Transition Metals Coordination by Bis-imidazole-calix[4]arene Ligands with and Without Pyrene Units Grafted at the Large Rim
Abstract
1. Introduction
2. Results
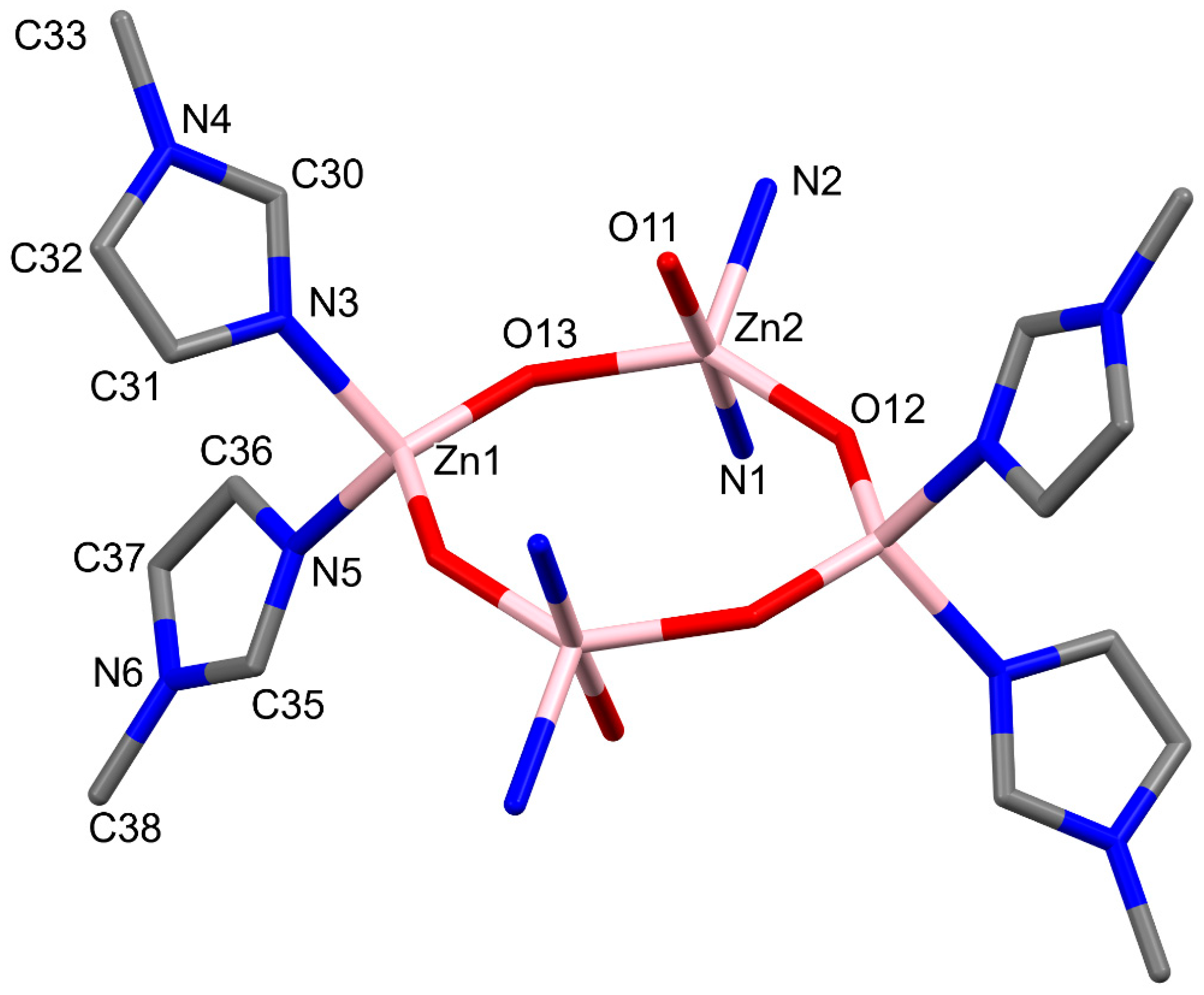
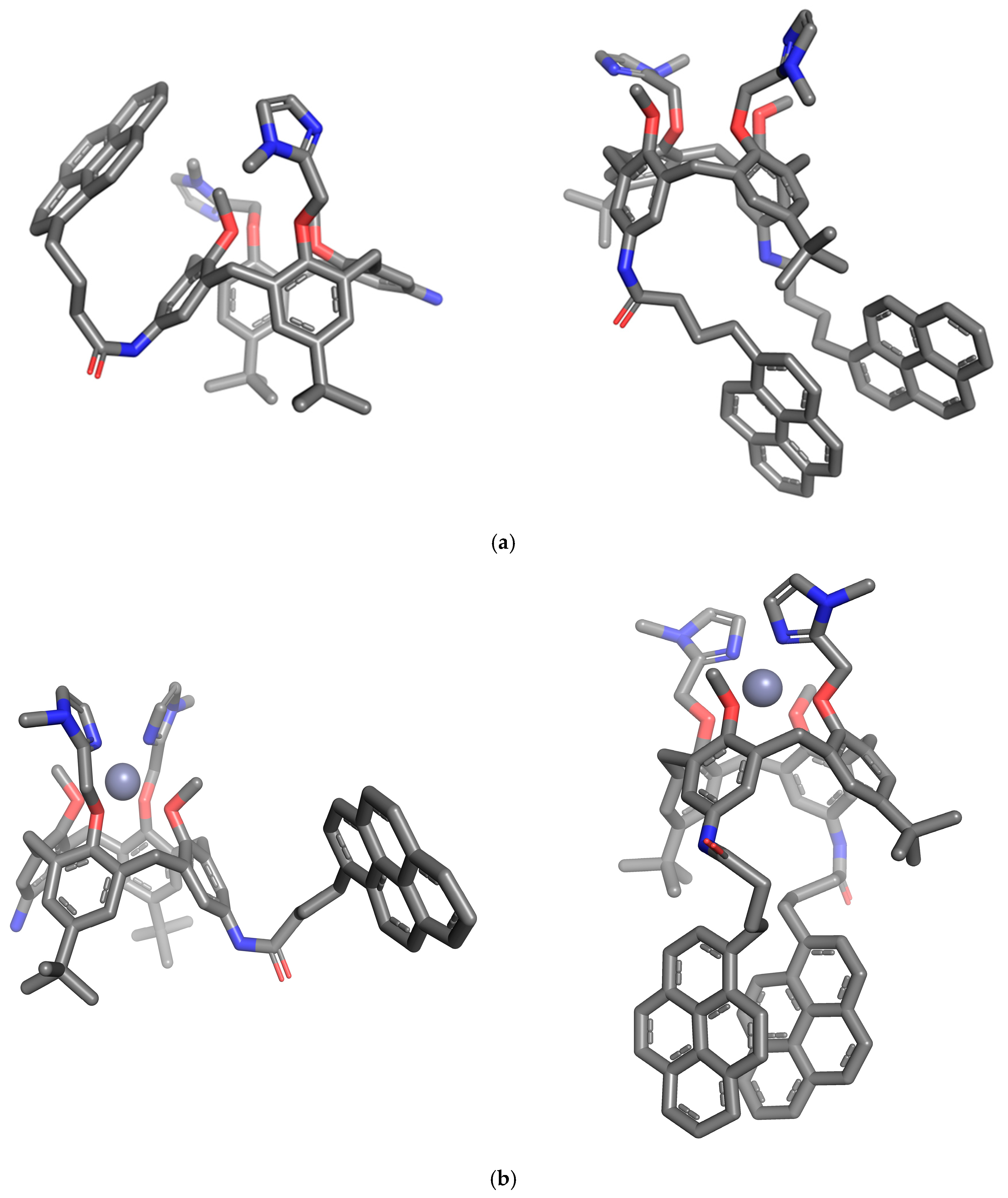
2.1. Molecular and Crystal Structures
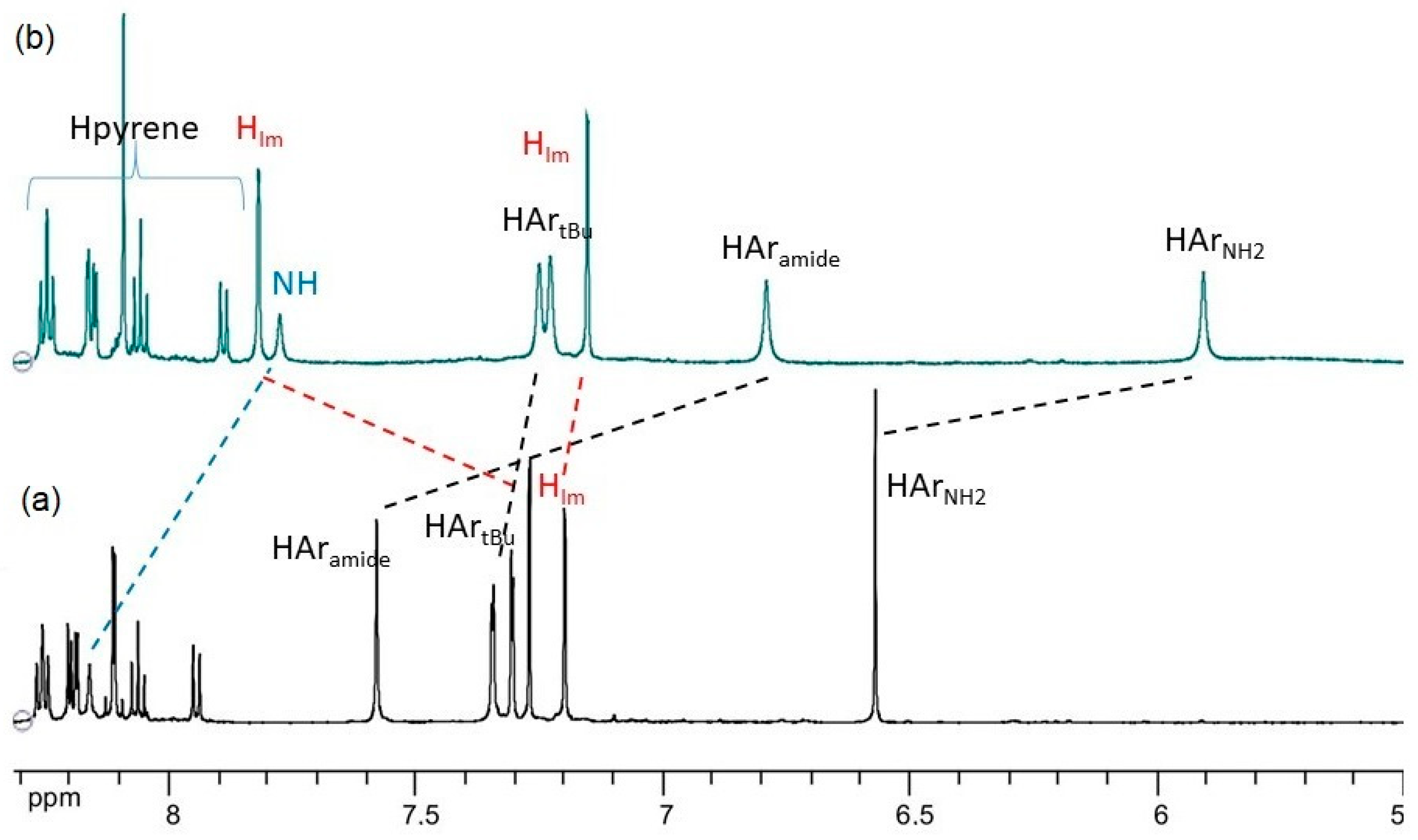
2.2. NMR Studies
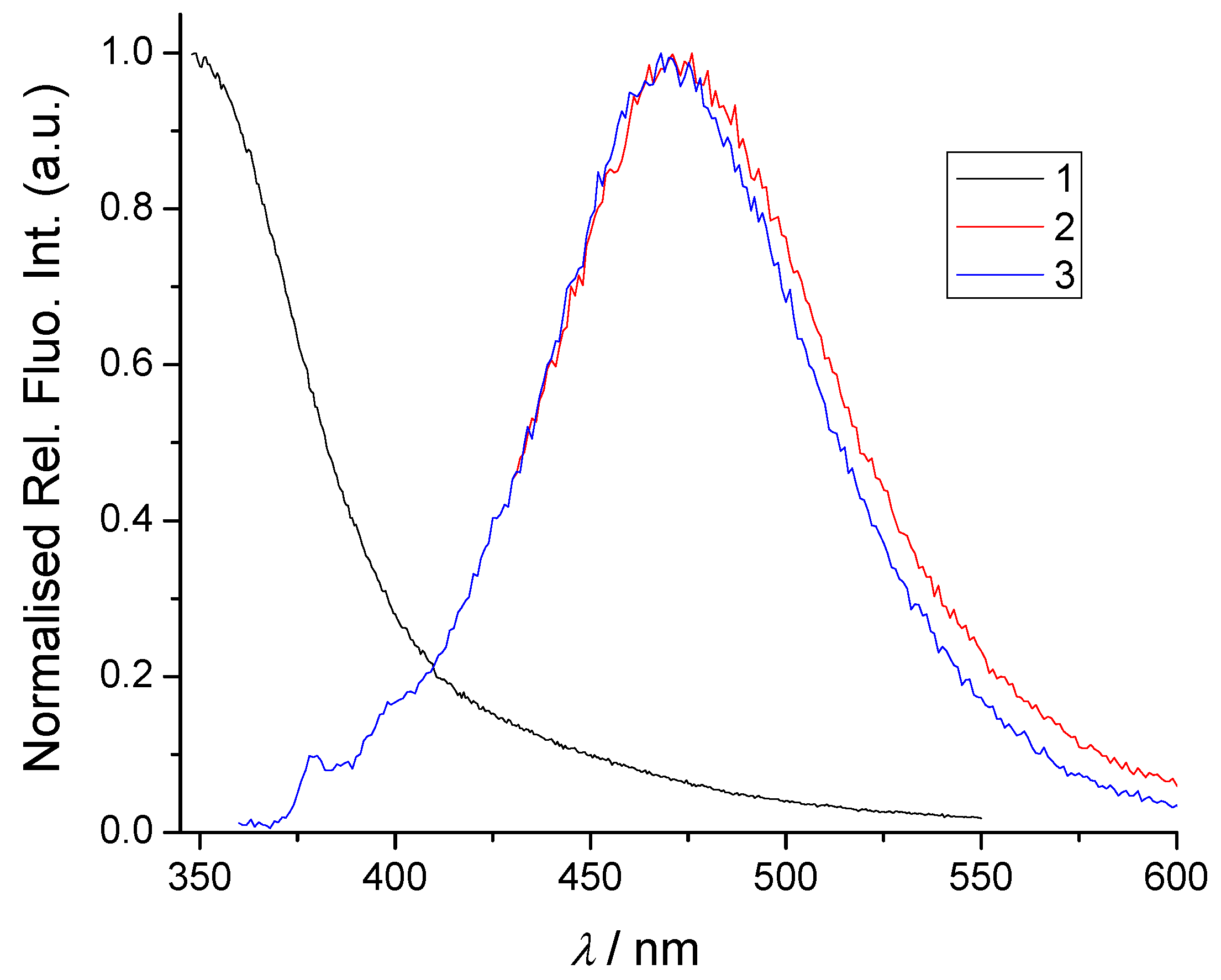
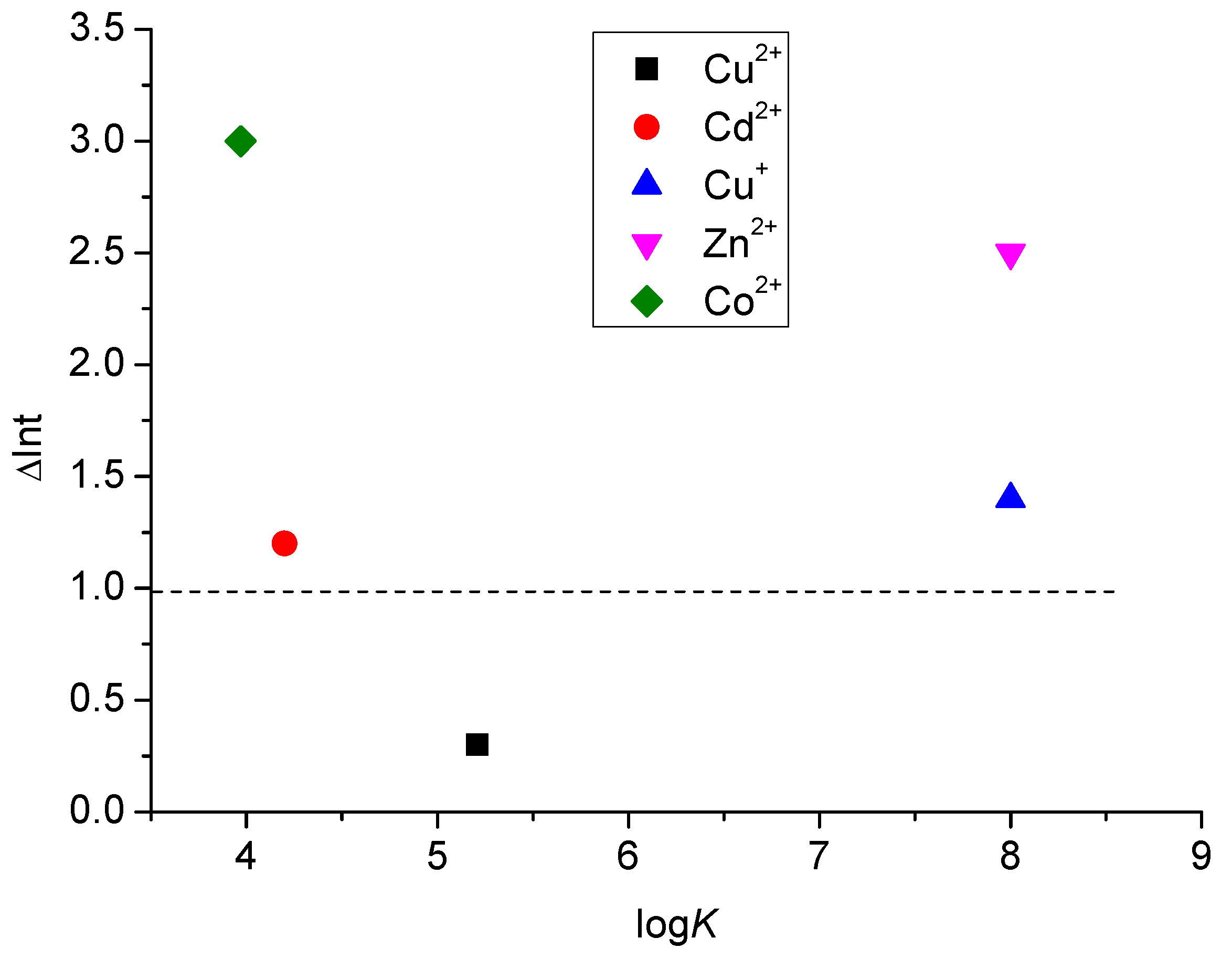
2.3. Fluorimetric Titrations
2.4. Molecular Modelling
3. Conclusions
4. Materials and Methods
4.1. Chemicals for Synthesis and Physicochemical Investigations
4.2. Synthesis
4.3. Fluorimetric Studies
4.4. Crystallizations
4.5. X-ray Diffraction Studies
4.6. NMR Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Komor, A.C.; Barton, J.K. The path for metal complexes to a DNA target. Chem. Commun. 2013, 49, 3617–3630. [Google Scholar] [CrossRef] [PubMed]
- Meggers, E. Targeting proteins with metal complexes. Chem. Commun. 2009, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Archibald, S.J.; Smith, R. Protein-Binding Metal Complexes: Noncovalent and Coordinative Interactions. In Comprehensive Inorganic Chemistry II (Second Edition): From Elements to Applications; Reedijk, J., Poeppelmeier, K., Eds.; Elsevier: Calgary, AB, Canada, 2013; Volume 3, pp. 661–682. [Google Scholar]
- Alsaedi, S.; Babgi, B.A.; Abdellatif, M.H.; Emwas, A.-H.; Jaremko, M.; Humphrey, M.G.; Hussien, M.A. Effect of Net Charge on DNA-Binding, Protein-Binding and Anticancer Properties of Copper(I) Phosphine-Diimine Complexes. J. Inorg. Organomet. Polym. 2021, 31, 3943–3952. [Google Scholar] [CrossRef]
- Krošl, I.; Otković, E.; Nikšić-Franjić, I.; Colasson, B.; Reinaud, O.; Višnjevac, A.; Piantanida, I. Impact of positive charge and ring-size on interactions of calixarenes with DNA, RNA and nucleotides. New J. Chem. 2022, 46, 6860–6869. [Google Scholar] [CrossRef]
- Bertini, I.; Gray, H.B.; Stiefel, E.I.; Valentine, J.S. (Eds.) Biological Inorganic Chemistry—Structure & Reactivity; University Science Book: Sausalito, CA, USA, 2007. [Google Scholar]
- Nakamura, T.; Kaneko, Y.; Nishibori, E.; Nabeshima, T. Molecular recognition by multiple metal coordination inside wavy-stacked macrocycles. Nat. Commun. 2017, 8, 129. [Google Scholar] [CrossRef]
