Neodymium-Facilitated Visualization of Extreme Phosphate Accumulation in Fibroblast Filopodia: Implications for Intercellular and Cell–Matrix Interactions
Abstract
1. Introduction
2. Results
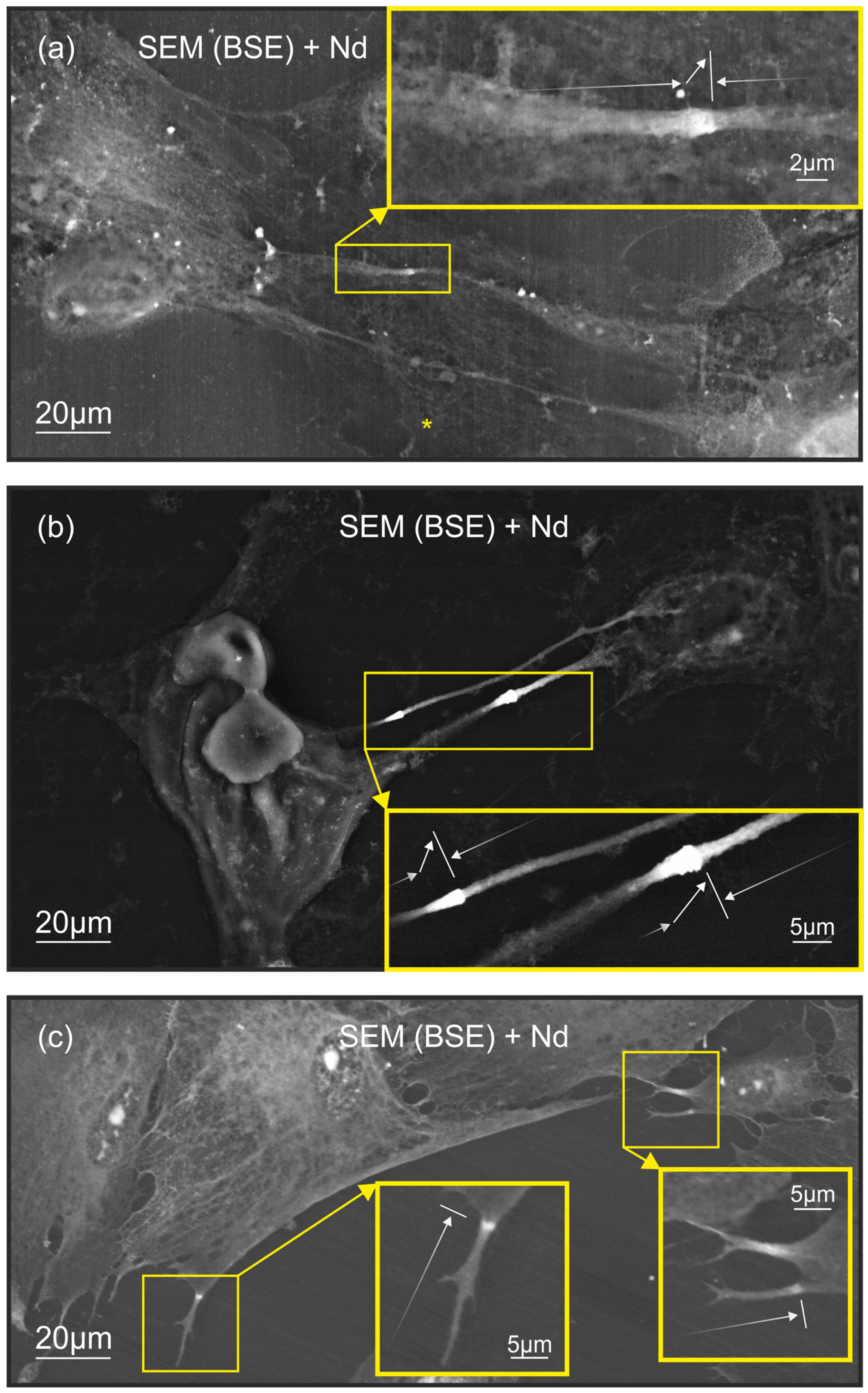
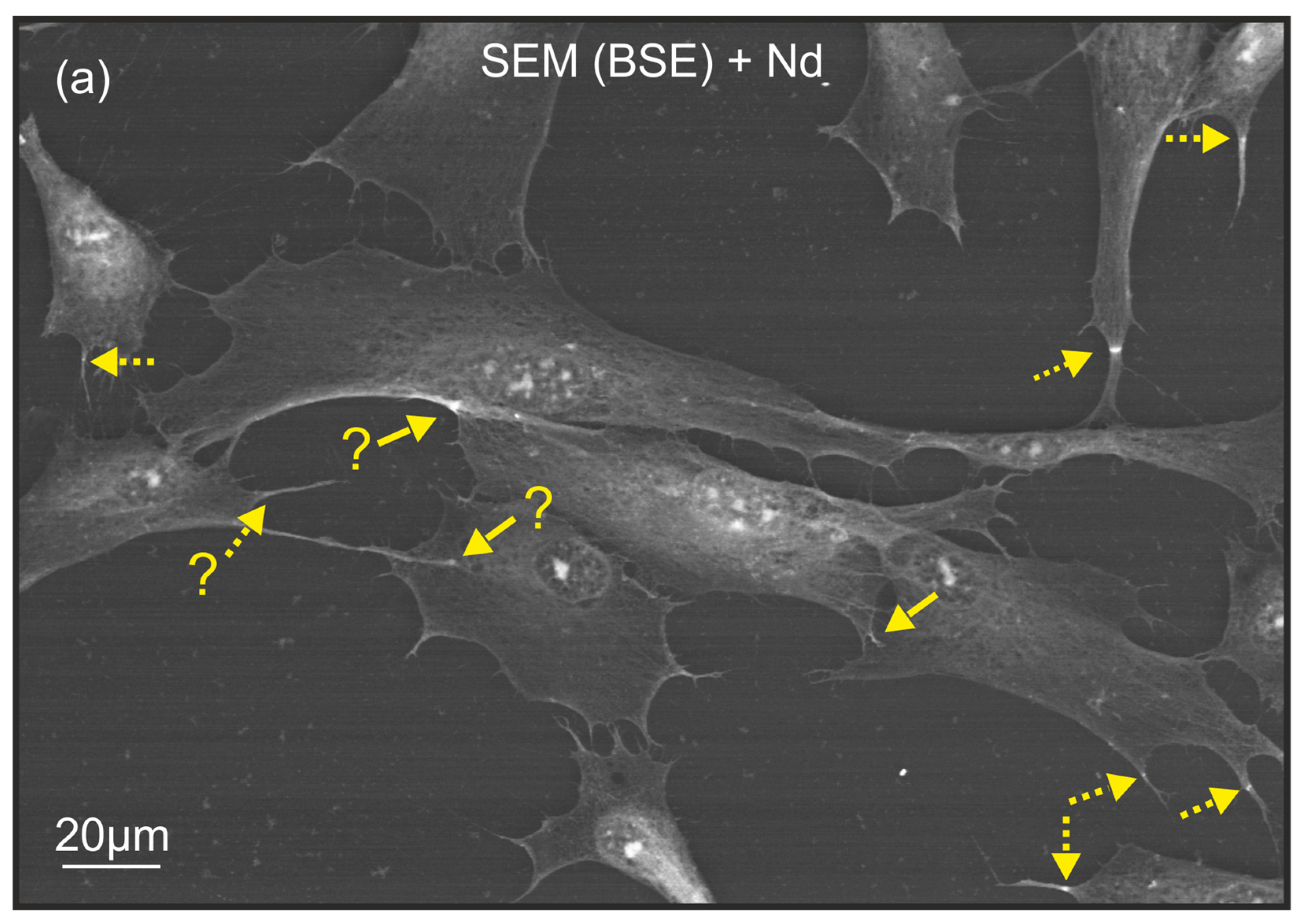
2.1. Characteristics of Neodymium Staining of Filopodia in Cell Culture
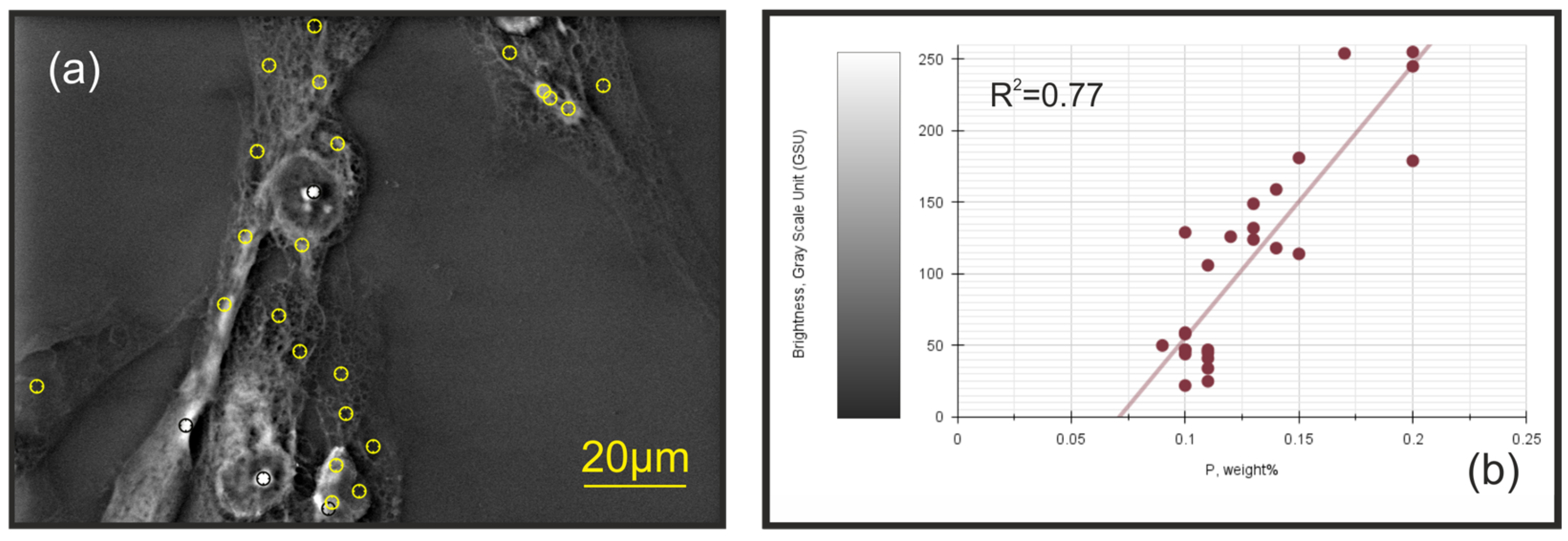
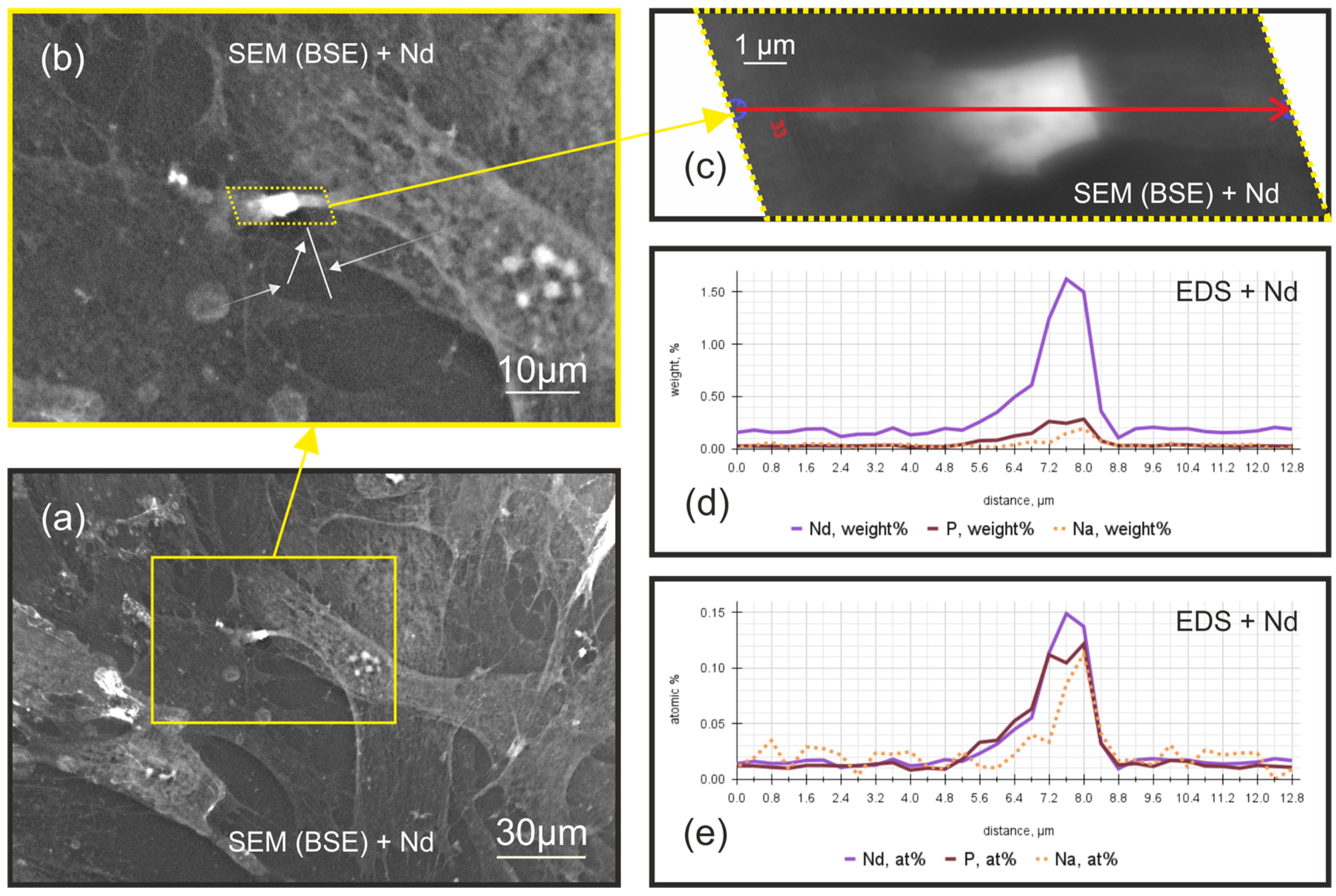
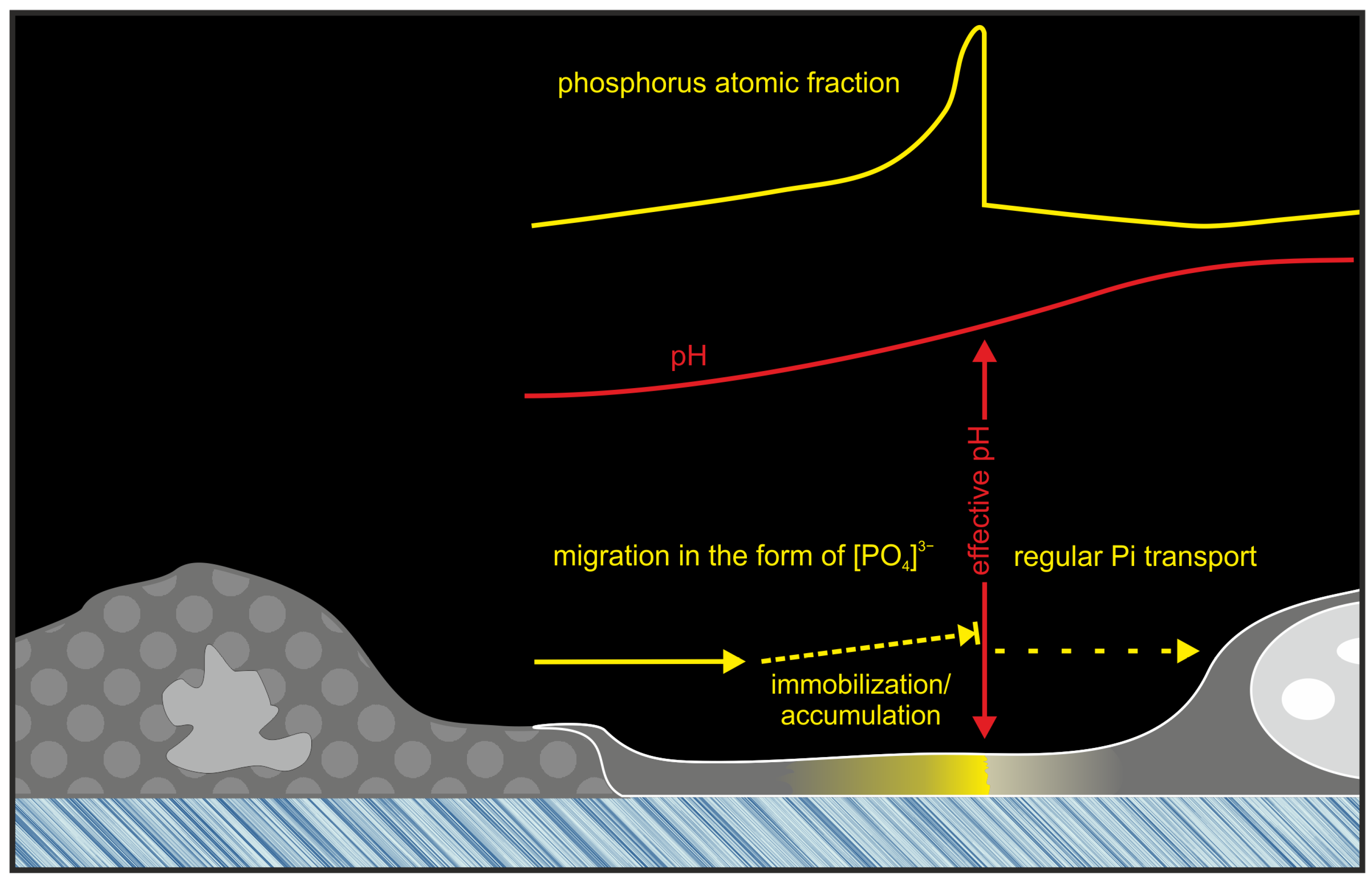
2.2. The Decoding of the (Bio)Chemical Nature of Extreme Contrast Zones in Filopodia—Data from Semi-Quantitative Chemical Microanalysis Using Scanning Electron Microscopy (SEM) Coupled with Energy-Dispersive X-ray Spectroscopy (EDS)
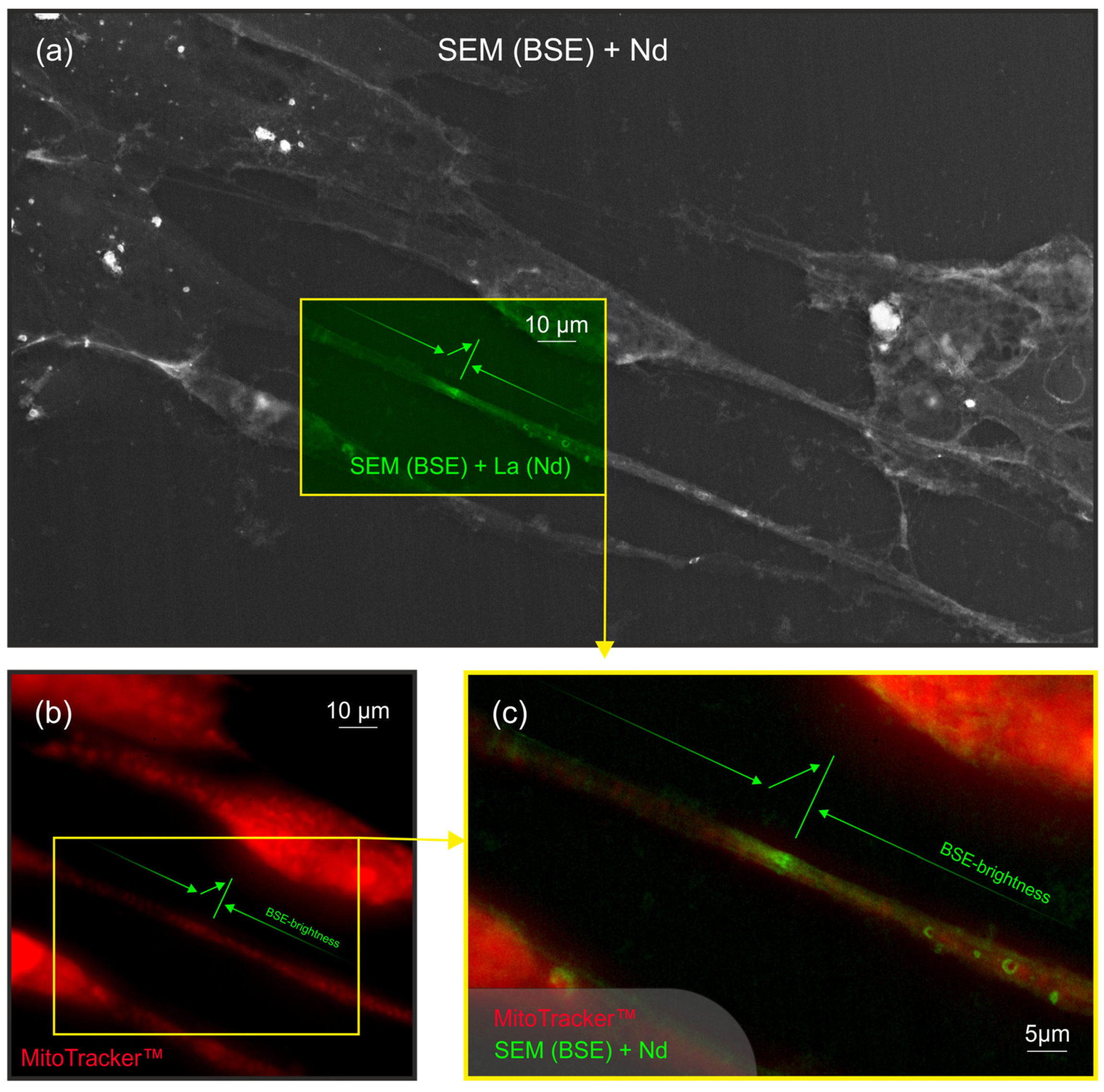
2.3. Non-Colocalization of Mitochondria and Neodymium in Keratocyte Filopodia
