Expression Profiles of Fatty Acid Transporters and the Role of n-3 and n-6 Polyunsaturated Fatty Acids in the Porcine Endometrium
Abstract
1. Introduction
2. Results
2.1. The mRNA Expression of FA Transporters in the Endometrium of Cyclic and Early Pregnant Gilts
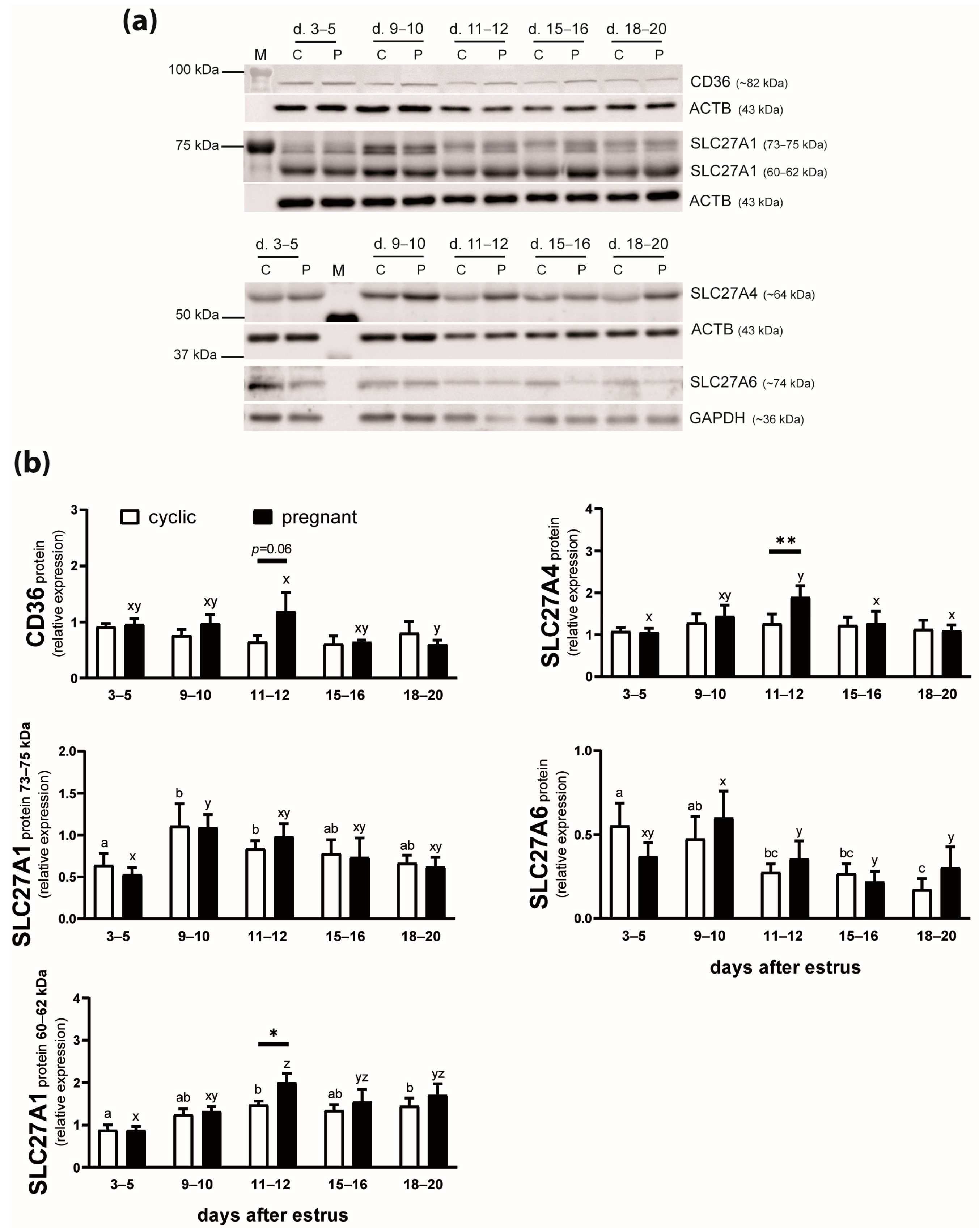
2.2. The Protein Expression of FA Transporters in the Endometrium of Cyclic and Early Pregnant Gilts
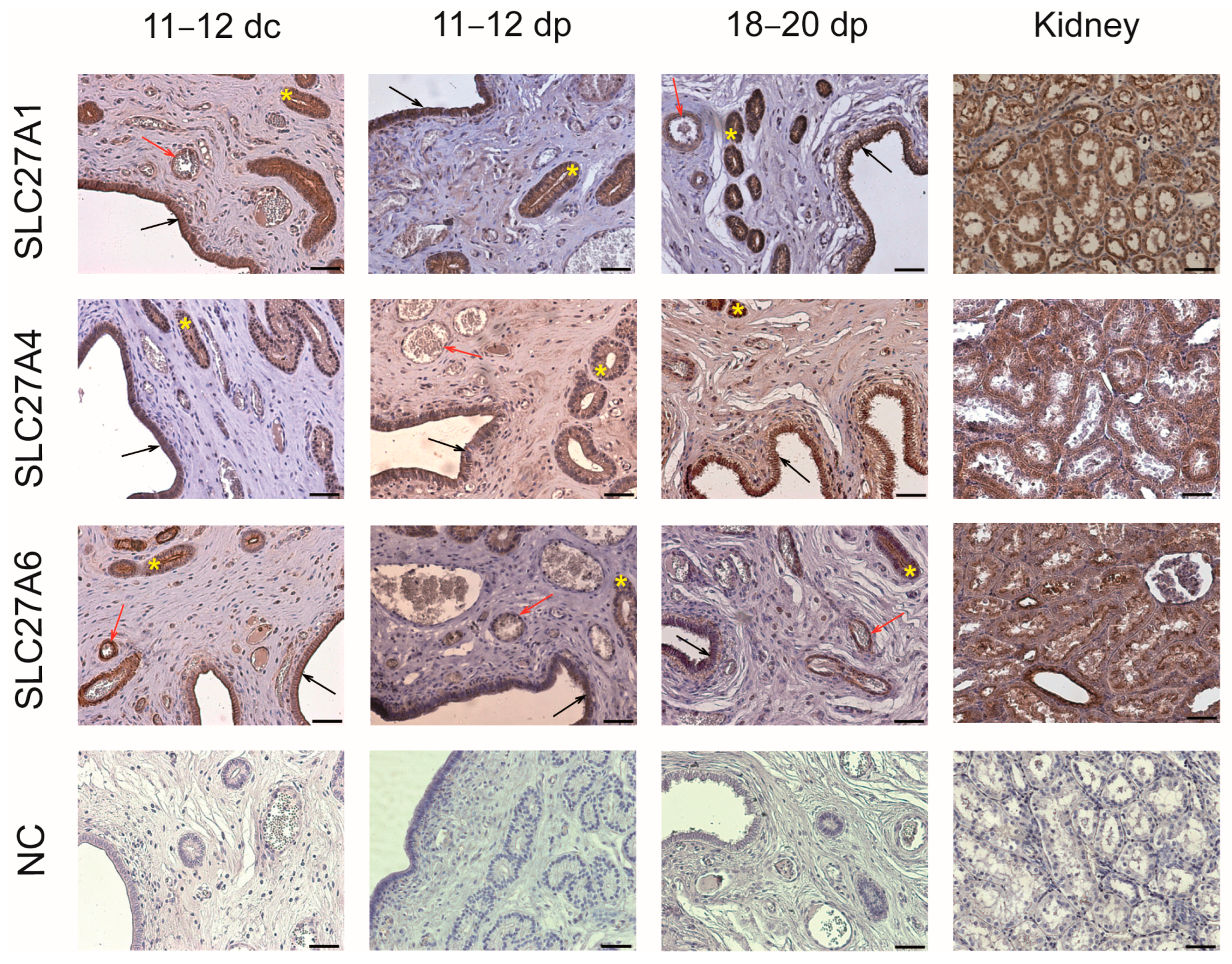
2.3. Localization of FA Transporters in the Endometrium
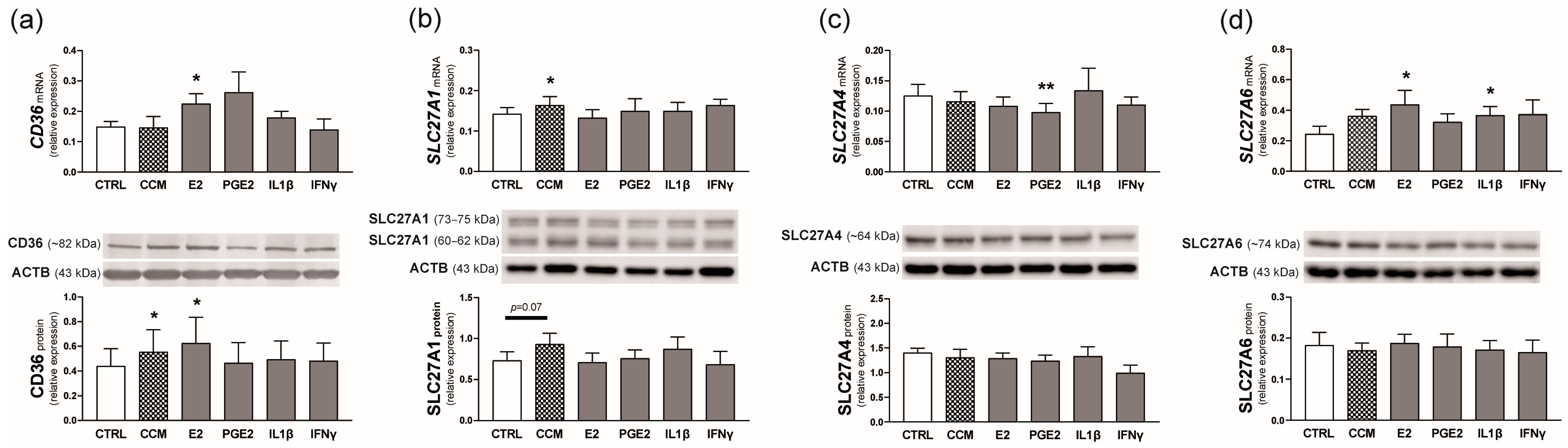
2.4. Experiment 1. Effect of Conceptus Signals on the Expression of FA Transporters in the Endometrium
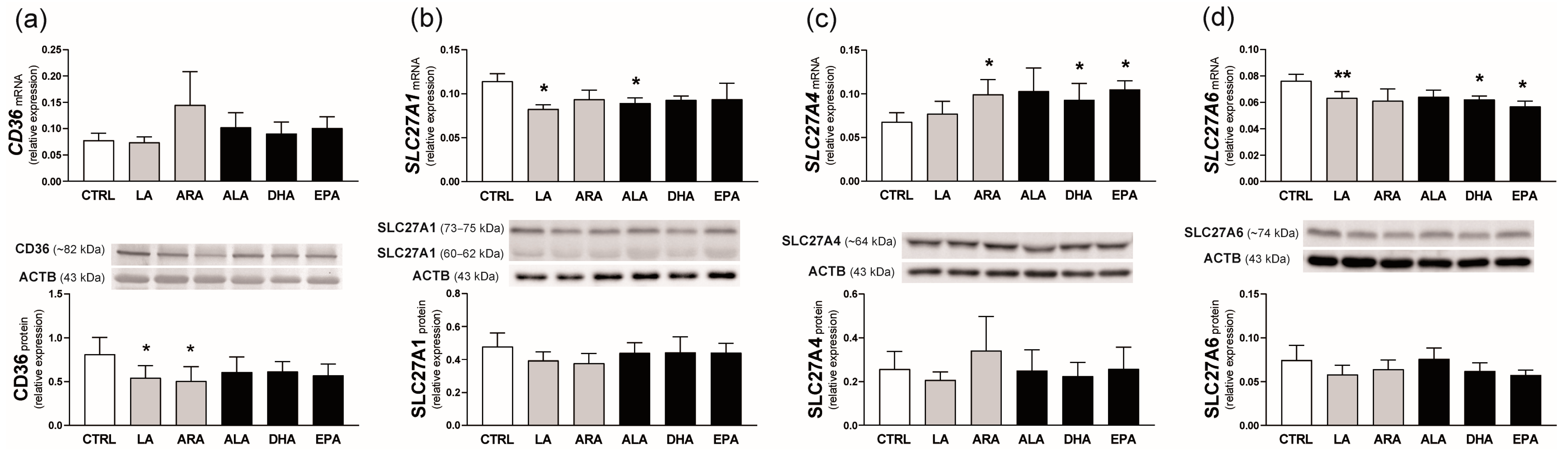
2.5. Experiment 2. Effect of n-6 and n-3 PUFAs on FA Transporter Expression in the Endometrium
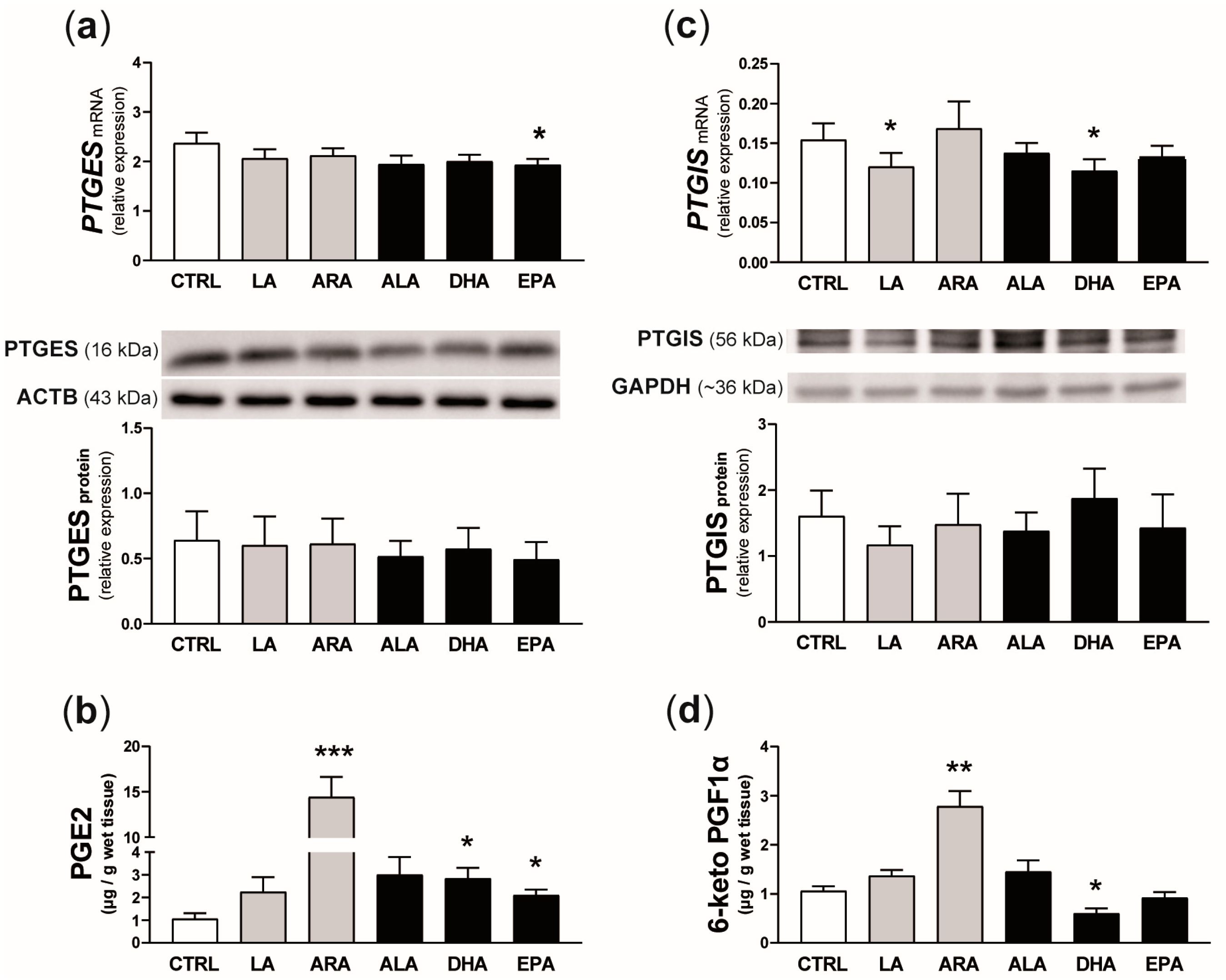
2.6. Experiment 3. Effect of n-6 and n-3 PUFAs on PGE2 and PGI2 Synthesis and the Concentration of Selected Transcripts in the Endometrium
3. Discussion
4. Materials and Methods
4.1. Animals and Sample Collection
4.2. Experiment 1. Effect of Conceptus Signals on the Expression of FA Transporters in the Endometrium
4.3. Experiment 2. Effect of n-6 and n-3 PUFAs on the Expression of FA Transporters in the Endometrium
4.4. Experiment 3. Effect of n-6 and n-3 PUFAs on PGE2 and PGI2 Synthesis and the Abundance of Selected Transcripts in the Endometrium
4.5. Total RNA Isolation and Real-Time PCR
4.6. Western Blot Analysis
4.7. Immunostaining of the Uterine Endometrium
4.8. Immunoassay
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaskins, A.J.; Chavarro, J.E. Diet and fertility: A review. Am. J. Obstet. Gynecol. 2018, 218, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Silvestris, E.; Lovero, D.; Palmirotta, R. Nutrition and Female Fertility: An Interdependent Correlation. Front. Endocrinol. 2019, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Koga, F.; Kitagami, S.; Izumi, A.; Uemura, T.; Takayama, O.; Koga, T.; Mizoguchi, T. Relationship between nutrition and reproduction. Reprod. Med. Biol. 2020, 19, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Skoracka, K.; Ratajczak, A.E.; Rychter, A.M.; Dobrowolska, A.; Krela-Kaźmierczak, I. Female Fertility and the Nutritional Approach: The Most Essential Aspects. Adv. Nutr. 2021, 12, 2372–2386. [Google Scholar] [CrossRef] [PubMed]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef]
- Herrera, E. Implications of Dietary Fatty Acids During Pregnancy on Placental, Fetal and Postnatal Development—A Review. Placenta 2002, 23, S9–S19. [Google Scholar] [CrossRef]
- Jump, D.B. Fatty Acid Regulation of Gene Transcription. Crit. Rev. Clin. Lab. Sci. 2004, 41, 41–78. [Google Scholar] [CrossRef]
