beta and oxygen deprivation. A novel Egr-1-dependent pathway for fibrin deposition in hypoxemic vasculature. J. Biol. Chem. 2000, 275, 11921–11928. [Google Scholar] [CrossRef]
- Lo, L.W.; Cheng, J.J.; Chiu, J.J.; Wung, B.S.; Liu, Y.C.; Wang, D.L. Endothelial exposure to hypoxia induces Egr-1 expression involving PKCalpha-mediated Ras/Raf-1/ERK1/2 pathway. J. Cell Physiol. 2001, 188, 304–312. [Google Scholar] [CrossRef]
- Hsieh, K.Y.; Wei, C.K.; Wu, C.C. YC-1 prevents tumor-associated tssue factor expression and procoagulant activity in hypoxic conditions by inhibiting p38/NF-kB signaling pathway. Int. J. Mol. Sci. 2019, 20, 244. [Google Scholar] [CrossRef]
- Liao, H.; Hyman, M.C.; Lawrence, D.A.; Pinsky, D.J. Molecular regulation of the PAI-1 gene by hypoxia: Contributions of Egr-1, HIF-1alpha, and C/EBPalpha. FASEB J. 2007, 21, 935–949. [Google Scholar] [CrossRef]
- Bikov, A.; Meszaros, M.; Schwarz, E.I. Coagulation and fibrinolysis in obstructive sleep apnoea. Int. J. Mol. Sci. 2021, 22, 2834. [Google Scholar] [CrossRef]
- Donnally, C.J.; Vakharia, A.M.; Sheu, J.I.; Vakharia, R.M.; Damodar, D.; Shenoy, K.; Gjolaj, J.P. High altitude is an independent risk factor for developing a pulmonary embolism, but not a deep vein thrombosis following a 1- to 2-level lumbar fusion. Glob. Spine J. 2019, 9, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C.; Franchini, M. Sleep apnea and venous thromboembolism. A systematic review. Thromb. Haemost. 2015, 114, 958–963. [Google Scholar] [PubMed]
- Trzepizur, W.; Gervès-Pinquié, C.; Heudes, B.; Blanchard, M.; Meslier, N.; Jouvenot, M.; Kerbat, S.; Mao, R.L.; Magois, E.; Racineux, J.L.; et al. Sleep apnea and incident unprovoked venous thromboembolism: Data from the Pays de la Loire Sleep Cohort. Thromb. Haemost. 2023, 123, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Treml, B.; Wallner, B.; Blank, C.; Fries, D.; Schobersberger, W. The influence of environmental hypoxia on hemostasis—A systematic review. Front. Cardiovasc. Med. 2022, 9, 813550. [Google Scholar] [CrossRef]
- Brooks, E.G.; Trotman, W.; Wadsworth, M.P.; Taatjes, D.J.; Evans, M.F.; Ittleman, F.P.; Callas, P.W.; Esmon, C.T.; Bovill, E.G. Valves of the deep venous system: An overlooked risk factor. Blood 2009, 114, 1276–1279. [Google Scholar] [CrossRef][Green Version]
- Trotman, W.E.; Taatjes, D.J.; Callas, P.W.; Bovill, E.G. The endothelial microenvironment in the venous valvular sinus: Thromboresistance trends and inter-individual variation. Histochem. Cell Biol. 2011, 135, 141–152. [Google Scholar] [CrossRef]
- Brill, A.; Fuchs, T.A.; Chauhan, A.K.; Yang, J.J.; De Meyer, S.F.; Köllnberger, M.; Wakefield, T.W.; Lämmle, B.; Massberg, S.; Wagner, D.D. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood 2011, 117, 1400–1407. [Google Scholar] [CrossRef]
- von Brühl, M.L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Köllnberger, M.; et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef]
- Desch, K.C.; Ozel, A.B.; Halvorsen, M.; Jacobi, P.M.; Golden, K.; Underwood, M.; Germain, M.; Tregouet, D.A.; Reitsma, P.H.; Kearon, C.; et al. Whole-exome sequencing identifies rare variants in STAB2 associated with venous thromboembolic disease. Blood 2020, 136, 533–541. [Google Scholar] [CrossRef]
- Hathcock, J.J. Flow effects on coagulation and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 41–48. [Google Scholar] [CrossRef]
