Antiviral Potential of Chiococca alba (L.) Hitchc. Plant Extracts Against Chikungunya and Mayaro Viruses
Abstract
1. Introduction
2. Results
2.1. Total Chemical Profiles of C. alba CAH21 and CAH24 Extracts
2.2. Partial Chemical Profiles of C. alba CAH21 and CAH24 Extracts
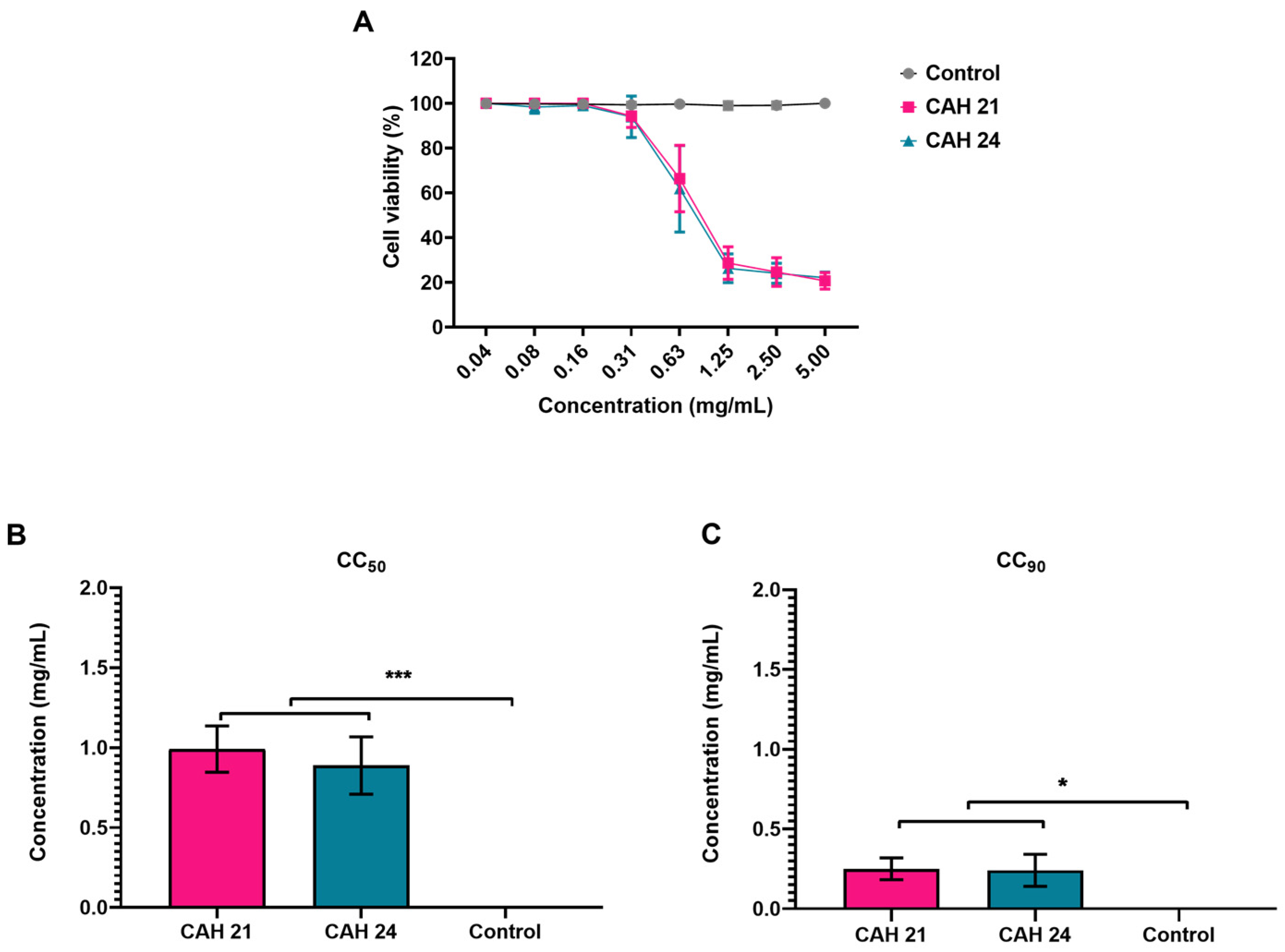
2.3. Cytotoxic Assay
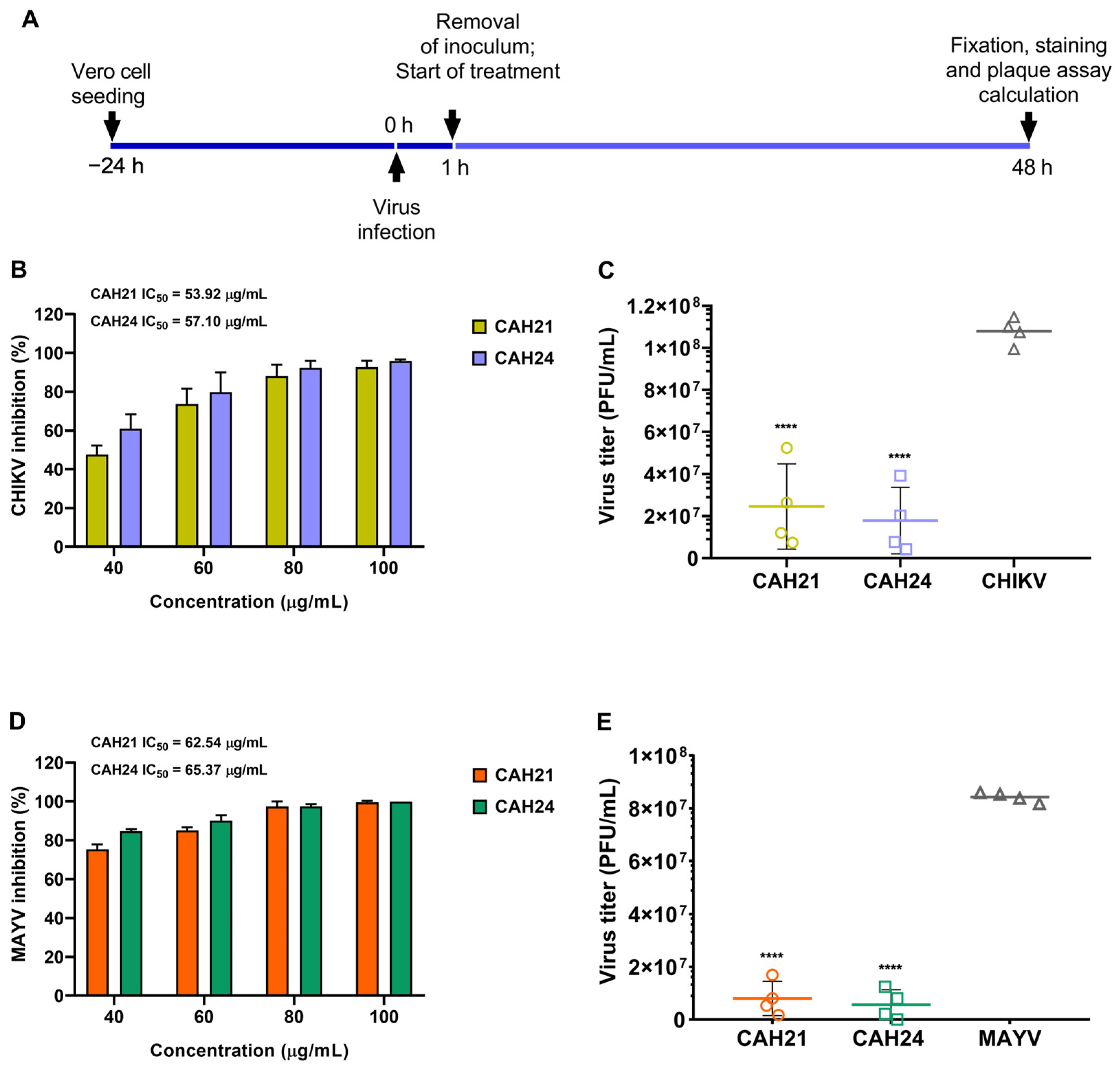
2.4. Antiviral Activity Analysis
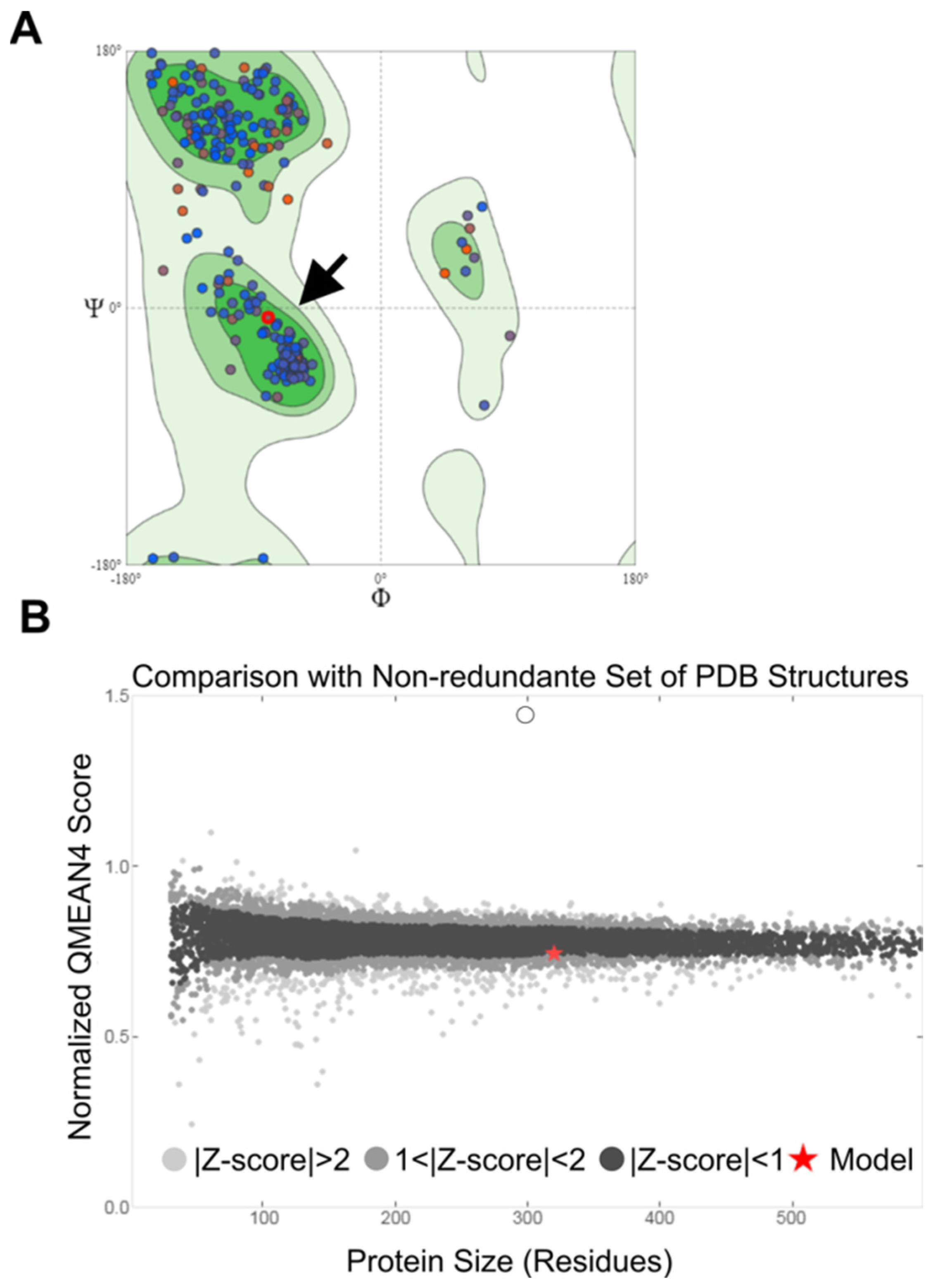
2.5. Modeling and Validation of the MAYV nsP2 Structure
2.6. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Extracts
4.3. Chemical Analysis of Extracts
4.4. Cells and Viruses
4.5. Viral Titration by Plaque Assay
4.6. Cytotoxicity Assay
4.7. Antiviral Assays
4.8. In Silico Analysis of the Interaction between Molecules of C. alba and the nsP2 Protease of CHIKV and MAYV
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cleton, N.; Koopmans, M.; Reimerink, J.; Godeke, G.J.; Reusken, C. Come fly with me: Review of clinically important arboviruses for global travelers. J. Clin. Virol. 2012, 55, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.N.; Carvalho, F.D.; De Mendonça, S.F.; Rocha, M.N.; Moreira, L.A. Vector competence of Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus mosquitoes for Mayaro virus. PLoS Neglected Trop. Dis. 2020, 14, e0007518. [Google Scholar] [CrossRef] [PubMed]
- Contigiani, M.S.; Diaz, L.A. Togaviridae. In Arthropod Borne Diseases; Springer: Cham, Switzerland, 2017; pp. 115–135. [Google Scholar] [CrossRef]
- Pego, P.N.; Gomes, L.P.; Provance, D.W., Jr.; De-Simone, S.G. Mayaro virus Disease. J. Hum. Virol. Retrovirol. 2014, 1, 00018. [Google Scholar] [CrossRef]
- Kielian, M.; Chanel-Vos, C.; Liao, M. Alphavirus Entry and Membrane Fusion. Viruses 2010, 2, 796–825. [Google Scholar] [CrossRef]
- Carey, B.D.; Bakovic, A.; Callahan, V.; Narayanan, A.; Kehn-Hall, K. New World Alphavirus protein interactomes from a therapeutic perspective. Antivir. Res. 2019, 163, 125–139. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Yu, H.; Keller, P.A. Identification of Chikungunya virus nsP2 protease inhibitors using structure-base approaches. J. Mol. Graph. Model. 2015, 57, 1–8. [Google Scholar] [CrossRef]
- Abu Bakar, F.; Ng, L.F.P. Nonstructural Proteins of Alphavirus-Potential Targets for Drug Development. Viruses 2018, 10, 71. [Google Scholar] [CrossRef]
- Rashad, A.A.; Mahalingam, S.; Keller, P.A. Chikungunya virus: Emerging targets and new opportunities for medicinal chemistry. J. Med. Chem. 2014, 57, 1147–1166. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.T.; Hsu, W.C.; Lin, C.C. Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 2014, 4, 24–35. [Google Scholar] [CrossRef]