- Nakamura, M.T.; Cheon, Y.; Li, Y.; Nara, T.Y. Mechanisms of regulation of gene expression by fatty acids. Lipids 2004, 39, 1077–1083. [Google Scholar] [CrossRef]
- Calder, P.C. Long-chain fatty acids and inflammation. Proc. Nutr. Soc. 2012, 71, 284–289. [Google Scholar] [CrossRef]
- Georgiadi, A.; Kersten, S. Mechanisms of Gene Regulation by Fatty Acids. Adv. Nutr. 2012, 3, 127–134. [Google Scholar] [CrossRef]
- Basak, S.; Duttaroy, A.K. Maternal PUFAs, Placental Epigenetics, and Their Relevance to Fetal Growth and Brain Development. Reprod. Sci. 2023, 30, 408–427. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.-S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Duttaroy, A.K.; Basak, S. Maternal dietary fatty acids and their roles in human placental development. Prostaglandins, Leukot. Essent. Fatty Acids 2020, 155, 102080. [Google Scholar] [CrossRef] [PubMed]
- Kamp, F.; Hamilton, J.A. How fatty acids of different chain length enter and leave cells by free diffusion. Prostaglandins, Leukot. Essent. Fatty Acids 2006, 75, 149–159. [Google Scholar] [CrossRef]
- Larqué, E.; Demmelmair, H.; Gil-Sánchez, A.; Prieto-Sánchez, M.T.; Blanco, J.E.; Pagán, A.; Faber, F.L.; Zamora, S.; Parrilla, J.J.; Koletzko, B. Placental transfer of fatty acids and fetal implications. Am. J. Clin. Nutr. 2011, 94, S1908–S1913. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Luiken, J.J.F.P.; Bonen, A. Membrane Fatty Acid Transporters as Regulators of Lipid Metabolism: Implications for Metabolic Disease. Physiol. Rev. 2010, 90, 367–417. [Google Scholar] [CrossRef]
- Duttaroy, A.K. Transport of fatty acids across the human placenta: A review. Prog. Lipid Res. 2009, 48, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Mark, P.J.; Waddell, B.J. Maternal dietary omega-3 fatty acids and placental function. Reproduction 2014, 147, R143–R152. [Google Scholar] [CrossRef]
- Campbell, F.M.; Bush, P.G.; Veerkamp, J.H.; Dutta-Roy, A.K. Detection and cellular localization of plasma membrane-associated and cytoplasmic fatty acid-binding proteins in human placenta. Placenta 1998, 19, 409–415. [Google Scholar] [CrossRef]
- Larqué, E.; Krauss-Etschmann, S.; Campoy, C.; Hartl, D.; Linde, J.; Klingler, M.; Demmelmair, H.; Caño, A.; Gil, A.; Bondy, B.; et al. Docosahexaenoic acid supply in pregnancy affects placental expression of fatty acid transport proteins. Am. J. Clin. Nutr. 2006, 84, 853–861. [Google Scholar] [CrossRef]
