OsNAL11 and OsGASR9 Regulate the Low-Temperature Germination of Rice Seeds by Affecting GA Content
Abstract
1. Introduction
2. Results
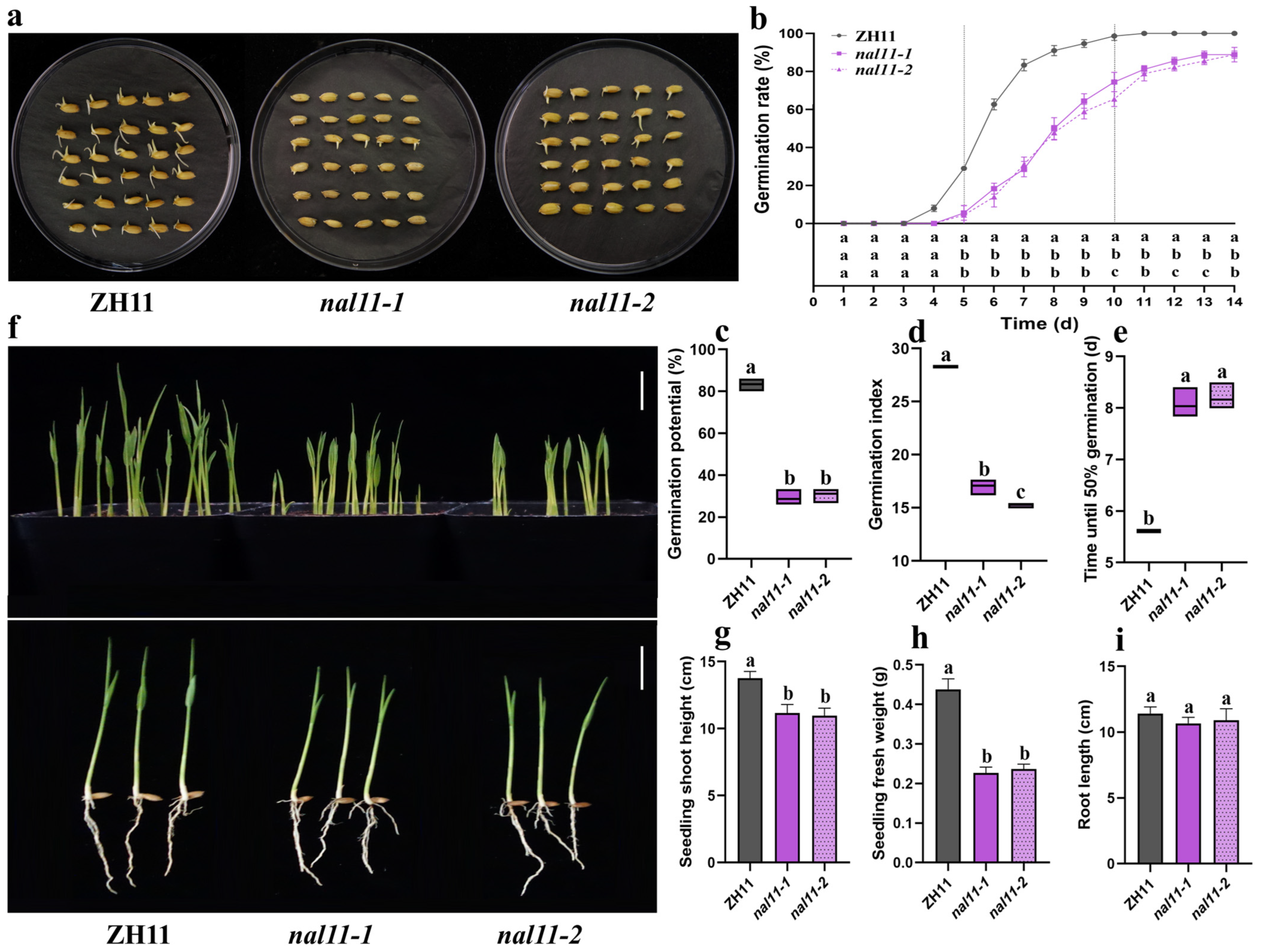
2.1. OsNAL11 Gene Affected Seed Germination and Seedling Growth Under Low-Temperature Conditions
2.2. Physiological Indices of Low-Temperature Germinated Seeds Were Altered in nal11 Mutants
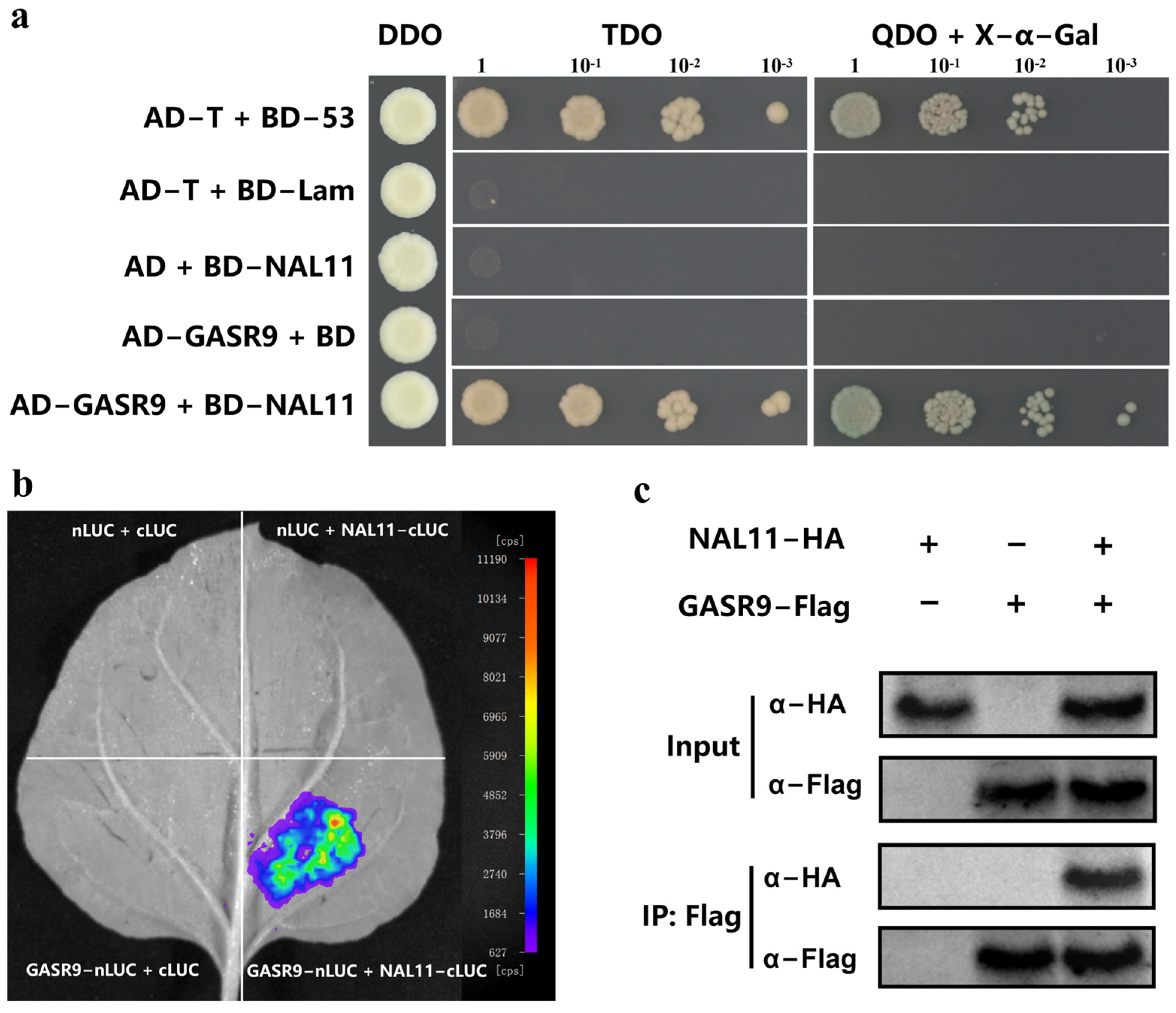
2.3. OsNAL11 Interacts with OsGASR9
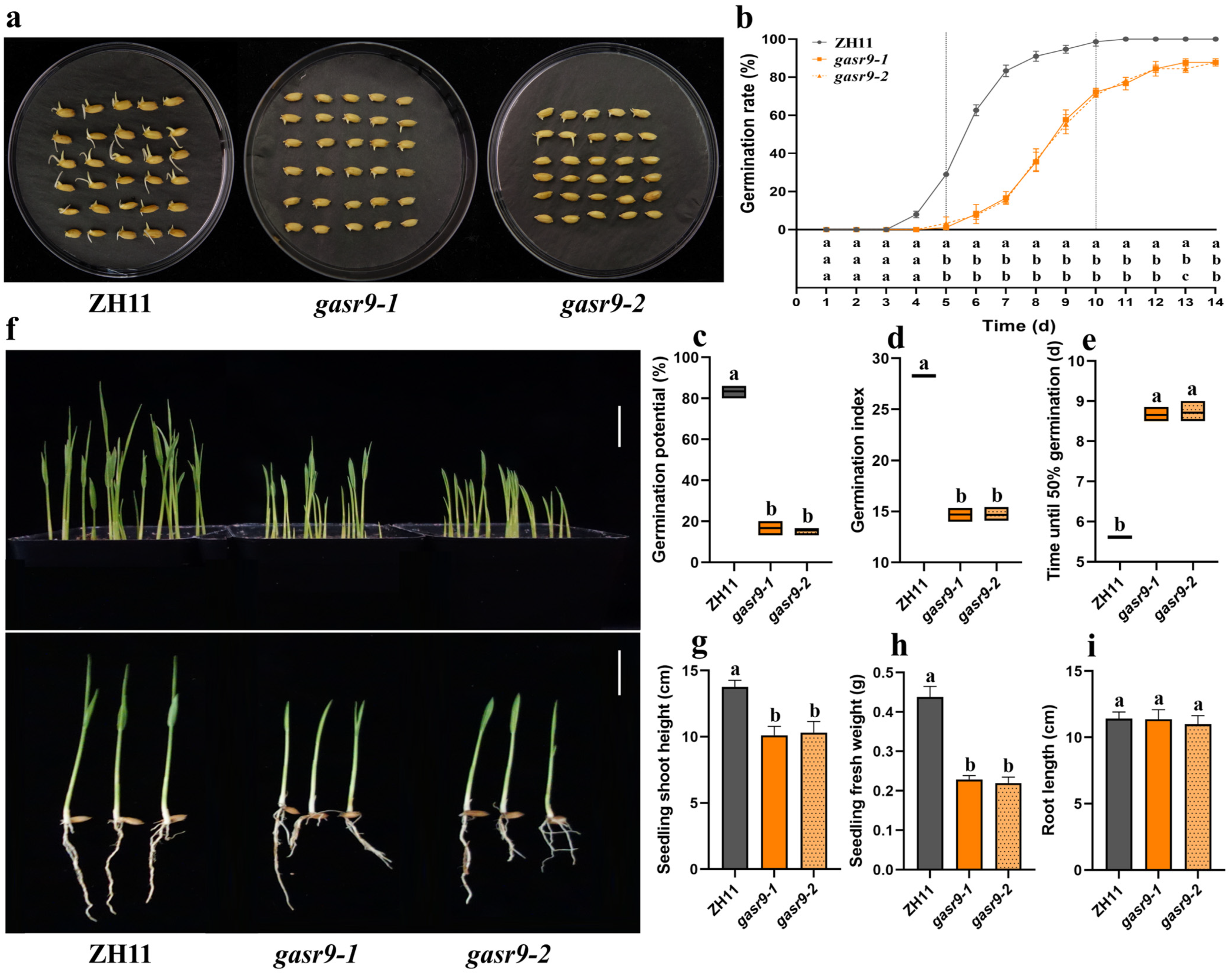
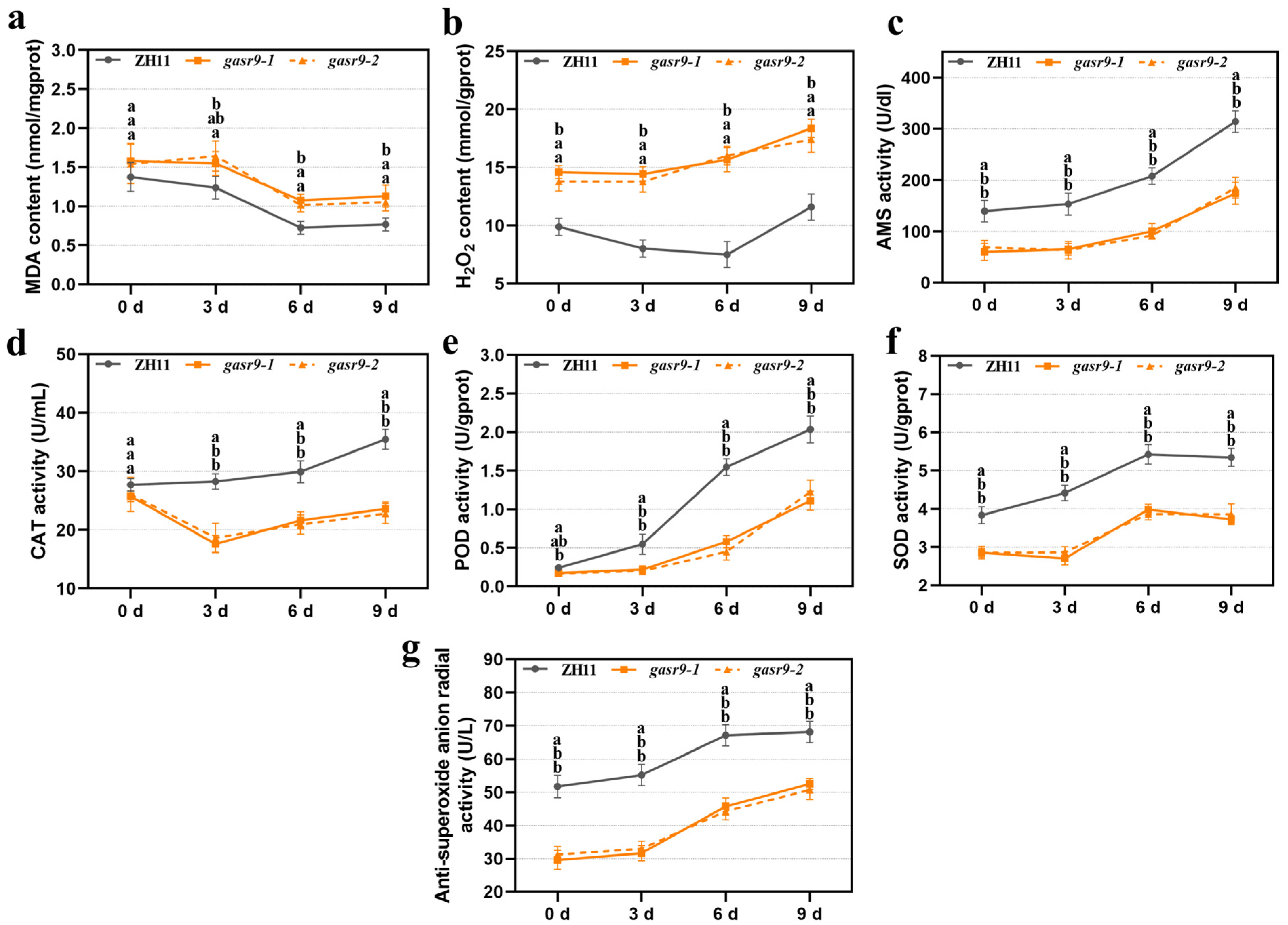
2.4. The gasr9 Mutants Showed Reduced Seed Germination and Seedling Growth Under Low-Temperature Conditions
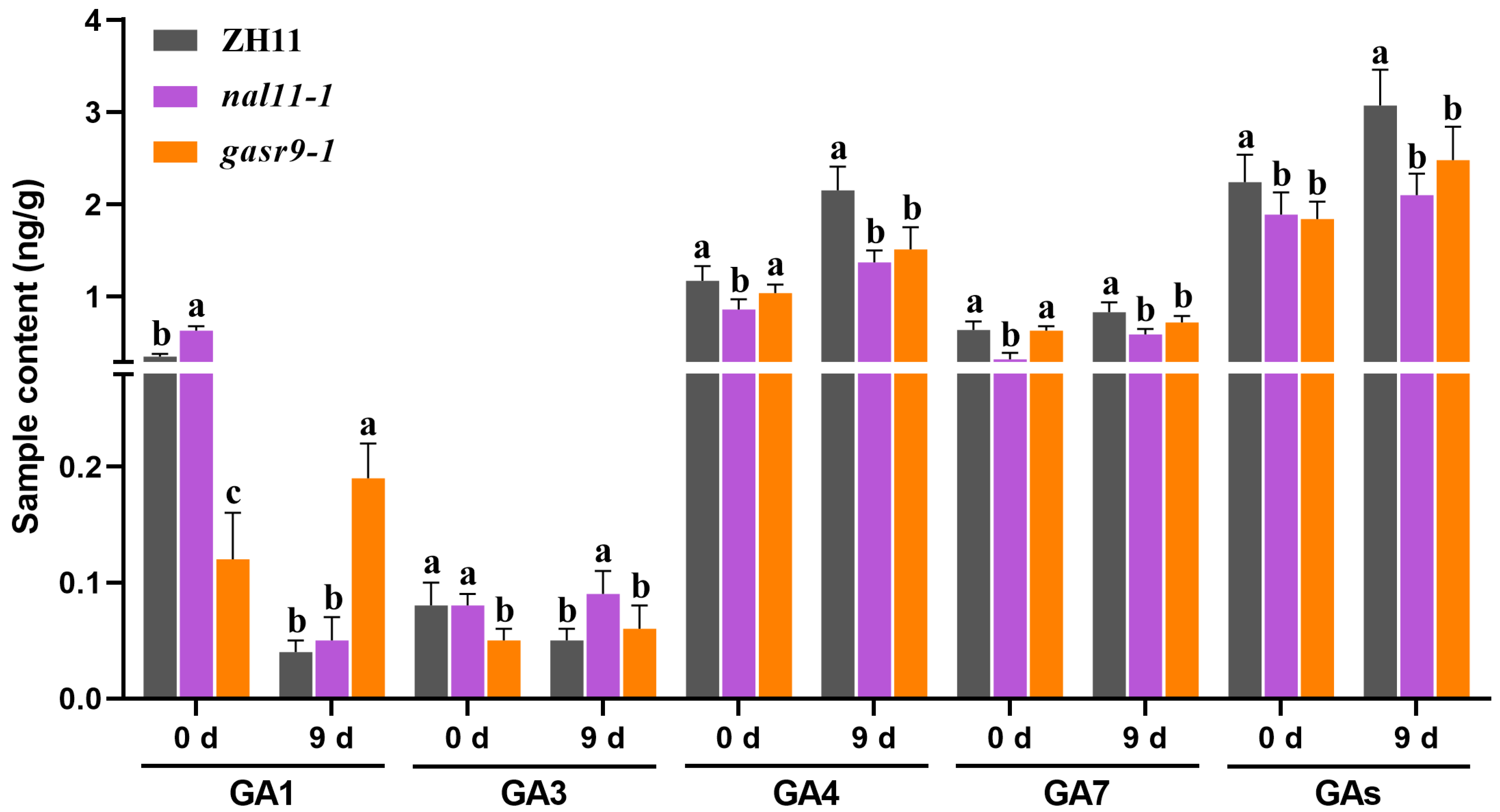
2.5. The nal11 and gasr9 Mutants Reduced the GA Levels in Seeds Germinated at Low Temperatures
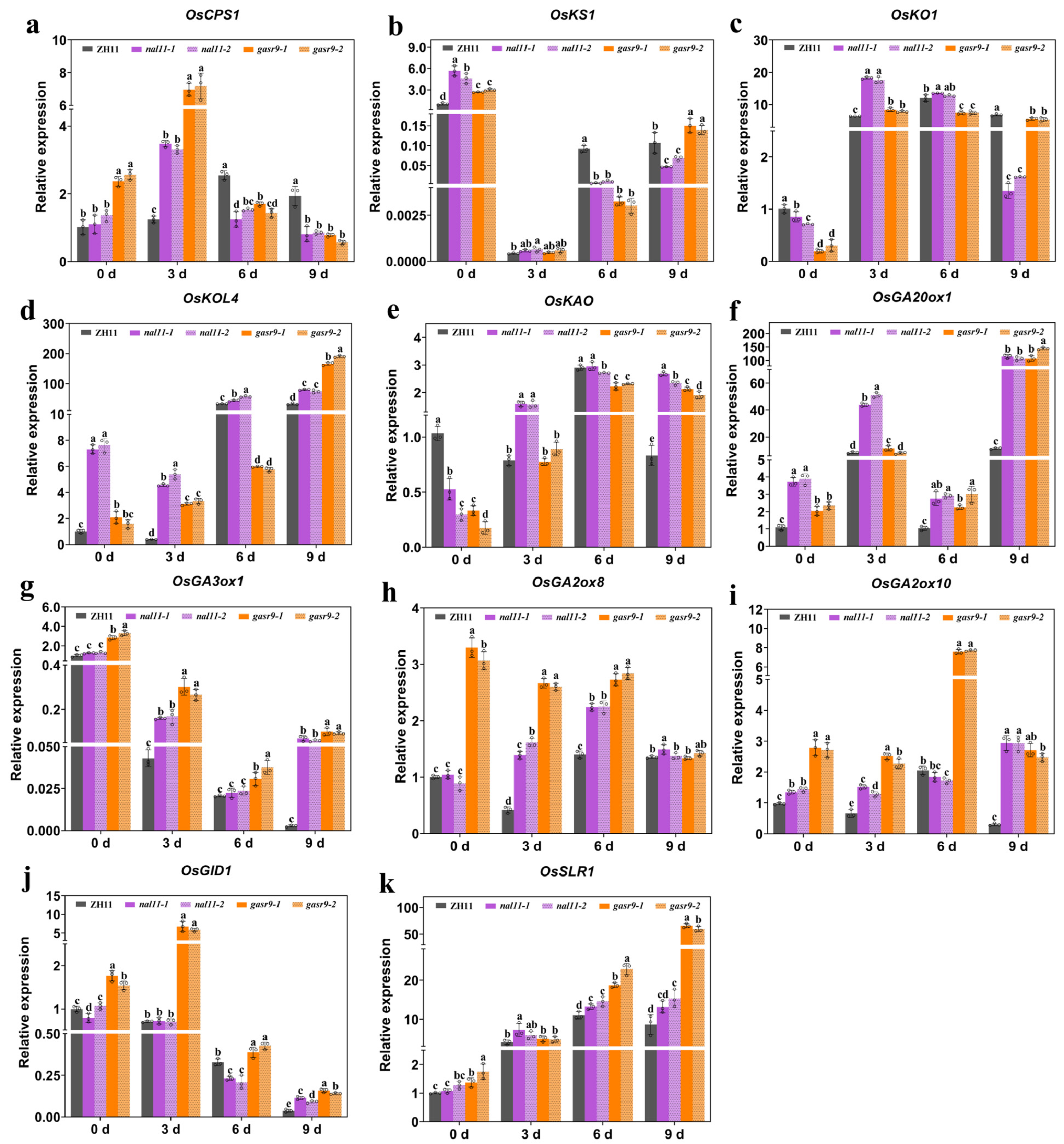
2.6. Both the OsNAL11 and OsGASR9 Genes Were Involved in Regulating the Expression of GA Pathway-Related Genes During Low-Temperature Germination
3. Discussion
4. Materials and Methods
4.1. Experimental Materials and Processing
4.2. Measurement of Physiological Indices
4.3. Gene Expression Analysis
4.4. Yeast Two-Hybrid (Y2H) Assays
4.5. Luciferase (LUC) Complementation Imaging Assays
4.6. Co-Immunoprecipitation (Co-IP) Assays
4.7. Statistical Analysis and Data Plotting
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhong, Y.; Luo, Y.; Sun, J.; Qin, X.; Gan, P.; Zhou, Z.; Qian, Y.; Zhao, R.; Zhao, Z.; Cai, W. Pan-transcriptomic analysis reveals alternative splicing control of cold tolerance in rice. Plant Cell 2024, 36, 2117–2139. [Google Scholar] [CrossRef]
- Najeeb, S.; Ali, J.; Mahender, A.; Pang, Y.L.; Zilhas, J.; Murugaiyan, V.; Vemireddy, L.R.; Li, Z. Identification of main-effect quantitative trait loci (QTLs) for low-temperature stress tolerance germination-and early seedling vigor-related traits in rice (Oryza sativa L.). Mol. Breed. 2020, 40, 10. [Google Scholar] [CrossRef]