- Sharma, P.; Manchanda, R.; Goswami, R.; Chawla, S. Biodiversity and therapeutic potential of medicinal plants. In Environmental Concerns and Sustainable Development: Volume 2: Biodiversity, Soil and Waste Management; Springer: Singapore, 2020; pp. 27–44. [Google Scholar] [CrossRef]
- Akram, M.; Tahir, I.M.; Shah, S.M.A.; Mahmood, Z.; Altaf, A.; Ahmad, K.; Munir, N.; Daniyal, M.; Nasir, S.; Mehboob, H. Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: A systematic review. Phytother. Res. 2018, 32, 811–822. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising natural compounds against viral infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef] [PubMed]
- Aro, A.O.; Dzoyem, J.P.; Goddard, A.; Fonteh, P.; Kayoka-Kabongo, P.N.; McGaw, L.J. In vitro Antimycobacterial, Apoptosis-Inducing Potential, and Immunomodulatory Activity of Some Rubiaceae Species. Front. Pharmacol. 2019, 10, 185. [Google Scholar] [CrossRef]
- Rivero-Cruz, I.; Cristians, S.; Ovalle-Magallanes, B.; Mata, R. Mexican copalchis of the Rubiaceae family: More than a century of pharmacological and chemical investigations. Phytochem. Rev. 2019, 18, 1435–1455. [Google Scholar] [CrossRef]
- Martins, D.; Nunez, C.V. Secondary metabolites from Rubiaceae species. Molecules 2015, 20, 13422–13495. [Google Scholar] [CrossRef]
- Gazda, V.E.; Gomes-Carneiro, M.R.; Barbi, N.S.; Paumgartten, F.J. Toxicological evaluation of an ethanolic extract from Chiococca alba roots. J. Ethnopharmacol. 2006, 105, 187–195. [Google Scholar] [CrossRef]
- Bhattacharyya, J.; Cunha, E.V. A triterpenoid from the root-bark of Chiococca alba. Phytochemistry 1992, 31, 2546–2547. [Google Scholar] [CrossRef]
- Comerford, S.C. Medicinal plants of two mayan healers from San Andrés, Petén, Guatemala. Econ. Bot. 1996, 50, 327–336. [Google Scholar] [CrossRef]
- Rico-Gray, V.; Chemás, A.; Mandujano, S. Uses of tropical deciduous forest species by the Yucatecan Maya. Agrofor. Syst. 1991, 14, 149–161. [Google Scholar] [CrossRef]
- Hernández-Bolio, G.I.; Ruiz-Vargas, J.A.; Peña-Rodríguez, L.M. Natural Products from the Yucatecan Flora: Structural Diversity and Biological Activity. J. Nat. Prod. 2019, 82, 647–656. [Google Scholar] [CrossRef]
- Borges, R.M.; Valença, S.S.; Lopes, A.A.; dos Santos Barbi, N.; da Silva, A.J.R. Saponins from the roots of Chiococca alba and their in vitro anti-inflammatory activity. Phytochem. Lett. 2013, 6, 96–100. [Google Scholar] [CrossRef]