- Basak, S.; Duttaroy, A.K. Effects of fatty acids on angiogenic activity in the placental extravillious trophoblast cells. Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Leroy, C.; Tobin, K.A.R.; Basak, S.; Staff, A.C.; Duttaroy, A.K. Fatty acid-binding protein3 expression in BeWo cells, a human placental choriocarcinoma cell line. Prostaglandins Leukot. Essent. Fatty Acids 2017, 120, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Salehi, R.; Ambrose, D.J. Prepartum maternal diets supplemented with oilseeds alter the fatty acid profile in bovine neonatal plasma possibly through reduced placental expression of fatty acid transporter protein 4 and fatty acid translocase. Reprod. Fertil. Dev. 2017, 29, 1846–1855. [Google Scholar] [CrossRef]
- Steinhauser, C.B.; Askelson, K.; Lambo, C.A.; Hobbs, K.C.; Bazer, F.W.; Satterfield, M.C. Lipid metabolism is altered in maternal, placental, and fetal tissues of ewes with small for gestational age fetuses†. Biol. Reprod. 2021, 104, 170–180. [Google Scholar] [CrossRef]
- McNeel, A.K.; Chen, C.; Schroeder, S.; Sonstegard, T.; Dawson, H.; Vallet, J.L. Application of RNA-seq transcriptomic analysis to reproductive physiology of the pig: Insights into differential trophoblast function within the late gestation porcine placenta. Control. Pig Reprod. IX 2019, 175, 183. [Google Scholar] [CrossRef]
- Tian, L.; Dong, S.S.; Hu, J.; Yao, J.J.; Yan, P.S. The effect of maternal obesity on fatty acid transporter expression and lipid metabolism in the full-term placenta of lean breed swine. J. Anim. Physiol. Anim. Nutr. 2018, 102, E242–E253. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.; Wu, X.; Zhou, J.; Gu, T.; Wang, X.; Shi, J.; Zhao, C.; Cai, G.; Zheng, E.; Liu, D.; et al. Cloned pig fetuses exhibit fatty acid deficiency from impaired placental transport. Mol. Reprod. Dev. 2019, 86, 1569–1581. [Google Scholar] [CrossRef]
- Achache, H.; Revel, A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum. Reprod. Update 2006, 12, 731–746. [Google Scholar] [CrossRef]
- Bazer, F.W.; Spencer, T.E.; Johnson, G.A.; Burghardt, R.C. Uterine receptivity to implantation of blastocysts in mammals. Front. Biosci. 2011, S3, 745–767. [Google Scholar] [CrossRef]
- Garratt, J.; Rahmati, M. Assessing the endometrium: An update on current and potential novel biomarkers of receptivity. J. Reprod. Immunol. 2023, 160, 104162. [Google Scholar] [CrossRef]
- Spencer, T.E.; Johnson, G.A.; Burghardt, R.C.; Bazer, F.W. Progesterone and Placental Hormone Actions on the Uterus: Insights from Domestic Animals. Biol. Reprod. 2004, 71, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Ziecik, A.J.; Waclawik, A.; Kaczmarek, M.M.; Blitek, A.; Jalali, B.M.; Andronowska, A. Mechanisms for the Establishment of Pregnancy in the Pig. Reprod. Domest. Anim. 2011, 46, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Johnson, G.A. Pig blastocyst–uterine interactions. Differentiation 2014, 87, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Waclawik, A.; Kaczmarek, M.M.; Blitek, A.; Kaczynski, P.; Ziecik, A.J. Embryo-maternal dialogue during pregnancy establishment and implantation in the pig. Mol. Reprod. Dev. 2017, 84, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Samborski, A.; Graf, A.; Krebs, S.; Kessler, B.; Bauersachs, S. Deep Sequencing of the Porcine Endometrial Transcriptome on Day 14 of Pregnancy. Biol. Reprod. 2013, 88, 84. [Google Scholar] [CrossRef]
