Intravitreal Metformin Protects Against Choroidal Neovascularization and Light-Induced Retinal Degeneration
Abstract
1. Introduction
2. Results
2.1. Metformin Suppresses Vessel Growth Area in a Dose-Dependent Manner in an Ex Vivo Model of Choroidal Sprouting
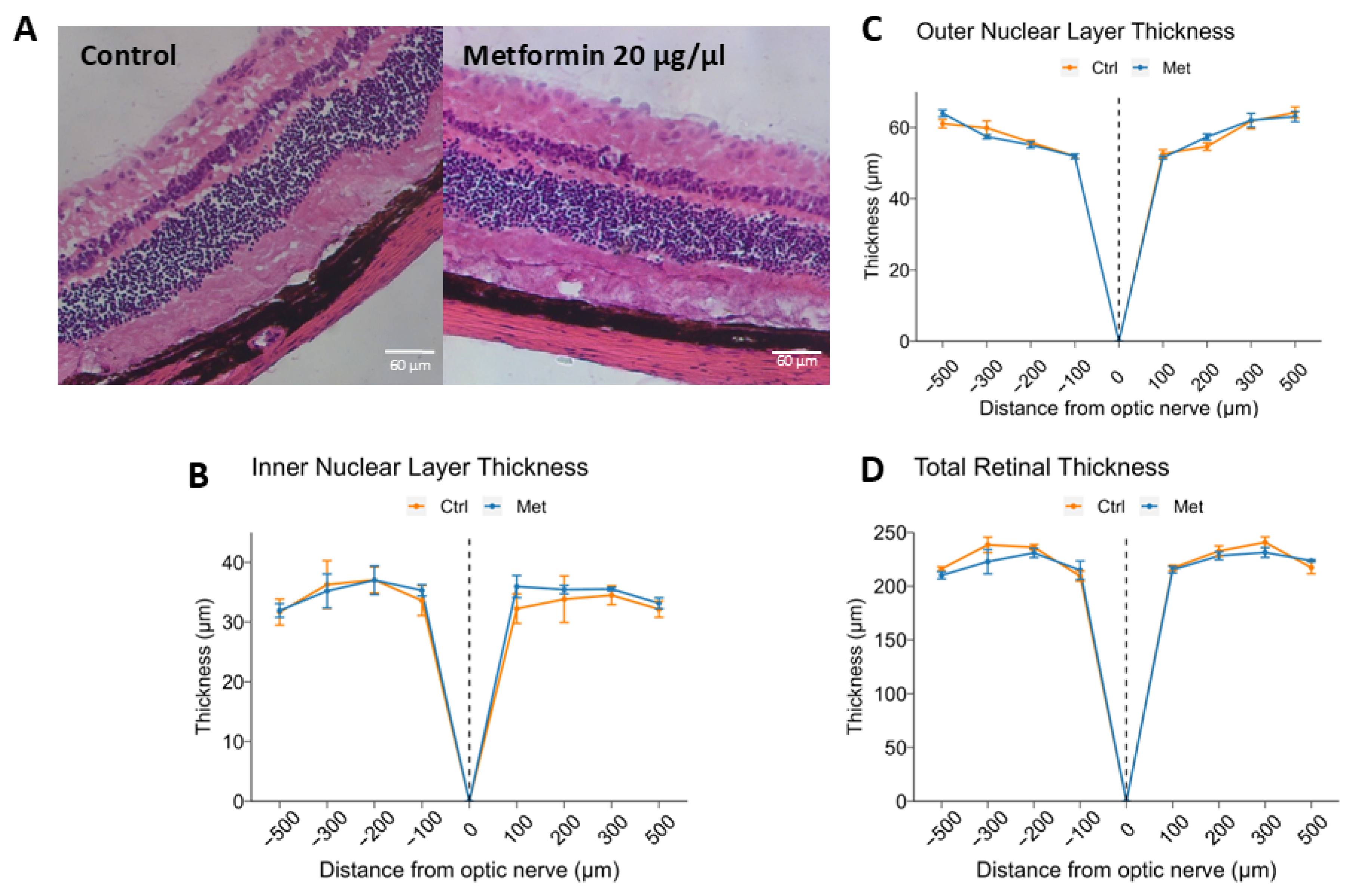
2.2. Intravitreal Metformin Does Not Significantly Change Retinal Morphology or Thickness
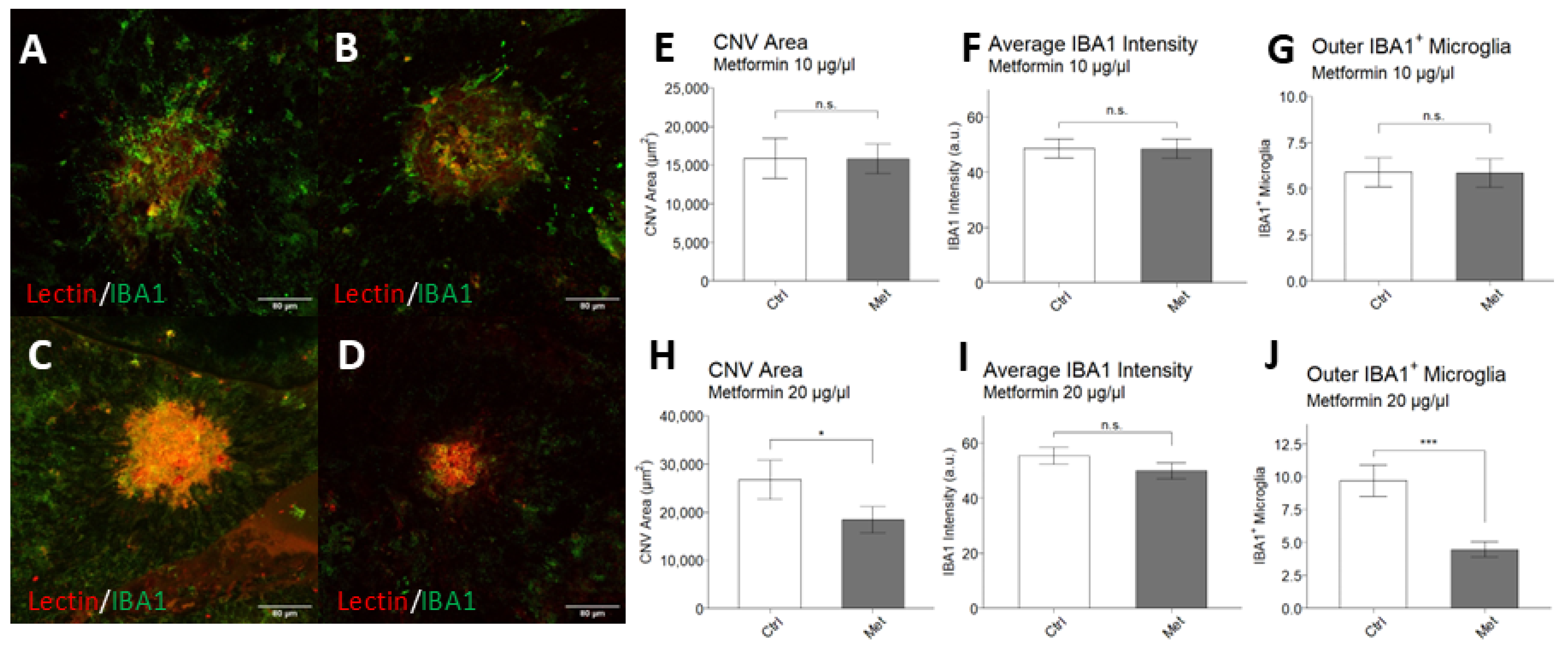
2.3. Intravitreal Metformin Suppresses Choroidal Neovascularization and Infiltration of IBA1+ Macrophages/Microglia
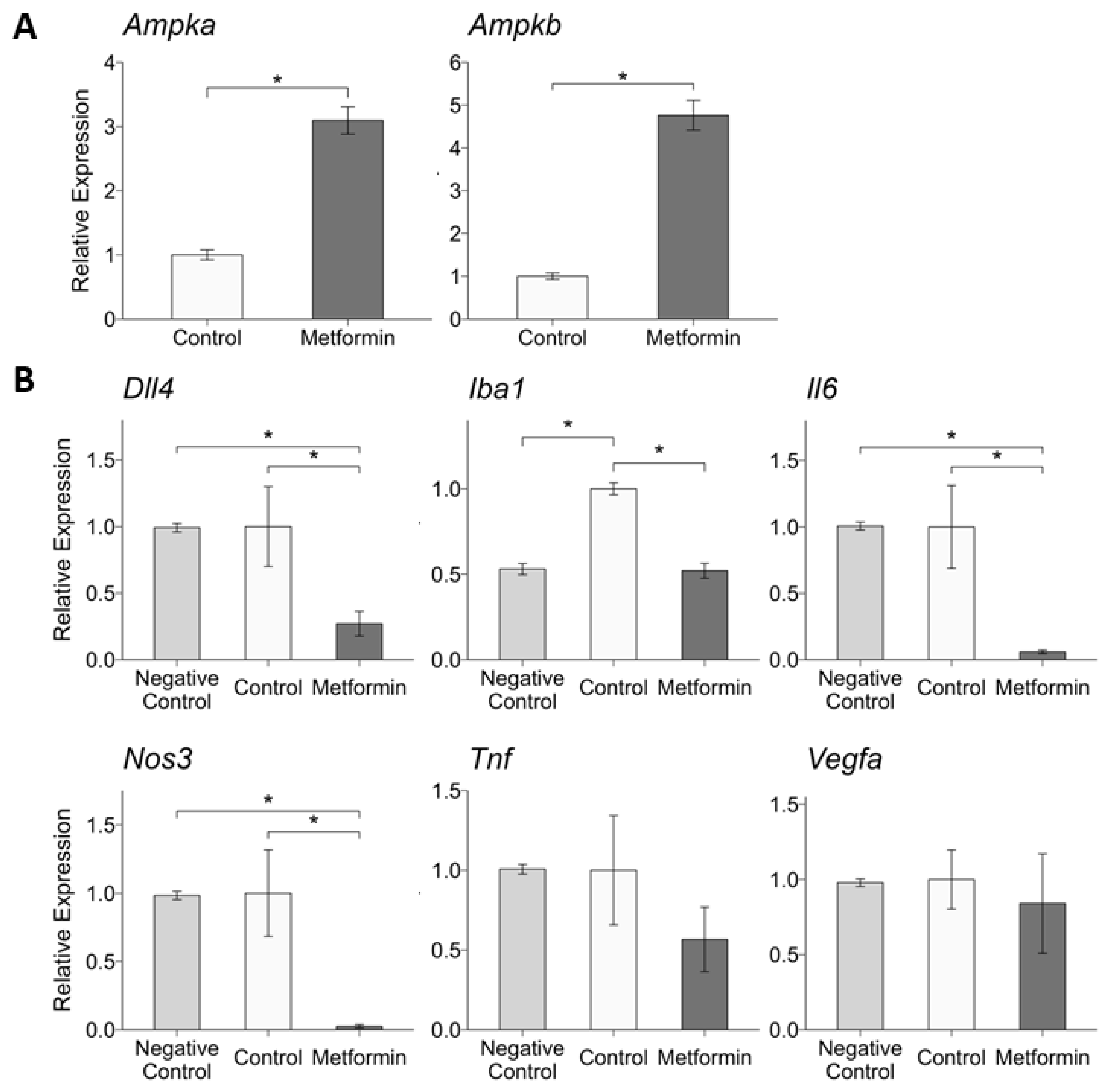
2.4. Intravitreal Metformin Alters RPE and Choroid Expression of Genes Related to Angiogenesis and Inflammation
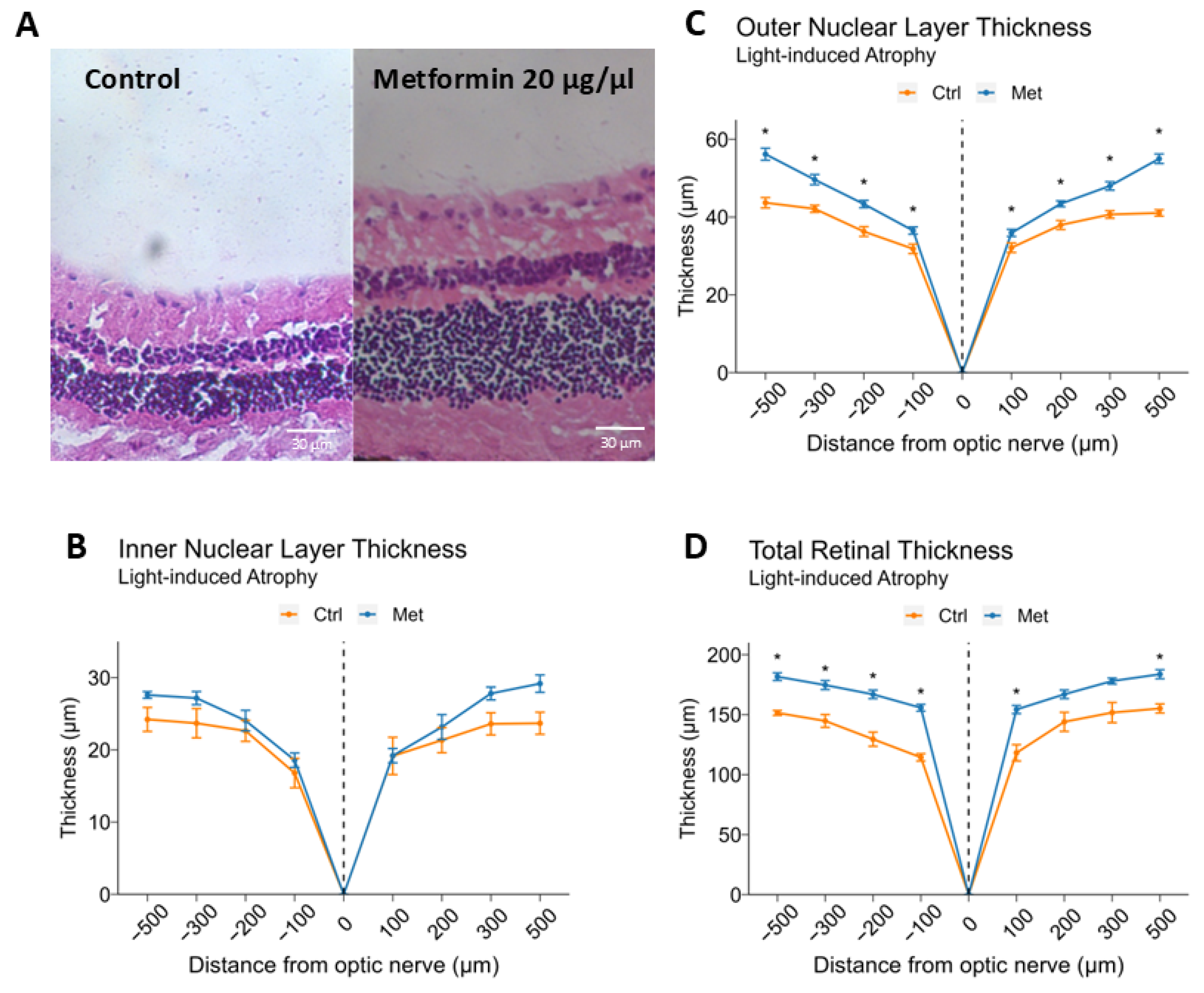
2.5. Intravitreal Metformin Protects Against Light-Induced Retinal Degeneration
3. Discussion
4. Materials & Methods
4.1. Animals
4.2. Choroidal Sprouting Assay
4.3. Laser-Induced Choroidal Neovascularization
4.4. Intravitreal Injection
4.5. Choroid/RPE Flatmounts and Immunohistochemistry
4.6. Visualization and Quantification of Choroid/RPE Flatmounts
4.7. RNA Extraction and Quantitative Real-Time Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
4.8. Light-Induction of Retinal Degeneration
4.9. Histology
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of Blindness and Vision Impairment in 2020 and Trends over 30 Years, and Prevalence of Avoidable Blindness in Relation to VISION 2020: The Right to Sight: An Analysis for the Global Burden of Disease Study. Lancet Glob Health 2021, 9, e144–e160. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Bressler, S.B. Introduction: Understanding the Role of Angiogenesis and Antiangiogenic Agents in Age-Related Macular Degeneration. Ophthalmology 2009, 116, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Yeo, N.J.Y.; Chan, E.J.J.; Cheung, C. Choroidal Neovascularization: Mechanisms of Endothelial Dysfunction. Front. Pharmacol. 2019, 10, 1363. [Google Scholar] [CrossRef]
- Ferris, F.L.; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R. Beckman Initiative for Macular Research Classification Committee Clinical Classification of Age-Related Macular Degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef]
- Rein, D.B.; Wittenborn, J.S.; Burke-Conte, Z.; Gulia, R.; Robalik, T.; Ehrlich, J.R.; Lundeen, E.A.; Flaxman, A.D. Prevalence of Age-Related Macular Degeneration in the US in 2019. JAMA Ophthalmol. 2022, 140, 1202–1208. [Google Scholar] [CrossRef]
- Hobbs, S.D.; Tripathy, K.; Pierce, K. Wet Age-Related Macular Degeneration (AMD). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ferris, F.L.; Fine, S.L.; Hyman, L. Age-Related Macular Degeneration and Blindness Due to Neovascular Maculopathy. Arch. Ophthalmol. 1984, 102, 1640–1642. [Google Scholar] [CrossRef]
- Domalpally, A.; Danis, R.P.; Trane, R.; Blodi, B.A.; Clemons, T.E.; Chew, E.Y. Atrophy in Neovascular Age-Related Macular Degeneration: Age-Related Eye Disease Study 2 Report Number 15. Ophthalmol. Retin. 2018, 2, 1021–1027. [Google Scholar] [CrossRef]
- Cheng, F.-F.; Liu, Y.-L.; Du, J.; Lin, J.-T. Metformin’s Mechanisms in Attenuating Hallmarks of Aging and Age-Related Disease. Aging Dis. 2022, 13, 970–986. [Google Scholar] [CrossRef]
- Sunjaya, A.P.; Sunjaya, A.F. Targeting Ageing and Preventing Organ Degeneration with Metformin. Diabetes Metab. 2021, 47, 101203. [Google Scholar] [CrossRef]
- Saraei, P.; Asadi, I.; Kakar, M.A.; Moradi-Kor, N. The Beneficial Effects of Metformin on Cancer Prevention and Therapy: A Comprehensive Review of Recent Advances. Cancer Manag. Res. 2019, 11, 3295–3313. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, A.H.; Abbaszadeh, A.; Mir, S.; Hasanvand, A. Metformin and Its Anti-Inflammatory and Anti-Oxidative Effects; New Concepts. J. Ren. Inj. Prev. 2018, 8, 54–61. [Google Scholar] [CrossRef]
