Melatonin Mediates Cardiac Tissue Damage under Septic Conditions Induced by Lipopolysaccharide
Abstract
1. Introduction
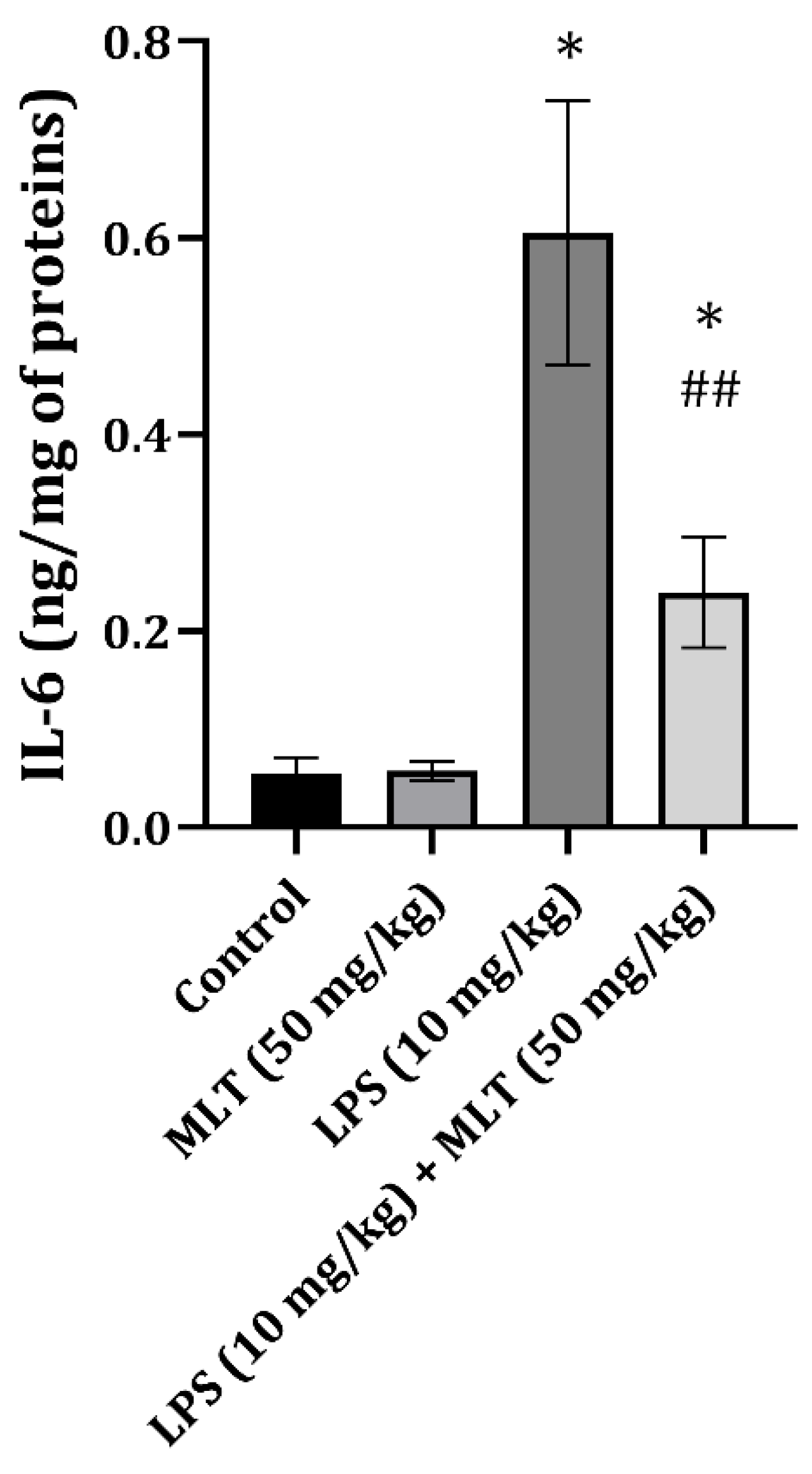
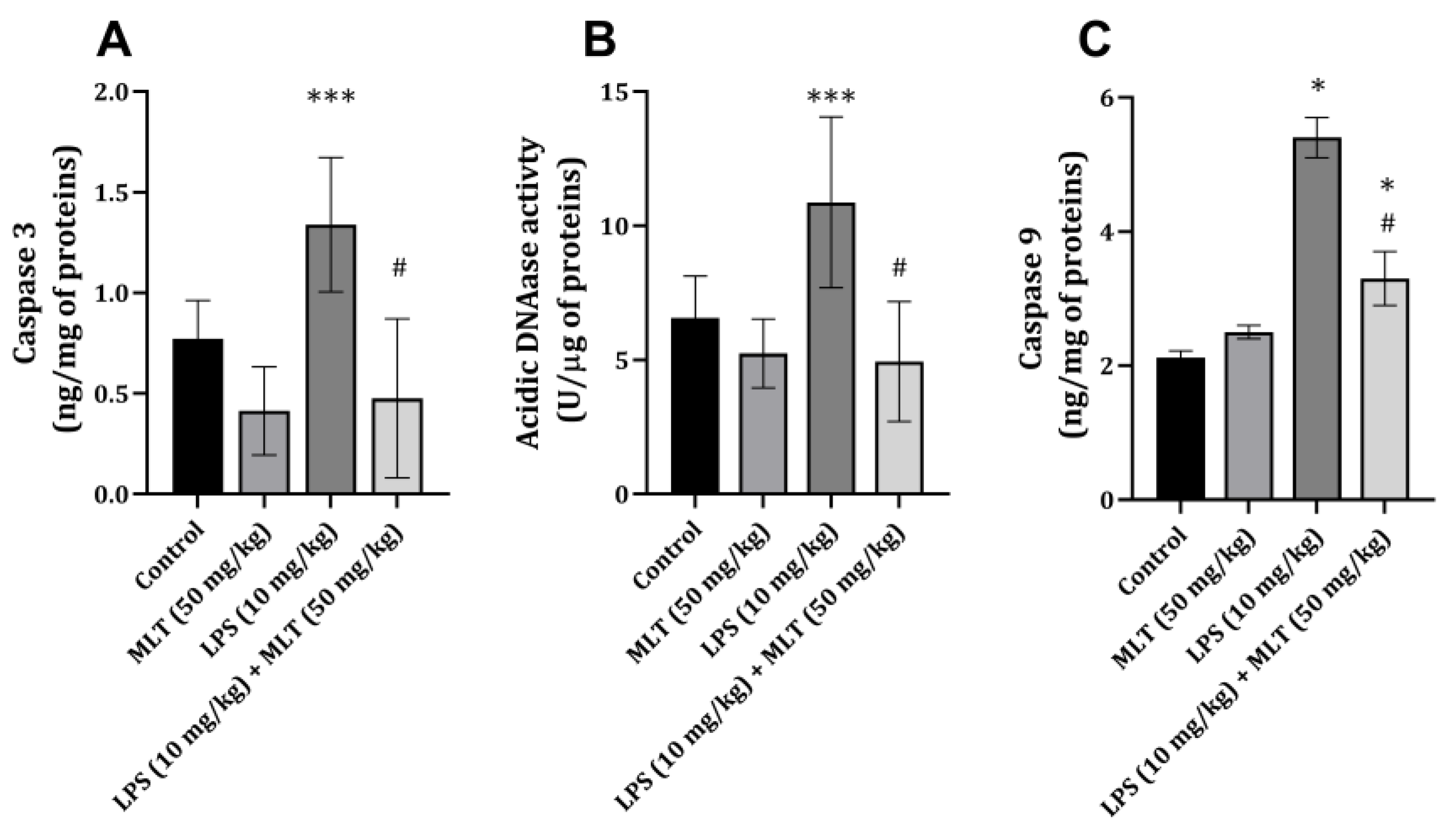
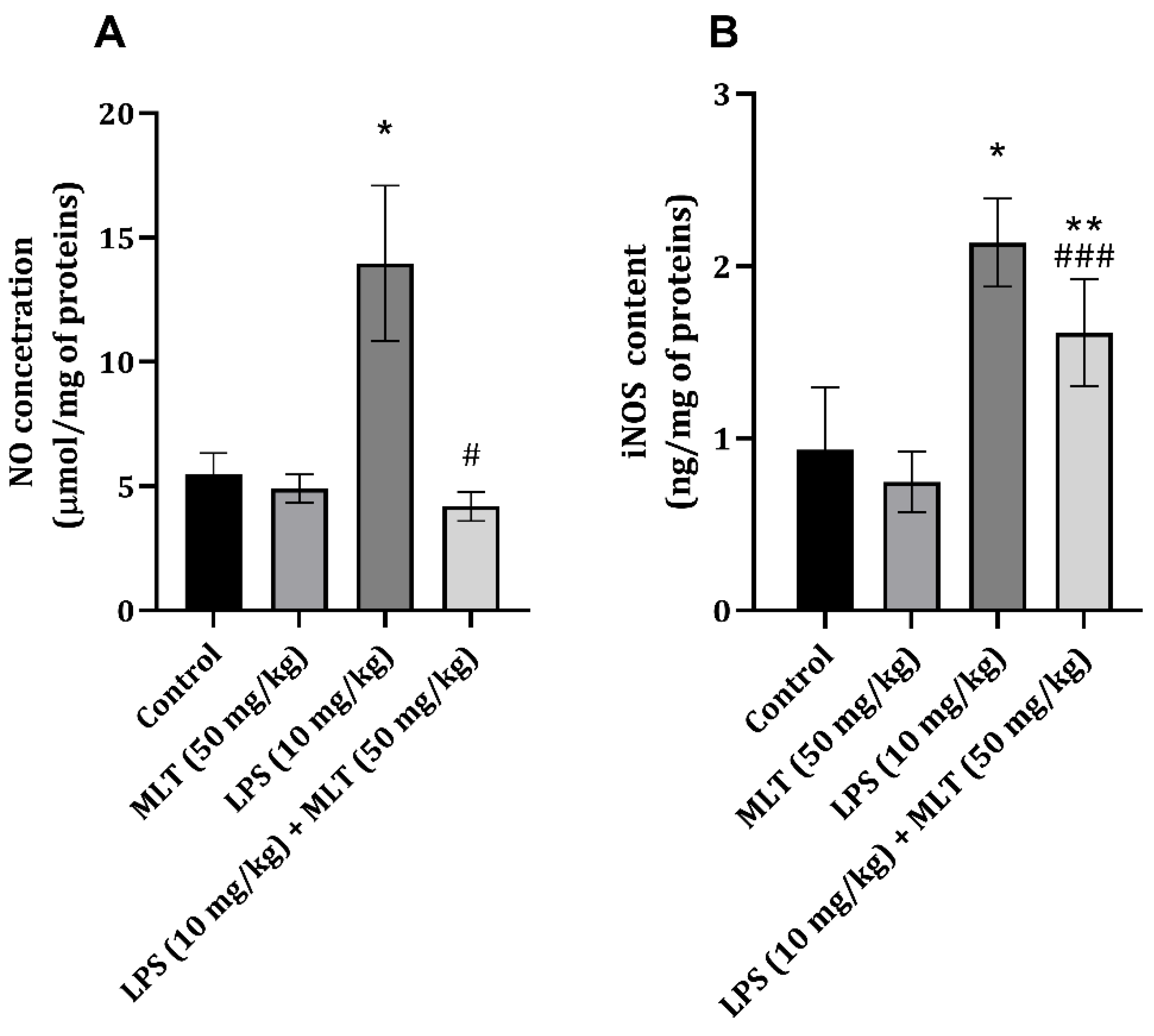
2. Results
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Animals and Housing
4.3. Experimental Design
4.4. Tissue Isolation and Homogenate Preparation
4.5. Oxidative Tissue Damage Determination
4.6. Inflammatory Cytokine Determination
4.7. Apoptosis Estimation
4.8. Nitric Oxide and iNOS Determination
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koc, F.; Tekeli, M.Y.; Kanbur, M.; Karayigit, M.Ö.; Liman, B.C. The effects of chrysin on lipopolysaccharide-induced sepsis in rats. J. Food Biochem. 2020, 44, e13359. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Leelahavanichkul, A.; Yuen, P.S.; Star, R.A. Animal models of sepsis and sepsis-induced kidney injury. J. Clin. Investig. 2009, 119, 2868–2878. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of clinical criteria for sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef]
- Brealey, D.; Brand, M.; Hargreaves, I.; Heales, S.; Land, J.; Smolenski, R.; Davies, N.A.; Cooper, C.E.; Singer, M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002, 360, 219–223. [Google Scholar] [CrossRef]
- Pravda, J. Hydrogen peroxide and disease: Towards a unified system of pathogenesis and therapeutics. Mol. Med. 2020, 26, 41. [Google Scholar] [CrossRef]
- Ni, J.; Zhao, Y.; Su, J.; Liu, Z.; Fang, S.; Li, L.; Deng, J.; Fan, G. Toddalolactone protects lipopolysaccharide-induced sepsis and attenuates lipopolysaccharide-induced inflammatory response by modulating HMGB1-NF-κB translocation. Front. Pharmacol. 