Systematic Investigation on Acid-Catalyzed Truncation of N-Acylated Peptoids
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Procedure for Peptoid Synthesis
3.2. General Procedure for Acetylation of N-Terminal of Peptoids
3.3. General Procedure for TFA-Assisted Cleavage and Reverse-Phase HPLC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zuckermann, R.N.; Kerr, J.M.; Kent, S.B.H.; Moos, W.H. Efficient Method for the Preparation of Peptoids [Oligo(N-substituted glycines)] by Submonomer Solid-Phase Synthesis. J. Am. Chem. Soc. 1992, 114, 10646–10647. [Google Scholar] [CrossRef]
- Olivos, H.J.; Alluri, P.G.; Reddy, M.M.; Salony, D.; Kodadek, T. Microwave-Assisted Solid-Phase Synthesis of Peptoids. Org. Lett. 2002, 4, 4057–4059. [Google Scholar] [CrossRef]
- Culf, A.S.; Ouellette, R.J. Solid-Phase Synthesis of N-Substituted Glycine Oligomers (α-Peptoids) and Derivatives. Molecules 2010, 15, 5282–5335. [Google Scholar] [CrossRef] [PubMed]
- Clapperton, A.M.; Babi, J.; Tran, H. A Field Guide to Optimizing Peptoid Synthesis. ACS Polym. Au 2022, 2, 417–429. [Google Scholar] [CrossRef]
- Mannige, R.V.; Haxton, T.K.; Proulx, C.; Robertson, E.J.; Battigelli, A.; Butterfoss, G.L.; Zuckermann, R.N.; Whitelam, S. Peptoid Nanosheets Exhibit a New Secondary-Structure Motif. Nature 2015, 526, 415–420. [Google Scholar] [CrossRef]
- Kwon, Y.U.; Kodadek, T. Quantitative Evaluation of the Relative Cell Permeability of Peptoids and Peptides. J. Am. Chem. Soc. 2007, 129, 1508–1509. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.M.; Simmon, R.J.; Ng, S.; Zuckermann, R.N.; Kerr, J.M.; Moos, W.H. Comparison of the Proteolytic Susceptibilities of Homologous L-Amino Acid, D-Amino Acid, and N-Substituted Glycine Peptide and Peptoid Oligomers. Drug Dev. Res. 1995, 35, 20–32. [Google Scholar] [CrossRef]
- Schettini, R.; Riccardis, F.D.; Sala, G.D.; Izzo, I. Enantioselective Alkylation of Amino Acid Derivatives Promoted by Cyclic Peptoids under Phase-Transfer Conditions. J. Org. Chem. 2016, 81, 2494–2505. [Google Scholar] [CrossRef]