- Goel, M.S.; Diamond, S.L. Adhesion of normal erythrocytes at depressed venous shear rates to activated neutrophils, activated platelets, and fibrin polymerized from plasma. Blood 2002, 100, 3797–3803. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Russell, J.; Senchenkova, E.Y.; Almeida Paula, L.D.; Granger, D.N. Interleukin-1beta mediates the extra-intestinal thrombosis associated with experimental colitis. Am. J. Pathol. 2010, 177, 2774–2781. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Ghani, S.E.; Hamed, R.M.R.; Eid, R.A.; Ibrahim, A.Y.M.; Abdel-Hamid, H.M.; Abdelrahman, W.; Ibrahim, R.E.; Abdel-Aziz, M.M.; Mohamed, M.S. Serum interleukin 1b and sP-selectin as biomarkers of inflammation and thrombosis, could they be predictors of disease severity in COVID 19 Egyptian patients? (A cross-sectional study). Thromb. J. 2022, 20, 77. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yu, M.; Zhu, F.; Zhang, S.; Ding, P.; Wang, M. IL-9 promotes the development of deep venous thrombosis by facilitating platelet function. Thromb. Haemost. 2018, 118, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Zhang, S.; Yu, M.; Feng, Y.; Long, Q.; Yang, H.; Li, J.; Wang, M. IL-17A promotes the formation of deep vein thrombosis in a mouse model. Int. Immunopharmacol. 2018, 57, 132–138. [Google Scholar] [CrossRef]
- Ding, J.; Pan, L.; Lan, D.; Chen, Z.; Wang, Z.; Zou, M.; Meng, R. Inflammatory markers differentiate cerebral venous sinus thrombosis from mimics. Thromb. Haemost. 2023, 123, 326–335. [Google Scholar] [CrossRef]
- Bittar, L.F.; Mazetto Bde, M.; Orsi, F.L.; Collela, M.P.; De Paula, E.V.; Annichino-Bizzacchi, J.M. Long-term increased factor VIII levels are associated to interleukin-6 levels but not to post-thrombotic syndrome in patients with deep venous thrombosis. Thromb. Res. 2015, 135, 497–501. [Google Scholar] [CrossRef]
- Du, Y.Q.; Tang, J.; Zhang, Z.X.; Bian, J. Correlation of interleukin-18 and high-sensitivity c-reactive protein with perioperative deep vein thrombosis in patients with ankle fracture. Ann. Vasc. Surg. 2019, 54, 282–289. [Google Scholar] [CrossRef]
- Mutlu, G.M.; Green, D.; Bellmeyer, A.; Baker, C.M.; Burgess, Z.; Rajamannan, N.; Christman, J.W.; Foiles, N.; Kamp, D.W.; Ghio, A.J.; et al. Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. J. Clin. Investig. 2007, 117, 2952–2961. [Google Scholar] [CrossRef]
- Nemmar, A.; Hoet, P.H.; Vermylen, J.; Nemery, B.; Hoylaerts, M.F. Pharmacological stabilization of mast cells abrogates late thrombotic events induced by diesel exhaust particles in hamsters. Circulation 2004, 110, 1670–1677. [Google Scholar] [CrossRef]
- Lucking, A.J.; Lundback, M.; Mills, N.L.; Faratian, D.; Barath, S.L.; Pourazar, J.; Cassee, F.R.; Donaldson, K.; Boon, N.A.; Badimon, J.J.; et al. Diesel exhaust inhalation increases thrombus formation in man. Eur. Heart J. 2008, 29, 3043–3051. [Google Scholar] [CrossRef] [PubMed]
- Dales, R.E.; Cakmak, S.; Vidal, C.B. Air pollution and hospitalization for venous thromboembolic disease in Chile. J. Thromb. Haemost. 2010, 8, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Mengoli, C.; Cruciani, M.; Bonfanti, C.; Mannucci, P.M. Association between particulate air pollution and venous thromboembolism: A systematic literature review. Eur. J. Intern. Med. 2016, 27, 10–13. [Google Scholar] [CrossRef]