2020, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Martinez de Tejada, G.; Heinbockel, L.; Ferrer-Espada, R.; Heine, H.; Alexander, C.; Bárcena-Varela, S.; Goldmann, T.; Correa, W.; Wiesmüller, K.H.; Gisch, N.; et al. Lipoproteins/peptides are sepsis-inducing toxins from bacteria that can be neutralized by synthetic anti-endotoxin peptides. Sci. Rep. 2015, 5, 14292. [Google Scholar] [CrossRef]
- Chang, P.C.; Chen, L.J.; Cheng, J.T. Role of peroxisome proliferator-activated receptors δ (PPARδ) in rats showing endotoxemic heart failure. J. Appl. Biomed. 2014, 12, 79–85. [Google Scholar] [CrossRef]
- Kuo, F.Y.; Lee, S.P.; Cheng, J.T.; Wu, M.C. Molecular mechanisms regarding potassium bromate-induced cardiac hypertrophy without apoptosis in H9c2 cells. Mol. Med. Rep. 2018, 18, 4700–4708. [Google Scholar] [CrossRef]
- Tavener, S.A.; Long, E.M.; Robbins, S.M.; McRae, K.M.; Van Remmen, H.; Kubes, P. Immune cell Toll-like receptor 4 is required for cardiac myocyte impairment during endotoxemia. Circ. Res. 2004, 95, 700–707. [Google Scholar] [CrossRef]
- Wang, H.Y.; Liu, X.Y.; Han, G.; Wang, Z.Y.; Li, X.X.; Jiang, Z.M.; Jiang, C.M. LPS induces cardiomyocyte injury through calcium-sensing receptor. Mol. Cell Biochem. 2013, 379, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Kuo, F.Y.; Lee, S.P.; Cheng, J.T.; Wu, M.C. The direct effect of lipopolysaccharide on an isolated heart is different from the effect on cardiac myocytes in vitro. Arch. Med. Sci. 2019, 19, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Mundigler, G.; Delle-Karth, G.; Koreny, M.; Zehetgruber, M.; Steindl-Munda, P.; Marktl, W.; Ferti, L.; Siostrzonek, P. We impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit. Care Med. 2002, 30, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Sokolović, D.T.; Lilić, L.; Milenković, V.; Stefanović, R.; Ilić, T.P.; Mekić, B. Effects of melatonin on oxidative stress parameters