Role of Exogenous Pyruvate in Maintaining Adenosine Triphosphate Production under High-Glucose Conditions through PARP-Dependent Glycolysis and PARP-Independent Tricarboxylic Acid Cycle
Abstract
1. Introduction
2. Results
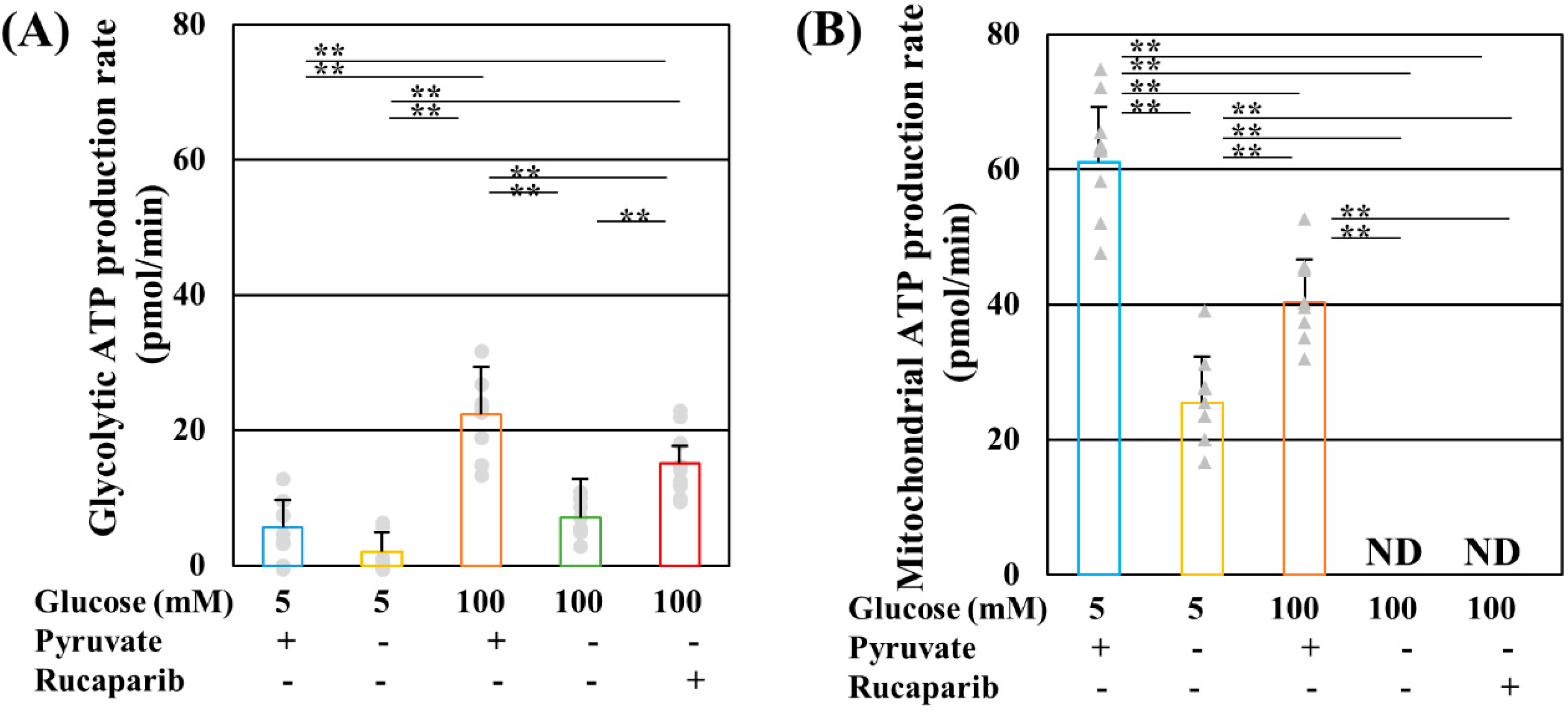
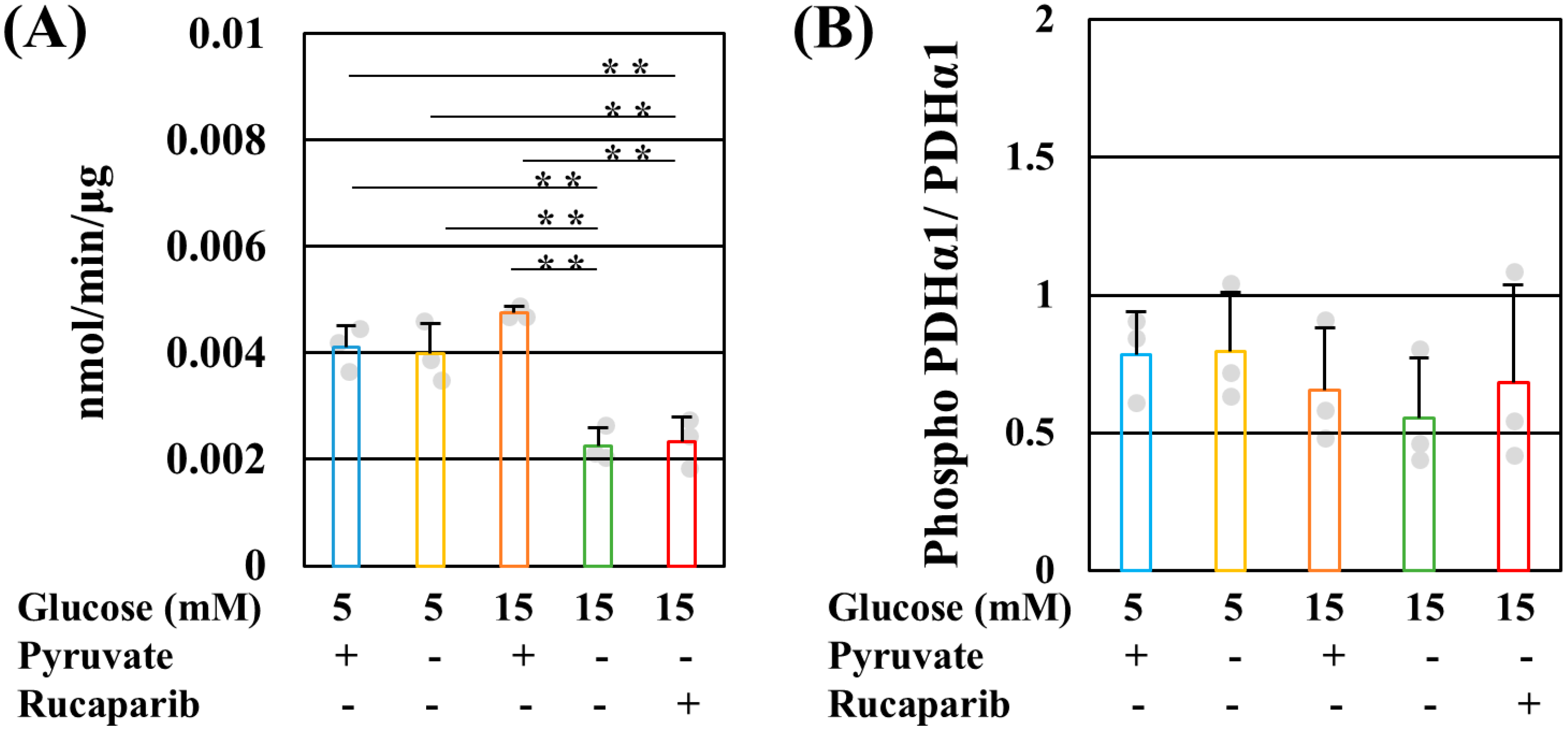
2.1. Pyruvate Starvation under High-Glucose Conditions Decreased Mitochondrial ATP Production by Impaired PDH Activity in PDK- and PARP-Independent Manners
2.2. GAPDH Did Not Receive Poly-ADP-ribosylation under High-Glucose Pyruvate-Starved Conditions
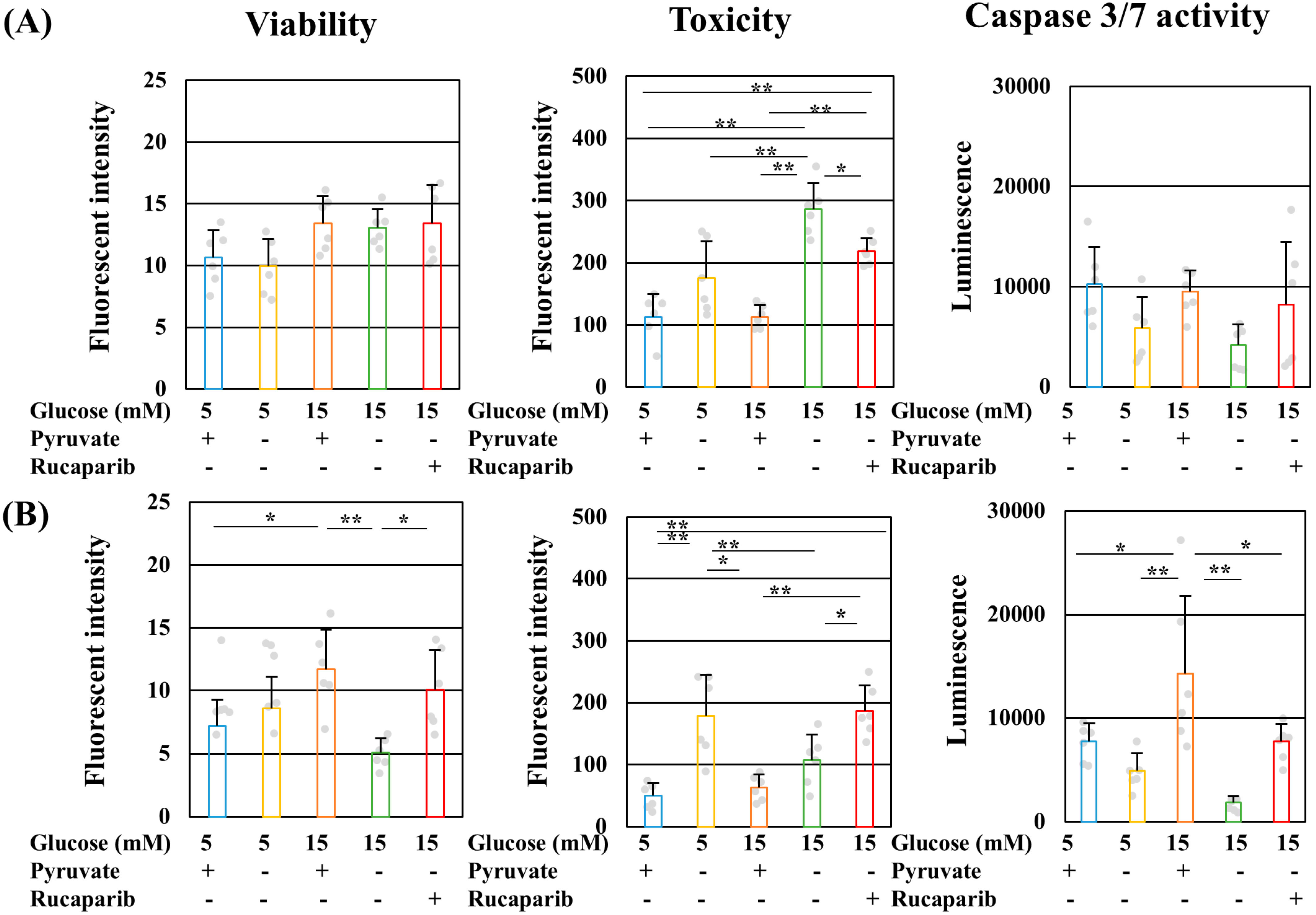
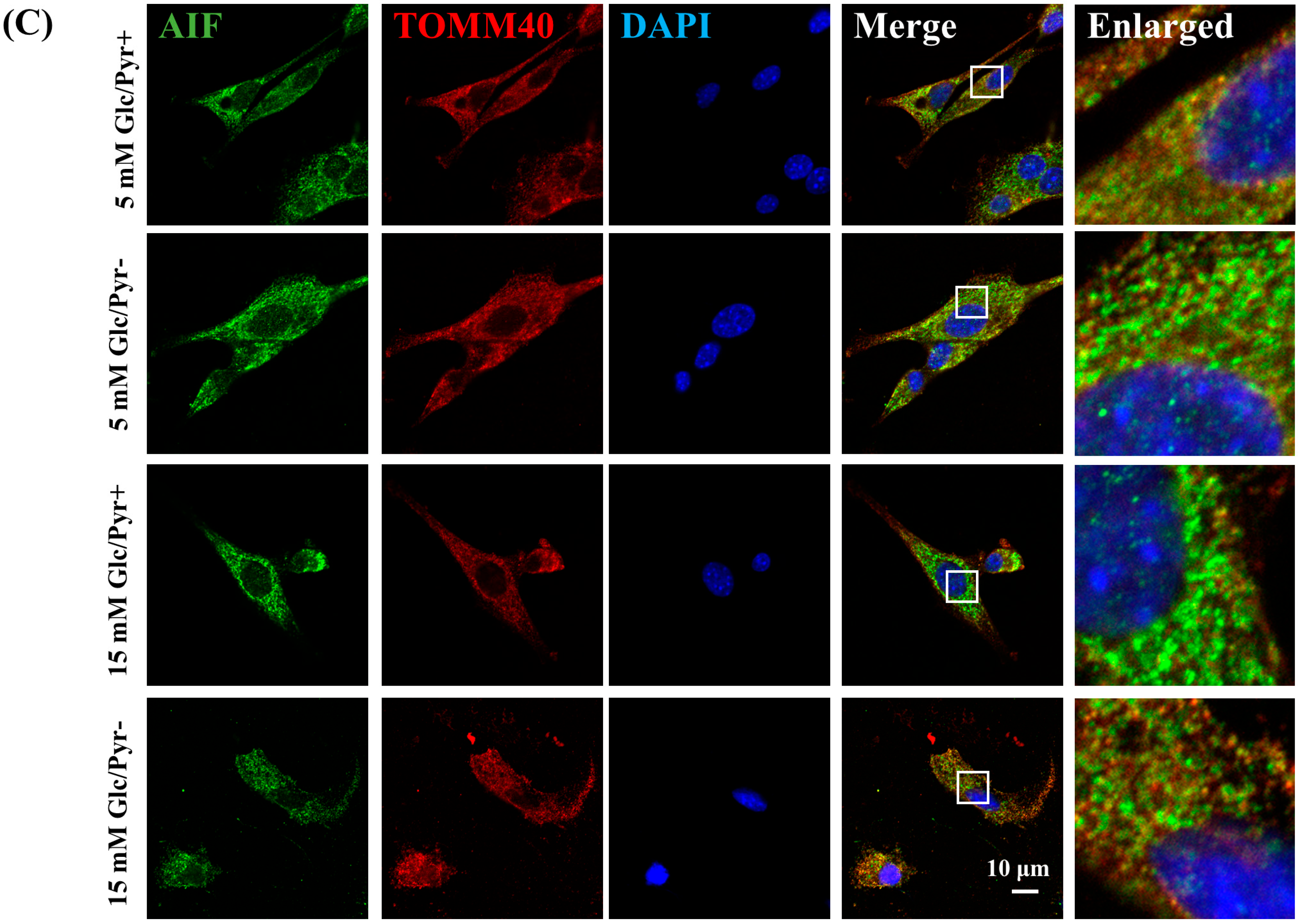
2.3. Pyruvate-Starvation-Induced Necrosis-like Schwann Cell Death under High-Glucose Conditions
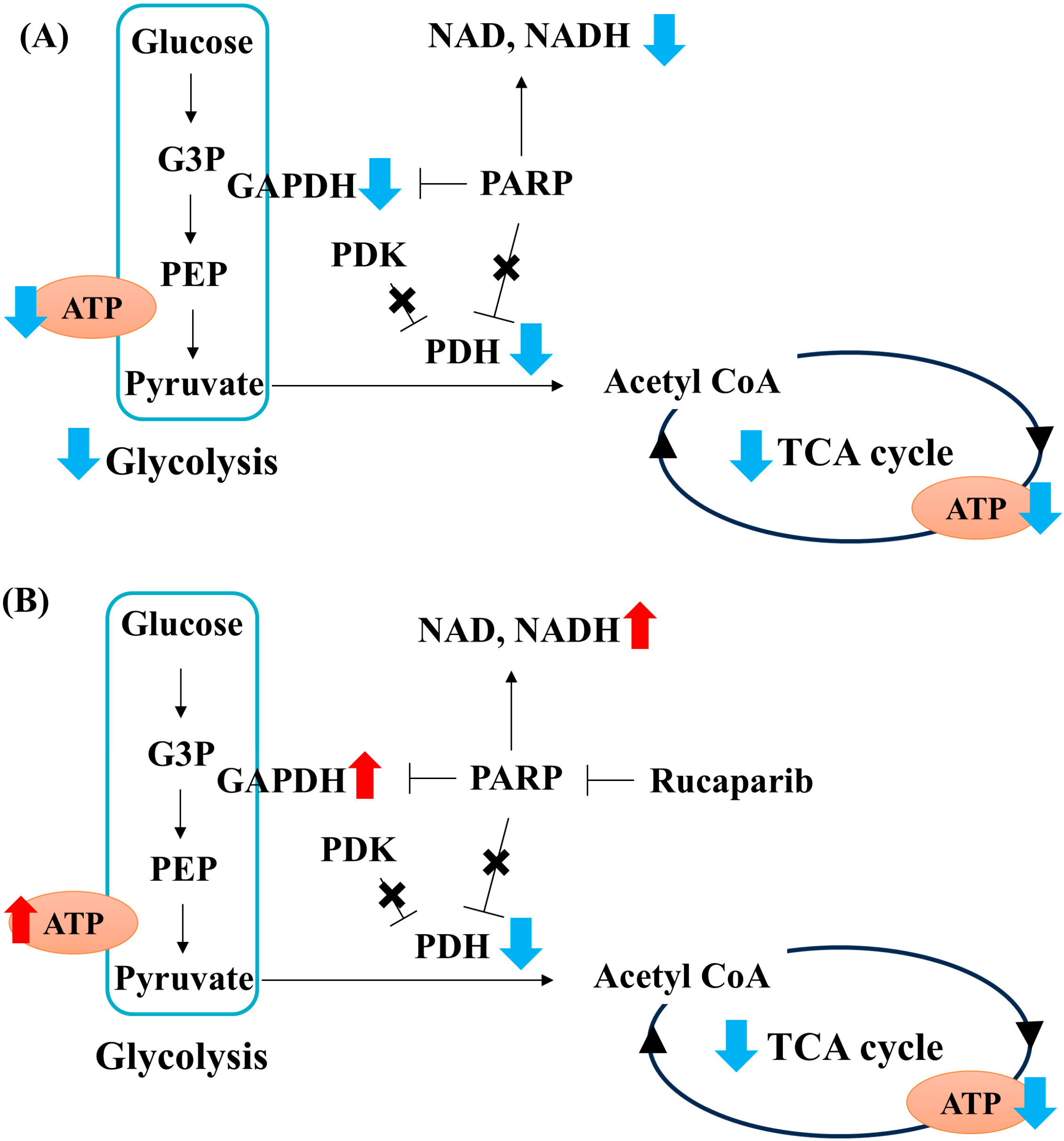
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Measurement of Glycolytic and Mitochondrial ATP Production
4.3. Measurement of Cell Viability, Toxicity, and Caspase 3/7 Activity
4.4. MTS Assay
4.5. Western Blotting
4.6. Immunoprecipitation
4.7. Immunocytochemistry
4.8. Mitochondrial Isolation and Blue Native PAGE
4.9. Measurement of PDH and Complex I Activity
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamiya, H.; Himeno, T.; Watarai, A.; Baba, M.; Nishimura, R.; Tajima, N.; Nakamura, J. Prevalence and characteristics of diabetic symmetric sensorimotor polyneuropathy in Japanese patients with type 2 diabetes: The Japan Diabetes Complication and its Prevention Prospective study (JDCP study 10). J. Diabetes Investig. 2024, 15, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Akamine, T.; Takaku, S.; Suzuki, M.; Niimi, N.; Yako, H.; Matoba, K.; Kawanami, D.; Utsunomiya, K.; Nishimura, R.; Sango, K. Glycolaldehyde induces sensory neuron death through activation of the c-Jun N-terminal kinase and p-38 MAP kinase pathways. Histochem. Cell Biol. 2020, 153, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Niimi, N.; Yako, H.; Takaku, S.; Kato, H.; Matsumoto, T.; Nishito, Y.; Watabe, K.; Ogasawara, S.; Mizukami, H.; Yagihashi, S.; et al. A spontaneously immortalized Schwann cell line from aldose reductase-deficient mice as a useful tool for studying polyol pathway and aldehyde metabolism. J. Neurochem. 