Identification of Genetic Variants Associated with Hereditary Thoracic Aortic Diseases (HTADs) Using Next Generation Sequencing (NGS) Technology and Genotype–Phenotype Correlations
Abstract
1. Introduction
1.1. Epidemiology
1.2. Etiology of HTAD
Genetic Variants Associated with HTADs
1.3. Clinical Manifestations of Syndromic HTADs
1.4. Genetic Counseling
2. Results
3. Discussion
3.1. Genotype–Phenotype Correlations in Marfan Syndrome
3.2. Are Genetic Factors the Cause of Faster Aortic Dilatation? The Importance of Genetic Testing and Genetic Counseling in Syndromic Forms of HTADs
3.3. Genomic Insights into HTADs
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| HTAD | Hereditary thoracic aorta disease |
| TAA | Thoracic aortic aneurysm |
| TAD | Thoracic aortic dissection |
| CTA | Computed tomographic angiography |
| MRA | Magnetic resonance angiography |
| WES | Whole exome sequencing |
| MLPA | Multiplex ligation-dependent probe amplification |
| WGS | Whole genome sequencing |
| AA | Aortic aneurysms |
| AAD | Acute aortic dissection |
| AAA | Abdominal aortic aneurysm |
| AD | Autosomal dominant |
| VUS | Variant of unknown significance |
| VSMCs | Vascular smooth muscle cells |
| LOF | Loss-of-function |
| LP | Likely pathogenic |
| FBN1 | Fibrillin 1 |
| TGFBR2 | Transforming growth factor-beta receptor, type II |
| SLC2A10 | Solute carrier family 2 (facilitated glucose transporter), member 10 |
| ACGM | American College of Medical Genetics and Genomics |
References
- De Backer, J.; Bondue, A.; Budts, W.; Evangelista, A.; Gallego, P.; Jondeau, G.; Loeys, B.; Peña, M.L.; Teixido-Tura, G.; van de Laar, I.; et al. Genetic counselling and testing in adults with congenital heart disease: A consensus document of the ESC Working Group of Grown-Up Congenital Heart Disease, the ESC Working Group on Aorta and Peripheral Vascular Disease and the European Society of Human Genetics. Eur. J. Prev. Cardiol. 2020, 27, 1423–1435. [Google Scholar]
- Duarte, V.E.; Yousefzai, R.; Singh, M.N. Genetically Triggered Thoracic Aortic Disease: Who Should be Tested? Methodist Debakey Cardiovasc. J. 2023, 19, 24–28. [Google Scholar] [CrossRef]
- Waldmüller, S.; Müller, M.; Warnecke, H.; Rees, W.; Schöls, W.; Walterbusch, G.; Ennker, J.; Scheffold, T. Genetic testing in patients with aortic aneurysms/dissections: A novel genotype/phenotype correlation? Eur. J. Cardiothorac. Surg. 2007, 31, 970–975. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Hamilton Black, J., 3rd; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, e334–e482. [Google Scholar] [CrossRef]
- Levy, D.; Goyal, A.; Grigorova, Y.; Levy, D.; Farci, F.; Le, J.K. Aortic Dissection. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024; Updated 23 April 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441963/ (accessed on 26 June 2024).
- Fletcher, A.J.; Syed, M.B.J.; Aitman, T.J.; Newby, D.E.; Walker, N.L. Inherited Thoracic Aortic Disease: New Insights and Translational Targets. Circulation 2020, 141, 1570–1587. [Google Scholar] [CrossRef]
- Chou, E.; Pirruccello, J.P.; Ellinor, P.T.; Lindsay, M.E. Genetics and mechanisms of thoracic aortic disease. Nat. Rev. Cardiol. 2023, 20, 168–180. [Google Scholar] [CrossRef]
- Luan, J.; Mao, L.; Zhu, Z.; Fu, W.; Zhu, T. New indicators for systematic assessment of aortic morphology: A narrative review. J. Thorac. Dis. 2021, 13, 372–383. [Google Scholar] [CrossRef]
- Zhou, Z.; Cecchi, A.C.; Prakash, S.K.; Milewicz, D.M. Risk Factors for Thoracic Aortic Dissection. Genes 2022, 13, 1814. [Google Scholar] [CrossRef]
- Monda, E.; Lioncino, M.; Verrillo, F.; Rubino, M.; Caiazza, M.; Mauriello, A.; Guarnaccia, N.; Fusco, A.; Cirillo, A.; Covino, S.; et al. The Role of Genetic Testing in Patients with Heritable Thoracic Aortic Diseases. Diagnostics 2023, 13, 772. [Google Scholar] [CrossRef]
- Acharya, M.; Maselli, D.; Mariscalco, G. Genetic screening in heritable thoracic aortic disease-rationale, potentials and pitfalls. Indian J. Thorac. Cardiovasc. Surg. 2022, 38 (Suppl. S1), 24–35. [Google Scholar] [CrossRef]
- Online Mendelian Inheritance in Man—OMIM. Available online: https://www.omim.org/ (accessed on 23 July 2024).
- Renard, M.; Francis, C.; Ghosh, R.; Scott, A.F.; Witmer, P.D.; Ades, L.C.; Andelfinger, G.U.; Arnaud, P.; Boileau, C.; Callewaert, B.L.; et al. Clinical Validity of Genes for Heritable Thoracic Aortic Aneurysm and Dissection. J. Am. Coll. Cardiol. 2018, 72, 605–615. [Google Scholar] [CrossRef]
- Pinard, A.; Jones, G.T.; Milewicz, D.M. Genetics of Thoracic and Abdominal Aortic Diseases. Circ. Res. 2019, 124, 588–606. [Google Scholar] [CrossRef]
- Milewicz, D.M.; Guo, D.; Hostetler, E.; Marin, I.; Pinard, A.C.; Cecchi, A.C. Update on the genetic risk for thoracic aortic aneurysms and acute aortic dissections: Implications for clinical care. J. Cardiovasc. Surg. 2021, 62, 203–210. [Google Scholar] [CrossRef]
- Nakashima, Y. Pathogenesis of aortic dissection: Elastic fiber abnormalities and aortic medial weakness. Ann. Vasc. Dis. 2010, 3, 28–36. [Google Scholar] [CrossRef]
- Michel, J.-B.; Jondeau, G.; Milewicz, D.M. From Genetics to Response to Injury: Vascular Smooth Muscle Cells in Aneurysms and Dissections of the Ascending Aorta. Cardiovasc. Res. 2018, 114, 578–589. [Google Scholar] [CrossRef]
- De Cario, R.; Giannini, M.; Cassioli, G.; Kura, A.; Gori, A.M.; Marcucci, R.; Nistri, S.; Pepe, G.; Giusti, B.; Sticchi, E. Tracking an Elusive Killer: State of the Art of Molecular-Genetic Knowledge and Laboratory Role in Diagnosis and Risk Stratification of Thoracic Aortic Aneurysm and Dissection. Diagnostics 2022, 12, 1785. [Google Scholar] [CrossRef]
- Daugherty, A.; Chen, Z.; Sawada, H.; Rateri, D.L.; Sheppard, M.B. Transforming Growth Factor-β in Thoracic Aortic Aneurysms: Good, Bad, or Irrelevant? J. Am. Heart Assoc. 2017, 6, e005221. [Google Scholar] [CrossRef]
- MacFarlane, E.G.; Parker, S.J.; Shin, J.Y.; Ziegler, S.G.; Creamer, T.J.; Bagirzadeh, R.; Bedja, D.; Chen, Y.; Calderon, J.F.; Weissler, K.; et al. Lineage-Specific Events Underlie Aortic Root Aneurysm Pathogenesis in Loeys-Dietz Syndrome. J. Clin. Investig. 2019, 129, 659–675. [Google Scholar] [CrossRef]
- Dietz, H. FBN1-Related Marfan Syndrome. In GeneReviews® 1993–2024; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2001; Updated 17 February 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1335/ (accessed on 29 June 2024).