- Samborski, A.; Graf, A.; Krebs, S.; Kessler, B.; Reichenbach, M.; Reichenbach, H.-D.; Ulbrich, S.E.; Bauersachs, S. Transcriptome Changes in the Porcine Endometrium During the Preattachment Phase. Biol. Reprod. 2013, 89, 134. [Google Scholar] [CrossRef]
- Chen, X.; Li, A.; Chen, W.; Wei, J.; Fu, J.; Wang, A. Differential Gene Expression in Uterine Endometrium During Implantation in Pigs. Biol. Reprod. 2015, 92, 52. [Google Scholar] [CrossRef]
- Uzbekova, S.; Bertevello, P.S.; Dalbies-Tran, R.; Elis, S.; Labas, V.; Monget, P.; Teixeira-Gomes, A.-P. Metabolic exchanges between the oocyte and its environment: Focus on lipids. Reprod. Fertil. Dev. 2021, 34, 1–26. [Google Scholar] [CrossRef]
- Knapp, P.; Chabowski, A.; Posmyk, R.; Górski, J. Expression of the energy substrate transporters in uterine fibroids. Prostaglandins Other Lipid Mediat. 2016, 123, 9–15. [Google Scholar] [CrossRef]
- Knapp, P.; Chabowski, A.; Harasiuk, D.; Górski, J. Reversed Glucose and Fatty Acids Transporter Expression in Human Endometrial Cancer. Horm. Metab. Res. 2012, 44, 436–441. [Google Scholar] [CrossRef]
- Forde, N.; Carter, F.; Spencer, T.E.; Bazer, F.W.; Sandra, O.; Mansouri-Attia, N.; Okumu, L.A.; McGettigan, P.A.; Mehta, J.P.; McBride, R.; et al. Conceptus-Induced Changes in the Endometrial Transcriptome: How Soon Does the Cow Know She Is Pregnant? Biol. Reprod. 2011, 85, 144–156. [Google Scholar] [CrossRef]
- Zeng, S.; Ulbrich, S.E.; Bauersachs, S. Spatial organization of endometrial gene expression at the onset of embryo attachment in pigs. BMC Genom. 2019, 20, 895. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Anderson, L.L.; Henricks, D.M. Progesterone in Ovarian Venous Plasma and Corpora Lutea of the Pig1. Endocrinology 1967, 80, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Ziecik, A.J.; Przygrodzka, E.; Kaczmarek, M.M. Corpus Luteum Regression and Early Pregnancy Maintenance in Pigs. The Life Cycle of the Corpus Luteum; Springer International Publishing: Cham, Switzerland, 2017; pp. 227–248. ISBN 9783319432380.2. [Google Scholar]
- Acharya, R.; Shetty, S.S.; Kumari N, S. Fatty acid transport proteins (FATPs) in cancer. Chem. Phys. Lipids 2023, 250, 105269. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.A.; Bazer, F.W.; Burghardt, R.C.; Spencer, T.E.; Wu, G.; Bayless, K.J. Conceptus-uterus interactions in pigs: Endometrial gene expression in response to estrogens and interferons from conceptuses. Soc. Reprod. Fertiil Suppl. 2009, 66, 321–332. [Google Scholar] [CrossRef]