- Ren, Y.; Luo, H. Metformin: The next Angiogenesis Panacea? SAGE Open Med. 2021, 9, 20503121211001641. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.E.; Ball, J.D.; Chen, Z.; Khurshid, G.S.; Prosperi, M.; Ash, J.D. The Common Antidiabetic Drug Metformin Reduces Odds of Developing Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1470–1477. [Google Scholar] [CrossRef]
- Stewart, J.M.; Lamy, R.; Wu, F.; Keenan, J.D. Relationship between Oral Metformin Use and Age-Related Macular Degeneration. Ophthalmol. Retin. 2020, 4, 1118–1119. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Shen, Y.-C.; Lai, Y.-J.; Wang, C.-Y.; Lin, K.-H.; Feng, S.-C.; Liang, C.-Y.; Wei, L.-C.; Chou, P. Association between Metformin and a Lower Risk of Age-Related Macular Degeneration in Patients with Type 2 Diabetes. J. Ophthalmol. 2019, 2019, 1649156. [Google Scholar] [CrossRef]
- Lee, H.; Jeon, H.L.; Park, S.J.; Shin, J.Y. Effect of Statins, Metformin, Angiotensin-Converting Enzyme Inhibitors, and Angiotensin II Receptor Blockers on Age-Related Macular Degeneration. Yonsei Med. J. 2019, 60, 679–686. [Google Scholar] [CrossRef]
- Blitzer, A.L.; Ham, S.A.; Colby, K.A.; Skondra, D. Association of Metformin Use With Age-Related Macular Degeneration: A Case-Control Study. JAMA Ophthalmol. 2021, 139, 302–309. [Google Scholar] [CrossRef]
- Romdhoniyyah, D.F.; Harding, S.P.; Cheyne, C.P.; Beare, N.A.V. Metformin, A Potential Role in Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis. Ophthalmol. Ther. 2021, 10, 245–260. [Google Scholar] [CrossRef]
- Peyman, G.A.; Lad, E.M.; Moshfeghi, D.M. Intravitreal Injection of Therapeutic Agents. Retina 2009, 29, 875–912. [Google Scholar] [CrossRef]
- Oubaha, M.; Miloudi, K.; Dejda, A.; Guber, V.; Mawambo, G.; Germain, M.-A.; Bourdel, G.; Popovic, N.; Rezende, F.A.; Kaufman, R.J.; et al. Senescence-Associated Secretory Phenotype Contributes to Pathological Angiogenesis in Retinopathy. Sci. Transl. Med. 2016, 8, 362ra144. [Google Scholar] [CrossRef] [PubMed]
- A, L.; Zou, T.; He, J.; Chen, X.; Sun, D.; Fan, X.; Xu, H. Rescue of Retinal Degeneration in Rd1 Mice by Intravitreally Injected Metformin. Front. Mol. Neurosci. 2019, 12, 102. [Google Scholar] [CrossRef]
- Shao, Z.; Friedlander, M.; Hurst, C.G.; Cui, Z.; Pei, D.T.; Evans, L.P.; Juan, A.M.; Tahir, H.; Duhamel, F.; Chen, J.; et al. Choroid Sprouting Assay: An Ex Vivo Model of Microvascular Angiogenesis. PLoS ONE 2013, 8, e69552. [Google Scholar] [CrossRef]
- McLeod, D.S.; Bhutto, I.; Edwards, M.M.; Silver, R.E.; Seddon, J.M.; Lutty, G.A. Distribution and Quantification of Choroidal Macrophages in Human Eyes With Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5843–5855. [Google Scholar] [CrossRef]
- Carozza, G.; Zerti, D.; Tisi, A.; Ciancaglini, M.; Maccarrone, M.; Maccarone, R. An Overview of Retinal Light Damage Models for Preclinical Studies on Age-Related Macular Degeneration: Identifying Molecular Hallmarks and Therapeutic Targets. Rev. Neurosci. 2023, 35, 303–330. [Google Scholar] [CrossRef] [PubMed]
- Grimm, C.; Remé, C.E. Light Damage as a Model of Retinal Degeneration. In Retinal Degeneration: Methods and Protocols; Weber, B.H.F., Langmann, T., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; pp. 87–97. ISBN 978-1-62703-080-9. [Google Scholar]
- Zhang, J.-H.; Zhang, X.-Y.; Sun, Y.-Q.; Lv, R.-H.; Chen, M.; Li, M. Metformin Use Is Associated with a Reduced Risk of Cognitive Impairment in Adults with Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front. Neurosci. 2022, 16, 984559. [Google Scholar] [CrossRef]