- Papatheodorou, E.; Degiannis, D.; Anastasakis, A. Genetics of Heritable Thoracic Aortic Disease. Cardiogenetics 2022, 12, 63–79. [Google Scholar] [CrossRef]
- Takeda, N.; Inuzuka, R.; Maemura, S.; Morita, H.; Nawata, K.; Fujita, D.; Taniguchi, Y.; Yamauchi, H.; Yagi, H.; Kato, M.; et al. Impact of Pathogenic FBN1 Variant Types on the Progression of Aortic Disease in Patients with Marfan Syndrome. Circ. Genom. Precis. Med. 2018, 11, e002058. [Google Scholar]
- Franken, R.; den Hartog, A.W.; Radonic, T.; Micha, D.; Maugeri, A.; van Dijk, F.S.; Meijers-Heijboer, H.E.; Timmermans, J.; Scholte, A.J.; van den Berg, M.P.; et al. Beneficial Outcome of Losartan Therapy Depends on Type of FBN1 Mutation in Marfan Syndrome. Circ. Cardiovasc. Genet. 2015, 8, 383–388. [Google Scholar] [CrossRef]
- Arnaud, P.; Milleron, O.; Hanna, N.; Ropers, J.; Ould Ouali, N.; Affoune, A.; Langeois, M.; Eliahou, L.; Arnoult, F.; Renard, P.; et al. Clinical relevance of genotype–phenotype correlations beyond vascular events in a cohort study of 1500 Marfan syndrome patients with FBN1 pathogenic variants. Genet. Med. 2021, 23, 1296. [Google Scholar] [CrossRef]
- De Backer, J.; Campens, L.; Muiño Mosquera, L. Looking for the Missing Links: Challenges in the Search for Genotype-Phenotype Correlation in Marfan Syndrome. Circ. Genom. Precis. Med. 2018, 11, e002185. [Google Scholar] [CrossRef]
- Baudhuin, L.M.; Kotzer, K.E.; Lagerstedt, S.A. Increased frequency of FBN1 truncating and splicing variants in Marfan syndrome patients with aortic events. Genet. Med. 2015, 17, 177–187. [Google Scholar] [CrossRef]
- Loeys, B.L.; Dietz, H.C. Loeys-Dietz Syndrome. In GeneReviews®, 1993–2024; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2008; Updated 1 March 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1133/ (accessed on 29 June 2024).
- Byers, P.H. Vascular Ehlers-Danlos Syndrome. In GeneReviews®, 1993–2024; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1999; Updated 21 February 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1494/ (accessed on 29 June 2024).
- Pepin, M.G.; Schwarze, U.; Rice, K.M.; Liu, M.; Leistritz, D.; Byers, P.H. Survival is affected by mutation type and molecular mechanism in vascular Ehlers-Danlos syndrome (EDS type IV). Genet. Med. 2014, 16, 881–888. [Google Scholar] [CrossRef]
- Frank, M.; Albuisson, J.; Ranque, B.; Golmard, L.; Mazzella, J.M.; Bal-Theoleyre, L.; Fauret, A.L.; Mirault, T.; Denarié, N.; Mousseaux, E.; et al. The type of variants at the COL3A1 gene associates with the phenotype and severity of vascular Ehlers-Danlos syndrome. Eur. J. Hum. Genet. 2015, 23, 1657–1664. [Google Scholar] [CrossRef]
- Callewaert, B.; De Paepe, A.; Coucke, P. Arterial Tortuosity Syndrome. In GeneReviews®, 1993–2024; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2014; Updated 23 February 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK253404/ (accessed on 29 June 2024).
- Salameh, M.J.; Black, J.H.; Ratchford, E.V. Thoracic Aortic Aneurysm. Vasc. Med. 2018, 23, 573–578. [Google Scholar] [CrossRef]
- Cecconi, M.; Manfrin, M.; Moraca, A.; Zanoli, R.; Colonna, P.L.; Bettuzzi, M.G.; Moretti, S.; Gabrielli, D.; Perna, G.P. Aortic Dimensions in Patients with Bicuspid Aortic Valve without Significant Valve Dysfunction. Am. J. Cardiol. 2005, 95, 292–294. [Google Scholar] [CrossRef]
- Bravo-Jaimes, K.M.; Prakash, S.K. Genetics in bicuspid aortic valve disease: Where are we? Prog. Cardiovasc. Dis. 2020, 63, 398–406. [Google Scholar] [CrossRef]
- Sticchi, E.; De Cario, R.; Magi, A.; Giglio, S.; Provenzano, A.; Nistri, S.; Pepe, G.; Giusti, B. Bicuspid Aortic Valve: Role of Multiple Gene Variants in Influencing the Clinical Phenotype. Biomed Res. Int. 2018, 2018, 8386123. [Google Scholar] [CrossRef]
- Pepe, G.; Nistri, S.; Giusti, B.; Sticchi, E.; Attanasio, M.; Porciani, C.; Abbate, R.; Bonow, R.O.; Yacoub, M.; Gensini, G.F. Identification of Fibrillin 1 Gene Mutations in Patients with Bicuspid Aortic Valve (BAV) without Marfan Syndrome. BMC Med. Genet. 2014, 15, 23. [Google Scholar] [CrossRef]
- Boyum, J.; Fellinger, E.K.; Schmoker, J.D.; Trombley, L.; McPartland, K.; Ittleman, F.P.; Howard, A.B. Matrix Metalloproteinase Activity in Thoracic Aortic Aneurysms Associated with Bicuspid and Tricuspid Aortic Valves. J. Thorac. Cardiovasc. Surg. 2004, 127, 686–691. [Google Scholar] [CrossRef]
- Rabkin, S.W. Differential Expression of MMP-2, MMP-9 and TIMP Proteins in Thoracic Aortic Aneurysm—Comparison with and without Bicuspid Aortic Valve: A Meta-Analysis. Vasa 2014, 43, 433–442. [Google Scholar] [CrossRef]
- Li, T.; Jiang, B.; Li, X.; Sun, H.; Li, X.; Jing, J.; Yang, J. Serum Matrix Metalloproteinase-9 Is a Valuable Biomarker for Identification of Abdominal and Thoracic Aortic Aneurysm: A Case-Control Study. BMC Cardiovasc. Disord. 2018, 18, 202. [Google Scholar] [CrossRef]
- Wilton, E.; Bland, M.; Thompson, M.; Jahangiri, M. Matrix Metalloproteinase Expression in the Ascending Aorta and Aortic Valve. Interact. Cardiovasc. Thorac. Surg. 2008, 7, 37–40. [Google Scholar] [CrossRef]
- Rodrigues Bento, J.; Meester, J.; Luyckx, I.; Peeters, S.; Verstraeten, A.; Loeys, B. The Genetics and Typical Traits of Thoracic Aortic Aneurysm and Dissection. Annu. Rev. Genom. Hum. Genet. 2022, 23, 223–253. [Google Scholar] [CrossRef]
- The UMD-FBN1 Mutations Database. Available online: http://www.umd.be/FBN1/ (accessed on 23 July 2024).