and pathohistological ch;anges in rat skeletal muscle tissue following carbon tetrachloride application. Saudi Pharm. J. 2018, 26, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Potić, M.; Ignjatović, I.; Ničković, V.P.; Živković, J.B.; Krdžić, J.D.; Mitić, J.S.; Popović, D.; Ilić, I.R.; Stojanović, N.M.; Sokolović, D. Two different melatonin treatment regimens prevent an increase in kidney injury marker-1 induced by carbon tetrachloride in rat kidneys. Can. J. Physiol. Pharmacal. 2019, 97, 422–428. [Google Scholar] [CrossRef]
- Stojanović, N.M.; Maslovarić, A.; Mihajlović, I.; Marković, A.; Randjelović, P.J.; Sokolović, D. Melatonin treatment prevents carbon-tetrachloride induced rat brain injury. Toxicol. Res. 2023, 12, 895–901. [Google Scholar] [CrossRef]
- Kamfar, W.W.; Khraiwesh, H.M.; Ibrahim, M.O.; Qadhi, A.H.; Azhar, W.F.; Ghafouri, K.J.; Alhussain, M.H.; AlShahrani, A.M.; Alghannam, A.F.; Abdulal, R.H.; et al. Comprehensive review of melatonin as a promising nutritional and nutraceutical supplement. Heliyon 2024, 10, e24266. [Google Scholar] [CrossRef]
- Santos-Ledo, A.; Luxán-Delgado, B.; Caballero, B.; Potes, Y.; Rodríguez-González, S.; Boga, J.A.; Coto-Montes, A.; García-Macia, M. Melatonin Ameliorates Autophagy Impairment in a Metabolic Syndrome Model. Antioxidants 2021, 10, 796. [Google Scholar] [CrossRef]
- López-Armas, G.; Flores-Soto, M.E.; Chaparro-Huerta, V.; Jave-Suarez, L.F.; Soto-Rodríguez, S.; Rusanova, I.; Acuña-Castroviejo, D.; González-Perez, O.; González-Castañeda, R.E. Prophylactic Role of Oral Melatonin Administration on Neurogenesis in Adult Balb/C Mice during REM Sleep Deprivation. Oxid. Med. Cell Longev. 2016, 2016, 2136902. [Google Scholar] [CrossRef]