- Khanna, S.; Shaw, L.; Hyman, M.J.; Zhang, J.; Hariprasad, S.; Soo, J.; Flores, A.; Skondra, D. Association of Metformin Use with Risk of Newly Onset Neovascular Age-Related Macular Degeneration Development. Retina 2022, 44, 205–213. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Xiao, J.; Xie, B.; Barba, H.; Boachie-Mensah, M.; Shah, R.N.; Nadeem, U.; Spedale, M.; Dylla, N.; Lin, H.; et al. Oral Metformin Inhibits Choroidal Neovascularization by Modulating the Gut-Retina Axis. Investig. Ophthalmol. Vis. Sci. 2023, 64, 21. [Google Scholar] [CrossRef]
- del Amo, E.M.; Rimpelä, A.-K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic Aspects of Retinal Drug Delivery. Progress. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef]
- Dallaglio, K.; Bruno, A.; Cantelmo, A.R.; Esposito, A.I.; Ruggiero, L.; Orecchioni, S.; Calleri, A.; Bertolini, F.; Pfeffer, U.; Noonan, D.M.; et al. Paradoxic Effects of Metformin on Endothelial Cells and Angiogenesis. Carcinogenesis 2014, 35, 1055–1066. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Wang, Y.; Tang, S.; Sun, X.; Feng, X.; Li, Y.; Bao, G.; Li, P.; Mao, X.; et al. Suppression of Tumor Angiogenesis by Metformin Treatment via a Mechanism Linked to Targeting of HER2/HIF-1α/VEGF Secretion Axis. Oncotarget 2015, 6, 44579–44592. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, Y.; Liu, X.; Zhou, T.; Sun, H.; Edwards, P.; Gao, H.; Yu, F.-S.; Qiao, X. Metformin Suppresses Retinal Angiogenesis and Inflammation in Vitro and in Vivo. PLoS ONE 2018, 13, e0193031. [Google Scholar] [CrossRef] [PubMed]
- Yagasaki, R.; Morita, A.; Mori, A.; Sakamoto, K.; Nakahara, T. The Anti-Diabetic Drug Metformin Suppresses Pathological Retinal Angiogenesis via Blocking the mTORC1 Signaling Pathway in Mice (Metformin Suppresses Pathological Angiogenesis). Curr. Eye Res. 2024, 49, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Fabian-Jessing, B.K.; Jakobsen, T.S.; Jensen, E.G.; Alsing, S.; Hansen, S.; Aagaard, L.; Askou, A.L.; Bek, T.; Corydon, T.J. Animal Models of Choroidal Neovascularization: A Systematic Review. Investig. Ophthalmol. Vis. Sci. 2022, 63, 11. [Google Scholar] [CrossRef]
- Ying, Y.; Ueta, T.; Jiang, S.; Lin, H.; Wang, Y.; Vavvas, D.; Wen, R.; Chen, Y.-G.; Luo, Z. Metformin Inhibits ALK1-Mediated Angiogenesis via Activation of AMPK. Oncotarget 2017, 8, 32794–32806. [Google Scholar] [CrossRef]
- LaVail, M.M.; White, M.P.; Gorrin, G.M.; Yasumura, D.; Porrello, K.V.; Mullen, R.J. Retinal Degeneration in the Nervous Mutant Mouse. I. Light Microscopic Cytopathology and Changes in the Interphotoreceptor Matrix. J. Comp. Neurol. 1993, 333, 168–181. [Google Scholar] [CrossRef]
- Vogl, W.-D.; Bogunović, H.; Waldstein, S.M.; Riedl, S.; Schmidt-Erfurth, U. Spatio-Temporal Alterations in Retinal and Choroidal Layers in the Progression of Age-Related Macular Degeneration (AMD) in Optical Coherence Tomography. Sci. Rep. 2021, 11, 5743. [Google Scholar] [CrossRef]
- Brandl, C.; Brücklmayer, C.; Günther, F.; Zimmermann, M.E.; Küchenhoff, H.; Helbig, H.; Weber, B.H.F.; Heid, I.M.; Stark, K.J. Retinal Layer Thicknesses in Early Age-Related Macular Degeneration: Results From the German AugUR Study. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1581–1594. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Baksheeva, V.E.; Tiulina, V.V.; Goriainov, S.V.; Azbukina, N.V.; Gancharova, O.S.; Arifulin, E.A.; Komarov, S.V.; Chistyakov, V.V.; Tikhomirova, N.K.; et al. Mechanisms and Treatment of Light-Induced Retinal Degeneration-Associated Inflammation: Insights from Biochemical Profiling of the Aqueous Humor. Int. J. Mol. Sci. 2020, 21, 704. [Google Scholar] [CrossRef]