- Sakai, L.Y.; Keene, D.R.; Renard, M.; De Backer, J. FBN1: The disease-causing gene for Marfan syndrome and other genetic disorders. Gene 2016, 591, 279–291. [Google Scholar] [CrossRef]
- Collod-Béroud, G.; Le Bourdelles, S.; Ades, L.; Ala-Kokko, L.; Booms, P.; Boxer, M.; Child, A.; Comeglio, P.; De Paepe, A.; Hyland, J.C.; et al. Update of the UMD-FBN1 mutation database and creation of an FBN1 polymorphism database. Hum. Mutat. 2003, 22, 199–208. [Google Scholar] [CrossRef]
- Murdoch, J.L.; Walker, B.A.; McKusick, V.A. Parental age effects on the occurrence of new mutations for the Marfan syndrome. Ann. Hum. Genet. 1972, 35, 331–336. [Google Scholar] [CrossRef]
- Du, Q.; Zhang, D.; Zhuang, Y.; Xia, Q.; Wen, T.; Jia, H. The Molecular Genetics of Marfan Syndrome. Int. J. Med. Sci. 2021, 18, 2752–2766. [Google Scholar] [CrossRef]
- Stengl, R.; Bors, A.; Ágg, B.; Pólos, M.; Matyas, G.; Molnár, M.J.; Fekete, B.; Csabán, D.; Andrikovics, H.; Merkely, B.; et al. Optimising the mutation screening strategy in Marfan syndrome and identifying genotypes with more severe aortic involvement. Orphanet J. Rare Dis. 2020, 15, 290. [Google Scholar] [CrossRef]
- Dietz, H.C.; Pyeritz, R.E.; Puffenberger, E.G.; Kendzior, R.J., Jr.; Corson, G.M.; Maslen, C.L.; Sakai, L.Y.; Francomano, C.A.; Cutting, G.R. Marfan phenotype variability in a family segregating a missense mutation in the epidermal growth factor-like motif of the fibrillin gene. J. Clin. Investig. 1992, 89, 1674–1680. [Google Scholar] [CrossRef]
- Schrijver, I.; Liu, W.; Brenn, T.; Furthmayr, H.; Francke, U. Cysteine substitutions in epidermal growth factor-like domains of fibrillin-1: Distinct effects on biochemical and clinical phenotypes. Am. J. Hum. Genet. 1999, 65, 1007–1020. [Google Scholar] [CrossRef]
- Wang, J.J.; Yu, B.; Sun, Y.; Song, X.; Wang, D.W.; Li, Z. FBN1 Splice-Altering Mutations in Marfan Syndrome: A Case Report and Literature Review. Genes 2022, 13, 1842. [Google Scholar] [CrossRef]
- Liu, W.; Qian, C.; Francke, U. Silent mutation induces exon skipping of fibrillin-1 gene in Marfan syndrome. Nat. Genet. 1997, 16, 328–329. [Google Scholar] [CrossRef]
- Dietz, H.C.; Valle, D.; Francomano, C.A.; Kendzior, R.J., Jr.; Pyeritz, R.E.; Cutting, G.R. The skipping of constitutive exons in vivo induced by nonsense mutations. Science 1993, 259, 680–683. [Google Scholar] [CrossRef]
- Schrijver, I.; Liu, W.; Odom, R.; Brenn, T.; Oefner, P.; Furthmayr, H.; Francke, U. Premature termination mutations in FBN1: Distinct effects on differential allelic expression and on protein and clinical phenotypes. Am. J. Hum. Genet. 2002, 71, 223–237. [Google Scholar] [CrossRef]
- Furtado, L.V.; Wooderchak-Donahue, W.; Rope, A.F.; Yetman, A.T.; Lewis, T.; Plant, P.; Bayrak-Toydemir, P. Characterization of large genomic deletions in the FBN1 gene using multiplex ligation-dependent probe amplification. BMC Med. Genet. 2011, 12, 119. [Google Scholar] [CrossRef]
- Lu, X.; Wang, R.; Li, M.; Zhang, B.; Rao, H.; Huang, X.; Chen, X.; Wu, Y. Identification of two novel large deletions in FBN1 gene by next-generation sequencing and multiplex ligation-dependent probe amplification. BMC Med. Genom. 2024, 17, 47. [Google Scholar] [CrossRef]
- Mátyás, G.; Alonso, S.; Patrignani, A.; Marti, M.; Arnold, E.; Magyar, I.; Henggeler, C.; Carrel, T.; Steinmann, B.; Berger, W. Large genomic fibrillin-1 (FBN1) gene deletions provide evidence for true haploinsufficiency in Marfan syndrome. Hum. Genet. 2007, 122, 23–32. [Google Scholar] [CrossRef]
- Tynan, K.; Comeau, K.; Pearson, M.; Wilgenbus, P.; Levitt, D.; Gasner, C.; Berg, M.A.; Miller, D.C.; Francke, U. Mutation screening of complete fibrillin-1 coding sequence: Report of five new mutations, including two in 8-cysteine domains. Hum. Mol. Genet. 1993, 2, 1813–1821. [Google Scholar] [CrossRef]
- Nijbroek, G.; Sood, S.; McIntosh, I.; Francomano, C.A.; Bull, E.; Pereira, L.; Ramirez, F.; Pyeritz, R.E.; Dietz, H.C. Fifteen novel FBN1 mutations causing Marfan syndrome detected by heteroduplex analysis of genomic amplicons. Am. J. Hum. Genet. 1995, 57, 8–21. [Google Scholar]
- Xu, S.; Li, L.; Fu, Y.; Wang, X.; Sun, H.; Wang, J.; Han, L.; Wu, Z.; Liu, Y.; Zhu, J.; et al. Increased frequency of FBN1 frameshift and nonsense mutations in Marfan syndrome patients with aortic dissection. Mol. Genet. Genom. Med. 2020, 8, e1041. [Google Scholar] [CrossRef]
- Yang, H.; Ma, Y.; Luo, M.; Zhao, K.; Zhang, Y.; Zhu, G.; Sun, X.; Luo, F.; Wang, L.; Shu, C.; et al. Identification of gross deletions in FBN1 gene by MLPA. Hum. Genom. 2018, 12, 46. [Google Scholar] [CrossRef]
- Buki, G.; Szalai, R.; Pinter, A.; Hadzsiev, K.; Melegh, B.; Rauch, T.; Bene, J. Correlation between large FBN1 deletions and severe cardiovascular phenotype in Marfan syndrome: Analysis of two novel cases and analytical review of the literature. Mol. Genet. Genom. Med. 2023, 11, e2166. [Google Scholar] [CrossRef]
- Singh, K.K.; Elligsen, D.; Liersch, R.; Schubert, S.; Pabst, B.; Arslan-Kirchner, M.; Schmidtke, J. Multi-exon out of frame deletion of the FBN1 gene leading to a severe juvenile onset cardiovascular phenotype in Marfan syndrome. J. Mol. Cell. Cardiol. 2007, 42, 352–356. [Google Scholar] [CrossRef]
- Chen, Z.X.; Jia, W.N.; Jiang, Y.X. Genotype-phenotype correlations of marfan syndrome and related fibrillinopathies: Phenomenon and molecular relevance. Front. Genet. 2022, 13, 943083. [Google Scholar] [CrossRef]
- Zarate, Y.A.; Morris, S.A.; Blackshare, A.; Algaze, C.A.; Connor, B.S.; Kim, A.J.; Yutzey, K.E.; Miller, E.M.; Weaver, K.N.; Collins, R.T., 2nd. A clinical scoring system for early onset (neonatal) Marfan syndrome. Genet. Med. 2022, 24, 1503–1511. [Google Scholar] [CrossRef]
- Li, J.; Wu, W.; Lu, C.; Liu, Y.; Wang, R.; Si, N.; Liu, F.; Zhou, J.; Zhang, S.; Zhang, X. Gross deletions in FBN1 results in variable phenotypes of Marfan syndrome. Clin. Chim. Acta 2017, 474, 54–59. [Google Scholar] [CrossRef]
- Available online: https://dare.uva.nl/search?identifier=420cb2ef-5ecf-4194-980e-8a7f5873aadc (accessed on 24 July 2024).