- Xu, Q.; Huang, J.; Sun, A.; Hong, X.; Zhu, L.; Cao, X.; Kong, Y.; Jin, Q.; Zhu, C.; Zhang, J. Effects of low temperature on the growth and development of rice plants and the advance of regulation pathways: A review. Chin. J. Rice Sci. 2022, 36, 118–130. [Google Scholar] [CrossRef]
- Guo, Z.; Cai, L.; Liu, C.; Chen, Z.; Guan, S.; Ma, W.; Pan, G. Low-temperature stress affects reactive oxygen species, osmotic adjustment substances, and antioxidants in rice (Oryza sativa L.) at the reproductive stage. Sci. Rep. 2022, 12, 6224. [Google Scholar] [CrossRef]
- Sugimura, Y.; Oikawa, K.; Sugihara, Y.; Utsushi, H.; Kanzaki, E.; Ito, K.; Ogasawara, Y.; Fujioka, T.; Takagi, H.; Shimizu, M. Impact of rice GENERAL REGULATORY FACTOR14h (GF14h) on low-temperature seed germination and its application to breeding. PLoS Genet. 2024, 20, e1011369. [Google Scholar] [CrossRef]
- Fujino, K.; Obara, M.; Sato, K. Diversification of the plant-specific hybrid glycine-rich protein (HyGRP) genes in cereals. Front. Plant Sci. 2014, 5, 489. [Google Scholar] [CrossRef]
- Wang, X.; Zou, B.; Shao, Q.; Cui, Y.; Lu, S.; Zhang, Y.; Huang, Q.; Huang, J.; Hua, J. Natural variation reveals that OsSAP16 controls low-temperature germination in rice. J. Exp. Bot. 2018, 69, 413–421. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, G.; Cui, Z.; Kong, X.; Yu, X.; Gui, R.; Han, Y.; Li, Z.; Lang, H.; Hua, Y. Regain flood adaptation in rice through a 14-3-3 protein OsGF14h. Nat. Commun. 2022, 13, 5664. [Google Scholar] [CrossRef]
- Yoshida, H.; Hirano, K.; Yano, K.; Wang, F.; Mori, M.; Kawamura, M.; Koketsu, E.; Hattori, M.; Ordonio, R.L.; Huang, P. Genome-wide association study identifies a gene responsible for temperature-dependent rice germination. Nat. Commun. 2022, 13, 5665. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Tian, X.; Lin, X.; Han, Y.; Han, Z.; Sha, H.; Liu, J.; Liu, J.; Zhang, J.; et al. A transposon insertion in the promoter of OsUBC12 enhances cold tolerance during japonica rice germination. Nat. Commun. 2024, 15, 2211. [Google Scholar] [CrossRef]