- Srinivasan, V.; Spence, D.W.; Pandi-Perumal, S.R.; Brown, G.M.; Cardinali, D.P. Melatonin in mitochondrial dysfunction and related disorders. Int. J. Alzheimers Dis. 2011, 2011, 326320. [Google Scholar] [CrossRef]
- Benedeto-Stojanov, D.; Ničković, V.P.; Petrović, G.; Rancić, A.; Grgov, I.; Nikolić, G.R.; Marčetić, Z.P.; Popović, M.R.; Lazarević, M.; Mitić, K.V.; et al. Melatonin as a Promising Anti-Inflammatory Agent in an In Vivo Animal Model of Sepsis-Induced Rat Liver Damage. Int. J. Mol. Sci. 2023, 25, 455. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Mayo, J.C.; Sainz, R.M.; Leon, J.; Czarnocki, Z. Melatonin as an antioxidant: Biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 2003, 50, 1129–1146. [Google Scholar] [CrossRef]
- Ničković, V.P.; Novaković, T.; Lazarević, S.; Šulović, L.; Živković, Z.; Živković, J.; Mladenović, B.; Stojanović, N.M.; Petrović, V.; Sokolović, D.T. Pre-vs. post-treatment with melatonin in CCl4-induced liver damage: Oxidative stress inferred from biochemical and pathohistological studies. Life Sci. 2018, 202, 28–34. [Google Scholar] [CrossRef]
- Naffaa, M.; Makhoul, B.F.; Tobia, A.; Kaplan, M.; Aronson, D.; Azzam, Z.S.; Saliba, W. Procalcitonin and interleukin 6 for predicting blood culture positivity in sepsis. Am. J. Emerg. Med. 2014, 32, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Lin, Y.; Zhu, T.; Huang, M.; Xu, Q. Interleukin 6 increases dysfunction of organs in sepsis rats through sirtuin 1. Int. J. Clin. Exp. Med. 2014, 7, 2593–2598. [Google Scholar] [PubMed]
- Fontes, J.A.; Rose, N.R.; Čiháková, D. The varying faces of IL-6: From cardiac protection to cardiac failure. Cytokine 2015, 74, 62–68. [Google Scholar] [CrossRef]
- Dawn, B.; Xuan, Y.T.; Guo, Y.; Rezazadeh, A.; Stein, A.B.; Hunt, G.; Wu, W.-J.; Tan, W.; Bolli, R. IL-6 plays an obligatory role in late preconditioning via JAK-STAT signaling and upregulation of iNOS and COX-2. Cardiovasc. Res. 2004, 64, 61–71. [Google Scholar] [CrossRef]