- Faivre, L.; Collod-Beroud, G.; Loeys, B.L.; Child, A.; Binquet, C.; Gautier, E.; Callewaert, B.; Arbustini, E.; Mayer, K.; Arslan-Kirchner, M.; et al. Effect of mutation type and location on clinical outcome in 1013 probands with Marfan syndrome or related phenotypes and FBN1 mutations: An international study. Am. J. Hum. Genet. 2007, 81, 454–466. [Google Scholar] [CrossRef]
- ClinVar Database. Available online: https://www.ncbi.nlm.nih.gov/clinvar/ (accessed on 26 July 2024).
- Genome Aggregation Database. Available online: https://gnomad.broadinstitute.org/ (accessed on 26 July 2024).
- Attanasio, M.; Lapini, I.; Evangelisti, L.; Lucarini, L.; Giusti, B.; Porciani, M.; Fattori, R.; Anichini, C.; Abbate, R.; Gensini, G.; et al. FBN1 mutation screening of patients with Marfan syndrome and related disorders: Detection of 46 novel FBN1 mutations. Clin. Genet. 2008, 74, 39–46. [Google Scholar] [CrossRef]
- Comeglio, P.; Johnson, P.; Arno, G.; Brice, G.; Evans, A.; Aragon-Martin, J.; da Silva, F.P.; Kiotsekoglou, A.; Child, A. The importance of mutation detection in Marfan syndrome and Marfan-related disorders: Report of 193 FBN1 mutations. Hum. Mutat. 2007, 28, 928. [Google Scholar] [CrossRef]
- Li, J.; Lu, C.; Wu, W.; Liu, Y.; Wang, R.; Si, N.; Meng, X.; Zhang, S.; Zhang, X. Application of next-generation sequencing to screen for pathogenic mutations in 123 unrelated Chinese patients with Marfan syndrome or a related disease. Sci. China Life Sci. 2019, 62, 1630–1637. [Google Scholar] [CrossRef]
- Proost, D.; Vandeweyer, G.; Meester, J.A.; Salemink, S.; Kempers, M.; Ingram, C.; Peeters, N.; Saenen, J.; Vrints, C.; Lacro, R.V.; et al. Performant Mutation Identification Using Targeted Next-Generation Sequencing of 14 Thoracic Aortic Aneurysm Genes. Hum. Mutat. 2015, 36, 808–814. [Google Scholar] [CrossRef]
- Söylen, B.; Singh, K.K.; Abuzainin, A.; Rommel, K.; Becker, H.; Arslan-Kirchner, M.; Schmidtke, J. Prevalence of dural ectasia in 63 gene-mutation-positive patients with features of Marfan syndrome type 1 and Loeys-Dietz syndrome and report of 22 novel FBN1 mutations. Clin. Genet. 2009, 75, 265–270. [Google Scholar] [CrossRef]
- Stheneur, C.; Collod-Béroud, G.; Faivre, L.; Buyck, J.F.; Gouya, L.; Le Parc, J.M.; Moura, B.; Muti, C.; Grandchamp, B.; Sultan, G.; et al. Identification of the minimal combination of clinical features in probands for efficient mutation detection in the FBN1 gene. Eur. J. Hum. Genet. 2009, 17, 1121–1128. [Google Scholar] [CrossRef]
- Loeys, B.L.; Chen, J.; Neptune, E.R.; Judge, D.P.; Podowski, M.; Holm, T.; Meyers, J.; Leitch, C.C.; Katsanis, N.; Sharifi, N.; et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 2005, 37, 275–281. [Google Scholar] [CrossRef]
- Loeys, B.L.; Schwarze, U.; Holm, T.; Callewaert, B.L.; Thomas, G.H.; Pannu, H.; De Backer, J.F.; Oswald, G.L.; Symoens, S.; Manouvrier, S.; et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N. Engl. J. Med. 2006, 355, 788–798. [Google Scholar] [CrossRef]
- Chen, J.; Li, B.; Yang, Y.; Hu, J.; Zhao, T.; Gong, Y.; Tan, Z. Mutations of the TGFBR2 gene in Chinese patients with Marfan-related syndrome. Clin. Investig. Med. 2010, 33, E14–E21. [Google Scholar] [CrossRef]
- LeMaire, S.A.; Pannu, H.; Tran-Fadulu, V.; Carter, S.A.; Coselli, J.S.; Milewicz, D.M. Severe aortic and arterial aneurysms associated with a TGFBR2 mutation. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, 167–171. [Google Scholar] [CrossRef]
- Stheneur, C.; Collod-Béroud, G.; Faivre, L.; Gouya, L.; Sultan, G.; Le Parc, J.M.; Moura, B.; Attias, D.; Muti, C.; Sznajder, M.; et al. Identification of 23 TGFBR2 and 6 TGFBR1 gene mutations and genotype-phenotype investigations in 457 patients with Marfan syndrome type I and II, Loeys-Dietz syndrome and related disorders. Hum. Mutat. 2008, 29, E284–E295. [Google Scholar] [CrossRef]
- Jamsheer, A.; Henggeler, C.; Wierzba, J.; Loeys, B.; De Paepe, A.; Stheneur, C.; Badziag, N.; Matuszewska, K.; Matyas, G.; Latos-Bielenska, A. A new sporadic case of early-onset Loeys-Dietz syndrome due to the recurrent mutation p.R528C in the TGFBR2 gene substantiates interindividual clinical variability. J. Appl. Genet. 2009, 50, 405–410. [Google Scholar] [CrossRef][Green Version]
- Horbelt, D.; Guo, G.; Robinson, P.N.; Knaus, P. Quantitative analysis of TGFBR2 mutations in Marfan-syndrome-related disorders suggests a correlation between phenotypic severity and Smad signaling activity. J. Cell Sci. 2010, 123 Pt 24, 4340–4350. [Google Scholar] [CrossRef]
- Human Gene Mutation Database (HGMD) Database. Available online: https://digitalinsights.qiagen.com/products-overview/clinical-insights-portfolio/human-gene-mutation-database/ (accessed on 23 August 2024).