- Müller, F.; Mutch, N.J.; Schenk, W.A.; Smith, S.A.; Esterl, L.; Spronk, H.M.; Schmidbauer, S.; Gahl, W.A.; Morrissey, J.H.; Renné, T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell 2009, 139, 1143–1156. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Brill, A.; Wagner, D.D. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1777–1783. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Pelayo, R.; Monestier, M.; Ammollo, C.T.; Semeraro, F.; Taylor, F.B.; Esmon, N.L.; Lupu, F.; Esmon, C.T. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009, 15, 1318–1321. [Google Scholar] [CrossRef]
- van Montfoort, M.L.; Stephan, F.; Lauw, M.N.; Hutten, B.A.; Van Mierlo, G.J.; Solati, S.; Middeldorp, S.; Meijers, J.C.; Zeerleder, S. Circulating nucleosomes and neutrophil activation as risk factors for deep vein thrombosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 147–151. [Google Scholar] [CrossRef]
- Bressan, A.; Faggin, E.; Donato, M.; Tonon, L.; Buso, R.; Nardin, C.; Tiepolo, M.; Cinetto, F.; Scarpa, R.; Agostini, C.; et al. NETosis in acute thrombotic disorders. Semin. Thromb. Hemost. 2023, 49, 709–715. [Google Scholar] [CrossRef]
- Higuchi, D.A.; Wun, T.C.; Likert, K.M.; Broze, G.J., Jr. The effect of leukocyte elastase on tissue factor pathway inhibitor. Blood 1992, 79, 1712–1719. [Google Scholar] [CrossRef]
- Ansari, S.A.; Pendurthi, U.R.; Rao, L.V.M. Role of cell surface lipids and thiol-disulphide exchange pathways in regulating the encryption and decryption of tissue factor. Thromb. Haemost. 2019, 119, 860–870. [Google Scholar] [CrossRef]
- Levy, J.H.; Iba, T.; Olson, L.B.; Corey, K.M.; Ghadimi, K.; Connors, J.M. COVID-19: Thrombosis, thromboinflammation, and anticoagulation considerations. Int. J. Lab. Hematol. 2021, 43, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.J.; Bradbury, C.; Abrams, S.T.; Wang, G.; Toh, C.H. COVID-19 and immunothrombosis: Emerging understanding and clinical management. Br. J. Haematol. 2021, 194, 518–529. [Google Scholar] [CrossRef]
- Xu, X.; Wu, Y.; Xu, S.; Yin, Y.; Ageno, W.; De Stefano, V.; Zhao, Q.; Qi, X. Clinical significance of neutrophil extracellular traps biomarkers in thrombosis. Thromb. J. 2022, 20, 63. [Google Scholar] [CrossRef]
- Schulman, S.; Arnold, D.M.; Bradbury, C.A.; Broxmeyer, L.; Connors, J.M.; Falanga, A.; Iba, T.; Kaatz, S.; Levy, J.H.; Middeldorp, S.; et al. International Society on Thrombosis and Haemostasis. J. Thromb. Haemost. 2024, 22, 1779–1797. [Google Scholar] [CrossRef]
- Pryzdial, E.L.G.; Leatherdale, A.; Conway, E.M. Coagulation and complement: Key innate defense participants in a seamless web. Front. Immunol. 2022, 13, 918775. [Google Scholar] [CrossRef]
- Nørgaard, I.; Nielsen, S.F.; Nordestgaard, B.G. Complement C3 and high risk of venous thromboembolism: 80,517 individuals from the Copenhagen General Population Study. Clin. Chem. 2016, 62, 525–534. [Google Scholar] [CrossRef]
- Høiland, I.I.; Liang, R.A.; Braekkan, S.K.; Pettersen, K.; Ludviksen, J.K.; Latysheva, N.; Snir, O.; Ueland, T.; Hindberg, K.; Mollnes, T.E.; et al. Complement activation assessed by the plasma terminal complement complex and future risk of venous thromboembolism. J. Thromb. Haemost. 2019, 17, 934–943. [Google Scholar] [CrossRef]
- Skjeflo, E.W.; Braekkan, S.K.; Ludviksen, J.K.; Snir, O.; Hindberg, K.; Mollnes, T.E.; Hansen, J.B. Elevated plasma concentration of complement factor C5 is associated with risk of future venous thromboembolism. Blood 2021, 138, 2129–2137. [Google Scholar] [CrossRef]