- Gong, D.; He, F.; Liu, J.; Zhang, C.; Wang, Y.; Tian, S.; Sun, C.; Zhang, X. Understanding of hormonal regulation in rice seed germination. Life 2022, 12, 1021. [Google Scholar] [CrossRef]
- Yang, B.; Chen, M.; Zhan, C.; Liu, K.; Cheng, Y.; Xie, T.; Zhu, P.; He, Y.; Zeng, P.; Tang, H. Identification of OsPK5 involved in rice glycolytic metabolism and GA/ABA balance for improving seed germination via genome-wide association study. J. Exp. Bot. 2022, 73, 3446–3461. [Google Scholar] [CrossRef]
- Xia, J.; Hao, X.; Wang, T.; Li, H.; Shi, X.; Liu, Y.; Luo, H. Seed priming with gibberellin regulates the germination of cotton seeds under low-temperature conditions. J. Plant Growth Regul. 2023, 42, 319–334. [Google Scholar] [CrossRef]
- Kim, S.; Huh, S.M.; Han, H.J.; Lee, G.S.; Hwang, Y.; Cho, M.H.; Kim, B.; Song, J.S.; Chung, J.H.; Nam, M.H. A rice seed-specific glycine-rich protein OsDOR1 interacts with GID1 to repress GA signaling and regulates seed dormancy. Plant Mol. Biol. 2023, 111, 523–539. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, Y.; Hu, G.; Wang, X.; Chen, H.; Shi, Q.; Xiang, J.; Zhang, Y.; Zhu, D.; Zhang, Y. Reduced bioactive gibberellin content in rice seeds under low temperature leads to decreased sugar consumption and low seed germination rates. Plant Physiol. Bioch 2018, 133, 1–10. [Google Scholar] [CrossRef]
- Luo, L.; Xie, Y.; Yu, S.; Yang, J.; Chen, S.; Yuan, X.; Guo, T.; Wang, H.; Liu, Y.; Chen, C. The DnaJ domain-containing heat-shock protein NAL11 determines plant architecture by mediating gibberellin homeostasis in rice (Oryza sativa). New Phytol. 2023, 237, 2163–2179. [Google Scholar] [CrossRef]