- Callewaert, B.L.; Willaert, A.; Kerstjens-Frederikse, W.S.; De Backer, J.; Devriendt, K.; Albrecht, B.; Ramos-Arroyo, M.A.; Doco-Fenzy, M.; Hennekam, R.C.; Pyeritz, R.E.; et al. Arterial tortuosity syndrome: Clinical and molecular findings in 12 newly identified families. Hum. Mutat. 2008, 29, 150–158. [Google Scholar] [CrossRef]
- Hardin, J.S.; Zarate, Y.A.; Callewaert, B.; Phillips, P.H.; Warner, D.B. Ophthalmic findings in patients with arterial tortuosity syndrome and carriers: A case series. Ophthalmic Genet. 2018, 39, 29–34. [Google Scholar] [CrossRef]
- Weerakkody, R.; Ross, D.; Parry, D.A.; Ziganshin, B.; Vandrovcova, J.; Gampawar, P.; Abdullah, A.; Biggs, J.; Dumfarth, J.; Ibrahim, Y.; et al. Targeted genetic analysis in a large cohort of familial and sporadic cases of aneurysm or dissection of the thoracic aorta. Genet. Med. 2018, 20, 1414–1422. [Google Scholar] [CrossRef]
- Beyens, A.; Albuisson, J.; Boel, A.; Al-Essa, M.; Al-Manea, W.; Bonnet, D.; Bostan, O.; Boute, O.; Busa, T.; Canham, N.; et al. Arterial tortuosity syndrome: 40 new families and literature review. Genet. Med. 2018, 20, 1236–1245. [Google Scholar] [CrossRef]
- Poninska, J.K.; Bilinska, Z.T.; Franaszczyk, M.; Michalak, E.; Rydzanicz, M.; Szpakowski, E.; Pollak, A.; Milanowska, B.; Truszkowska, G.; Chmielewski, P.; et al. Next-generation sequencing for diagnosis of thoracic aortic aneurysms and dissections: Diagnostic yield, novel mutations and genotype phenotype correlations. J. Transl. Med. 2016, 14, 115. [Google Scholar] [CrossRef]
- Hannuksela, M.; Stattin, E.L.; Johansson, B.; Carlberg, B. Screening for Familial Thoracic Aortic Aneurysms with Aortic Imaging Does Not Detect All Potential Carriers of the Disease. Aorta 2015, 3, 1–8. [Google Scholar] [CrossRef][Green Version]
- Harris, S.L.; Lindsay, M.E. Role of Clinical Genetic Testing in the Management of Aortopathies. Curr. Cardiol. Rep. 2021, 23, 10. [Google Scholar] [CrossRef]
- Caglayan, A.O.; Dundar, M. Inherited diseases and syndromes leading to aortic aneurysms and dissections. Eur. J. Cardiothorac. Surg. 2009, 35, 931–940. [Google Scholar] [CrossRef]
- Cecchi, A.C.; Drake, M.; Campos, C.; Howitt, J.; Medina, J.; Damrauer, S.M.; Shalhub, S.; Milewicz, D.M. Aortic Dissection Collaborative. Current state and future directions of genomic medicine in aortic dissection: A path to prevention and personalized care. Semin. Vasc. Surg. 2022, 35, 51–59. [Google Scholar] [CrossRef]
- Tarcă, E.; Cojocaru, E.; Roşu, S.T.; Butnariu, L.I.; Plămădeală, P.; Moisă, Ş.M. Differential diagnosis difficulties related to infantile hemangioma—Case report and literature review. Rom. J. Morphol. Embryol. 2019, 60, 1375–1379. [Google Scholar]