- Wu, Q.; Leung, J.Y.; Geng, X.; Chen, S.; Huang, X.; Li, H.; Huang, Z.; Zhu, L.; Chen, J.; Lu, Y. Heavy metal contamination of soil and water in the vicinity of an abandoned e-waste recycling site: Implications for dissemination of heavy metals. Sci. Total Environ. 2015, 506, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.S.; Matusch, A.; Depboylu, C.; Dobrowolska, J.; Zoriy, M.V. Quantitative Imaging of Selenium, Copper, and Zinc in Thin Sections of Biological Tissues (Slugs−Genus Arion) Measured by Laser Ablation Inductively Coupled Plasma Mass Spectrometry. Anal. Chem. 2007, 79, 6074–6080. [Google Scholar] [CrossRef]
- Sun, W.; Guo, S.; Hu, C.; Fan, J.; Peng, X. Recent Development of Chemosensors Based on Cyanine Platforms. Chem. Rev. 2016, 116, 7768–7817. [Google Scholar] [CrossRef]
- Yang, Y.M.; Zhao, Q.; Feng, W.; Li, F.Y. Luminescent Chemodosimeters for Bioimaging. Chem. Rev. 2013, 113, 192–270. [Google Scholar] [CrossRef]
- De Silva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T. Signaling Recognition Events with Fluorescent Sensors and Switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef]
- Quang, D.T.; Kim, J.S. Fluoro- and Chromogenic Chemodosimeters for Heavy Metal Ion Detection in Solution and Biospecimens. Chem. Rev. 2010, 110, 6280–6301. [Google Scholar] [CrossRef] [PubMed]
- Vendrell, M.; Zhai, D.T.; Er, J.C.; Chang, Y.T. Combinatorial Strategies in Fluorescent Probe Development. Chem. Rev. 2012, 112, 4391–4420. [Google Scholar] [CrossRef]
- Cotruvo, J.A., Jr.; Aron, A.T.; Ramos-Torres, K.M.; Chang, C.J. Synthetic fluorescent probes for studying copper in biological systems. Chem. Soc. Rev. 2015, 44, 4400–4414. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Shon, O.J.; Rim, J.A.; Kim, S.K.; Yoon, J. Pyrene-armed calix[4]azacrowns as new fluorescent ionophores: “Molecular taekowndo” process via fluorescence change. J. Org. Chem. 2002, 67, 2348–2351. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, M.; Wang, X.; Wang, Q. Density Functional Theory Study On the “Molecular Taekwondo” Process of Pyrene-Armed Calix[4]azacrowns. Comput. Theor. Chem. 2014, 1031, 40–49. [Google Scholar] [CrossRef]