2018, 144, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Tatsumi, Y.; Yako, H.; Sango, K.; Himeno, T.; Kondo, M.; Kato, Y.; Kamiya, H.; Nakamura, J.; Kato, K. Recurrent short-term hypoglycemia and hyperglycemia induce apoptosis and oxidative stress via the ER stress response in immortalized adult mouse Schwann (IMS32) cells. Neurosci. Res. 2019, 147, 26–32. [Google Scholar] [CrossRef]
- Yako, H.; Niimi, N.; Takaku, S.; Sango, K. Advantages of omics approaches for elucidating metabolic changes in diabetic peripheral neuropathy. Front. Endocrinol. 2023, 14, 1208441. [Google Scholar] [CrossRef]
- Nihei, W.; Kato, A.; Himeno, T.; Kondo, M.; Nakamura, J.; Kamiya, H.; Sango, K.; Kato, K. Hyperglycaemia Aggravates Oxidised Low-Density Lipoprotein-Induced Schwann Cell Death via Hyperactivation of Toll-like Receptor 4. Neurol. Int. 2024, 16, 370–379. [Google Scholar] [CrossRef]
- Li, F.; Szabo, C.; Pacher, P.; Southan, G.J.; Abatan, O.I.; Charniauskaya, T.; Stevens, M.J.; Obrosova, I.G. Evaluation of orally active poly(ADP-ribose) polymerase inhibitor in streptozotocin-diabetic rat model of early peripheral neuropathy. Diabetologia 2004, 47, 710–717. [Google Scholar] [CrossRef][Green Version]
- Obrosova, I.G.; Li, F.; Omorodola, I.A.; Forsell, M.A.; Komjáti, K.; Pacher, P.; Szabó, C.; Stevens, M.J. Role of Poly(ADP-Ribose) Polymerase Activation in Diabetic Neuropathy. Diabetes 2004, 53, 711–720. [Google Scholar] [CrossRef]
- Obrosova, I.G.; Drel, V.R.; Pacher, P.; Ilnytska, O.; Wang, Z.Q.; Stevens, M.J.; Yorek, M.A. Oxidative-nitrosative stress and poly(ADP-ribose) polymerase (PARP) activation in experimental diabetic neuropathy: The relation is revisited. Diabetes 2005, 54, 3435–3441. [Google Scholar] [CrossRef]
- Obrosova, I.G.; Pacher, P.; Szabo, C.; Zsengeller, Z.; Hirooka, H.; Stevens, M.J.; Yorek, M.A. Aldose reductase inhibition counteracts oxidative-nitrosative stress and poly(ADP-ribose) polymerase activation in tissue sites for diabetes complications. Diabetes 2005, 54, 234–242. [Google Scholar] [CrossRef]
- Ho, E.C.; Lam, K.S.; Chen, Y.S.; Yip, J.C.; Arvindakshan, M.; Yamagishi, S.; Yagihashi, S.; Oates, P.J.; Ellery, C.A.; Chung, S.S.; et al. Aldose reductase-deficient mice are protected from delayed motor nerve conduction velocity, increased c-Jun NH2-terminal kinase activation, depletion of reduced glutathione, increased superoxide accumulation, and DNA damage. Diabetes 2006, 55, 1946–1953. [Google Scholar] [CrossRef]