- Wenzel, A.; Grimm, C.; Samardzija, M.; Remé, C.E. Molecular Mechanisms of Light-Induced Photoreceptor Apoptosis and Neuroprotection for Retinal Degeneration. Prog. Retin. Eye Res. 2005, 24, 275–306. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.-H.; Do, J.Y.; Lee, J.Y.; Yanai, R.; Lee, I.; Suk, K.; Park, D.H. Key Role of Microglial Matrix Metalloproteinases in Choroidal Neovascularization. Front. Cell Neurosci. 2021, 15, 638098. [Google Scholar] [CrossRef] [PubMed]
- Bellver-Landete, V.; Bretheau, F.; Mailhot, B.; Vallières, N.; Lessard, M.; Janelle, M.-E.; Vernoux, N.; Tremblay, M.-È.; Fuehrmann, T.; Shoichet, M.S.; et al. Microglia Are an Essential Component of the Neuroprotective Scar That Forms after Spinal Cord Injury. Nat. Commun. 2019, 10, 518. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.H.; Fernandes, R.; Santiago, A.R.; Ambrósio, A.F. Microglia Contribution to the Regulation of the Retinal and Choroidal Vasculature in Age-Related Macular Degeneration. Cells 2020, 9, 1217. [Google Scholar] [CrossRef]
- Gupta, N.; Shyamasundar, S.; Patnala, R.; Karthikeyan, A.; Arumugam, T.V.; Ling, E.-A.; Dheen, S.T. Recent Progress in Therapeutic Strategies for Microglia-Mediated Neuroinflammation in Neuropathologies. Expert. Opin. Ther. Targets 2018, 22, 765–781. [Google Scholar] [CrossRef]
- Scholz, R.; Sobotka, M.; Caramoy, A.; Stempfl, T.; Moehle, C.; Langmann, T. Minocycline Counter-Regulates pro-Inflammatory Microglia Responses in the Retina and Protects from Degeneration. J. Neuroinflamm. 2015, 12, 209. [Google Scholar] [CrossRef]
- Rashid, K.; Akhtar-Schaefer, I.; Langmann, T. Microglia in Retinal Degeneration. Front. Immunol. 2019, 10, 1975. [Google Scholar] [CrossRef]
- Lobov, I.; Mikhailova, N. The Role of Dll4/Notch Signaling in Normal and Pathological Ocular Angiogenesis: Dll4 Controls Blood Vessel Sprouting and Vessel Remodeling in Normal and Pathological Conditions. J. Ophthalmol. 2018, 2018, 3565292. [Google Scholar] [CrossRef]
- Lobov, I.B.; Renard, R.A.; Papadopoulos, N.; Gale, N.W.; Thurston, G.; Yancopoulos, G.D.; Wiegand, S.J. Delta-like Ligand 4 (Dll4) Is Induced by VEGF as a Negative Regulator of Angiogenic Sprouting. Proc. Natl. Acad. Sci. USA 2007, 104, 3219–3224. [Google Scholar] [CrossRef]
- Dong, X.; Wang, Y.-S.; Dou, G.-R.; Hou, H.-Y.; Shi, Y.-Y.; Zhang, R.; Ma, K.; Wu, L.; Yao, L.-B.; Cai, Y.; et al. Influence of Dll4 via HIF-1α-VEGF Signaling on the Angiogenesis of Choroidal Neovascularization under Hypoxic Conditions. PLoS ONE 2011, 6, e18481. [Google Scholar] [CrossRef]
- Rana, U.; Callan, E.; Entringer, B.; Michalkiewicz, T.; Joshi, A.; Parchur, A.K.; Teng, R.-J.; Konduri, G.G. AMP-Kinase Dysfunction Alters Notch Ligands to Impair Angiogenesis in Neonatal Pulmonary Hypertension. Am. J. Respir. Cell Mol. Biol. 2020, 62, 719–731. [Google Scholar] [CrossRef]
- Yang, B.; Huang, C.-Z.; Yu, T.; Zhou, S.-N.; Liu, Q.; Liu, G.-J.; Chen, S.; Han, F.-H. Metformin Depresses Overactivated Notch1/Hes1 Signaling in Colorectal Cancer Patients with Type 2 Diabetes Mellitus. Anticancer Drugs 2017, 28, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Droho, S.; Cuda, C.M.; Perlman, H.; Lavine, J.A. Macrophage-Derived Interleukin-6 Is Necessary and Sufficient for Choroidal Angiogenesis. Sci. Rep. 2021, 11, 18084. [Google Scholar] [CrossRef] [PubMed]
- Nahavandipour, A.; Krogh Nielsen, M.; Sørensen, T.L.; Subhi, Y. Systemic Levels of Interleukin-6 in Patients with Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis. Acta Ophthalmol. 2020, 98, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Dingli, D. Metformin Inhibits IL-6 Signaling by Decreasing IL-6R Expression on Multiple Myeloma Cells. Leukemia 2019, 33, 2695–2709. [Google Scholar] [CrossRef]
- Isoda, K.; Young, J.L.; Zirlik, A.; MacFarlane, L.A.; Tsuboi, N.; Gerdes, N.; Schönbeck, U.; Libby, P. Metformin Inhibits Proinflammatory Responses and Nuclear Factor-kappaB in Human Vascular Wall Cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 611–617. [Google Scholar] [CrossRef]