- Borges, J.C.M.; Haddi, K.; Valbon, W.R.; Costa, L.T.M.; Ascêncio, S.D.; Santos, G.R.; Soares, I.M.; Barbosa, R.S.; Viana, K.F.; Silva, E.A.P.; et al. Methanolic Extracts of Chiococca alba in Aedes aegypti Biorational Management: Larvicidal and Repellent Potential, and Selectivity against Non-Target Organisms. Plants 2022, 11, 3298. [Google Scholar] [CrossRef]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar] [PubMed]
- Hobohm, U.; Sander, C. A sequence property approach to searching protein databases. J. Mol. Biol. 1995, 251, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Moura, W.S.; de Souza, S.R.; Campos, F.S.; Cangussu, A.S.R.; Santos, E.M.S.; Andrade, B.S.; Gomes, C.H.B.; Viana, K.F.; Haddi, K.; Oliveira, E.E.; et al. Antibacterial activity of Siparuna guianensis essential oil mediated by impairment of membrane permeability and replication of pathogenic bacteria. Ind. Crop. Prod. 2020, 146, 112–142. [Google Scholar] [CrossRef]
- Sobolev, O.V.; Afonine, P.V.; Moriarty, N.W.; Hekkelman, M.L.; Joosten, R.P.; Perrakis, A.; Adams, P.D. A Global Ramachandran Score Identifies Protein Structures with Unlikely Stereochemistry. Structure 2020, 28, 1249–1258.e2. [Google Scholar] [CrossRef]
- Zou, J.; Yin, J.; Fang, L.; Yang, M.; Wang, T.; Wu, W.; Bellucci, M.A.; Zhang, P. Computational Prediction of Mutational Effects on SARS-CoV-2 Binding by Relative Free Energy Calculations. J. Chem. Inf. Model. 2020, 60, 5794–5802. [Google Scholar] [CrossRef]
- Schnitzler, P.; Neuner, A.; Nolkemper, S.; Zundel, C.; Nowack, H.; Sensch, K.H.; Reichling, J. Antiviral Activity and Mode of Action of Propolis Extracts and Selected Compounds. Phytother. Res. 2009, 24, S20–S28. [Google Scholar] [CrossRef]
- De Castro, M.L.; García-Ayuso, L.E. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal. Chim. Acta 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Onwubuya, E.I.; Oladejo, A.A. Cardio-Protective Effect of the Leaf Extract of Andrographis paniculata in Isoproterenol-Induced Myocardial Infarction. Cardiol. E Angiol. Um Periódico Int. 2022, 11, 467–478. [Google Scholar] [CrossRef]
- Sarumathy, K.; Rajan, M.D.; Vijay, T.; Jayakanthi, J. Evaluation of phytoconstituents, nephro-protective and antioxidant activities of Clitoria ternatea. J. Appl. Pharm. Sci. 2011, 1, 164–172. [Google Scholar] [CrossRef]
- Novak, J.; Novak, S.; Bitsch, C.; Franz, C.M. Essential oil composition of underground parts of Valeriana celtica ssp. from Austria and Italy. Flavour Fragr. J. 2000, 15, 40–42. [Google Scholar] [CrossRef]