- Li, F.; Drel, V.R.; Szabo, C.; Stevens, M.J.; Obrosova, I.G. Low-dose poly(ADP-ribose) polymerase inhibitor-containing combination therapies reverse early peripheral diabetic neuropathy. Diabetes 2005, 54, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Negi, G.; Kumar, A.; Sharma, S.S. Concurrent targeting of nitrosative stress-PARP pathway corrects functional, behavioral and biochemical deficits in experimental diabetic neuropathy. Biochem. Biophys. Res. Commun. 2010, 391, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Drel, V.R.; Lupachyk, S.; Shevalye, H.; Vareniuk, I.; Xu, W.; Zhang, J.; Delamere, N.A.; Shahidullah, M.; Slusher, B.; Obrosova, I.G. New therapeutic and biomarker discovery for peripheral diabetic neuropathy: PARP inhibitor, nitrotyrosine, and tumor necrosis factor-alpha. Endocrinology 2010, 151, 2547–2555. [Google Scholar] [CrossRef][Green Version]
- Zilberter, Y.; Gubkina, O.; Ivanov, A.I. A unique array of neuroprotective effects of pyruvate in neuropathology. Front. Neurosci. 2015, 9, 17. [Google Scholar] [CrossRef]
- Guarino, V.A.; Oldham, W.M.; Loscalzo, J.; Zhang, Y.Y. Reaction rate of pyruvate and hydrogen peroxide: Assessing antioxidant capacity of pyruvate under biological conditions. Sci. Rep. 2019, 9, 19568. [Google Scholar] [CrossRef]
- Hegde, K.R.; Varma, S.D. Prevention of cataract by pyruvate in experimentally diabetic mice. Mol. Cell. Biochem. 2005, 269, 115–120. [Google Scholar] [CrossRef]
- Hegde, K.; Kovtun, S.; Varma, S. Prevention of cataract in diabetic mice by topical pyruvate. Clin. Ophthalmol. 2011, 5, 1141–1145. [Google Scholar] [CrossRef][Green Version]
- Ju, K.D.; Shin, E.K.; Cho, E.J.; Yoon, H.B.; Kim, H.S.; Kim, H.; Yang, J.; Hwang, Y.H.; Ahn, C.; Oh, K.H. Ethyl pyruvate ameliorates albuminuria and glomerular injury in the animal model of diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2012, 302, F606–F613. [Google Scholar] [CrossRef]
- Zhang, X.M.; Deng, H.; Tong, J.D.; Wang, Y.Z.; Ning, X.C.; Yang, X.H.; Zhou, F.Q.; Jin, H.M. Pyruvate-Enriched Oral Rehydration Solution Improves Glucometabolic Disorders in the Kidneys of Diabetic db/db Mice. J. Diabetes Res. 2020, 2020, 2817972. [Google Scholar] [CrossRef]