- Yamamoto, N.; Oyaizu, T.; Enomoto, M.; Horie, M.; Yuasa, M.; Okawa, A.; Yagishita, K. VEGF and bFGF Induction by Nitric Oxide Is Associated with Hyperbaric Oxygen-Induced Angiogenesis and Muscle Regeneration. Sci. Rep. 2020, 10, 2744. [Google Scholar] [CrossRef]
- Toma, C.; De Cillà, S.; Palumbo, A.; Garhwal, D.P.; Grossini, E. Oxidative and Nitrosative Stress in Age-Related Macular Degeneration: A Review of Their Role in Different Stages of Disease. Antioxidants 2021, 10, 653. [Google Scholar] [CrossRef]
- Qi, X.; Ricart, K.; Ahmed, K.A.; Patel, R.P.; Boulton, M.E. Supplemental Nitrite Increases Choroidal Neovascularization in Mice. Nitric Oxide 2021, 117, 7–15. [Google Scholar] [CrossRef]
- Sambe, T.; Mason, R.P.; Dawoud, H.; Bhatt, D.L.; Malinski, T. Metformin Treatment Decreases Nitroxidative Stress, Restores Nitric Oxide Bioavailability and Endothelial Function beyond Glucose Control. Biomed. Pharmacother. 2018, 98, 149–156. [Google Scholar] [CrossRef]
- Kalariya, N.M.; Shoeb, M.; Ansari, N.H.; Srivastava, S.K.; Ramana, K.V. Antidiabetic Drug Metformin Suppresses Endotoxin-Induced Uveitis in Rats. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3431–3440. [Google Scholar] [CrossRef]
- Joe, S.G.; Yoon, Y.H.; Choi, J.A.; Koh, J.-Y. Anti-Angiogenic Effect of Metformin in Mouse Oxygen-Induced Retinopathy Is Mediated by Reducing Levels of the Vascular Endothelial Growth Factor Receptor Flk-1. PLoS ONE 2015, 10, e0119708. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.-Y.; Deng, G.; Chen, N.; Bai, Z.-S.; Yuan, J.-S.; Wu, G.-H.; Wang, Y.-W.; Wu, S.-J. Metformin Inhibits Development of Diabetic Retinopathy through Inducing Alternative Splicing of VEGF-A. Am. J. Transl. Res. 2016, 8, 3947–3954. [Google Scholar] [PubMed]
- Usuba, R.; Pauty, J.; Soncin, F.; Matsunaga, Y.T. EGFL7 Regulates Sprouting Angiogenesis and Endothelial Integrity in a Human Blood Vessel Model. Biomaterials 2019, 197, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Collazos-Alemán, J.D.; Gnecco-González, S.; Jaramillo-Zarama, B.; Jiménez-Mora, M.A.; Mendivil, C.O. The Role of Angiopoietins in Neovascular Diabetes-Related Retinal Diseases. Diabetes Ther. 2022, 13, 1811–1821. [Google Scholar] [CrossRef]
- Giani, A.; Thanos, A.; Roh, M.I.; Connolly, E.; Trichonas, G.; Kim, I.; Gragoudas, E.; Vavvas, D.; Miller, J.W. In Vivo Evaluation of Laser-Induced Choroidal Neovascularization Using Spectral-Domain Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3880–3887. [Google Scholar] [CrossRef]
- Yang, X.-M.; Wang, Y.-S.; Zhang, J.; Li, Y.; Xu, J.-F.; Zhu, J.; Zhao, W.; Chu, D.-K.; Wiedemann, P. Role of PI3K/Akt and MEK/ERK in Mediating Hypoxia-Induced Expression of HIF-1α and VEGF in Laser-Induced Rat Choroidal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1873–1879. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, M.; Liang, J.; Chen, Y.; Wang, Y.; Wang, Y.; Xiao, Y.; Zhao, Z.; Wan, X.; Jiang, M.; et al. SLC7A11 Reduces Laser-Induced Choroidal Neovascularization by Inhibiting RPE Ferroptosis and VEGF Production. Front. Cell Dev. Biol. 2021, 9, 639851. [Google Scholar] [CrossRef]
- Hara, C.; Kasai, A.; Gomi, F.; Satooka, T.; Sakimoto, S.; Nakai, K.; Yoshioka, Y.; Yamamuro, A.; Maeda, S.; Nishida, K. Laser-Induced Choroidal Neovascularization in Mice Attenuated by Deficiency in the Apelin-APJ System. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4321–4329. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMP-Activated Protein Kinase (AMPK) Signaling Pathway Coordinates Cell Growth, Autophagy, & Metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Yan, H.; Zhou, H.; Hu, Y.; Pham, C.T.N. Suppression of Experimental Arthritis through AMP-Activated Protein Kinase Activation and Autophagy Modulation. J. Rheum. Dis. Treat. 2015, 1, 5. [Google Scholar] [CrossRef]