- Sati, S.; Mathela, C.S. Volatile Constituents in the Root and Leaf Oils of Valeriana pyrolaefolia Decne. J. Essent. Oil Res. 2006, 18, 29–31. [Google Scholar] [CrossRef]
- Gu, H.; Foong, S.Y.; Lam, S.S.; Yue, X.; Yang, J.; Peng, W. Characterization and potential utilization of extracts and pyrolyzates from Jasminum nudiflorum Lindl. Bark. J. Anal. Appl. Pyrolysis 2021, 155, 105092. [Google Scholar] [CrossRef]
- Cao, G.; Zhang, Y.; Feng, J.; Cai, H.; Zhang, C.; Ding, M.; Cong, X.; Cai, B. A rapid and sensitive assay for determining the main components in processed fructus Corni by UPLC–Q-TOF-MS. Chromatographia 2011, 73, 135–141. [Google Scholar] [CrossRef]
- Zou, H.; Wu, T.; Wang, Y.; Kang, Y.; Shan, Q.; Xu, L.; Jiang, Z.; Lin, X.; Ye, X.Y.; Xie, T.; et al. 5-Hydroxymethylfurfural enhances the antiviral immune response in macrophages through the modulation of RIG-I-mediated interferon production and the JAK/STAT signaling pathway. ACS Omega 2021, 6, 28019–28030. [Google Scholar] [CrossRef]
- Sriphana, U.; Yenjai, C.; Tungnoi, S.; Srirapa, J.; Junsongduang, A. Flavonoids from Milletia leucantha and Their Cytotoxicity. Nat. Prod. Commun. 2018, 13, 961–962. [Google Scholar] [CrossRef]
- Solnier, J.; Fladerer, J.P. Flavonoids: A complementary approach to conventional therapy of COVID-19? Phytochem. Rev. 2020, 20, 773–795. [Google Scholar] [CrossRef]
- Arthanari, S.K.; Vanitha, J.; Ganesh, M.; Venkateshwaran, K.; Clercq, D. Evaluation of antiviral and cytotoxic activities of methanolic extract of S. grandiflora (Fabaceae) flowers. Asian Pac. J. Trop. Biomed. 2012, 2, S855–S858. [Google Scholar] [CrossRef]
- Ferreira, P.G.; Ferraz, A.C.; Figueiredo, J.E.; Lima, C.F.; Rodrigues, V.G.; Taranto, A.G.; Ferreira, J.M.S.; Brandão, G.C.; Vieira-Filho, S.A.; Duarte, L.P.; et al. Detection of the antiviral activity of epicatechin isolated from Salacia crassifolia (Celastraceae) against Mayaro virus based on protein C homology modelling and virtual screening. Arch. Virol. 2018, 163, 1567–1576. [Google Scholar] [CrossRef]
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral properties of flavonoids and delivery strategies. Nutrients 2020, 12, 2534. [Google Scholar] [CrossRef]
- Goh, V.S.L.; Mok, C.K.; Chu, J.J.H. Antiviral natural products for arbovirus infections. Molecules 2020, 25, 2796. [Google Scholar] [CrossRef] [PubMed]
- Jayadevappa, M.K.; Karkera, P.R.; Siddappa, R.Y.; Telkar, S.; Karunakara, P. Investigation of plant flavonoids as potential dengue protease inhibitors. J. Herbmed Pharmacol. 2020, 9, 366–373. [Google Scholar] [CrossRef]