- Larsen, B.D.; Rampalli, S.; Burns, L.E.; Brunette, S.; Dilworth, F.J.; Megeney, L.A. Caspase 3/caspase-activated DNase promotes cell differentiation by inducing DNA strand breaks. Proc. Natl. Acad. Sci. USA 2010, 107, 4230–4235. [Google Scholar] [CrossRef]
- Sano, E.; Kazaana, A.; Tadakuma, H.; Takei, T.; Yoshimura, S.; Hanashima, Y.; Ozawa, Y.; Yoshino, A.; Suzuki, Y.; Ueda, T. Interleukin-6 sensitizes TNF-α and TRAIL/Apo2L dependent cell death through upregulation of death receptors in human cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119037. [Google Scholar] [CrossRef] [PubMed]
- Mehrzadi, S.; Pourhanifeh, M.H.; Mirzaei, A.; Moradian, F.; Hosseinzadeh, A. An updated review of mechanistic potentials of melatonin against cancer: Pivotal roles in angiogenesis, apoptosis, autophagy, endoplasmic reticulum stress, and oxidative stress. Cancer Cell Int. 2021, 21, 188. [Google Scholar] [CrossRef]
- Ćirić Zdravković, S.; Kostić, T.; Marcetić, Z.P.; Šulović, L.S.; Nedeljković, B.M.; Preljević, A.; Toskić, D.; Sokolović, D. Melatonin modulates acute cardiac muscle damage induced by carbon tetrachloride—Involvement of oxidative damage, glutathione, and arginine and nitric oxide metabolism. Can. J. Physiol. Pharmacol. 2021, 99, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Gilad, E.; Wong, H.R.; Zingarelli, B.; Virág, L.; O’Connor, M.; Salzman, A.L.; Szabó, C. Melatonin inhibits expression of the inducible isoform of nitric oxide synthase in murine macrophages: Role of inhibition of NF-kappaB activation. FASEB J. 1998, 12, 685–693. [Google Scholar] [CrossRef]
- Liu, Y.H.; Carretero, O.A.; Cingolani, O.H.; Liao, T.D.; Sun, Y.; Xu, J.; Li, L.Y.; Pagano, P.J.; Yang, J.J.; Yang, X.P. Role of inducible nitric oxide synthase in cardiac function and remodeling in mice with heart failure due to myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2616–H2623. [Google Scholar] [CrossRef]