- Blitek, A.; Morawska, E.; Kiewisz, J.; Ziecik, A.J. Effect of conceptus secretions on HOXA10 and PTGS2 gene expression, and PGE2 release in co-cultured luminal epithelial and stromal cells of the porcine endometrium at the time of early implantation. Theriogenology 2011, 76, 954–966. [Google Scholar] [CrossRef]
- Geisert, R.D.; Johnson, G.A.; Burghardt, R.C. Implantation and Establishment of Pregnancy in the Pig. Adv. Anat. Embryol. Cell. Biol. 2015, 216, 137–163. [Google Scholar] [CrossRef]
- Kayser, J.-P.R.; Kim, J.G.; Cerny, R.L.; Vallet, J.L. Global characterization of porcine intrauterine proteins during early pregnancy. Reproduction 2006, 131, 379–388. [Google Scholar] [CrossRef]
- Stewart, F.; Kennedy, M.W.; Suire, S. A novel uterine lipocalin supporting pregnancy in equids. Cell. Mol. Life Sci. 2000, 57, 1373–1378. [Google Scholar] [CrossRef]
- Miles, J.R.; Walsh, S.C.; Rempel, L.A.; Pannier, A.K. Mechanisms regulating the initiation of porcine conceptus elongation. Mol. Reprod. Dev. 2023, 90, 646–657. [Google Scholar] [CrossRef]
- Kappen, C.; Kruger, C.; Jones, S.; Herion, N.J.; Salbaum, J.M. Maternal diet modulates placental nutrient transporter gene expression in a mouse model of diabetic pregnancy. PLoS ONE 2019, 14, e0224754. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Duan, Y.; Li, Y.; Tang, Y.; Geng, M.; Oladele, O.A.; Kim, S.W.; Yin, Y. Effects of dietary n-6:n-3 PUFA ratio on fatty acid composition, free amino acid profile and gene expression of transporters in finishing pigs. Br. J. Nutr. 2015, 113, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Tanghe, S.; De Smet, S. Does sow reproduction and piglet performance benefit from the addition of n-3 polyunsaturated fatty acids to the maternal diet? Vet. J. 2013, 197, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Rosero, D.S.; Boyd, R.D.; McCulley, M.; Odle, J.; van Heugten, E. Essential fatty acid supplementation during lactation is required to maximize the subsequent reproductive performance of the modern sow. Anim. Reprod. Sci. 2016, 168, 151–163. [Google Scholar] [CrossRef]
- Kennedy, T.G.; Gillio-Meina, C.; Phang, S.H. Prostaglandins and the initiation of blastocyst implantation and decidualization. Reproduction 2007, 134, 635–643. [Google Scholar] [CrossRef]
- Smith, W.L.; Garavito, R.M.; DeWitt, D.L. Prostaglandin Endoperoxide H Synthases (Cyclooxygenases)-1 and -2. J. Biol. Chem. 1996, 271, 33157–33160. [Google Scholar] [CrossRef]
- Petit, H.V.; Germiquet, C.; Lebel, D. Effect of Feeding Whole, Unprocessed Sunflower Seeds and Flaxseed on Milk Production, Milk Composition, and Prostaglandin Secretion in Dairy Cows. J. Dairy Sci. 2004, 87, 3889–3898. [Google Scholar] [CrossRef]