- Butnariu, L.I.; Gorduza, E.V.; Florea, L.; Țarcă, E.; Moisă, Ș.M.; Trandafir, L.M.; Stoleriu, S.; Bădescu, M.C.; Luca, A.-C.; Popa, S.; et al. The Genetic Architecture of Vascular Anomalies: Current Data and Future Therapeutic Perspectives Correlated with Molecular Mechanisms. Int. J. Mol. Sci. 2022, 23, 12199. [Google Scholar] [CrossRef]
- Levy, L.E.; Zak, M.; Glotzbach, J.P. Current understanding of the genetics of thoracic aortic disease. Vessel Plus 2024, 8, 4. [Google Scholar] [CrossRef]
- Klarin, D.; Devineni, P.; Sendamarai, A.K.; Angueira, A.R.; Graham, S.E.; Shen, Y.H.; Levin, M.G.; Pirruccello, J.P.; Surakka, I.; Karnam, P.R.; et al. Genome-wide association study of thoracic aortic aneurysm and dissection in the Million Veteran Program. Nat. Genet. 2023, 55, 1106–1115. [Google Scholar] [CrossRef]
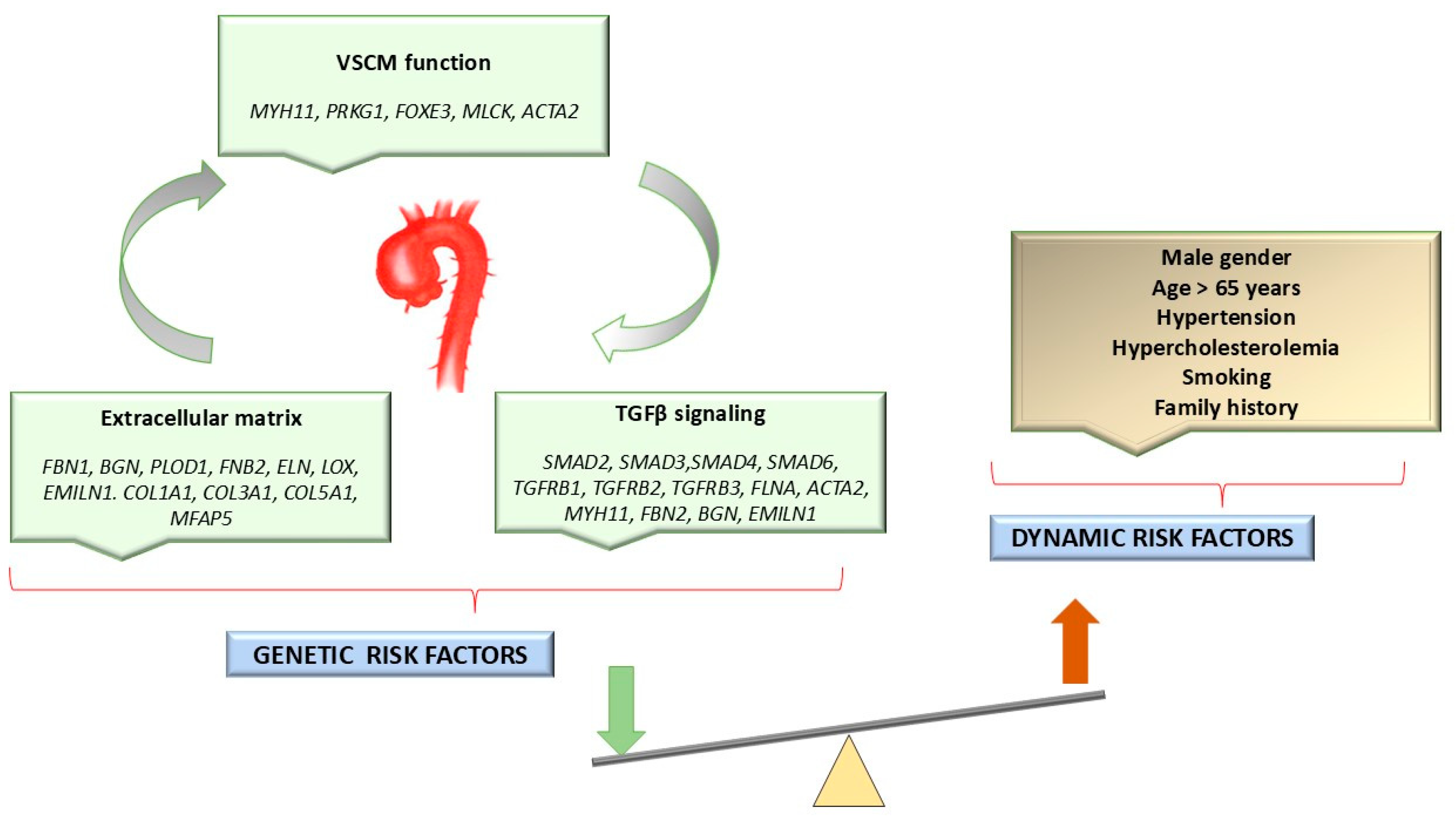
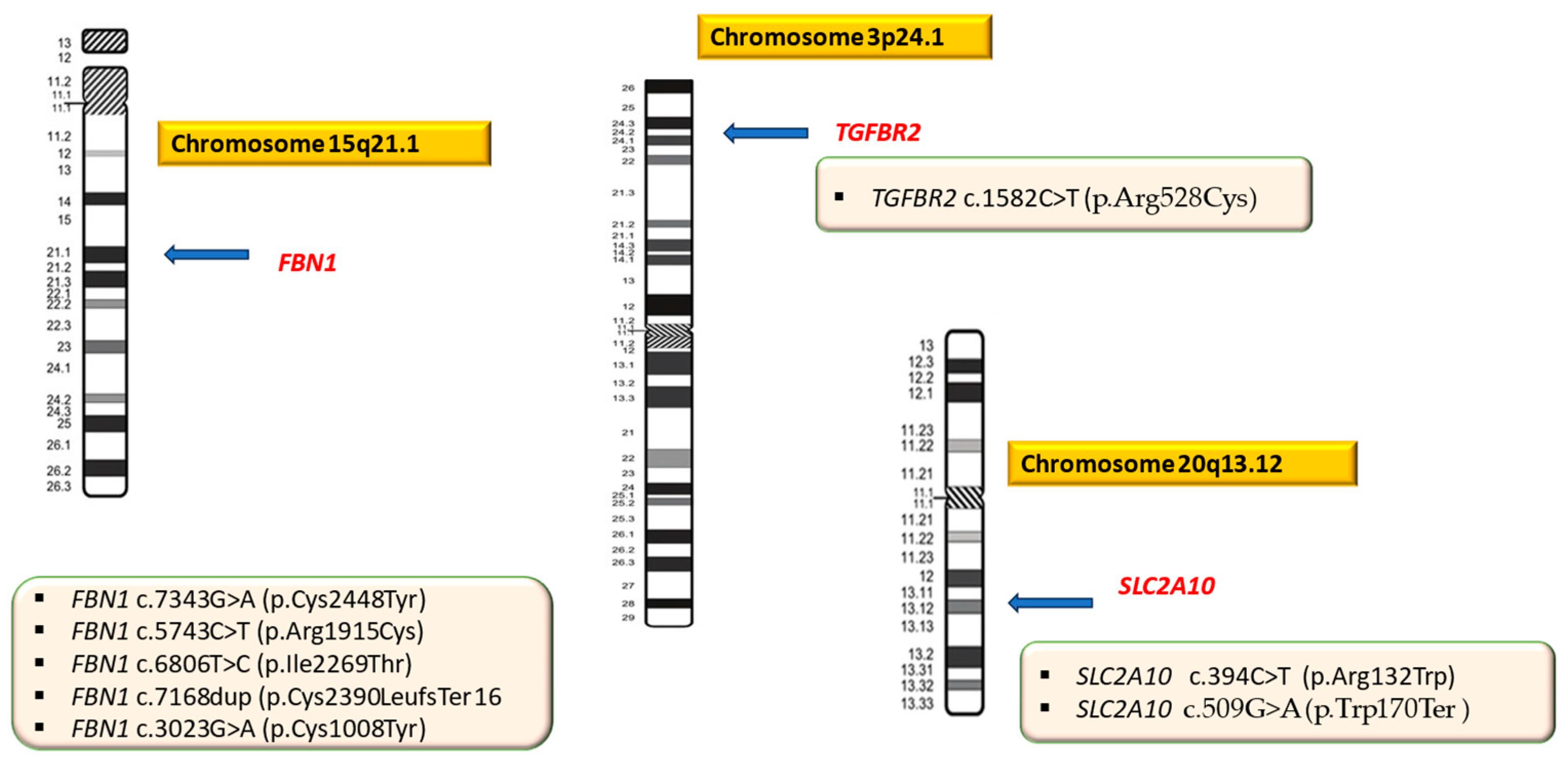
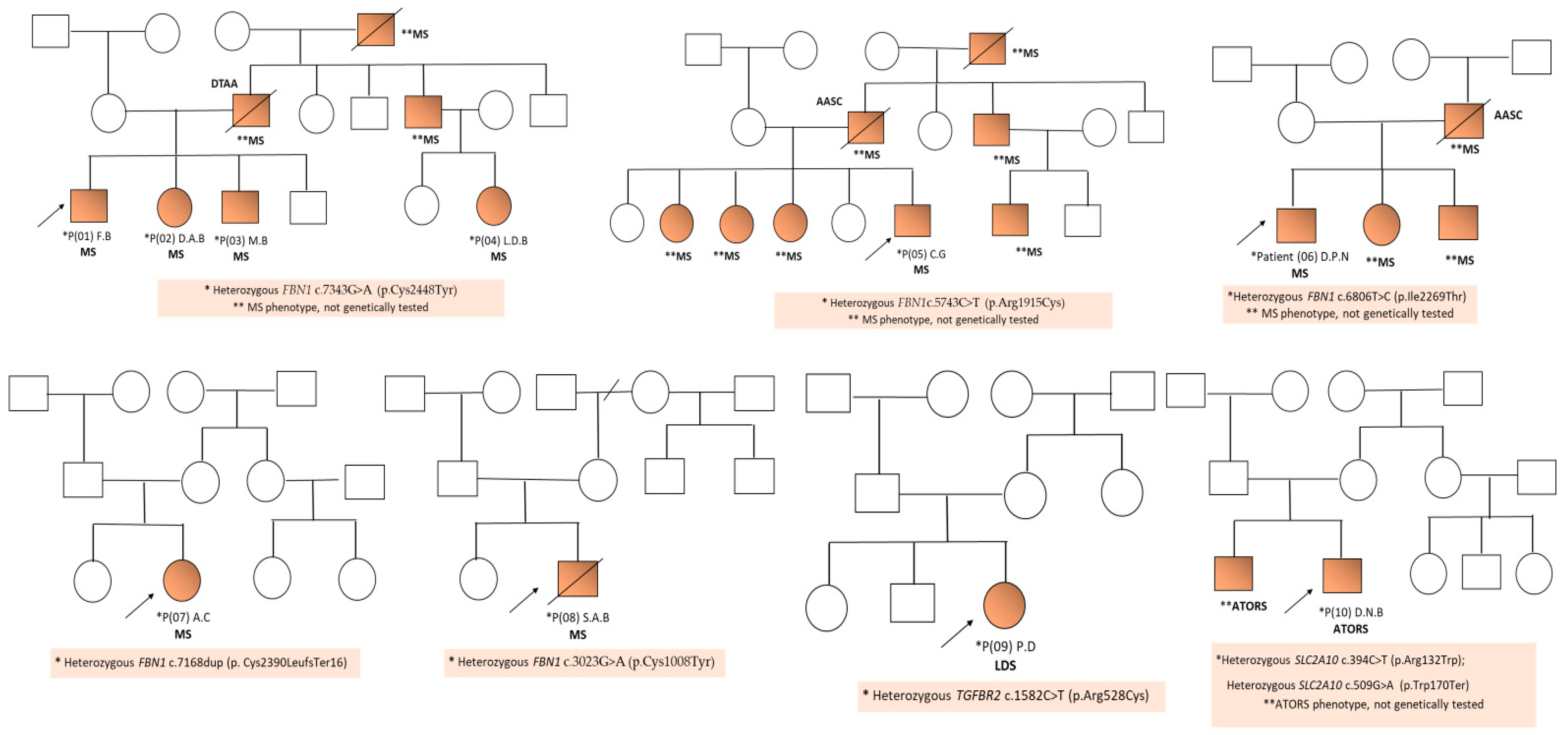
| Gene | Locus | OMIM | Protein | Syndrome | Inheritance Pattern | Clinical Features | References |
|---|---|---|---|---|---|---|---|
| Syndromic TAA/TAD | |||||||
| FBN1 | 15q21.1 | 154700 | Fibrillin 1 | MS | AD | PE/PC, SC, PP, EL, MVP, ARD, ARH, TS, WT, and CFD | [1,6,7,12,13] |
| TGFBR2 | 3p24.1 | 190182 | Transforming growth factor beta receptor 2 | LDS | AD | AT, AA, hypertelorism, and bifid uvula or cleft palate | [1,6,7,12,13] |
| TGFBR1 | 9q22.33 | 609192 | Transforming growth factor beta receptor 1 | LDS | AD | [1,6,7,12,13] | |
| SMAD3 | 15q22.33 | 603109 | MADH3 | LDS3 | AD | [1,6,7,12,13] | |
| COL3A1 | 2q32.2 | 130050 | Collagen, type III, alpha-1 | EDSVASC | AD | Arterial and bowel rupture; uterine rupture during pregnancy; easy bruising, thin skin with visible veins, CFD; LH and SH are minimal or absent | [1,6,7,12,13] |
| SLC2A10 | 20q13.12 | 208050 | Solute carrier family 2 member 10 | ATORS | AR | Tortuosity, elongation, stenosis, and aneurysms of the major arteries; LH or contractures, SH, IH; CFD (micrognathia, elongated face, high palate, beaked nose); LVH | [1,6,7,12,13] |
| BGN | Xq28 | 300989 | Biglycan | MLS | LX | Early-onset AA and AAD; PE/PC; hypertelorism, LH or, contractures, and mild SD | [1,6,7,12,13] |
| LTBP3 | 11q13.1 | 601216 | Latent TGF-b-binding protein | DASS | AR | SS with brachyolmia, HAI with almost absent enamel; MVP, ARD or other arterial aneurysms | [1,6,7,12,13] |
| Non-syndromic/isolated TAA/TAD | |||||||
| Unknown | 11q23.3-q24 | 607086 | AAT1 | AD | Medial necrosis’ or ‘Erdheim cystic medial necrosis’ | [1,6,7,12,13] | |
| Unknown | 5q13-q14 | 607087 | AAT2 | AD | TAA/TAD | [1,6,7,12,13] | |
| MYH11 | 16p13.11 | 132900 | Myosin heavy chain 11 | AAT4 | AD | TAA and/or PDA | [1,6,7,12,13] |
| MYLK | 3q21.1 | 613780 | Myosin light chain kinase | AAT7 | AD | TAA/TAD | [1,6,7,12,13] |
| PRKG1 | 10q11.23-q21.1 | 176894 | CGMP-dependent protein kinase 1 | AAT8 | AD | TAA/AD | [1,6,7,12,13] |