- Grover, S.P.; Kawano, T.; Wan, J.; Tanratana, P.; Polai, Z.; Shim, Y.J.; Snir, O.; Braekkan, S.; Dhrolia, S.; Kasthuri, R.R.; et al. C1 inhibitor deficiency enhances contact pathway-mediated activation of coagulation and venous thrombosis. Blood 2023, 141, 2390–2401. [Google Scholar] [CrossRef]
- Morange, P.E.; Bezemer, I.; Saut, N.; Bare, L.; Burgos, G.; Brocheton, J.; Durand, H.; Biron-Andreani, C.; Schved, J.F.; Pernod, G.; et al. A follow-up study of a genome-wide association scan identifies a susceptibility locus for venous thrombosis on chromosome 6p24. 1. Am. J. Hum. Genet. 2010, 86, 592–595. [Google Scholar] [CrossRef]
- Chew, H.K.; Wun, T.; Harvey, D.; Zhou, H.; White, R.H. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch. Intern. Med. 2006, 166, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Carrier, M.; Ay, C.; Di Nisio, M.; Hicks, L.K.; Khorana, A.A.; Leavitt, A.D.; Lee, A.Y.Y.; Macbeth, F.; Morgan, R.L.; et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: Prevention and treatment in patients with cancer. Blood Adv. 2021, 5, 927–974. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Thromboembolism is a leadin cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemost. 2007, 5, 632–634. [Google Scholar] [CrossRef]
- Levitan, N.; Dowlati, A.; Remick, S.C.; Tahsildar, H.I.; Sivinski, L.D.; Beyth, R.; Rimm, A.A. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine 1999, 78, 285–291. [Google Scholar] [CrossRef]
- Prandoni, P.; Lensing, A.W.; Piccioli, A.; Bernardi, E.; Simioni, P.; Girolami, B.; Marchiori, A.; Sabbion, P.; Prins, M.H.; Noventa, F.; et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002, 100, 3484–3488. [Google Scholar] [CrossRef]
- Ikushima, S.; Ono, R.; Fukuda, K.; Sakayori, M.; Awano, N.; Kondo, K. Trousseau’s syndrome: Cancer-associated thrombosis. Jpn. J. Clin. Oncol. 2016, 46, 204–208. [Google Scholar] [CrossRef]
- Cwikiel, M.; Eskilsson, J.; Albertsson, M.; Stavenow, L. The influence of 5-fluorouracil and methotrexate on vascular endothelium. An experimental study using endothelial cells in the culture. Ann. Oncol. 1996, 7, 731–737. [Google Scholar] [CrossRef]
- Sorrentino, M.F.; Kim, J.; Foderaro, A.E.; Truesdell, A.G. 5-fluorouracil induced cardiotoxicity: Review of the literature. Cardiol. J. 2012, 19, 453–458. [Google Scholar] [CrossRef]
- Kumar, D.; Warsha, F.; Mehta, A.; Deepak, V.; Jawad, W. 5-fluorouracil induced takotsubo cardiomyopathy complicated by left ventricular thrombosis. Cureus 2021, 13, e14049. [Google Scholar] [CrossRef]
- Kinhult, S.; Albertsson, M.; Eskilsson, J.; Cwikiel, M. Antithrombotic treatment in protection against thrombogenic effects of 5-fluorouracil on vascular endothelium: A scanning microscopy evaluation. Scanning 2001, 23, 1–8. [Google Scholar] [CrossRef]
- Weitz, I.C.; Israel, V.K.; Waisman, J.R.; Presant, C.A.; Rochanda, L.; Liebman, H.A. Chemotherapy-induced activation of hemostasis: Effect of a low molecular weight heparin (dalteparin sodium) on plasma markers of hemostatic activation. Thromb. Haemost. 2002, 88, 213–220. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Woodley-Cook, J.; Shin, L.Y.; Swystun, L.; Caruso, S.; Beaudin, S.; Liaw, P.C. Effects of the chemotherapeutic agent doxorubicin on the protein C anticoagulant pathway. Mol. Cancer Ther. 2006, 5, 3303–3311. [Google Scholar] [CrossRef] [PubMed]