- Li, X.; Shi, S.; Tao, Q.; Tao, Y.; Miao, J.; Peng, X.; Li, C.; Yang, Z.; Zhou, Y.; Liang, G. OsGASR9 positively regulates grain size and yield in rice (Oryza sativa). Plant Sci. 2019, 286, 17–27. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, C.; Zhong, Y.; Xing, M.; Li, B.; Yang, Y.; Yuan, X.; Wen, Y.; Shu, C.; Yang, Z. Relationship between seed germination physiological characteristics and germination percentages of direct-seeded hybrid Indica rice under low-temperature and anaerobic interaction. Seed Sci. Technol. 2022, 50, 241–256. [Google Scholar] [CrossRef]
- Yang, M.; Yang, J.; Su, L.; Sun, K.; Li, D.; Liu, Y.; Wang, H.; Chen, Z.; Guo, T. Metabolic profile analysis and identification of key metabolites during rice seed germination under low-temperature stress. Plant Sci. 2019, 289, 110282. [Google Scholar] [CrossRef]
- Nair, A.U.; Bhukya, D.P.N.; Sunkar, R.; Chavali, S.; Allu, A.D. Molecular basis of priming-induced acquired tolerance to multiple abiotic stresses in plants. J. Exp. Bot. 2022, 73, 3355–3371. [Google Scholar] [CrossRef]
- Lin, S.; Liu, Z.; Sun, S.; Xue, F.; Li, H.; Tursun, A.; Cao, L.; Zhang, L.; Wilson, Z.A.; Zhang, D. Rice HEAT SHOCK PROTEIN60-3B maintains male fertility under high temperature by starch granule biogenesis. Plant Physiol. 2023, 192, 2301–2317. [Google Scholar] [CrossRef]
- Ding, F.; Li, F.; Zhang, B. A plastid-targeted heat shock cognate 70-kDa protein confers osmotic stress tolerance by enhancing ROS scavenging capability. Front. Plant Sci. 2022, 13, 1012145. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X. Overexpression of GASA5 increases the sensitivity of Arabidopsis to heat stress. J. Plant Physiol. 2011, 168, 2093–2101. [Google Scholar] [CrossRef]
- Sun, S.; Wang, H.; Yu, H.; Zhong, C.; Zhang, X.; Peng, J.; Wang, X. GASA14 regulates leaf expansion and abiotic stress resistance by modulating reactive oxygen species accumulation. J. Exp. Bot. 2013, 64, 1637–1647. [Google Scholar] [CrossRef]
- Bouteraa, M.T.; Romdhane, W.B.; Hsouna, A.B.; Amor, F.; Ebel, C.; Saad, R.B. Genome-wide characterization and expression profiling of GASA gene family in Triticum turgidum ssp. durum (desf.) husn. (Durum wheat) unveils its involvement in environmental stress responses. Phytochemistry 2023, 206, 113544. [Google Scholar] [CrossRef]