- Rang, W.K.; Sheng, G.D.; Ping, J.B.; Yu, L. Highly selective fluorescent chemosensor for Na+ based on pyrene-modified calix[4]arene derivative. Sci. China B Chem. 2009, 52, 513–517. [Google Scholar] [CrossRef]
- Nishimura, Y.; Takemura, T.; Arai, S. Effective fluorescent sensing of Na+ ion by calix[4]arene bearing pyrene and perylene based on energy transfer. ARKIVOC 2007, 2007, 259–268. [Google Scholar] [CrossRef]
- Jeon, N.J.; Ryu, B.J.; Nam, K.C. Pb2+ On-Off Switchable 1,3-Alternate Calix[4]arene Chemosensor Containing Urea and Pyrene Moieties. Bull. Korean Chem. Soc. 2012, 33, 3129–3132. [Google Scholar] [CrossRef]
- Clainche, L.; Giorgi, M.; Reinaud, O. Synthesis and Characterization of a Novel Calix[4]arene-Based Two-Coordinate Copper(I) Complex That Is Unusually Resistant to Dioxygen. Eur. J. Inorg. Chem. 2000, 9, 1931–1933. [Google Scholar] [CrossRef]
- Maurin, A.; Varatharajan, S.; Colasson, B.; Reinaud, O. A water-soluble calix[4]arene-based ligand for the selective linear coordination and stabilization of copper(I) ion in aerobic conditions. Org. Lett. 2014, 16, 5426–5429. [Google Scholar] [CrossRef]
- Renier, N.; Reinaud, O.; Jabin, I.; Valkenier, H. Transmembrane transport of copper(i) by imidazole-functionalised calix[4]arenes. Chem. Commun. 2020, 56, 8206–8209. [Google Scholar] [CrossRef] [PubMed]
- Nikšić-Franjić, I.; Colasson, B.; Reinaud, O.; Višnjevac, A.; Piantanida, I.; Pavlović Saftić, D. Novel Pyrene-Calix[4]arene Derivatives as Highly Sensitive Sensors for Nucleotides, DNA and RNA. RSC Adv. 2023, 13, 27423–27433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jiao, N. Copper-catalyzed aerobic oxidative dehydrogenative coupling of anilines leading to aromatic azo compounds using dioxygen as an oxidant. Angew. Chem. Int. Ed. 2010, 49, 6174–6177. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Crystallogr. Sect. B Struct. Sci. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Wagner, C.L.; Tao, L.; Thompson, E.J.; Stich, T.A.; Guo, J.; Fettinger, J.C.; Berben, L.A.; Britt, D.R.; Nagase, S.; Power, P.P. Dispersion Force-assisted Disproportionation: A Stable Two Coordinate Copper(II) Complex. Angew. Chem. Int. Ed. 2016, 55, 10444–10447. [Google Scholar] [CrossRef]