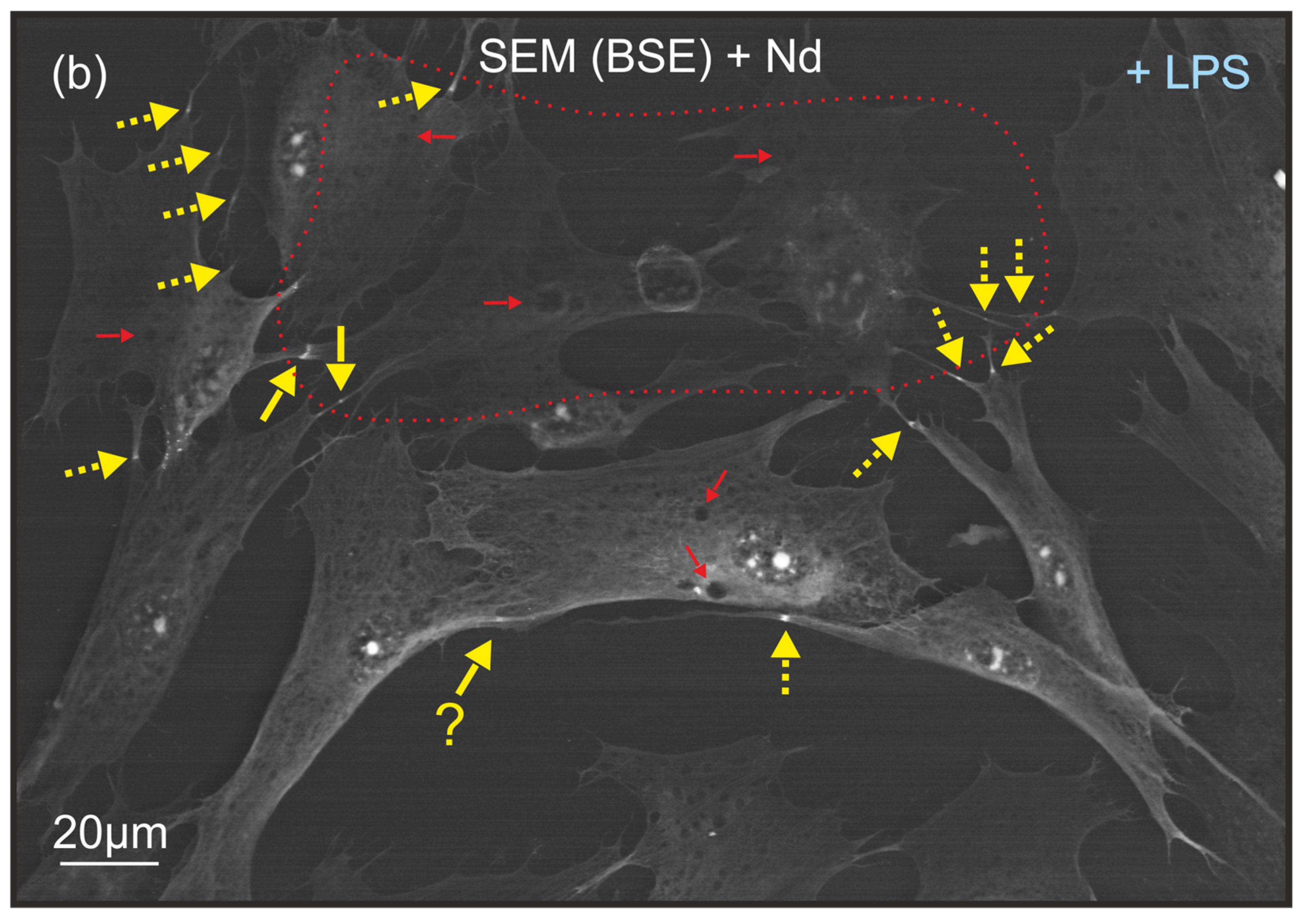
2.4. The Frequency of Filopodia Observations with Zones of Extreme Contrast in Culture with Induced Apoptosis
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Preparation, Visualization Using SEM, and Semi-Quantitative Chemical Microanalysis
4.3. Determination of the Mitochondrial Localization in Cell Culture
4.4. Combining Images from Scanning Electron Microscopy and Fluorescence Microscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wood, W.; Martin, P. Structures in focus—Filopodia. Int. J. Biochem. Cell Biol. 2002, 34, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Gallop, J.L. Filopodia and their links with membrane traffic and cell adhesion. Semin. Cell Dev. Biol. 2020, 102, 81–89. [Google Scholar] [CrossRef]