- Kretzschmar, T.; Pelayo, M.A.F.; Trijatmiko, K.R.; Gabunada, L.F.M.; Alam, R.; Jimenez, R.; Mendioro, M.S.; Slamet-Loedin, I.H.; Sreenivasulu, N.; Bailey-Serres, J. A trehalose-6-phosphate phosphatase enhances anaerobic germination tolerance in rice. Nat. Plants 2015, 1, 15124. [Google Scholar] [CrossRef]
- Trong, L.V.; Thinh, B.B.; Chinh, H.V.; Giang, T.V. Effects of Low Temperature and Potassium Chloride on Physiological and Biochemical Indices of Rice (Oryza sativa L.) at the Seedling Stage. J. Agric. Sci. Tech-Iran. 2022, 24, 847–859. [Google Scholar]
- Yu, H.; Teng, Z.; Liu, B.; Lv, J.; Chen, Y.; Qin, Z.; Peng, Y.; Meng, S.; He, Y.; Duan, M. Transcription factor OsMYB30 increases trehalose content to inhibit α-Amylase and seed germination at low temperature. Plant Physiol. 2024, 194, 1815–1833. [Google Scholar] [CrossRef]
- Nie, L.; Song, S.; Yin, Q.; Zhao, T.; Liu, H.; He, A.; Wang, W. Enhancement in seed priming-induced starch degradation of rice seed under chilling stress via GA-mediated α-Amylase expression. Rice 2022, 15, 19. [Google Scholar] [CrossRef]
- Ravindran, P.; Kumar, P.P. Regulation of seed germination: The involvement of multiple forces exerted via gibberellic acid signaling. Mol. Plant 2019, 12, 24–26. [Google Scholar] [CrossRef]
- Tuan, P.A.; Kumar, R.; Rehal, P.K.; Toora, P.K.; Ayele, B.T. Molecular mechanisms underlying abscisic acid/gibberellin balance in the control of seed dormancy and germination in cereals. Front. Plant Sci. 2018, 9, 668. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, A. Comparative studies on seed germination of two rice genotypes with different tolerances to low temperature. Environ. Exp. Bot. 2020, 179, 104216. [Google Scholar] [CrossRef]