| MFAP5 | 12p13.31 | 601103 | Microfibril-associated protein 5 | AAT9 | AD | TAA | [1,6,7,12,13] |
| LOX | 5q23.1 | 153455 | Lysyl oxidase | AAT10 | AD | TAA | [1,6,7,12,13] |
| ACTA2 | 10q23.31 | 611788 | Actin alpha 2, smooth muscle (ACTA2) | AAT6 | AD | TAA leading to TAD, livedo reticularis visible on arms and legs; iris flocculi; PDA; BAV | [1,6,7,12,13] |
| FOXE3 | 1p33 | 601094 | Forkhead box protein E3 | AAT11 | AD | TAA/TAD | [1,6,7,12,13] |
| MAT2A glu344ala (E344A); (R356H) variants | 2p11.2 | 601468 | Methionine adenosyltransferase | VUS | AD | TAA/TAD | [1,6,7,12,13] |
| NOTCH1 | 9q34.3 | 190730 | Neurogenic locus notch homolog protein 1 | AOVD1 | AD | AV calcification, stenosis and insufficiency; HLHS | [1,6,7,12,13] |
| FBN1 | 15q21.1 | 154700 | Fibrillin 1 | MS | AD | PE/PC, SC, PP, EL, MVP, ARD, ARH, TS, WT, and CFD | [1,6,7,12,13] |
| Patient ID | Mutation | Locus | OMIM | Transcript | Protein | Genotype | Effect of the Mutation/Pathogenicity | Variant Previously Reported | Syndrome |
|---|---|---|---|---|---|---|---|---|---|
| P01 (F.B) | FBN1 c.7343G>A | 15q21.1 | 134797 | NM_000138.4 | p.Cys2448Tyr | Hz | Missense/LP | new | MS |
| P02 (D.A.B) | FBN1 c.7343G>A | 15q21.1 | 134797 | NM_000138.4 | p.Cys2448Tyr | Hz | Missense/LP | new | MS |
| P03 (M.B) | FBN1 c.7343G>A | 15q21.1 | 134797 | NM_000138.4 | p.Cys2448Tyr | Hz | Missense/LP | new | MS |
| P04 (L.D.B.) | FBN1 c.7343G>A | 15q21.1 | 134797 | NM_000138.4 | p.Cys2448Tyr | Hz | Missense/LP | new | MS |
| P05 (C.G) | FBN1 c.5743C>T | 15q21.1 | 134797 | NM_000138.4 | p.Arg1915Cys | Hz | Missense/LP | new | MS |
| P06 (D.P.N) | FBN1 c.6806T>C | 15q21.1 | 134797 | NM_000138.4 | p.Ile2269Thr | Hz | Missense/LP | known | MS |
| P07 (A.C) | FBN1 c.7168dup | 15q21.1 | 134797 | NM_000138.4 | p. Cys2390LeufsTer16 | Hz | Frameshift/LP | new | MS |
| P08 (S.A.B) | FBN1 c.3023G>A | 15q21.1 | 134797 | NM_000138.4 | p.Cys1008Tyr | Hz | Missense/LP | new | MS |
| P09 (P.D) | TGFBR2 c.1582C>T | 3p24.1 | 190182 | NM_003242.6 | p.Arg528Cys | Hz | Missense/Pathogenic | known | LDS |
| P10 (D.N.B) | SLC2A10 c.394C>T | 20q13.12 | 606145 | NM_030777.3 | p.Arg132Trp | Hz | Missense/Pathogenic | known | ATORS |
| SLC2A10 c.509G>A | 20q13.12 | 606145 | NM_030777.3 | p.Trp170Ter | Hz | Nonsense/Pathogenic | new | ATORS |
| Patient ID | Age * (m/y) | Sex M/F | Family History of AAD | Cardiac Features | Ao dil/Ao dis | Aortic Root Z Score ≥ 2 | Aortic/Arterial Tortuosity | Skelethal Features | Ocular Findings | CTD Features | Other Clinical Features | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P01 (F.B) | 12 y | M | + | MVP, MI | dilated CS | - | - | TS; PE, ARH, SC, CV, WT | - | Striae, inguinal hernia | CFD, phimosis | MS |
| P02 (D.A.B) | 9 y | F | + | MVP, MI | dilated CS | - | - | PE, ARH, PP, | Myopic astigmatism | - | CFD | MS |