- Oliveira, A.F.C.D.S.; Teixeira, R.R.; Oliveira, A.S.D.; Souza, A.P.M.D.; Silva, M.L.D.; Paula, S.O.D. Potential antivirals: Natural products targeting replication enzymes of dengue and chikungunya viruses. Molecules 2017, 22, 505. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Hassandarvish, P.; Lani, R.; Yadollahi, P.; Jokar, A.; Bakar, S.A.; Zandi, K. Inhibition of Chikungunya virus replication by hesperetin and naringenin. RSC Adv. 2016, 6, 69421–69430. [Google Scholar] [CrossRef]
- Zandi, K.; Teoh, B.T.; Sam, S.S.; Wong, P.F.; Mustafa, M.R.; Abu Bakar, S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol. J. 2011, 8, 560. [Google Scholar] [CrossRef]
- Sanchéz, I.; Gómez-Garibay, F.; Taboada, J.; Ruiz, B.H. Antiviral effect of flavonoids on the dengue virus. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2000, 14, 89–92. [Google Scholar] [CrossRef]
- Grienke, U.; Richter, M.; Walther, E.; Hoffmann, A.; Kirchmair, J.; Makarov, V.; Nietzsche, S.; Schmidtke, M.; Rollinger, J.M. Discovery of prenylated flavonoids with dual activity against Influenza virus and Streptococcus pneumoniae. Sci. Rep. 2016, 6, 27156. [Google Scholar] [CrossRef]
- Frabasile, S.; Koishi, A.C.; Kuczera, D.; Silveira, G.F.; Verri, W.A., Jr.; Dos Santos, C.N.D.; Bordignon, J. The citrus flavanone naringenin impairs dengue virus replication in human cells. Sci. Rep. 2017, 7, 41864. [Google Scholar] [CrossRef]
- dos Santos, A.E.; Kuster, R.M.; Yamamoto, K.A.; Salles, T.S.; Campos, R.; de Meneses, M.D.; Soares, M.R.; Ferreira, D. Quercetin and quercetin 3-O-glycosides from Bauhinia longifolia (Bong.) Steud. show anti-Mayaro virus activity. Parasites Vectors 2014, 7, 41864. [Google Scholar] [CrossRef]
- Tang, L.I.; Ling, A.P.; Koh, R.Y.; Chye, S.M.; Voon, K.G. Screening of anti-dengue activity in methanolic extracts of medicinal plants. BMC Complement. Altern. Med. 2012, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Lalani, S.; Poh, C.L. Flavonoids as antivirals agents for Enterovirus A71 (EV-A71). Viruses 2020, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.G.; Leu, Y.L.; Cheng, M.L.; Ting, S.C.; Liu, C.C.; Wang, S.D.; Yang, C.H.; Hung, C.Y.; Sakurai, H.; Chen, K.H.; et al. Anti-enterovirus 71 activities of Melissa officinalis extract and its biologically active constituent rosmarinic acid. Sci. Rep. 2017, 7, 12264. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, J.F.; Lima, C.S.; Pereira, C.M.; Bittar, C.; Batista, M.N.; Nazaré, A.C. Flavonoids from Pterogyne nitens inhibit hepatitis C virus entry. Sci. Rep. 2017, 7, 16127. [Google Scholar] [CrossRef] [PubMed]