- Kim, N.; Jan, R.; Park, J.; Asif, S.; Zhao, D.; Kim, E.; Jang, Y.; Eom, G.; Lee, G.; Kim, K. QTL mapping and candidate gene analysis for seed germination response to low temperature in Rice. Int. J. Mol. Sci. 2022, 23, 7379. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Dong, S.; Beckles, D.M.; Miao, H.; Sun, J.; Liu, X.; Wang, W.; Zhang, S.; Gu, X. The qLTG1.1 candidate gene CsGAI regulates low temperature seed germination in cucumber. Theor. Appl. Genet. 2022, 135, 2593–2607. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Mao, Q.; Hong, J.; Shi, L.; Ran, X.; Liaquat, F.; Uzair, M.; Liang, W.; Fernie, A.R.; Shi, J. HSP70-16 and VDAC3 jointly inhibit seed germination under cold stress in Arabidopsis. Plant Cell Environ. 2021, 44, 3616–3627. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Jiang, H.; Du, J.; Shi, L.; Bin, Q.; Yang, X.; Wang, L. Variations in physiological response and expression profiles of proline metabolism-related genes and heat shock transcription factor genes in petunia subjected to heat stress. Sci. Hortic. 2019, 258, 108811. [Google Scholar] [CrossRef]
- Rajan, V.B.V.; D’Silva, P. Arabidopsis thaliana J-class heat shock proteins: Cellular stress sensors. Funct. Integr. Genomic 2009, 9, 433–446. [Google Scholar] [CrossRef]
- Page, D.; Gouble, B.; Valot, B.; Bouchet, J.; Callot, C.; Kretzschmar, A.; Causse, M.; Renard, C.; Faurobert, M. Protective proteins are differentially expressed in tomato genotypes differing for their tolerance to low-temperature storage. Planta 2010, 232, 483–500. [Google Scholar] [CrossRef]
- Ukaji, N.; Kuwabara, C.; Kanno, Y.; Seo, M.; Takezawa, D.; Arakawa, K.; Fujikawa, S. Endoplasmic reticulum-localized small heat shock protein that accumulates in mulberry tree (Morus bombycis Koidz.) during seasonal cold acclimation is responsive to abscisic acid. Tree Physiol. 2010, 30, 502–513. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, C.; Peng, J.; Sun, S.; Wang, X. GASA5, a regulator of flowering time and stem growth in Arabidopsis thaliana. Plant Mol. Biol. 2009, 69, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Rubinovich, L.; Ruthstein, S.; Weiss, D. The Arabidopsis cysteine-rich GASA5 is a redox-active metalloprotein that suppresses gibberellin responses. Mol. Plant 2014, 7, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Rubinovich, L.; Weiss, D. The Arabidopsis cysteine-rich protein GASA4 promotes GA responses and exhibits redox activity in bacteria and in planta. Plant J. 2010, 64, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Xu, H.; Ye, S.; Wang, S.; Li, L.; Zhang, S.; Wang, X. Gibberellic acid-stimulated Arabidopsis6 serves as an integrator of gibberellin, abscisic acid, and glucose signaling during seed germination in Arabidopsis. Plant Physiol. 2015, 169, 2288–2303. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Han, S.; Kim, S. Salt-and ABA-inducible OsGASR1 is involved in salt tolerance. J. Plant Biol. 2015, 58, 96–101. [Google Scholar] [CrossRef]
- Yang, Q.; Niu, Q.; Tang, Y.; Ma, Y.; Yan, X.; Li, J.; Tian, J.; Bai, S.; Teng, Y. PpyGAST1 is potentially involved in bud dormancy release by integrating the GA biosynthesis and ABA signaling in ‘Suli’pear (Pyrus pyrifolia White Pear Group). Environ. Exp. Bot. 2019, 162, 302–312. [Google Scholar] [CrossRef]
- Sakata, T.; Oda, S.; Tsunaga, Y.; Shomura, H.; Kawagishi-Kobayashi, M.; Aya, K.; Saeki, K.; Endo, T.; Nagano, K.; Kojima, M. Reduction of gibberellin by low temperature disrupts pollen development in rice. Plant Physiol. 2014, 164, 2011–2019. [Google Scholar] [CrossRef]
- Sarkar, N.K.; Thapar, U.; Kundnani, P.; Panwar, P.; Grover, A. Functional relevance of J-protein family of rice (Oryza sativa). Cell Stress. Chaperones 2013, 18, 321–331. [Google Scholar] [CrossRef]
- Bouteraa, M.T.; Ben Romdhane, W.; Baazaoui, N.; Alfaifi, M.Y.; Chouaibi, Y.; Ben Akacha, B.; Ben Hsouna, A.; Kačániová, M.; Ćavar Zeljković, S.; Garzoli, S. GASA proteins: Review of their functions in plant environmental stress tolerance. Plants 2023, 12, 2045. [Google Scholar] [CrossRef]