- Wender, P.A.; Mitchell, D.J.; Pattabiraman, K.; Pelkey, E.T.; Steinman, L.; Rothbard, J.B. The Design, Synthesis, and Evaluation of Molecules that Enable or Enhance Cellular Uptake: Peptoid Molecular Transporters. Proc. Natl. Acad. Sci. USA 2000, 97, 13003–13008. [Google Scholar] [CrossRef]
- Rosalba, T.P.F.; Matos, G.D.R.; Salvador, C.E.M.; Andrade, C.K.Z. Rational Design and Multicomponent Synthesis of Lipid–Peptoid Nanocomposites towards a Customized Drug Delivery System Assembly. Molecules 2023, 28, 5725. [Google Scholar] [CrossRef]
- Robertson, E.J.; Battigelli, A.; Proulx, C.; Mannige, R.V.; Haxton, T.K.; Yun, L.; Whitelam, S.; Zuckermann, R.N. Design, Synthesis, Assembly, and Engineering of Peptoid Nanosheets. Acc. Chem. Res. 2016, 49, 379–389. [Google Scholar] [CrossRef]
- Giorgio, A.; Del Gatto, A.; Pennacchio, S.; Salviano, M.; Zaccarro, L. Peptoids: Smart and Emerging Candidates for the Diagnosis of Cancer, Neurological and Autoimmune Disorders. Int. J. Mol. Sci. 2023, 24, 16333. [Google Scholar] [CrossRef]
- Bolt, H.L.; Eggimann, G.A.; Jahoda, C.A.B.; Zuckermann, R.N.; Sharples, G.J.; Cobb, S.L. Exploring the Links between Peptoid Antibacterial Activity and Toxicity. MedChemComm 2017, 8, 886–896. [Google Scholar] [CrossRef]
- Molchanova, N.; Hansen, P.R.; Franzyk, H. Advances in Development of Antimicrobial Peptidomimetics as Potential Drugs. Molecules 2017, 22, 1430. [Google Scholar] [CrossRef]
- Sun, J.; Zuckermann, R.N. Peptoid Polymers: A Highly Designable Bioinspired Material. ACS Nano 2013, 7, 4715–4732. [Google Scholar] [CrossRef]
- Creighton, C.J.; Romoff, T.T.; Bu, J.H.; Goodman, M. Mechanistic Studies of an Unusual Amide Bond Scission. J. Am. Chem. Soc. 1999, 121, 6786–6791. [Google Scholar] [CrossRef]
- Fang, W.J.; Bennett, M.A.; Aldrich, J.V. Deletion of Ac-NMePhe1 from [NMePhe1]arodyn under Acidic Conditions, Part 2: Effects of Cleavage Conditions and N-Terminal Functionality. Biopolymers 2011, 96, 97–102. [Google Scholar] [CrossRef]