- Calland, N.; Albecka, A.; Belouzard, S.; Wychowski, C.; Duverlie, G.; Descamps, V.; Hober, D.; Dubuisson, J.; Rouillé, Y.; Séron, K. (−)-Epigallocatechin-3-gallate is a new inhibitor of hepatitis C virus entry. Hepatology 2012, 55, 720–729. [Google Scholar] [CrossRef]
- Miao, M.; Xiang, L. Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol. 2020, 87, 71–88. [Google Scholar] [CrossRef]
- Bissoyi, A.; Pattanayak, S.K.; Bit, A.; Patel, A.; Singh, A.K.; Behera, S.S.; Satpathy, D. Alphavirus nonstructural proteases and their inhibitors. In Viral Proteases and Their Inhibitors; Academic Press: Cambridge, MA, USA, 2017; pp. 77–104. [Google Scholar] [CrossRef]
- Seyedi, S.S.; Shukri, M.; Hassandarvish, P.; Oo, A.; Shankar, E.M.; Abubakar, S.; Zandi, K. Computational approach towards exploring potential anti-chikungunya activity of selected flavonoids. Sci. Rep. 2016, 6, 24027. [Google Scholar] [CrossRef]
- Narwal, M.; Singh, H.; Pratap, S.; Malik, A.; Kuhn, R.J.; Kumar, P.; Tomar, S. Crystal structure of Chikungunya virus nsP2 cysteine protease reveals a putative flexible loop blocking its active site. Int. J. Biol. Macromol. 2018, 116, 451–462. [Google Scholar] [CrossRef]
- Tripathi, P.K.; Singh, J.; Gaurav, N.; Garg, D.K.; Patel, A.K. In-silico and biophysical investigation of biomolecular interaction between naringin and nsP2 of the Chikungunya virus. Int. J. Biol. Macromol. 2020, 160, 1061–1065. [Google Scholar] [CrossRef]
- Camini, F.C.; da Silva, T.F.; da Silva Caetano, C.C.; Almeida, L.T.; Ferraz, A.C.; Vitoreti, V.M.A.; de Mello Silva, B.; de Queiroz Silva, S.; de Magalhães, J.C.; de Brito Magalhães, C.L. Antiviral activity of silymarin against Mayaro virus and protective effect in virus-induced oxidative stress. Antivir. Res. 2018, 158, 8–12. [Google Scholar] [CrossRef]
- Lani, R.; Hassandarvish, P.; Chiam, C.W.; Moghaddam, E.; Chu, J.J.H.; Rausalu, K.; Merits, A.; Higgs, S.; Vanlandingham, D.; Abu Bakar, S.; et al. Antiviral activity of silymarin against chikungunya virus. Sci. Rep. 2015, 5, 11421. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.H. Trends in ethnopharmacology. J. Ethnopharmacol. 2005, 100, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Pirintsos, S.; Panagiotopoulos, A.; Bariotakis, M.; Daskalakis, V.; Lionis, C.; Sourvinos, G.; Karakasiliotis, I.; Kampa, M.; Castanas, E. From traditional ethnopharmacology to modern natural drug discovery: A methodology discussion and specific examples. Molecules 2022, 27, 4060. [Google Scholar] [CrossRef] [PubMed]