- Yako, H.; Niimi, N.; Kato, A.; Takaku, S.; Tatsumi, Y.; Nishito, Y.; Kato, K.; Sango, K. Role of pyruvate in maintaining cell viability and energy production under high-glucose conditions. Sci. Rep. 2021, 11, 18910. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Fabuel, I.; Le Douce, J.; Logan, A.; James, A.M.; Bonvento, G.; Murphy, M.P.; Almeida, A.; Bolanos, J.P. Complex I assembly into supercomplexes determines differential mitochondrial ROS production in neurons and astrocytes. Proc. Natl. Acad. Sci. USA 2016, 113, 13063–13068. [Google Scholar] [CrossRef] [PubMed]
- Maranzana, E.; Barbero, G.; Falasca, A.I.; Lenaz, G.; Genova, M.L. Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I. Antioxid. Redox Signal. 2013, 19, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.; Genova, M.L.; Parenti Castelli, G.; Lenaz, G. The mitochondrial respiratory chain is partially organized in a supercomplex assembly: Kinetic evidence using flux control analysis. J. Biol. Chem. 2004, 279, 36562–36569. [Google Scholar] [CrossRef]
- Lapuente-Brun, E.; Moreno-Loshuertos, R.; Acin-Perez, R.; Latorre-Pellicer, A.; Colas, C.; Balsa, E.; Perales-Clemente, E.; Quiros, P.M.; Calvo, E.; Rodriguez-Hernandez, M.A.; et al. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 2013, 340, 1567–1570. [Google Scholar] [CrossRef]
- Jha, P.; Wang, X.; Auwerx, J. Analysis of Mitochondrial Respiratory Chain Supercomplexes Using Blue Native Polyacrylamide Gel Electrophoresis (BN-PAGE). Curr. Protoc. Mouse Biol. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Park, S.; Jeon, J.H.; Min, B.K.; Ha, C.M.; Thoudam, T.; Park, B.Y.; Lee, I.K. Role of the Pyruvate Dehydrogenase Complex in Metabolic Remodeling: Differential Pyruvate Dehydrogenase Complex Functions in Metabolism. Diabetes Metab. J. 2018, 42, 270–281. [Google Scholar] [CrossRef]
- Rahman, M.H.; Jha, M.K.; Kim, J.H.; Nam, Y.; Lee, M.G.; Go, Y.; Harris, R.A.; Park, D.H.; Kook, H.; Lee, I.K.; et al. Pyruvate Dehydrogenase Kinase-mediated Glycolytic Metabolic Shift in the Dorsal Root Ganglion Drives Painful Diabetic Neuropathy. J. Biol. Chem. 2016, 291, 6011–6025. [Google Scholar] [CrossRef]