- Alonso, M.L.; Montaña, F.P.; Miranda, M.; Castillo, C.; Hernández, J.; Benedito, J.L. Interactions between toxic (As, Cd, Hg and Pb) and nutritional essential (Ca, Co, Cr, Cu, Fe, Mn, Mo, Ni, Se, Zn) elements in the tissues of cattle from NW Spain. Biometals 2004, 17, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006. [Google Scholar]
- Maity, D.; Chakraborty, A.; Gunupuru, R.; Paul, P. Calix[4]arene based molecular sensors with pyrene as fluoregenic unit: Effect of solvent in ion selectivity and colorimetric detection of fluoride. Inorg. Chim. Acta 2011, 372, 126–135. [Google Scholar] [CrossRef]
- Gampp, H.; Maeder, M.; Meyer, C.J.; Zuberbuhler, A.D. Calculation of equilibrium-constants from multiwavelength spectroscopic data–II: SPECFIT: Two user-friendly programs in basic and standard FORTRAN 77. Talanta 1985, 32, 257–264. [Google Scholar] [CrossRef]
- Maeder, M.; Zuberbuhler, A.D. Nonlinear least-squares fitting of multivariate absorption data. Anal. Chem. 1990, 62, 2220–2224. [Google Scholar] [CrossRef]
- Persson, I. Hydrated metal ions in aqueous solution: How regular are their structures? Pure Appl. Chem. 2010, 82, 1901–1917. [Google Scholar] [CrossRef]
- Liu, C.-M.; Sun, R.; Wang, B.-W.; Hao, X.; Li, X.-L. Effects of counterions, coordination anions, and coordination solvent molecules on single-molecule magnetic behaviors and nonlinear optical properties of chiral Zn2Dy Schiff base complexes. Inorg. Chem. 2022, 61, 18510–18523. [Google Scholar] [CrossRef] [PubMed]
- Hare, D.J.; New, E.J.; de Jongee, M.D.; McColl, G. Imaging metals in biology: Balancing sensitivity, selectivity and spatial resolution. Chem. Soc. Rev. 2015, 44, 5941–5958. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cao, X.; Deng, Y.; Ji, X.; Sun, W.; Xia, S.; Wan, S.; Zhang, H.; Xing, R.; Ding, J.; et al. An overview on recent advances of reversible fluorescent probes and their biological applications. Talanta 2024, 268, 125275. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Raghavachari, K. Perspective on “Density functional thermochemistry. III. The role of exact exchange“ Becke AD (1993) J Chem Phys 98: 5648–52. Theor. Chem. Acc. 2000, 103, 361–363. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Chiodo, S.; Russo, N.; Sicilia, E. LANL2DZ basis sets recontracted in the framework of density functional theory. J. Chem. Phys. 2006, 125, 104107. [Google Scholar] [CrossRef]