- Peng, X.; Luo, L.; Cui, H.; Wang, H.; Guo, T.; Liu, Y.; Wang, J.; Huang, M.; Yang, G.; Chen, Z. Characterization and fine mapping of a leaf wilt mutant, m3, induced by heavy ion irradiation of rice. Crop Sci. 2019, 59, 2679–2688. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Li, F.; Wang, H.; Yao, Y.; Shu, J.; Ying, M. Cryptotanshinone protects against adriamycin-induced mitochondrial dysfunction in cardiomyocytes. Pharm. Biol. 2016, 54, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Fu, J.X.; Li, J.; Cheng, X.L.; Li, F.; Dong, J.F.; Liu, Z.L.; Zhuang, C.X. A novel co-immunoprecipitation protocol based on protoplast transient gene expression for studying protein-protein interactions in rice. Plant Mol. Biol. Rep. 2014, 32, 153–161. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Yuan, X.; Tian, M.; Chen, J.; Chen, C.; Luo, Z.; Guo, T.; Huo, X.; Xiao, W. OsNAL11 and OsGASR9 Regulate the Low-Temperature Germination of Rice Seeds by Affecting GA Content. Int. J. Mol. Sci. 2024, 25, 11291. https://doi.org/10.3390/ijms252011291
Liu J, Yuan X, Tian M, Chen J, Chen C, Luo Z, Guo T, Huo X, Xiao W. OsNAL11 and OsGASR9 Regulate the Low-Temperature Germination of Rice Seeds by Affecting GA Content. International Journal of Molecular Sciences. 2024; 25(20):11291. https://doi.org/10.3390/ijms252011291
Chicago/Turabian StyleLiu, Jinzhao, Xi Yuan, Mengqing Tian, Jialing Chen, Chun Chen, Zengtong Luo, Tao Guo, Xing Huo, and Wuming Xiao. 2024. "OsNAL11 and OsGASR9 Regulate the Low-Temperature Germination of Rice Seeds by Affecting GA Content" International Journal of Molecular Sciences 25, no. 20: 11291. https://doi.org/10.3390/ijms252011291
APA StyleLiu, J., Yuan, X., Tian, M., Chen, J., Chen, C., Luo, Z., Guo, T., Huo, X., & Xiao, W. (2024). OsNAL11 and OsGASR9 Regulate the Low-Temperature Germination of Rice Seeds by Affecting GA Content. International Journal of Molecular Sciences, 25(20), 11291. https://doi.org/10.3390/ijms252011291