- Du, X.; Matsumura, T.; Edelstein, D.; Rossetti, L.; Zsengeller, Z.; Szabo, C.; Brownlee, M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J. Clin. Investig. 2003, 112, 1049–1057. [Google Scholar] [CrossRef]
- Vyas, S.; Chesarone-Cataldo, M.; Todorova, T.; Huang, Y.H.; Chang, P. A systematic analysis of the PARP protein family identifies new functions critical for cell physiology. Nat. Commun. 2013, 4, 2240. [Google Scholar] [CrossRef]
- Weaver, A.N.; Yang, E.S. Beyond DNA Repair: Additional Functions of PARP-1 in Cancer. Front. Oncol. 2013, 3, 290. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, N.S.; Haince, J.F.; Kang, H.C.; David, K.K.; Andrabi, S.A.; Poirier, G.G.; Dawson, V.L.; Dawson, T.M. Poly(ADP-ribose) (PAR) binding to apoptosis-inducing factor is critical for PAR polymerase-1-dependent cell death (parthanatos). Sci. Signal. 2011, 4, ra20. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Kubo, T.; Ihara, H.; Hikida, T.; Danjo, T.; Nakatsuji, M.; Shahani, N.; Itakura, M.; Ono, Y.; Azuma, Y.T.; et al. Nuclear-translocated Glyceraldehyde-3-phosphate Dehydrogenase Promotes Poly(ADP-ribose) Polymerase-1 Activation during Oxidative/Nitrosative Stress in Stroke. J. Biol. Chem. 2015, 290, 14493–14503. [Google Scholar] [CrossRef] [PubMed]
- Wahlberg, E.; Karlberg, T.; Kouznetsova, E.; Markova, N.; Macchiarulo, A.; Thorsell, A.G.; Pol, E.; Frostell, A.; Ekblad, T.; Oncu, D.; et al. Family-wide chemical profiling and structural analysis of PARP and tankyrase inhibitors. Nat. Biotechnol. 2012, 30, 283–288. [Google Scholar] [CrossRef]
- Mayo, E.; Fabrizio, G.; Scarpa, E.; Stilla, A.; Dani, N.; Chiacchiera, F.; Kleine, H.; Attanasio, F.; Lüscher, B.; Di Girolamo, M. ARTD10/PARP10 Induces ADP-Ribosylation of GAPDH and Recruits GAPDH into Cytosolic Membrane-Free Cell Bodies When Overexpressed in Mammalian Cells. Challenges 2018, 9, 22. [Google Scholar] [CrossRef]
- Leist, M.; Single, B.; Naumann, H.; Fava, E.; Simon, B.; Kuhnle, S.; Nicotera, P. Inhibition of mitochondrial ATP generation by nitric oxide switches apoptosis to necrosis. Exp. Cell Res. 1999, 249, 396–403. [Google Scholar] [CrossRef]