- Chartrand, R.; Matte, J.J.; Lessard, M.; Chouinard, P.Y.; Giguère, A.; Laforest, J.P. Effect of dietary fat sources on systemic and intrauterine synthesis of prostaglandins during early pregnancy in gilts. J. Anim. Sci. 2003, 81, 726–734. [Google Scholar] [CrossRef]
- Coyne, G.S.; Kenny, D.A.; Childs, S.; Sreenan, J.M.; Waters, S.M. Dietary n-3 polyunsaturated fatty acids alter the expression of genes involved in prostaglandin biosynthesis in the bovine uterus. Theriogenology 2008, 70, 772–782. [Google Scholar] [CrossRef]
- Gokuldas, P.P.; Singh, S.K.; Tamuli, M.K.; Naskar, S.; Vashi, Y.; Thomas, R.; Barman, K.; Pegu, S.R.; Chethan, S.G.; Agarwal, S.K. Dietary supplementation of n-3 polyunsaturated fatty acid alters endometrial expression of genes involved in prostaglandin biosynthetic pathway in breeding sows (Sus scrofa). Theriogenology 2018, 110, 201–208. [Google Scholar] [CrossRef]
- Morawska, E.; Kaczmarek, M.M.; Blitek, A. Regulation of prostacyclin synthase expression and prostacyclin content in the pig endometrium. Theriogenology 2012, 78, 2071–2086. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, M.; Blitek, A. Diverse effects of prostacyclin on angiogenesis-related processes in the porcine endometrium. Sci. Rep. 2023, 13, 14133. [Google Scholar] [CrossRef] [PubMed]
- Slonina, D.; Kowalik, M.K.; Subocz, M.; Kotwica, J. The effect of ovarian steroids on oxytocin-stimulated secretion and synthesis of prostaglandins in bovine myometrial cells. Prostaglandins Other Lipid Mediat. 2009, 90, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Abayasekara, D.R.E.; Ward, F.; Preece, D.M.; Raheem, K.A.; Wathes, D.C. Altering n-3 to n-6 polyunsaturated fatty acid ratios affects prostaglandin production by ovine uterine endometrium. Anim. Reprod. Sci. 2013, 143, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhao, J.; Liu, F.; Li, Y. Lipid metabolism and endometrial receptivity. Hum. Reprod. Update 2022, 28, 858–889. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, Z.; Shi, J.; Shao, J. Insight on Polyunsaturated Fatty Acids in Endometrial Receptivity. Biomolecules 2021, 12, 36. [Google Scholar] [CrossRef]
- Smathers, R.L.; Petersen, D.R. The human fatty acid-binding protein family: Evolutionary divergences and functions. Hum. Genom. 2011, 5, 170–191. [Google Scholar] [CrossRef]
- Zhu, Q.; Jin, Y.; Wang, P.; Wang, H.; Lu, B.; Wang, Z.; Dong, M. Expression and function of fatty acid-binding protein 4 in epithelial cell of uterine endometrium. Cell Biol. Int. 2015, 39, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhu, Q.; Peng, H.; Du, M.; Dong, M.; Wang, H. Fatty Acid-Binding Protein 4 in Endometrial Epithelium Is Involved in Embryonic Implantation. Cell. Physiol. Biochem. 2017, 41, 501–509. [Google Scholar] [CrossRef]