- Mennucci, B. Polarizable continuum model. WIREs Comput. Mol. Sci. 2012, 2, 386–404. [Google Scholar] [CrossRef]
- Zheng, S.; Tang, Q.; He, J.; Du, S.; Xu, S.; Wang, C.; Xu, Y.; Lin, F. VFFDT: A new software for preparing AMBER force field parameters for metal-containing molecular systems. J. Chem. Inf. 2016, 56, 811–818. [Google Scholar] [CrossRef]
- Bayly, C.I.; Cieplak, P.; Cornell, W.; Kollman, P.A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Vassetti, D.; Paglia, M.; Procacci, P. Assessment of GAFF2 and OPLS-AA General Force Fields in Combination with the Water Models TIP3P, SPCE, and OPC3 for the Solvation Free Energy of Druglike Organic Molecules. J. Chem. Theory Comput. 2019, 15, 1983–1995. [Google Scholar] [CrossRef] [PubMed]
- Price, D.J.; Brooks, C.L. A modified TIP3P water potential for simulation with Ewald summation. J. Chem. Phys. 2004, 121, 10096–100103. [Google Scholar] [CrossRef]
- Goga, N.; Rzepiela, A.J.; de Vries, A.H.; Marrink, S.J.; Berendsen, H.J.C. Efficient Algorithms for Langevin and DPD Dynamics. J. Chem. Theory Comput. 2012, 8, 3637–3649. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅ log (N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Kräutler, V.; Van Gunsteren, W.F.; Hünenberger, P.H. A fast SHAKE algorithm to solve distance constraint equations for small molecules in molecular dynamics simulations. J. Comput. Chem. 2001, 22, 501–508. [Google Scholar] [CrossRef]
- Case, D.A.; Aktulga, H.M.; Belfon, K.; Ben-Shalom, I.Y.; Berryman, J.T.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E., III; Cisneros, G.A.; Cruzeiro, V.W.D.; et al. Amber 2022; University of California: San Francisco, CA, USA, 2022. [Google Scholar]
- Schrodinger, L. The PyMOL Molecular Graphics System. Version 1.8; Schrödinger: New York, NY, USA, 2015. [Google Scholar]
- Chaturvedi, J.; Haldar, C.; Bisht, R.; Pandey, G.; Chattopadhyay, B. Supplementary materials for meta selective C–H borylation of sterically biased and unbiased substrates directed by electrostatic interaction. J. Am. Chem. Soc. 2021, 143, 7604–7611. [Google Scholar] [CrossRef] [PubMed]
- CrysAlis CCD; Version 1.171.32.29 (Release 10-02008 CrysAlis171.NET); Oxford Diffraction Ltd.: Yarnton, UK, 2008.