- Lieberthal, W.; Menza, S.A.; Levine, J.S. Graded ATP depletion can cause necrosis or apoptosis of cultured mouse proximal tubular cells. Am. J. Physiol. 1998, 274, F315–F327. [Google Scholar] [CrossRef]
- Andrabi, S.A.; Umanah, G.K.; Chang, C.; Stevens, D.A.; Karuppagounder, S.S.; Gagne, J.P.; Poirier, G.G.; Dawson, V.L.; Dawson, T.M. Poly(ADP-ribose) polymerase-dependent energy depletion occurs through inhibition of glycolysis. Proc. Natl. Acad. Sci. USA 2014, 111, 10209–10214. [Google Scholar] [CrossRef]
- Zamaraeva, M.V.; Sabirov, R.Z.; Maeno, E.; Ando-Akatsuka, Y.; Bessonova, S.V.; Okada, Y. Cells die with increased cytosolic ATP during apoptosis: A bioluminescence study with intracellular luciferase. Cell Death Differ. 2005, 12, 1390–1397. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Z.; Kolwicz, S.C., Jr.; Abell, L.; Roe, N.D.; Kim, M.; Zhou, B.; Cao, Y.; Ritterhoff, J.; Gu, H.; et al. Defective Branched-Chain Amino Acid Catabolism Disrupts Glucose Metabolism and Sensitizes the Heart to Ischemia-Reperfusion Injury. Cell Metab. 2017, 25, 374–385. [Google Scholar] [CrossRef]
- Mizukami, H.; Osonoi, S.; Takaku, S.; Yamagishi, S.; Ogasawara, S.; Sango, K.; Chung, S.; Yagihashi, S. Role of glucosamine in development of diabetic neuropathy independent of the aldose reductase pathway. Brain Commun. 2020, 2, fcaa168. [Google Scholar] [CrossRef] [PubMed]
- Hammes, H.P.; Du, X.; Edelstein, D.; Taguchi, T.; Matsumura, T.; Ju, Q.; Lin, J.; Bierhaus, A.; Nawroth, P.; Hannak, D.; et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat. Med. 2003, 9, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Chen, Y.; Tishkoff, D.X.; Peng, C.; Tan, M.; Dai, L.; Xie, Z.; Zhang, Y.; Zwaans, B.M.; Skinner, M.E.; et al. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol. Cell 2013, 50, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Antoun, G.; McMurray, F.; Thrush, A.B.; Patten, D.A.; Peixoto, A.C.; Slack, R.S.; McPherson, R.; Dent, R.; Harper, M.E. Impaired mitochondrial oxidative phosphorylation and supercomplex assembly in rectus abdominis muscle of diabetic obese individuals. Diabetologia 2015, 58, 2861–2866. [Google Scholar] [CrossRef]