- Scifres, C.M.; Chen, B.; Nelson, D.M.; Sadovsky, Y. Fatty Acid Binding Protein 4 Regulates Intracellular Lipid Accumulation in Human Trophoblasts. J. Clin. Endocrinol. Metab. 2011, 96, E1083–E1091. [Google Scholar] [CrossRef]
- Basak, S.; Das, M.K.; Duttaroy, A.K. Fatty acid-induced angiogenesis in first trimester placental trophoblast cells: Possible roles of cellular fatty acid-binding proteins. Life Sci. 2013, 93, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta 2011, 1812, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Wu, D. Modulation of immune and inflammatory responses by dietary lipids. Curr. Opin. Lipidol. 2004, 15, 43–47. [Google Scholar] [CrossRef]
- Blitek, A.; Szymanska, M. Regulation of expression and role of peroxisome proliferator-activated receptors (PPARs) in luminal epithelial and stromal cells of the porcine endometrium. Theriogenology 2019, 127, 88–101. [Google Scholar] [CrossRef]
- Kim, S.T.; Marquard, K.; Stephens, S.; Louden, E.; Allsworth, J.; Moley, K.H. Adiponectin and adiponectin receptors in the mouse preimplantation embryo and uterus. Hum. Reprod. 2011, 26, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, G.M.; Basak, S.; Weedon-Fekjær, M.S.; Staff, A.C.; Duttaroy, A.K. Docosahexaenoic acid stimulates tube formation in first trimester trophoblast cells, HTR8/SVneo. Placenta 2011, 32, 626–632. [Google Scholar] [CrossRef]
- Godhamgaonkar, A.A.; Wadhwani, N.S.; Joshi, S.R. Exploring the role of LC-PUFA metabolism in pregnancy complications. Prostaglandins Leukot. Essent. Fatty Acids 2020, 163, 102203. [Google Scholar] [CrossRef]
- Joshi, N.P.; Madiwale, S.D.; Sundrani, D.P.; Joshi, S.R. Fatty acids, inflammation and angiogenesis in women with gestational diabetes mellitus. Biochimie 2023, 212, 31–40. [Google Scholar] [CrossRef]
- Chen, X.; Stein, T.P.; Steer, R.A.; Scholl, T.O. Individual free fatty acids have unique associations with inflammatory biomarkers, insulin resistance and insulin secretion in healthy and gestational diabetic pregnant women. BMJ Open Diabetes Res. Care 2019, 7, e000632. [Google Scholar] [CrossRef]
- Blitek, A.; Ziecik, A.J. Role of Tumour Necrosis Factor α in Stimulation of Prostaglandins F2α and E2 Release by Cultured Porcine Endometrial Cells. Reprod. Domest. Anim. 2006, 41, 562–567. [Google Scholar] [CrossRef]
- Franczak, A.; Zmijewska, A.; Kurowicka, B.; Wojciechowicz, B.; Kotwica, G. Interleukin 1β-induced synthesis and secretion of prostaglandin E₂ in the porcine uterus during various periods of pregnancy and the estrous cycle. J. Physiol. Pharmacol. 2010, 61, 733–742. [Google Scholar]