- Crespo, E.; Macías, M.; Pozo, D.; Escames, G.; Martín, M.; Vives, F.; Guerrero, J.M.; Acuña-Castroviejo, D. Melatonin inhibits expression of the inducible NO synthase II in liver and lung and prevents endotoxemia in lipopolysaccharide-induced multiple organ dysfunction syndrome in rats. FASEB J. 1999, 13, 1537–1546. [Google Scholar] [CrossRef]
- Turjanski, A.G.; Leonik, F.; Estrin, D.A.; Rosenstein, R.E.; Doctorovich, F. Scavenging of NO by Melatonin. J. Am. Chem. Soc. 2000, 122, 10468–10469. [Google Scholar] [CrossRef]
- Sokolović, D.; Lazarević, M.; Milić, D.; Stanojković, Z.; Petković, M.N.; Stojanović, N.M.; Sokolović, D.T. Melatonin reduces lipopolysaccharide-induced kidney damage. Acta Medica Median. 2023, 62, 15–20. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Stojanović, N.M.; Randjelović, P.J.; Mladenović, M.Z.; Ilić, I.R.; Petrović, V.; Stojiljković, N.; Ilić, S.; Radulović, N.S. Toxic essential oils, part VI: Acute oral toxicity of lemon balm (Melissa officinalis L.) essential oil in BALB/c mice. Food Chem. Toxicol. 2019, 133, 110794. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Bartholeyns, J.; Peeters-Joris, C.; Reychler, H.; Baudhuin, P. Hepatic Nucleases. Methods for the specific determination and characterization in rat liver. Eur. J. Biochem. 1975, 57, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Mladenović, B.; Mladenović, N.; Brzački, V.; Petrović, N.; Kamenov, A.; Golubović, M.; Ničković, V.; Stojanović, N.M.; Sokolović, D.T. Exogenous putrescine affects polyamine and arginine metabolism in rat liver following bile ductus ligation. Can. J. Physiol. Pharmacol. 2018, 96, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