- Jacquemet, G.; Stubb, A.; Saup, R.; Miihkinen, M.; Kremneva, E.; Hamidi, H.; Ivaska, J. Filopodome Mapping Identifies p130Cas as a Mechanosensitive Regulator of Filopodia Stability. Curr. Biol. 2019, 29, 202–216.e7. [Google Scholar] [CrossRef]
- Chang, M.; Oh, J.; Doh, J.; Lee, J.B. F-actin dynamics transform filopodial bridges into intercellular nanotubes capable of distant cell communication. BioRxiv 2018, 405340. [Google Scholar] [CrossRef]
- Korenkova, O.; Pepe, A.; Zurzolo, C. Fine intercellular connections in development: TNTs, cytonemes, or intercellular bridges. Cell Stress 2020, 4, 30–43. [Google Scholar] [CrossRef]
- Cordero Cervantes, D.; Zurzolo, C. Peering into tunneling nanotubes—The path forward. EMBO J. 2021, 40, e105789. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, X. Opportunities and Challenges in Tunneling Nanotubes Research: How Far from Clinical Application. Int. J. Mol. Sci. 2021, 22, 2306. [Google Scholar] [CrossRef] [PubMed]
- Eugenin, E.; Camporesi, E.; Peracchia, C. Direct Cell-Cell Communication via Membrane Pores, Gap Junction Channels, and Tunneling Nanotubes: Medical Relevance of Mitochondrial Exchange. Int. J. Mol. Sci. 2022, 23, 6133. [Google Scholar] [CrossRef]
- Guan, F.; Wu, X.; Zhou, J.; Lin, Y.; He, Y.; Fan, C.; Zeng, Z.; Xiong, W. Mitochondrial transfer in tunneling nanotubes-a new target for cancer therapy. J. Exp. Clin. Cancer Res. 2024, 43, 147. [Google Scholar] [CrossRef]
- Delage, E.; Cervantes, D.C.; Pénard, E.; Schmitt, C.; Syan, S.; Disanza, A.; Scita, G.; Zurzolo, C. Differential identity of Filopodia and Tunneling Nanotubes revealed by the opposite functions of actin regulatory complexes. Sci. Rep. 2016, 6, 39632. [Google Scholar] [CrossRef]