- Chiamenti, L.; Silva, F.P.D.; Schallemberger, K.; Demoliner, M.; Rigotto, C.; Fleck, J.D. Cytotoxicity and antiviral activity evaluation of Cymbopogon spp hydroethanolic extracts. Braz. J. Pharm. Sci. 2019, 55, e18063. [Google Scholar] [CrossRef]
- Troian, E.A.; Schallenberger, K.; Da Silva, F.P.; Dietrich, G.K.; Ferreira Chiesa, F.; Olivaro, C.; Wallace, F.; Fleck, J.; Verza, S. Screening for antiviral activity of two purified saponin fractions of Quillaja spp. against Yellow fever virus and Chikungunya virus. Int. J. Innov. Educ. Res. 2020, 8, 205–214. [Google Scholar] [CrossRef]
- Repetto, G.; Del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125. [Google Scholar] [CrossRef]
- Burleson, F.G.; Chambers, T.M.; Wiedbrauk, D.L. Techniques for evaluating antiviral agents. In Virology: A Laboratory Manual; Burleson, F.G., Ed.; Elsevier Academic Press: San Diego, CA, USA, 1994. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Borges, J.C.M.; Haddi, K.; Oliveira, E.E.; Andrade, B.S.; Nascimento, V.L.; Melo, T.S.; Didonet, J.; Carvalho, J.C.T.; Cangussu, A.S.; Soares, I.M.; et al. Mosquiticidal and repellent potential of formulations containing wood residue extracts of a Neotropical plant, Tabebuia heptaphylla. Ind. Crop. Prod. 2019, 129, 424–433. [Google Scholar] [CrossRef]
- Schrodinger LLC. The PyMOL Molecular Graphics System, Version 2.2.2; Schrodinger LLC: New York, NY, USA, 2018. [Google Scholar]
- Dassault Systems BIOVIA. Discovery Studio Modeling Environment, Release 2017; Dassault Systems: San Diego, CA, USA, 2017. [Google Scholar]

| CAH21 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Peak | Compound | Chemical Structure | Retention Time (min) | Molecular Formula | Molar Mass (g/mol) | Area (%) | PubChem CID | CAS Number |
| 9 | Naringin | 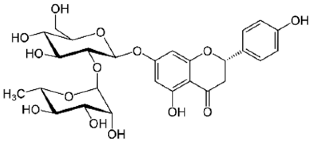 | 31.670 | C27H32O14 | 580.54 | 1.28 | 442428 | 10236-47-2 |
| CAH24 | ||||||||
| 5 | Syringic acid | 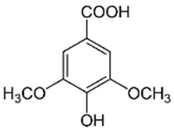 | 24.25 | C9H10O5 | 198.17 | 6.27 | 10742 | 530-57-4 |
| 6 | Chlorogenic acid | 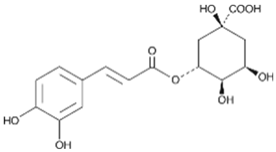 | 25.30 | C16H18O9 | 354.31 | 0.53 | 1794427 | 327-97-9 |
| 10 | Vitexin | 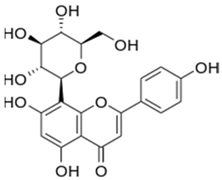 | 36.57 | C21H20O10 | 432.38 | 13.01 | 5280441 | 3681-93-4 |
| 12 | Myricetin | 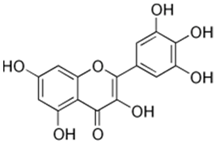 | 65.23 | C15H10O8 | 318.23 | 4.13 | 5281672 | 529-44-2 |
| 14 | Quercetin | 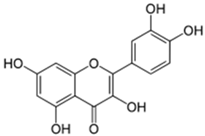 | 75.25 | C15H10O7 | 302.23 | 16.86 | 5280343 | 117-39-5 |