- Postler, T.S.; Peng, V.; Bhatt, D.M.; Ghosh, S. Metformin Selectively Dampens the Acute Inflammatory Response through an AMPK-Dependent Mechanism. Sci. Rep. 2021, 11, 18721. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tang, Y.; Jin, X.; Chen, C.; Lu, Y.; Liu, L.; Shen, C. Metformin Inhibits Advanced Glycation End Products-Induced Inflammatory Response in Murine Macrophages Partly through AMPK Activation and RAGE/NFκB Pathway Suppression. J. Diabetes Res. 2016, 2016, 4847812. [Google Scholar] [CrossRef]
- Karar, J.; Maity, A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Ashabi, G.; Khalaj, L.; Khodagholi, F.; Goudarzvand, M.; Sarkaki, A. Pre-Treatment with Metformin Activates Nrf2 Antioxidant Pathways and Inhibits Inflammatory Responses through Induction of AMPK after Transient Global Cerebral Ischemia. Metab. Brain Dis. 2015, 30, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Culmsee, C.; Monnig, J.; Kemp, B.E.; Mattson, M.P. AMP-Activated Protein Kinase Is Highly Expressed in Neurons in the Developing Rat Brain and Promotes Neuronal Survival Following Glucose Deprivation. J. Mol. Neurosci. 2001, 17, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Cheng, Y.-C.; Nicol, C.J.; Lin, K.-H.; Yen, C.-H.; Chiang, M.-C. Activation of AMPK Is Neuroprotective in the Oxidative Stress by Advanced Glycosylation End Products in Human Neural Stem Cells. Exp. Cell Res. 2017, 359, 367–373. [Google Scholar] [CrossRef]
- Lu, M.; Su, C.; Qiao, C.; Bian, Y.; Ding, J.; Hu, G. Metformin Prevents Dopaminergic Neuron Death in MPTP/P-Induced Mouse Model of Parkinson’s Disease via Autophagy and Mitochondrial ROS Clearance. Int. J. Neuropsychopharmacol. 2016, 19, pyw047. [Google Scholar] [CrossRef]
- Han, X.; Tai, H.; Wang, X.; Wang, Z.; Zhou, J.; Wei, X.; Ding, Y.; Gong, H.; Mo, C.; Zhang, J.; et al. AMPK Activation Protects Cells from Oxidative Stress-induced Senescence via Autophagic Flux Restoration and Intracellular NAD + Elevation. Aging Cell 2016, 15, 416–427. [Google Scholar] [CrossRef]
- Cantó, C.; Auwerx, J. AMP-Activated Protein Kinase and Its Downstream Transcriptional Pathways. Cell Mol. Life Sci. 2010, 67, 3407–3423. [Google Scholar] [CrossRef]
- Sekar, P.; Hsiao, G.; Hsu, S.-H.; Huang, D.-Y.; Lin, W.-W.; Chan, C.-M. Metformin Inhibits Methylglyoxal-Induced Retinal Pigment Epithelial Cell Death and Retinopathy via AMPK-Dependent Mechanisms: Reversing Mitochondrial Dysfunction and Upregulating Glyoxalase 1. Redox Biol. 2023, 64, 102786. [Google Scholar] [CrossRef]
- Csaky, K.; Do, D.V. Safety Implications of Vascular Endothelial Growth Factor Blockade for Subjects Receiving Intravitreal Anti-Vascular Endothelial Growth Factor Therapies. Am. J. Ophthalmol. 2009, 148, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Falavarjani, K.; Nguyen, Q.D. Adverse Events and Complications Associated with Intravitreal Injection of Anti-VEGF Agents: A Review of Literature. Eye 2013, 27, 787–794. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.; Fleming, G.A.; Chen, K.; Bicsak, T.A. Metformin-Associated Lactic Acidosis: Current Perspectives on Causes and Risk. Metabolism 2016, 65, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, J.; Sun, X. Resistance to Anti-VEGF Therapy in Neovascular Age-Related Macular Degeneration: A Comprehensive Review. Drug Des. Devel Ther. 2016, 10, 1857–1867. [Google Scholar] [CrossRef]
- Sharma, D.; Zachary, I.; Jia, H. Mechanisms of Acquired Resistance to Anti-VEGF Therapy for Neovascular Eye Diseases. Investig. Ophthalmol. Vis. Sci. 2023, 64, 28. [Google Scholar] [CrossRef]