- Guo, L.E.; Zhang, J.F.; Liu, X.Y.; Zhang, L.M.; Zhang, H.L.; Chen, J.H.; Xie, X.G.; Zhou, Y.; Luo, K.; Yoon, J. Phosphate ion targeted colorimetric and fluorescent probe and its use to monitor endogeneous phosphate ion in a hemichannel-closed cell. Anal. Chem. 2015, 87, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Sartori-Rupp, A.; Cordero Cervantes, D.; Pepe, A. Correlative cryo-electron microscopy reveals the structure of TNTs in neuronal cells. Nat. Commun. 2019, 10, 342. [Google Scholar] [CrossRef]
- Weissenberger, G.; Henderikx, R.J.M.; Peters, P.J. Understanding the invisible hands of sample preparation for cryo-EM. Nat. Methods 2021, 18, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Novikov, I.A.; Subbot, A.M.; Fedorov, A.A.; Griboedova, I.G.; Antonov, E.N.; Vakhrushev, I.V. Supravital lanthanoid staining for scanning electron microscopy of biological objects. Genes Cells 2015, 10, 90–96. [Google Scholar]
- Novikov, I.A.; Subbot, A.M.; Pak, O.A.; Chebotar, I.V. A two-step labeling of biological object’s ultrastructures by neodymium and lead for scanning electron microscopy. Analytics 2018, 4, 358–363. [Google Scholar] [CrossRef]
- Novikov, I.A.; Subbot, A.M.; Turenok, A.; Mayansky, N.A.; Chebotar, I.V. A rapid method of whole cell sample preparation for scanning electron microscopy using neodymium chloride. Micron 2019, 124, 102687. [Google Scholar] [CrossRef] [PubMed]