- Sheldrick, G.M. SHELXT: Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; PidZnck, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. New features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Faruggia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- van der Sluis, P.; Spek, A.L. BYPASS: An effective method for the refinement of crystal structures containing disordered solvent regions. Acta Crystallogr. Sect. A Found. Adv. 1990, 46, 194–201. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]

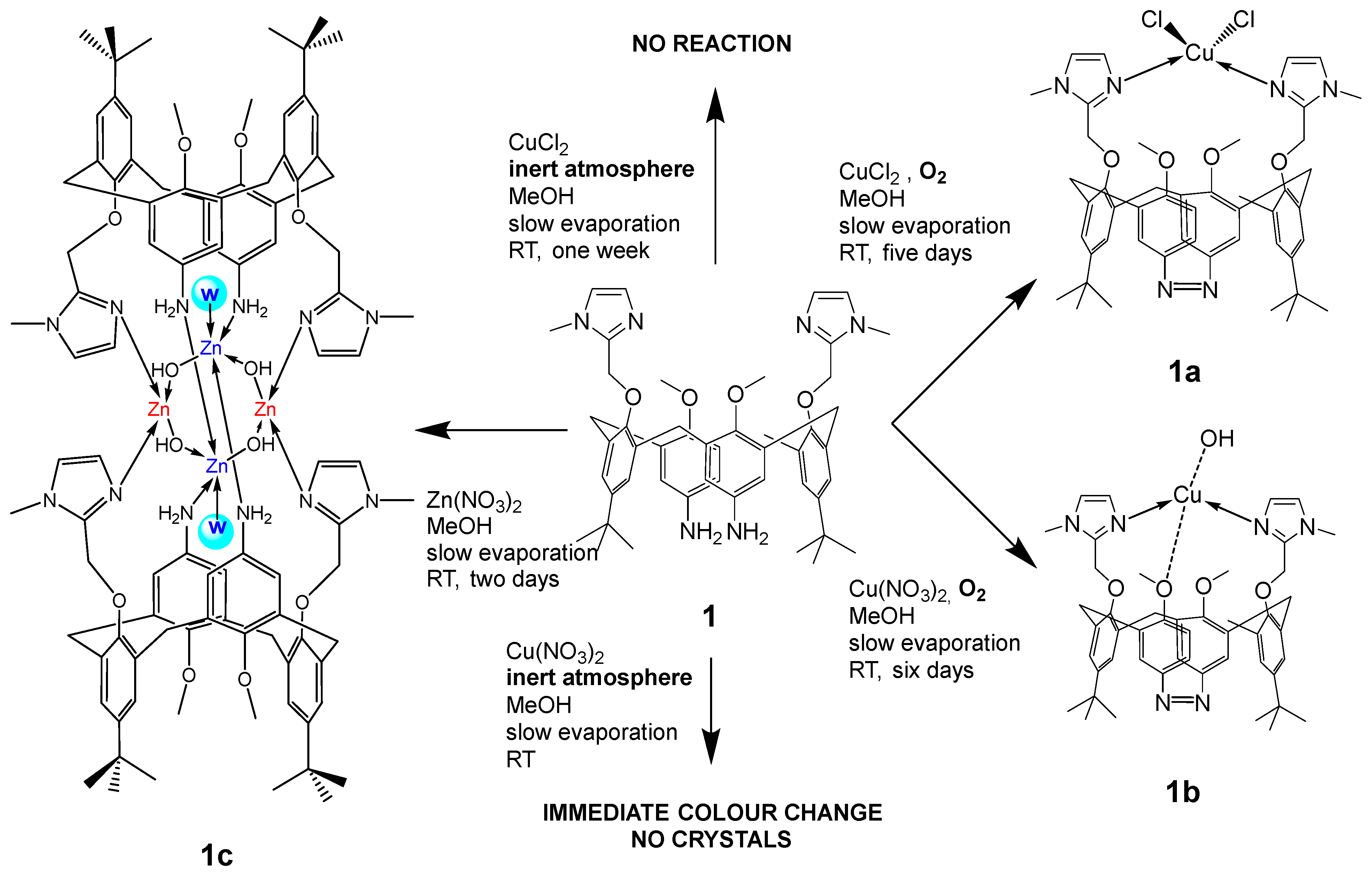
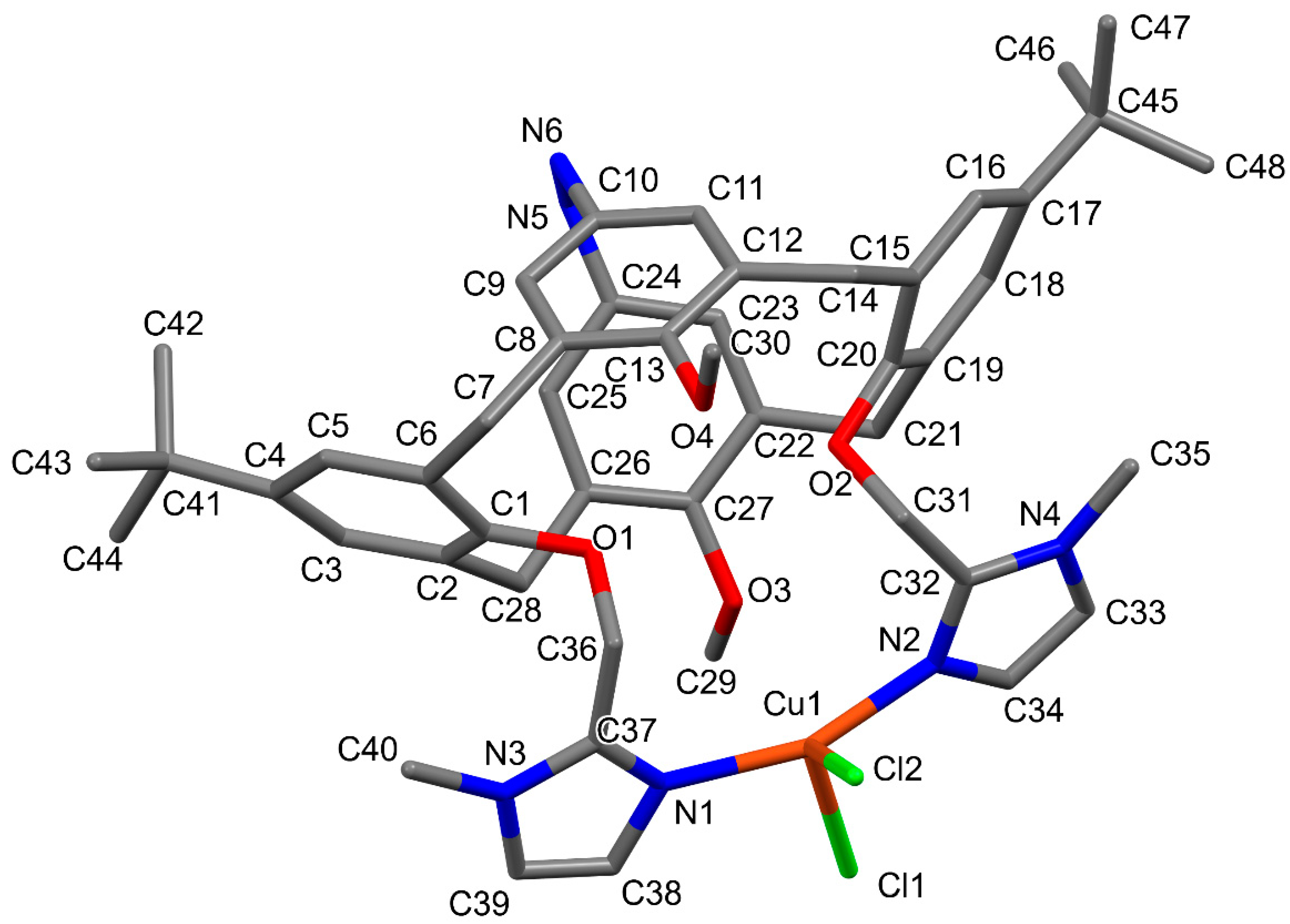
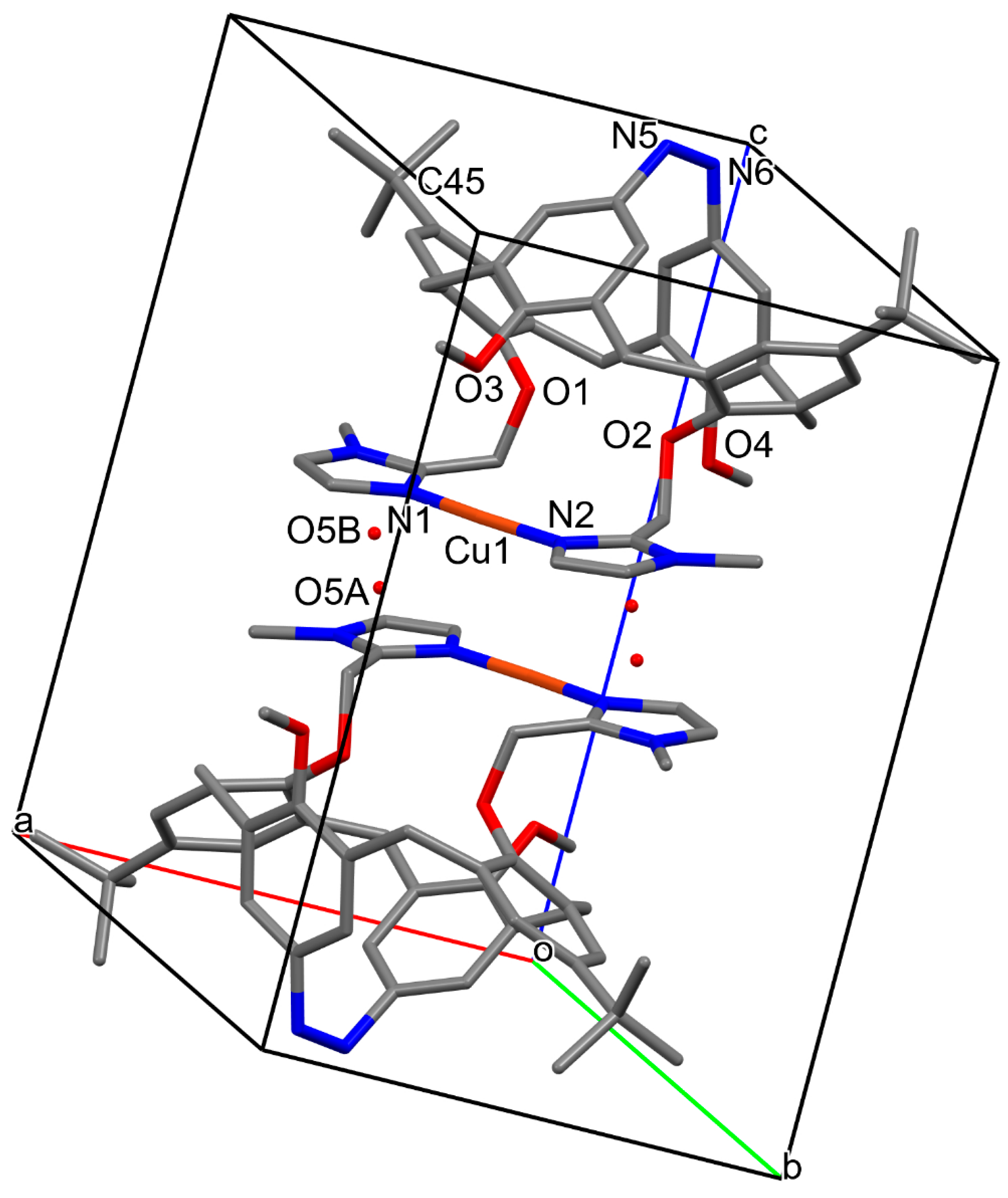
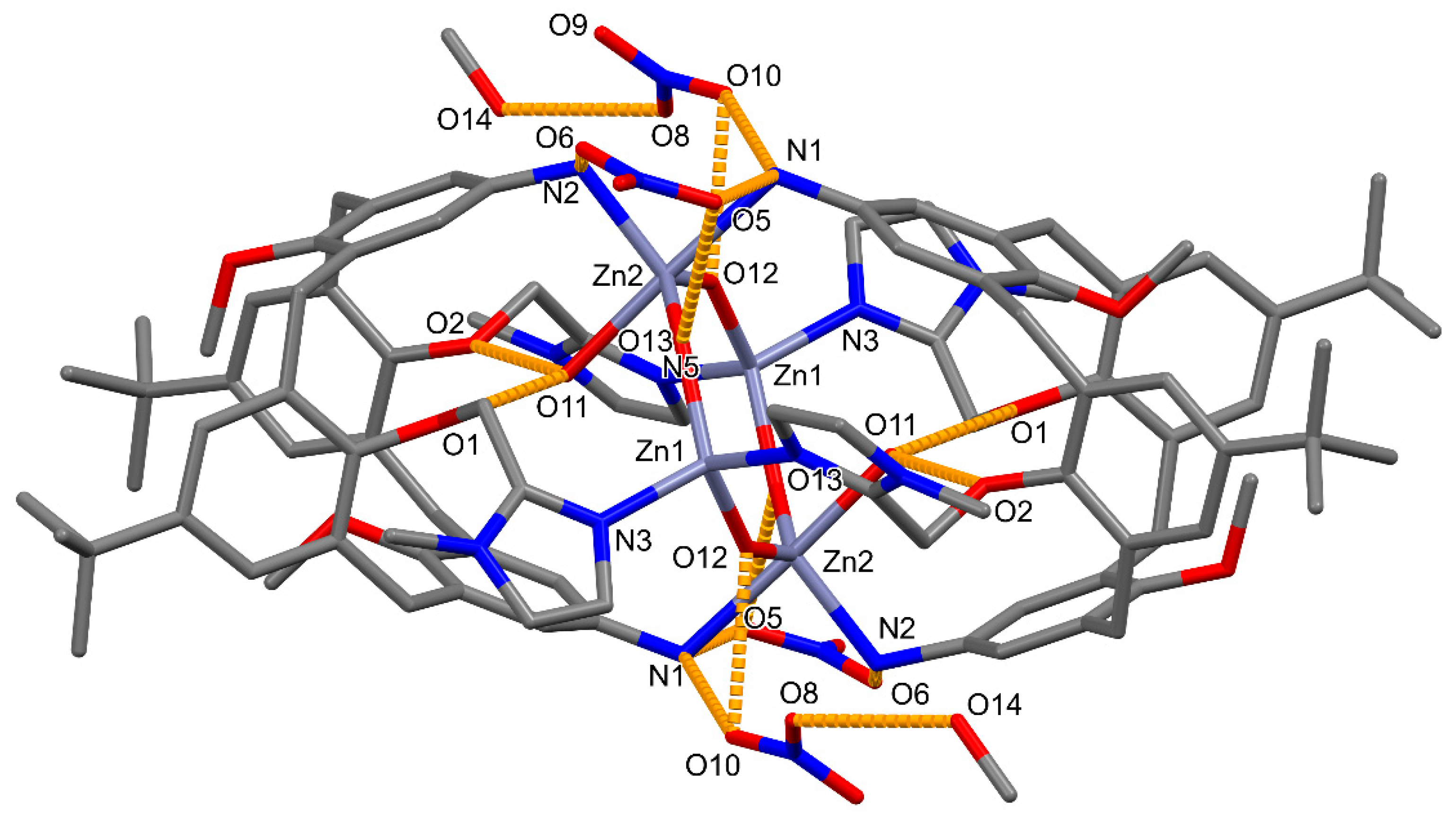
| Bond (Å) | 1a | 1b |
| Cu–N1 | 1.967 (11) | 1.884 (3) |
| Cu–N2 | 1.961 (13) | 1.884 (3) |
| Cu–Cl1/03 | 2.362 (4) | 2.774 (2) |
| Cu–Cl2/O5A (O5B) | 2.286 (4) | 2.766 (7) [2.608 (8)] |
| Angle (°) | 1a | 1b |
| N1–Cu–N2 | 159.4 (4) | 173.67 (11) |
| N1–Cu–Cl1 | 97.6 (4) | |
| N1–Cu–Cl2 | 95.0 (3) | |
| N2–Cu–Cl1 | 93.1 (4) | |
| N2–Cu–Cl2 | 94.3 (3) | |
| Cl1–Cu–Cl2 | 121.34 (17) |
| Struct. Fragment | Nsf | Cu–N (Å) | Cu–O (Å) | N–Cu–N (°) |
|---|---|---|---|---|
| N–Cu+–N | 419 | 1.88 (4) | 173 (7) | |
| N–Cu2+–N | 24 | 1.93 (7) | ||
| NNOO–Cu+ | 849 | 1.97 (4) | 1.94 (9) |
| Bond (Å) | Zn1 | Zn2 |
| Zn–N3/N1 | 2.039 (2) | 2.340 (2) |
| Zn–N5i/N2i | 2.047 (2) | 2.114 (2) |
| Zn–O12 | 1.919 (1) | 1.923 (1) |
| Zn–O13/O13i | 1.919(2) | 1.925(2) |
| Zn–O11 | - | 2.184 (2) |
| Angle (°) | Zn1 | Zn2 |