| P03 (M.B) | 7 y | M | + | MVP, MI | AB ectasia | - | - | TS, SC, PE, ARH, PP, WT | Severe myopia | - | CFD, MC, sleep disorder | MS |
| P04 (L.D.B.) | 12 y | F | + | MVP | - | - | - | PC, PP, ETT | Severe myopia | Inguinal hernia | CFD | MS |
| P05 (C.G) | 9 y | M | + | MVP, TI, PI | - | - | - | TS, PE, ARH, PP, WT, SC | Myopia | Striae | CFD, Accessory spleen | MS |
| P06 (D.P.N) | 6 y | M | + | MVP | AB ectasia | + (Z score 3.55) | - | TS, PE, PP, ARH, | - | LH | CFD | MS |
| P07 (A.C) | 20 y | F | - | MVP (annuloplasty), MI, AI | AB ectasia | + (Z score 5.24) | - | TS, ARH, PC, SC, WT | Severe myopia | Striae | CFD | MS |
| P08 (S.A.B) | 8 y | M | - | MVP, AI, MI, AH, SA | AB ectasia | + (Z score 3.2) | - | TS, ARH, PP, WT, SC | Ectopia lentis, congenital cataract | Inguinal–scrotal hernia | CFD | MS |
| P09 (P.D) | 4 y | F | - | TI, left AAH, PAD | - | - | - | PC, SC | - | - | CFD | LDS |
| P10 (D.N.B) | 3 m | M | - | AAH, LVDD, AI, LVH, PAS, ATORS | - | - | + | LH | - | Inguinal–scrotal hernia, SH | ATORS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butnariu, L.I.; Russu, G.; Luca, A.-C.; Sandu, C.; Trandafir, L.M.; Vasiliu, I.; Popa, S.; Ghiga, G.; Bălănescu, L.; Țarcă, E. Identification of Genetic Variants Associated with Hereditary Thoracic Aortic Diseases (HTADs) Using Next Generation Sequencing (NGS) Technology and Genotype–Phenotype Correlations. Int. J. Mol. Sci. 2024, 25, 11173. https://doi.org/10.3390/ijms252011173
Butnariu LI, Russu G, Luca A-C, Sandu C, Trandafir LM, Vasiliu I, Popa S, Ghiga G, Bălănescu L, Țarcă E. Identification of Genetic Variants Associated with Hereditary Thoracic Aortic Diseases (HTADs) Using Next Generation Sequencing (NGS) Technology and Genotype–Phenotype Correlations. International Journal of Molecular Sciences. 2024; 25(20):11173. https://doi.org/10.3390/ijms252011173
Chicago/Turabian StyleButnariu, Lăcrămioara Ionela, Georgiana Russu, Alina-Costina Luca, Constantin Sandu, Laura Mihaela Trandafir, Ioana Vasiliu, Setalia Popa, Gabriela Ghiga, Laura Bălănescu, and Elena Țarcă. 2024. "Identification of Genetic Variants Associated with Hereditary Thoracic Aortic Diseases (HTADs) Using Next Generation Sequencing (NGS) Technology and Genotype–Phenotype Correlations" International Journal of Molecular Sciences 25, no. 20: 11173. https://doi.org/10.3390/ijms252011173
APA StyleButnariu, L. I., Russu, G., Luca, A.-C., Sandu, C., Trandafir, L. M., Vasiliu, I., Popa, S., Ghiga, G., Bălănescu, L., & Țarcă, E. (2024). Identification of Genetic Variants Associated with Hereditary Thoracic Aortic Diseases (HTADs) Using Next Generation Sequencing (NGS) Technology and Genotype–Phenotype Correlations. International Journal of Molecular Sciences, 25(20), 11173. https://doi.org/10.3390/ijms252011173