- Swystun, L.L.; Shin, L.Y.; Beaudin, S.; Liaw, P.C. Chemotherapeutic agents doxorubicin and epirubicin induce a procoagulant phenotype on endothelial cells and blood monocytes. J. Thromb. Haemost. 2009, 7, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Boles, J.C.; Williams, J.C.; Hollingsworth, R.M.; Wang, J.G.; Glover, S.L.; Owens, A.P., 3rd; Barcel, D.A.; Kasthuri, R.S.; Key, N.S.; Mackman, N. Anthracycline treatment of the human monocytic leukemia cell line THP-1 increases phosphatidylserine exposure and tissue factor activity. Thromb. Res. 2012, 129, 197–203. [Google Scholar] [CrossRef]
- Swystun, L.L.; Mukherjee, S.; Liaw, P.C. Breast cancer chemotherapy induces the release of cell-free DNA, a novel procoagulant stimulus. J. Thromb. Haemost. 2011, 9, 2313–2321. [Google Scholar] [CrossRef]
- Chow, A.Y.; Chin, C.; Dahl, G.; Rosenthal, D.N. Anthracyclines cause endothelial injury in pediatric cancer patients: A pilot study. J. Clin. Oncol. 2006, 24, 925–928. [Google Scholar] [CrossRef]
- Zangari, M.; Barlogie, B.; Thertulien, R.; Jacobson, J.; Eddleman, P.; Fink, L.; Fassas, A.; Van Rhee, F.; Talamo, G.; Lee, C.K.; et al. Thalidomide and deep vein thrombosis in multiple myeloma: Risk factors and effect on survival. Clin. Lymphoma 2003, 4, 32–35. [Google Scholar] [CrossRef]
- Anderson, N.; Lokich, J.J.; Tullis, J.L. L-asparaginase effect on antithrombin-III levels. Med. Pediatr. Oncol. 1979, 7, 335–340. [Google Scholar] [CrossRef]
- Caruso, V.; Iacoviello, L.; Di Castelnuovo, A.; Storti, S.; Mariani, G.; de Gaetano, G.; Donati, M.B. Thrombotic complications in childhood acute lymphoblastic leukemia: A meta-analysis of 17 prospective studies comprising 1752 pediatric patients. Blood 2006, 108, 2216–2222. [Google Scholar] [CrossRef]
- Conard, J.; Cazenave, B.; Maury, J.; Horellou, M.H.; Samama, M. L-asparaginase, antithrombin III, and thrombosis. Lancet 1980, 1, 1091. [Google Scholar] [CrossRef]
- Conard, J.; Horellou, M.H.; Van Dreden, P.; Potevin, F.; Zittoun, R.; Samama, M. Decrease in protein C in L-asparaginase-treated patients. Br. J. Haematol. 1985, 59, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Hunault-Berger, M.; Chevallier, P.; Delain, M.; Bulabois, C.E.; Bologna, S.; Bernard, M.; Lafon, I.; Cornillon, J.; Maakaroun, A.; Tizon, A.; et al. Changes in antithrombin and fibrinogen levels during induction chemotherapy with L-asparaginase in adult patients with acute lymphoblastic leukemia or lymphoblastic lymphoma. Use of supportive coagulation therapy and clinical outcome: The CAPELAL study. Haematologica 2008, 93, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, H.R.; Geczy, C.L. Induction of macrophage procoagulant expression by cisplatin, daunorubicin and doxorubicin. Int. J. Cancer 1990, 46, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Lechner, D.; Kollars, M.; Gleiss, A.; Kyrle, P.A.; Weltermann, A. Chemotherapy-induced thrombin generation via procoagulant endothelial microparticles is independent of tissue factor activity. J. Thromb. Haemost. 2007, 5, 2445–2452. [Google Scholar] [CrossRef]
- Ma, R.; Bi, Y.; Kou, J.; Zhou, J.; Shi, J. Enhanced procoagulant activity of platelets after chemotherapy in non-small cell lung cancer. Cancer Biol. Ther. 2017, 18, 627–634. [Google Scholar] [CrossRef]