- Samaritoni, J.G.; Copes, A.T.; Crews, D.K.; Glos, C.; Thompson, A.L.; Wilson, C.; O’Donnell, M.J.; Scott, W.L. Unexpected Hydrolytic Instability of N-Acylated Amino Acid Amides and Peptides. J. Org. Chem. 2014, 79, 3140–3151. [Google Scholar] [CrossRef]
- Proulx, C.; Noё, F.; Yoo, S.; Connolly, M.D.; Zuckermann, R.N. On-Resin N-Terminal Peptoid Degradation: Toward Mild Sequencing Conditions. Biopolymers 2016, 106, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Biswas, G.; Park, S.; Kim, A.; Park, H.; Park, E.; Kim, J.; Kwon, Y.U. Unusual truncation of N-acylated peptoids under acidic conditions. Org. Biomol. Chem. 2014, 12, 5222–5226. [Google Scholar] [CrossRef] [PubMed]
- Kalita, D.; Sahariah, B.; Mookerjee, S.P.; Sarma, B.K. Strategies to Control the Cis-Trans Isomerization of Peptoid Amide Bonds. Chem. Asian J. 2022, 17, e202200149. [Google Scholar] [CrossRef]
- Li, H.; Hah, J.M.; Lawrence, D.S. Light-Mediated Liberation of Enzymatic Activity: “Small Molecule” Caged Protein Equivalents. J. Am. Chem. Soc. 2008, 130, 10474–10475. [Google Scholar] [CrossRef]
- Gorske, B.C.; Bastian, B.L.; Geske, G.D.; Blackwell, H.E. Local and Tunable n→π* Interactions Regulate Amide Isomerism in the Peptoid Backbone. J. Am. Chem. Soc. 2007, 129, 8928–8929. [Google Scholar] [CrossRef]
- Gorske, B.C.; Stringer, J.R.; Bastian, B.L.; Fowler, S.A.; Blackwell, H.E. New Strategies for the Design of Folded Peptoids Revealed by a Survey of Noncovalent Interactions in Model Systems. J. Am. Chem. Soc. 2009, 131, 16555–16567. [Google Scholar] [CrossRef]
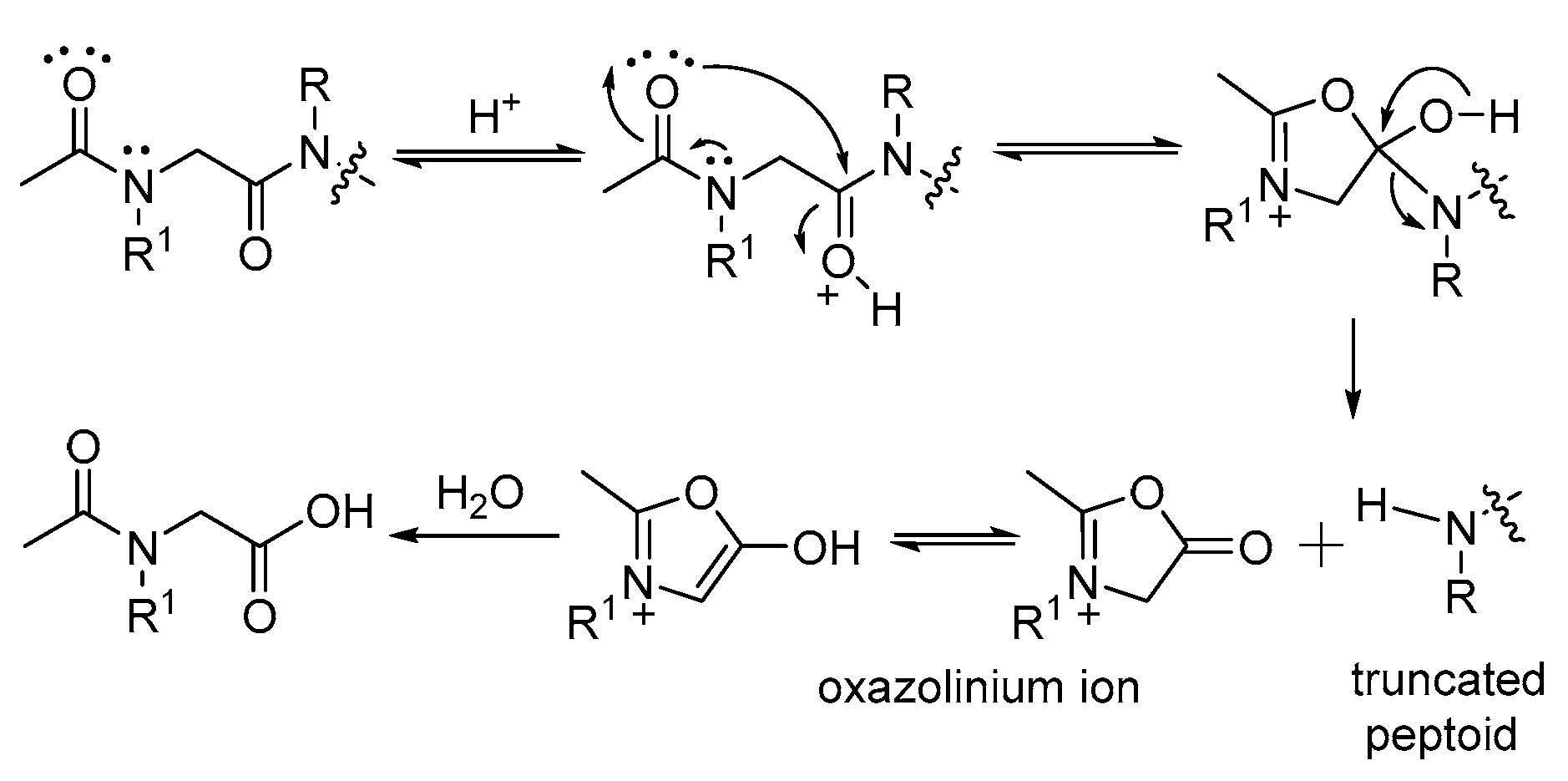

 | ||
|---|---|---|
| Entry | Peptoid | Truncated Peptoid (6) (%) b |
| 1 | 1A′ | 100 |
| 2 | 2A′ | 100 |
| 3 | 3A′ | 100 |
| 4 | 4A′ | 100 |
| 5 | 5A′ | 93.4 (100) c |
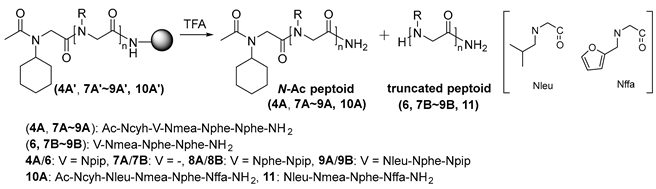 | |||||
|---|---|---|---|---|---|
| Entry | Peptoid | Truncated Peptoid (%) a | |||
| Cond. A b | Cond. B c | ||||
| 1 h | 3 h | 1 h | 3 h | ||
| 1 | 7A′ | 100 | - | 100 | - |