- Alejandra Sanchez-Munoz, M.; Valdez-Solana, M.A.; Campos-Almazan, M.I.; Flores-Herrera, O.; Esparza-Perusquia, M.; Olvera-Sanchez, S.; Garcia-Arenas, G.; Avitia-Dominguez, C.; Tellez-Valencia, A.; Sierra-Campos, E. Streptozotocin-Induced Adaptive Modification of Mitochondrial Supercomplexes in Liver of Wistar Rats and the Protective Effect of Moringa oleifera Lam. Biochem. Res. Int. 2018, 2018, 5681081. [Google Scholar] [CrossRef]
- Jeoung, N.H.; Harris, C.R.; Harris, R.A. Regulation of pyruvate metabolism in metabolic-related diseases. Rev. Endocr. Metab. Disord. 2014, 15, 99–110. [Google Scholar] [CrossRef]
- Hinder, L.M.; Vivekanandan-Giri, A.; McLean, L.L.; Pennathur, S.; Feldman, E.L. Decreased glycolytic and tricarboxylic acid cycle intermediates coincide with peripheral nervous system oxidative stress in a murine model of type 2 diabetes. J. Endocrinol. 2013, 216, 1–11. [Google Scholar] [CrossRef]
- Konrad, T.; Vicini, P.; Kusterer, K.; Hoflich, A.; Assadkhani, A.; Bohles, H.J.; Sewell, A.; Tritschler, H.J.; Cobelli, C.; Usadel, K.H. alpha-Lipoic acid treatment decreases serum lactate and pyruvate concentrations and improves glucose effectiveness in lean and obese patients with type 2 diabetes. Diabetes Care 1999, 22, 280–287. [Google Scholar] [CrossRef]
- Lin, H.T.; Cheng, M.L.; Lo, C.J.; Lin, G.; Lin, S.F.; Yeh, J.T.; Ho, H.Y.; Lin, J.R.; Liu, F.C. 1H Nuclear Magnetic Resonance (NMR)-Based Cerebrospinal Fluid and Plasma Metabolomic Analysis in Type 2 Diabetic Patients and Risk Prediction for Diabetic Microangiopathy. J. Clin. Med. 2019, 8, 874. [Google Scholar] [CrossRef]
- Jin, Q.; Ma, R.C.W. Metabolomics in Diabetes and Diabetic Complications: Insights from Epidemiological Studies. Cells 2021, 10, 2832. [Google Scholar] [CrossRef]
- Gao, H.; Jiang, Q.; Ji, H.; Ning, J.; Li, C.; Zheng, H. Type 1 diabetes induces cognitive dysfunction in rats associated with alterations of the gut microbiome and metabolomes in serum and hippocampus. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 165541. [Google Scholar] [CrossRef] [PubMed]
- Diao, C.; Zhao, L.; Guan, M.; Zheng, Y.; Chen, M.; Yang, Y.; Lin, L.; Chen, W.; Gao, H. Systemic and characteristic metabolites in the serum of streptozotocin-induced diabetic rats at different stages as revealed by a 1H-NMR based metabonomic approach. Mol. Biosyst. 2014, 10, 686–693. [Google Scholar] [CrossRef]