- Seng, S.; Liu, Z.; Chiu, S.K.; Proverbs-Singh, T.; Sonpavde, G.; Choueiri, T.K.; Tsao, C.K.; Yu, M.; Hahn, N.M.; Oh, W.K.; et al. Risk of venous thromboembolism in patients with cancer treated with Cisplatin: A systematic review and meta-analysis. J. Clin. Oncol. 2012, 30, 4416–4426. [Google Scholar] [CrossRef]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J. Urol. 2002, 168, 9–12. [Google Scholar] [CrossRef]
- Crawford, E.D.; Heidenreich, A.; Lawrentschuk, N.; Tombal, B.; Pompeo, A.C.L.; Mendoza-Valdes, A.; Miller, K.; Debruyne, F.M.J.; Klotz, L. Androgen-targeted therapy in men with prostate cancer: Evolving practice and future considerations. Prostate Cancer Prostatic Dis. 2019, 22, 24–38. [Google Scholar] [CrossRef]
- Cosman, F.; Baz-Hecht, M.; Cushman, M.; Vardy, M.D.; Cruz, J.D.; Nieves, J.W.; Zion, M.; Lindsay, R. Short-term effects of estrogen, tamoxifen and raloxifene on hemostasis: A randomized-controlled study and review of the literature. Thromb. Res. 2005, 116, 1–13. [Google Scholar] [CrossRef]
- Rühl, H.; Schröder, L.; Müller, J.; Fimmers, R.; Sukhitashvili, S.; Welz, J.; Kuhn, W.C.; Oldenburg, J.; Rudlowski, C.; Pötzsch, B. Tamoxifen induces resistance to activated protein C. Thromb. Res. 2014, 133, 886–891. [Google Scholar] [CrossRef]
- Shah, V.P.; Chegini, H.A.; Vishneski, S.R.; Weatherman, R.V.; Blackmore, P.F.; Dobrydneva, Y. Tamoxifen promotes superoxide production in platelets by activation of PI3-kinase and NADPH oxidase pathways. Thromb. Res. 2012, 129, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Jung, E.A.; Kim, Z.; Kim, B.Y. Risk of cardiovascular events and lipid profile change in patients with breast cancer taking aromatase inhibitor: A systematic review and meta-analysis. Curr. Oncol. 2023, 30, 1831–1843. [Google Scholar] [CrossRef] [PubMed]
- Blondon, M.; Bodmer, A.; Thouvenin, L.; Lecompte, T.; Righini, M.; Fontana, P.; Casini, A. Differential impact of tamoxifen and aromatase inhibitors on thrombin generation: The prospective HEMOBREAST cohort. Blood Adv. 2022, 6, 2884–2892. [Google Scholar] [CrossRef] [PubMed]
- Pather, K.; Dix-Peek, T.; Duarte, R.; Chetty, N.; Augustine, T.N. Breast cancer cell-induced platelet activation is compounded by tamoxifen and anastrozole in vitro. Thromb. Res. 2019, 177, 51–58. [Google Scholar] [CrossRef]
- Robak, M.; Treliński, J.; Chojnowski, K. Hemostatic changes after 1 month of thalidomide and dexamethasone therapy in patients with multiple myeloma. Med. Oncol. 2012, 29, 3574–3580. [Google Scholar] [CrossRef][Green Version]
- El Accaoui, R.N.; Shamseddeen, W.A.; Taher, A.T. Thalidomide and thrombosis. A meta-analysis. Thromb. Haemost. 2007, 97, 1031–1036. [Google Scholar]
- Miroddi, M.; Sterrantino, C.; Simmonds, M.; Caridi, L.; Calapai, G.; Phillips, R.S.; Stewart, L.A. Systematic review and meta-analysis of the risk of severe and life-threatening thromboembolism in cancer patients receiving anti-EGFR monoclonal antibodies (cetuximab or panitumumab). Int. J. Cancer 2016, 139, 2370–2380. [Google Scholar] [CrossRef]
- Young, K.; Paz-Ares, L.G.; Thatcher, N.; Spigel, D.R.; Shahidi, J.; Soldatenkova, V.; Grau, G.; Kurek, R.; Shepherd, F.A. Pooled analysis of venous thromboembolism (VTE) from four trials of necitumumab and chemotherapy for stage IV non-small cell lung cancer (NSCLC). Pooled analysis of venous thromboembolism (VTE) from four trials of neci-tumumab and chemotherapy for stage IV non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2016, 34, e20534. [Google Scholar]