| N3/N1–Zn–N5i/N2 i | 103.62 (9) | 82.92 (8) |
| N3/N1–Zn–O12 | 104.24 (9) | 92.34 (8) |
| N3/N1–Zn–O13/O13 i | 110.60 (9) | 90.92 (9) |
| N1–Zn–O11 | 177.88 (8) | |
| N5i/N2i–Zn–O12 | 110.64 (8) | 112.48 (9) |
| N5i/N i–Zn–O13/O13i | 103.43 (9) | 111.54 (9) |
| N i–Zn–O11 | 98.78 (8) | |
| O12–Zn–O13/O13i | 122.86 (8) | 135.92 (8) |
| O12–Zn–O11 | 88.18 (8) | |
| O13i–Zn–O11 | 87.28 (9) |
| D-H⋯A | D-H (Å) | H⋯A (Å) | D-H⋯A (Å) | D-H⋯A (°) | Sym. on A |
|---|---|---|---|---|---|
| N1–H1c ⋯ O5i | 0.84 (4) | 2.42 (4) | 3.128 (4) | 142 (3) | 1 − x, 1 − y, 1 − z |
| N1–H1d ⋯ O10i | 0.83 (4) | 2.34 (4) | 3.054 (5) | 145 (3) | 1 − x, 1 − y, 1 − z |
| N2–H2b ⋯ O6 | 0.87 (4) | 2.07 (4) | 2.922 (4) | 169 (4) | |
| O11–H11a ⋯ O1i | 0.89 (5) | 1.97 (5) | 2.850 (3) | 166 (4) | 1 − x, 1 − y, 1 − z |
| O11–H11b ⋯ O2i | 0.75 (4) | 2.13 (4) | 2.867 (3) | 169 (3) | 1 − x, 1 − y, 1 − z |
| O12–H12a ⋯ O10i | 0.78 (5) | 2.13 (4) | 2.858 (4) | 155 (4) | 1 − x, 1 − y, 1 − z |
| O13–H13 ⋯ O5 | 0.69 (4) | 2.27 (5) | 2.957 (4) | 173 (4) | |
| O14–H14a ⋯ O8 | 0.84 (6) | 2.09 (6) | 2.840 (4) | 149 (5) |
| Cation | Anion | 1 | 2 | 3 |
|---|---|---|---|---|
| Cu2+ | Cl− ClO4− | 5.21 ± 0.06 (0.3) nd | 5.41 ± 0.03 (0.3) 6.07 ± 0.07 | 5.29 ± 0.02 (0.4) nd |
| Cu+ | I− | c >8 (1.4) | 5.93 ± 0.04 (0.3) | 5.69 ± 0.02 (0.5) |
| Cd2+ | Cl− ClO4− | nd 4.2 (1.2) | 6.63 ± 0.06 (0.4) 5.63 ± 0.1 | 6.36 ± 0.06 (0.6) nd |
| Co2+ | Cl− ClO4− | 3.97 ± 0.07 (3) nd | c >8 (0.8) c >8 | c >8 (0.6) nd |