- Das, B.K.; Jayalakshmi, K.; Gadad, P.C. 1H-NMR-based serum metabolomic study to evaluate the effect of asarone and metformin on experimentally induced diabetic hepatocellular carcinoma in rats. Bull. Natl. Res. Cent. 2022, 46. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, X.; Xie, L.; Gao, H.; Lin, D. 1H NMR-based metabonomic analysis of metabolic changes in streptozotocin-induced diabetic rats. Anal. Sci. 2010, 26, 1277–1282. [Google Scholar] [CrossRef]
- Rawat, A.; Misra, G.; Saxena, M.; Tripathi, S.; Dubey, D.; Saxena, S.; Aggarwal, A.; Gupta, V.; Khan, M.Y.; Prakash, A. 1H NMR Based Serum Metabolic Profiling Reveals Differentiating Biomarkers in Patients with Diabetes and Diabetes Comorbidity. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 13, 290–298. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yako, H.; Niimi, N.; Takaku, S.; Kato, A.; Kato, K.; Sango, K. Role of Exogenous Pyruvate in Maintaining Adenosine Triphosphate Production under High-Glucose Conditions through PARP-Dependent Glycolysis and PARP-Independent Tricarboxylic Acid Cycle. Int. J. Mol. Sci. 2024, 25, 11089. https://doi.org/10.3390/ijms252011089
Yako H, Niimi N, Takaku S, Kato A, Kato K, Sango K. Role of Exogenous Pyruvate in Maintaining Adenosine Triphosphate Production under High-Glucose Conditions through PARP-Dependent Glycolysis and PARP-Independent Tricarboxylic Acid Cycle. International Journal of Molecular Sciences. 2024; 25(20):11089. https://doi.org/10.3390/ijms252011089
Chicago/Turabian StyleYako, Hideji, Naoko Niimi, Shizuka Takaku, Ayako Kato, Koichi Kato, and Kazunori Sango. 2024. "Role of Exogenous Pyruvate in Maintaining Adenosine Triphosphate Production under High-Glucose Conditions through PARP-Dependent Glycolysis and PARP-Independent Tricarboxylic Acid Cycle" International Journal of Molecular Sciences 25, no. 20: 11089. https://doi.org/10.3390/ijms252011089
APA StyleYako, H., Niimi, N., Takaku, S., Kato, A., Kato, K., & Sango, K. (2024). Role of Exogenous Pyruvate in Maintaining Adenosine Triphosphate Production under High-Glucose Conditions through PARP-Dependent Glycolysis and PARP-Independent Tricarboxylic Acid Cycle. International Journal of Molecular Sciences, 25(20), 11089. https://doi.org/10.3390/ijms252011089