- Young, K.; Paz-Ares, L.; Thatcher, N.; Spigel, D.R.; Shahidi, J.; Soldatenkova, V.; Grau, G.; Kurek, R.; Shepherd, F.A. Venous thromboembolism with EGFR monoclonal antibody necitumumab in stage IV non-small cell lung cancer: A retrospective cohort analysis. Thromb. Res. 2018, 167, 50–56. [Google Scholar] [CrossRef]
- Chen, N.; Ren, M.; Li, R.; Deng, X.; Li, Y.; Yan, K.; Xiao, L.; Yang, Y.; Wang, L.; Luo, M.; et al. Bevacizumab promotes venous thromboembolism through the induction of PAI-1 in a mouse xenograft model of human lung carcinoma. Mol. Cancer 2015, 14, 140. [Google Scholar] [CrossRef]
- Hurwitz, H.I.; Saltz, L.B.; Van Cutsem, E.; Cassidy, J.; Wiedemann, J.; Sirzén, F.; Lyman, G.H.; Rohr, U.P. Venous thromboembolic events with chemotherapy plus bevacizumab: A pooled analysis of patients in randomized phase II and III studies. J. Clin. Oncol. 2011, 29, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Kanukula, R.; Ganta, S.; Sirumalla, Y.; Salam, A.; Baddam, R.; Pasupuleti, B.C. Risk of venous thromboembolic events in patients with cancer treated with aflibercept: A systematic review and meta-analysis of randomized controlled trials. Am. J. Ther. 2019, 26, e549–e552. [Google Scholar] [CrossRef] [PubMed]
- Latifi, Y.; Moccetti, F.; Wu, M.; Xie, A.; Packwood, W.; Qi, Y.; Ozawa, K.; Shentu, W.; Brown, E.; Shirai, T.; et al. Thrombotic microangiopathy as a cause of cardiovascular toxicity from the BCR-ABL1 tyrosine kinase inhibitor ponatinib. Blood 2019, 133, 1597–1606. [Google Scholar] [CrossRef]
- Haguet, H.; Douxfils, J.; Mullier, F.; Chatelain, C.; Graux, C.; Dogén, J.M. Risk of arterial and venous occlusive events in chronic myeloid leukemia patients treated with new generation BCR-ABL tyrosine kinase inhibitors: A systematic review and meta-analysis. Expert Opin. Drug Saf. 2017, 16, 5–12. [Google Scholar] [CrossRef]
- Thein, K.Z.; Htut, T.W.; Ball, S.; Swarup, S.; Sultan, A.; Oo, T.H. Venous thromboembolism risk in patients with hormone receptor-positive HER2-negative metastatic breast cancer treated with combined CDK 4/6 inhibitors plus endocrine therapy versus endocrine therapy alone: A systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res. Treat. 2020, 183, 479–487. [Google Scholar]
- Mincu, R.I.; Mahabadi, A.A.; Michel, L.; Mrotzek, S.M.; Schadendorf, D.; Rassaf, T.; Totzeck, M. Cardiovascular adverse events associated with BRAF and MEK inhibitors: A systematic review and meta-analysis. JAMA Netw. Open 2019, 2, e198890. [Google Scholar] [CrossRef]
- Jie, Q.; Li, Y.; Jing, L.; Chen, J.; Li, Y. Adverse event profile differences between pralsetinib and selpercatinib: A real-world study based on the FDA adverse events reporting system. Front. Pharmacol. 2024, 15, 1424980. [Google Scholar] [CrossRef]
- Blom, J.W.; Osanto, S.; Rosendaal, F.R. The risk of a venous thrombotic event in lung cancer patients: Higher risk for adenocarcinoma than squamous cell carcinoma. J. Thromb. Haemost. 2004, 2, 1760–1765. [Google Scholar] [CrossRef]
- Wahrenbrock, M.; Borsig, L.; Le, D.; Varki, N.; Varki, A. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J. Clin. Investig. 2003, 112, 853–862. [Google Scholar] [CrossRef]
- Varki, A.; Kannagi, R.; Toole, B.; Stanley, P. Glycosylation Changes in Cancer. In Essentials of Glycobiology, 3rd ed.; Cold Sprin Hardor Laboratory Press: Cold Spring Harbor, NY, USA, 2017; Chapter 47; pp. 597–609. [Google Scholar]