| Group/Parameter | LDH (U/L) | CK-MB (U/L) |
|---|---|---|
| Control | 537 ± 85 | 1.09 ± 0.14 |
| Melatonin | 614 ± 94 | 1.23 ± 0.07 |
| LPS | 1588 ± 197 * | 1.90 ± 0.20 * |
| LPS + Melatonin | 1172 ± 70 # | 1.04 ± 0.27 # |
| Group/Parameter | TBARS (nM/mg of Protein) | AOPP (µM/mg of Protein) |
|---|---|---|
| Control | 1.97 ± 0.33 | 1.09 ± 0.14 |
| Melatonin | 2.29 ± 0.35 | 1.23 ± 0.07 |
| LPS | 5.25 ± 1.24 * | 1.90 ± 0.20 * |
| LPS + Melatonin | 2.49 ± 0.3 # | 1.04 ± 0.27 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarević, M.; Kostić, M.; Džopalić, T.; Sokolović, D.; Lazarević, Z.; Milovanović, J.; Ničković, V.; Sokolović, D. Melatonin Mediates Cardiac Tissue Damage under Septic Conditions Induced by Lipopolysaccharide. Int. J. Mol. Sci. 2024, 25, 11088. https://doi.org/10.3390/ijms252011088
Lazarević M, Kostić M, Džopalić T, Sokolović D, Lazarević Z, Milovanović J, Ničković V, Sokolović D. Melatonin Mediates Cardiac Tissue Damage under Septic Conditions Induced by Lipopolysaccharide. International Journal of Molecular Sciences. 2024; 25(20):11088. https://doi.org/10.3390/ijms252011088
Chicago/Turabian StyleLazarević, Milan, Miloš Kostić, Tanja Džopalić, Danka Sokolović, Zorica Lazarević, Jelena Milovanović, Vanja Ničković, and Dušan Sokolović. 2024. "Melatonin Mediates Cardiac Tissue Damage under Septic Conditions Induced by Lipopolysaccharide" International Journal of Molecular Sciences 25, no. 20: 11088. https://doi.org/10.3390/ijms252011088
APA StyleLazarević, M., Kostić, M., Džopalić, T., Sokolović, D., Lazarević, Z., Milovanović, J., Ničković, V., & Sokolović, D. (2024). Melatonin Mediates Cardiac Tissue Damage under Septic Conditions Induced by Lipopolysaccharide. International Journal of Molecular Sciences, 25(20), 11088. https://doi.org/10.3390/ijms252011088