- Kiewisz, J.; Krawczyński, K.; Lisowski, P.; Blitek, A.; Zwierzchowski, L.; Ziecik, A.J.; Kaczmarek, M.M. Global gene expression profiling of porcine endometria on Days 12 and 16 of the estrous cycle and pregnancy. Theriogenology 2014, 82, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Likszo, P.; Skarzynski, D.J.; Jalali, B.M. Changes in Porcine Corpus Luteum Proteome Associated with Development, Maintenance, Regression, and Rescue during Estrous Cycle and Early Pregnancy. Int. J. Mol. Sci. 2021, 22, 11740. [Google Scholar] [CrossRef] [PubMed]
- Szuszkiewicz, J.; Myszczynski, K.; Reliszko, Z.P.; Heifetz, Y.; Kaczmarek, M.M. Early steps of embryo implantation are regulated by exchange of extracellular vesicles between the embryo and the endometrium. FASEB J. 2022, 36, e22450. [Google Scholar] [CrossRef]
- Akins, E.L.; Morrissette, M.C. Gross ovarian changes during estrous cycle of swine. Am. J. Vet. Res. 1968, 29, 1953–1957. [Google Scholar] [PubMed]
- Anderson, L.L. Growth, protein content and distribution of early pig embryos. Anat. Rec. 1978, 190, 143–153. [Google Scholar] [CrossRef]
- Vallet, J.L.; Miles, J.R.; Freking, B.A. Development of the pig placenta. Soc. Reprod. Fertil. Suppl. 2009, 66, 265–279. [Google Scholar] [CrossRef]
- Zhao, S.; Fernald, R.D. Comprehensive Algorithm for Quantitative Real-Time Polymerase Chain Reaction. J. Comput. Biol. 2005, 12, 1047–1064. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Blitek, A.; Waclawik, A.; Kaczmarek, M.M.; Kiewisz, J.; Ziecik, A.J. Effect of estrus induction on prostaglandin content and prostaglandin synthesis enzyme expression in the uterus of early pregnant pigs. Theriogenology 2010, 73, 1244–1256. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blitek, A.; Szymanska, M. Expression Profiles of Fatty Acid Transporters and the Role of n-3 and n-6 Polyunsaturated Fatty Acids in the Porcine Endometrium. Int. J. Mol. Sci. 2024, 25, 11102. https://doi.org/10.3390/ijms252011102
Blitek A, Szymanska M. Expression Profiles of Fatty Acid Transporters and the Role of n-3 and n-6 Polyunsaturated Fatty Acids in the Porcine Endometrium. International Journal of Molecular Sciences. 2024; 25(20):11102. https://doi.org/10.3390/ijms252011102
Chicago/Turabian StyleBlitek, Agnieszka, and Magdalena Szymanska. 2024. "Expression Profiles of Fatty Acid Transporters and the Role of n-3 and n-6 Polyunsaturated Fatty Acids in the Porcine Endometrium" International Journal of Molecular Sciences 25, no. 20: 11102. https://doi.org/10.3390/ijms252011102
APA StyleBlitek, A., & Szymanska, M. (2024). Expression Profiles of Fatty Acid Transporters and the Role of n-3 and n-6 Polyunsaturated Fatty Acids in the Porcine Endometrium. International Journal of Molecular Sciences, 25(20), 11102. https://doi.org/10.3390/ijms252011102