- Gordon, S.G.; Franks, J.J.; Lewis, B. Cancer procoagulant A: A factor X activating procoagulant from malignant tissue. Thromb. Res. 1975, 6, 127–137. [Google Scholar] [CrossRef]
- Rondon, A.M.R.; Kroone, C.; Kapteijn, M.Y.; Versteeg, H.H.; Buijs, J.T. Role of tissue factor in tumor progression and cancer-associated thrombosis. Semin. Thromb. Hemost. 2019, 45, 396–412. [Google Scholar] [CrossRef] [PubMed]
- Reddel, C.J.; Tan, C.W.; Chen, V.M. Thrombin Generation and Cancer: Contributors and Consequences. Cancers 2019, 11, 100. [Google Scholar] [CrossRef]
- Egeberg, O. Inherited antithrombin deficiency causing thrombophilia. Thromb. Haemost. 1965, 13, 516–530. [Google Scholar] [CrossRef]
- Griffin, J.H.; Evatt, B.; Zimmerman, T.S.; Kleiss, A.J.; Wideman, C. Deficiency of protein C in congenital thrombotic disease. J. Clin. Investig. 1981, 68, 1370–1373. [Google Scholar] [CrossRef]
- Comp, P.; Esmon, C. Recurrent thromboembolism in patients with a partial deficiency of protein S. N. Engl. J. Med. 1984, 311, 1525–1528. [Google Scholar] [CrossRef]
- Schwarz, H.P.; Fischer, M.; Hopmeier, P.; Batard, M.A.; Griffin, J.H. Plasma protein S deficiency in familial thrombotic disease. Blood 1984, 64, 1297–1300. [Google Scholar] [CrossRef]
- Dahlbäck, B.; Carlsson, M.; Svensson, P.J. Familial thrombophilia due to a previously unrecognized mechanism characterized by poor anticoagulant response to activated protein C: Prediction of a cofactor to activated protein C. Proc. Natl. Acad. Sci. USA 1993, 90, 1004–1008. [Google Scholar] [CrossRef]
- Bertina, R.M.; Koeleman, B.P.; Koster, T.; Rosendaal, F.R.; Dirven, R.J.; De Ronde, H.; van der Velden, P.A.; Reitsma, P.H. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature 1994, 369, 64–67. [Google Scholar] [CrossRef]
- Poort, S.R.; Rosendaal, F.R.; Reitsma, P.H.; Bertina, R.M. A common genetic variation in the 3’-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood 1996, 88, 3698–3703. [Google Scholar] [CrossRef]
- Simioni, P.; Cagnin, S.; Sartorello, F.; Sales, G.; Pagani, L.; Bulato, C.; Gavasso, S.; Nuzzo, F.; Chemello, F.; Radu, C.M.; et al. Partial F8 gene duplication (factor VIII Padua) associated with high factor VIII levels and familial thrombophilia. Blood 2021, 187, 2383–2393. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulman, S.; Makatsariya, A.; Khizroeva, J.; Bitsadze, V.; Kapanadze, D. The Basic Principles of Pathophysiology of Venous Thrombosis. Int. J. Mol. Sci. 2024, 25, 11447. https://doi.org/10.3390/ijms252111447
Schulman S, Makatsariya A, Khizroeva J, Bitsadze V, Kapanadze D. The Basic Principles of Pathophysiology of Venous Thrombosis. International Journal of Molecular Sciences. 2024; 25(21):11447. https://doi.org/10.3390/ijms252111447
Chicago/Turabian StyleSchulman, Sam, Alexander Makatsariya, Jamilya Khizroeva, Victoria Bitsadze, and Daredzhan Kapanadze. 2024. "The Basic Principles of Pathophysiology of Venous Thrombosis" International Journal of Molecular Sciences 25, no. 21: 11447. https://doi.org/10.3390/ijms252111447
APA StyleSchulman, S., Makatsariya, A., Khizroeva, J., Bitsadze, V., & Kapanadze, D. (2024). The Basic Principles of Pathophysiology of Venous Thrombosis. International Journal of Molecular Sciences, 25(21), 11447. https://doi.org/10.3390/ijms252111447