- Du, M.-R.; Gao, Q.-Y.; Liu, C.-L.; Bai, L.-Y.; Li, T.; Wei, F.-L. Exploring the Pharmacological Potential of Metformin for Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 838173. [Google Scholar] [CrossRef]
- Loan, A.; Syal, C.; Lui, M.; He, L.; Wang, J. Promising Use of Metformin in Treating Neurological Disorders: Biomarker-Guided Therapies. Neural Regen. Res. 2023, 19, 1045–1055. [Google Scholar] [CrossRef]
- Moir, J.; Hyman, M.J.; Gonnah, R.; Flores, A.; Hariprasad, S.M.; Skondra, D. The Association Between Metformin Use and New-Onset ICD Coding of Geographic Atrophy. Investig. Ophthalmol. Vis. Sci. 2024, 65, 23. [Google Scholar] [CrossRef]
- Kaufmann, G.T.; Hyman, M.J.; Gonnah, R.; Hariprasad, S.; Skondra, D. Association of Metformin and Other Diabetes Medication Use and the Development of New-Onset Dry Age-Related Macular Degeneration: A Case-Control Study. Investig. Ophthalmol. Vis. Sci. 2023, 64, 22. [Google Scholar] [CrossRef]
- Spooner, K.L.; Mhlanga, C.T.; Hong, T.H.; Broadhead, G.K.; Chang, A.A. The Burden of Neovascular Age-Related Macular Degeneration: A Patient’s Perspective. Clin. Ophthalmol. 2018, 12, 2483–2491. [Google Scholar] [CrossRef]
- Reitan, G.; Kjellevold Haugen, I.B.; Andersen, K.; Bragadottir, R.; Bindesbøll, C. Through the Eyes of Patients: Understanding Treatment Burden of Intravitreal Anti-VEGF Injections for nAMD Patients in Norway. Clin. Ophthalmol. 2023, 17, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.V.; Khanna, S.; Parvar, S.P.; Shaw, L.T.; Dao, D.; Hariprasad, S.M.; Skondra, D. Metformin and Retinal Diseases in Preclinical and Clinical Studies: Insights and Review of Literature. Exp Biol Med. 2022, 247, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Ruesenberg, B.; Wiermann, A.; Bigdon, S.; Thill, M.; Grierson, R.; Richard, G.; Zeitz, O. Intravitreal Bevacizumab in a Mouse Model of Laser Induced CNV. Investig. Ophthalmol. Vis. Sci. 2009, 50, 74. [Google Scholar]
- Fernandez, P.; Recalde, S.; Hernandez, M.; Bezunartea, J.; Irache, J.M.; Luis de Redin, I.; Belza, I.; Rojas de Miguel, E.; Moreno, M.; Alonso, E.; et al. Intravitreal Bevacizumab-Loaded Nanoparticles Reduce Choroidal Neovascularization in Laser-Induced Animal Model. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1251. [Google Scholar]
- Irani, Y.; Scotney, P.; Nash, A.; Williams, K.A. Species Cross-Reactivity of Antibodies Used to Treat Ophthalmic Conditions. Investig. Ophthalmol. Vis. Sci. 2016, 57, 586–591. [Google Scholar] [CrossRef]
- Saishin, Y.; Saishin, Y.; Takahashi, K.; Lima e Silva, R.; Hylton, D.; Rudge, J.S.; Wiegand, S.J.; Campochiaro, P.A. VEGF-TRAP(R1R2) Suppresses Choroidal Neovascularization and VEGF-Induced Breakdown of the Blood-Retinal Barrier. J. Cell Physiol. 2003, 195, 241–248. [Google Scholar] [CrossRef]
- Shahid, H.; Khan, J.C.; Cipriani, V.; Sepp, T.; Matharu, B.K.; Bunce, C.; Harding, S.P.; Clayton, D.G.; Moore, A.T.; Yates, J.R.W.; et al. Age-Related Macular Degeneration: The Importance of Family History as a Risk Factor. Br. J. Ophthalmol. 2012, 96, 427–431. [Google Scholar] [CrossRef]
- Heesterbeek, T.J.; Lorés-Motta, L.; Hoyng, C.B.; Lechanteur, Y.T.E.; den Hollander, A.I. Risk Factors for Progression of Age-Related Macular Degeneration. Ophthalmic Physiol. Opt. 2020, 40, 140–170. [Google Scholar] [CrossRef]
- Cabral de Guimaraes, T.A.; Daich Varela, M.; Georgiou, M.; Michaelides, M. Treatments for Dry Age-Related Macular Degeneration: Therapeutic Avenues, Clinical Trials and Future Directions. Br. J. Ophthalmol. 2022, 106, 297–304. [Google Scholar] [CrossRef]
- Jonasson, F.; Fisher, D.E.; Eiriksdottir, G.; Sigurdsson, S.; Klein, R.; Launer, L.J.; Harris, T.; Gudnason, V.; Cotch, M.F. Five-Year Incidence, Progression and Risk Factors for Age-Related Macular Degeneration: The Age, Gene/Environment Susceptibility Study. Ophthalmology 2014, 121, 1766–1772. [Google Scholar] [CrossRef]