| Plant Extracts | CC50 (µg/mL) a | CC90 (µg/mL) b | µg/mL | CHIKV % Reduction c | SI e | MAYV % Reduction d | SI e |
|---|---|---|---|---|---|---|---|
| CAH21 | 991 ± 0.1 | 251 ± 0.1 | 40 | 47.6 ± 4.6 | 18.4 | 75.3 ± 2.6 | 15.8 |
| 60 | 73.6 ± 8.0 | 85.1 ± 1.5 | |||||
| 80 | 88.0 ± 5.9 | 97.4 ± 2.6 | |||||
| 100 | 92.6 ± 3.4 | 99.5 ± 0.8 | |||||
| CAH24 | 887 ± 0.2 | 241 ± 0.1 | 40 | 60.8 ± 7.5 | 15.5 | 84.6 ± 1.1 | 13.6 |
| 60 | 79.8 ± 10.1 | 90.1 ± 2.8 | |||||
| 80 | 92.3 ± 3.6 | 97.5 ± 1.2 | |||||
| 100 | 95.81 ± 0.77 | 100 ± 0.01 |
| Molecules | Receptor (kcal/mol) a | |
|---|---|---|
| nsP2 Protease—CHIKV | nsP2 Protease—MAYV | |
| Chlorogenic acid | −6.7 | −6.8 |
| Myricetin | −7.0 | −7.1 |
| Naringin | −7.9 | −8.5 |
| Quercetin | −7.1 | −7.3 |
| Syringic acid | −4.6 | −4.9 |
| Vitexin | −7.9 | −7.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, E.C.F.; da Silva, F.P.; Schallenberger, K.; Hermann, B.S.; Mallmann, L.; Moura, W.S.; Ascêncio, S.D.; Barbosa, R.d.S.; Soares, I.M.; Fleck, J.D.; et al. Antiviral Potential of Chiococca alba (L.) Hitchc. Plant Extracts Against Chikungunya and Mayaro Viruses. Int. J. Mol. Sci. 2024, 25, 11397. https://doi.org/10.3390/ijms252111397
Pires ECF, da Silva FP, Schallenberger K, Hermann BS, Mallmann L, Moura WS, Ascêncio SD, Barbosa RdS, Soares IM, Fleck JD, et al. Antiviral Potential of Chiococca alba (L.) Hitchc. Plant Extracts Against Chikungunya and Mayaro Viruses. International Journal of Molecular Sciences. 2024; 25(21):11397. https://doi.org/10.3390/ijms252111397
Chicago/Turabian StylePires, Ellen Caroline Feitoza, Francini Pereira da Silva, Karoline Schallenberger, Bruna Saraiva Hermann, Larissa Mallmann, Wellington Souza Moura, Sergio Donizeti Ascêncio, Robson dos Santos Barbosa, Ilsamar Mendes Soares, Juliane Deise Fleck, and et al. 2024. "Antiviral Potential of Chiococca alba (L.) Hitchc. Plant Extracts Against Chikungunya and Mayaro Viruses" International Journal of Molecular Sciences 25, no. 21: 11397. https://doi.org/10.3390/ijms252111397
APA StylePires, E. C. F., da Silva, F. P., Schallenberger, K., Hermann, B. S., Mallmann, L., Moura, W. S., Ascêncio, S. D., Barbosa, R. d. S., Soares, I. M., Fleck, J. D., de Oliveira, E. E., Smagghe, G., Ribeiro, B. M., & Aguiar, R. W. d. S. (2024). Antiviral Potential of Chiococca alba (L.) Hitchc. Plant Extracts Against Chikungunya and Mayaro Viruses. International Journal of Molecular Sciences, 25(21), 11397. https://doi.org/10.3390/ijms252111397