| 2 | 4A′ | 100 | - | 100 | - |
| 3 | 8A′ | 100 | - | 100 | - |
| 4 | 9A′ | 72.5 | 100 | 74.0 | 100 |
| 5 | 10A′ | 73.6 | 100 | 74.3 | 100 |
 | |||||
|---|---|---|---|---|---|
| Entry | Peptoid | pKa a | Cond. b | Truncated Peptoid (11) (%) c | |
| 1 h | 3 h | ||||
| 1 | 12A′ | 4.6 | A | 2.9 | 7.4 |
| 2 | B | 3.6 | 8.7 | ||
| 3 | 13A′ | 5.36 | A | 6.5 | 10.4 |
| 4 | B | 9.2 | 17.0 | ||
| 5 | 14A′ | 2.51 | A | 0.0 | 0.0 |
| 6 | B | 0.0 | 0.0 | ||
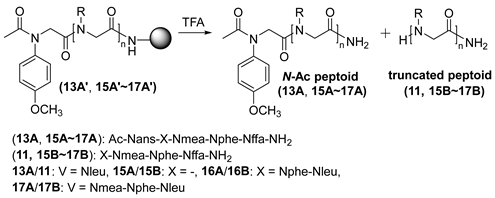 | |||||
|---|---|---|---|---|---|
| Entry | Peptoid | Truncated Peptoid (%) a | |||
| Cond. A b | Cond. B c | ||||
| 1 h | 3 h | 1 h | 3 h | ||
| 1 | 15A′ | 5.2 | 10.2 | 5.4 | 10.8 |
| 2 | 13A′ | 6.5 | 10.4 | 9.2 | 17.0 |
| 3 | 16A′ | 8.3 | 21.0 | 9.7 | 31.7 |
| 4 | 17A′ | 3.8 | 12.2 | 7.1 | 28.1 |
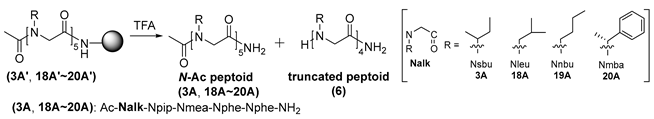 | |||||
|---|---|---|---|---|---|
| Entry | Peptoid | Truncated Peptoid (6) (%) a | |||
| Cond. A b | Cond. B c | ||||
| 1 h | 3 h | 1 h | 3 h | ||
| 1 | 3A′ | 100 | - | 100 | - |
| 2 | 18A′ | 65.5 | 84.0 | 69.8 | 85.8 |
| 3 | 19A′ | 44.0 | 76.7 | 69.4 | 78.5 |
| 4 | 20A′ | 70.0 | 86.7 | 86.9 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piao, R.; Kwon, Y.-U. Systematic Investigation on Acid-Catalyzed Truncation of N-Acylated Peptoids. Int. J. Mol. Sci. 2024, 25, 11390. https://doi.org/10.3390/ijms252111390
Piao R, Kwon Y-U. Systematic Investigation on Acid-Catalyzed Truncation of N-Acylated Peptoids. International Journal of Molecular Sciences. 2024; 25(21):11390. https://doi.org/10.3390/ijms252111390
Chicago/Turabian StylePiao, Ruiqi, and Yong-Uk Kwon. 2024. "Systematic Investigation on Acid-Catalyzed Truncation of N-Acylated Peptoids" International Journal of Molecular Sciences 25, no. 21: 11390. https://doi.org/10.3390/ijms252111390
APA StylePiao, R., & Kwon, Y.-U. (2024). Systematic Investigation on Acid-Catalyzed Truncation of N-Acylated Peptoids. International Journal of Molecular Sciences, 25(21), 11390. https://doi.org/10.3390/ijms252111390