- Shim, S.H.; Kim, S.-G.; Bae, J.H.; Yu, H.G.; Song, S.J. Risk Factors for Progression of Early Age-Related Macular Degeneration in Koreans. Ophthalmic Epidemiol. 2016, 23, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Sui, G.-Y.; Liu, G.-C.; Liu, G.-Y.; Gao, Y.-Y.; Deng, Y.; Wang, W.-Y.; Tong, S.-H.; Wang, L. Is Sunlight Exposure a Risk Factor for Age-Related Macular Degeneration? A Systematic Review and Meta-Analysis. Br. J. Ophthalmol. 2013, 97, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.M.J.; Colijn, J.M.; Cougnard-Grégoire, A.; de Koning-Backus, A.P.M.; Delyfer, M.-N.; Kiefte-de Jong, J.C.; Meester-Smoor, M.; Féart, C.; Verzijden, T.; Samieri, C.; et al. Mediterranean Diet and Incidence of Advanced Age-Related Macular Degeneration: The EYE-RISK Consortium. Ophthalmology 2019, 126, 381–390. [Google Scholar] [CrossRef]
- Jiang, H.; Shi, X.; Fan, Y.; Wang, D.; Li, B.; Zhou, J.; Pei, C.; Ma, L. Dietary Omega-3 Polyunsaturated Fatty Acids and Fish Intake and Risk of Age-Related Macular Degeneration. Clin. Nutr. 2021, 40, 5662–5673. [Google Scholar] [CrossRef]
- Parekh, N.; Voland, R.P.; Moeller, S.M.; Blodi, B.A.; Ritenbaugh, C.; Chappell, R.J.; Wallace, R.B.; Mares, J.A. Association between Dietary Fats and Age-Related Macular Degeneration (AMD) in the Carotenoids in Age-Related Eye Disease Study (CAREDS), an Ancillary Study of the Women’s Health Initiative. Arch. Ophthalmol. 2009, 127, 1483–1493. [Google Scholar] [CrossRef]
- Kaushik, S.; Wang, J.J.; Flood, V.; Tan, J.S.L.; Barclay, A.W.; Wong, T.Y.; Brand-Miller, J.; Mitchell, P. Dietary Glycemic Index and the Risk of Age-Related Macular Degeneration. Am. J. Clin. Nutr. 2008, 88, 1104–1110. [Google Scholar] [CrossRef]
- Chuang, J.-Z.; Yang, N.; Nakajima, N.; Otsu, W.; Fu, C.; Yang, H.H.; Lee, M.P.; Akbar, A.F.; Badea, T.C.; Guo, Z.; et al. Retinal Pigment Epithelium-Specific CLIC4 Mutant Is a Mouse Model of Dry Age-Related Macular Degeneration. Nat. Commun. 2022, 13, 374. [Google Scholar] [CrossRef]
- Pennesi, M.E.; Neuringer, M.; Courtney, R.J. Animal Models of Age Related Macular Degeneration. Mol. Asp. Med. 2012, 33, 487–509. [Google Scholar] [CrossRef]
- Droho, S.; Cuda, C.M.; Perlman, H.; Lavine, J.A. Monocyte-Derived Macrophages Are Necessary for Beta-Adrenergic Receptor-Driven Choroidal Neovascularization Inhibition. Investig. Ophthalmol. Vis. Sci. 2019, 60, 5059–5069. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.F.; Luo, W.; Mani, A.; Barba, H.; Solanki, A.; Droho, S.; Lavine, J.A.; Skondra, D. Intravitreal Metformin Protects Against Choroidal Neovascularization and Light-Induced Retinal Degeneration. Int. J. Mol. Sci. 2024, 25, 11357. https://doi.org/10.3390/ijms252111357
Xiao JF, Luo W, Mani A, Barba H, Solanki A, Droho S, Lavine JA, Skondra D. Intravitreal Metformin Protects Against Choroidal Neovascularization and Light-Induced Retinal Degeneration. International Journal of Molecular Sciences. 2024; 25(21):11357. https://doi.org/10.3390/ijms252111357
Chicago/Turabian StyleXiao, Jason F., Wendy Luo, Amir Mani, Hugo Barba, Aniruddhsingh Solanki, Steven Droho, Jeremy A. Lavine, and Dimitra Skondra. 2024. "Intravitreal Metformin Protects Against Choroidal Neovascularization and Light-Induced Retinal Degeneration" International Journal of Molecular Sciences 25, no. 21: 11357. https://doi.org/10.3390/ijms252111357
APA StyleXiao, J. F., Luo, W., Mani, A., Barba, H., Solanki, A., Droho, S., Lavine, J. A., & Skondra, D. (2024). Intravitreal Metformin Protects Against Choroidal Neovascularization and Light-Induced Retinal Degeneration. International Journal of Molecular Sciences, 25(21), 11357. https://doi.org/10.3390/ijms252111357