| Zn2+ | Cl− ClO4− | c >8 (2.5) nd | nd c >8 (0.1) | nd c >8 (0.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikšić-Franjić, I.; Pavlović Saftić, D.; Smrečki, V.; Colasson, B.; Reinaud, O.; Piantanida, I.; Višnjevac, A. Transition Metals Coordination by Bis-imidazole-calix[4]arene Ligands with and Without Pyrene Units Grafted at the Large Rim. Int. J. Mol. Sci. 2024, 25, 11314. https://doi.org/10.3390/ijms252011314
Nikšić-Franjić I, Pavlović Saftić D, Smrečki V, Colasson B, Reinaud O, Piantanida I, Višnjevac A. Transition Metals Coordination by Bis-imidazole-calix[4]arene Ligands with and Without Pyrene Units Grafted at the Large Rim. International Journal of Molecular Sciences. 2024; 25(20):11314. https://doi.org/10.3390/ijms252011314
Chicago/Turabian StyleNikšić-Franjić, Ivana, Dijana Pavlović Saftić, Vilko Smrečki, Benoit Colasson, Olivia Reinaud, Ivo Piantanida, and Aleksandar Višnjevac. 2024. "Transition Metals Coordination by Bis-imidazole-calix[4]arene Ligands with and Without Pyrene Units Grafted at the Large Rim" International Journal of Molecular Sciences 25, no. 20: 11314. https://doi.org/10.3390/ijms252011314
APA StyleNikšić-Franjić, I., Pavlović Saftić, D., Smrečki, V., Colasson, B., Reinaud, O., Piantanida, I., & Višnjevac, A. (2024). Transition Metals Coordination by Bis-imidazole-calix[4]arene Ligands with and Without Pyrene Units Grafted at the Large Rim. International Journal of Molecular Sciences, 25(20), 11314. https://doi.org/10.3390/ijms252011314