- Subbot, A.; Kondratieva, S.; Novikov, I.; Gogoleva, N.; Kozlova, O.; Chebotar, I.; Gazizova, G.; Ryabova, A.; Vorontsova, M.; Kikawada, T.; et al. Life-On-Hold: Lanthanoids Rapidly Induce a Reversible Ametabolic State in Mammalian Cells. Biology 2021, 10, 607. [Google Scholar] [CrossRef]
- Sahoo, J.; Jaiswar, S.; Chatterjee, P.B.; Subramanian, P.S.; Jena, H.S. Mechanistic Insight of Sensing Hydrogen Phosphate in Aqueous Medium by Using Lanthanoids(III)-Based Luminescent Probes. Nanomaterials 2021, 11, 53. [Google Scholar] [CrossRef]
- Sahoo, J.; Krishnaraj, C.; Sun, J.; Panda, B.B.; Subramanian, P.S.; Jena, H.S. Lanthanoids based inorganic phosphates and biological nucleotides sensor. Coord. Chem. Rev. 2022, 466, 214583. [Google Scholar] [CrossRef]
- Martinon, T.L.; Pierre, V.C. Luminescent lanthanoids probes for inorganic and organic phosphates. Chem. Asian J. 2022, 17, e202200495. [Google Scholar] [CrossRef]
- Kolodny, N.H.; Albert, D.M.; Epstein, J.; Ruzzo, M.; Sprengnether, M. Characterization of human uveal melanoma cells by phosphorus 31 nuclear magnetic resonance spectroscopy. Am. J. Ophthalmol. 1985, 100, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.C.P. NMR: Principles and Applications to Biomedical Research; Pettegrew, J.W., Ed.; Springer: New York, NY, USA, 1990; pp. 124–156. [Google Scholar]
- Greiner, J.V.; Kopp, S.J.; Glonek, T. Phosphorus nuclear magnetic resonance and ocular metabolism. Surv. Ophthalmol. 1985, 30, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Yamamoto, M.; Yamamoto, Y.; Tanaka, K. EXAFS study on the cause of enrichment of heavy REEs on bacterial cell surfaces. Geochim. Cosmochim. Acta 2010, 74, 5443–5462. [Google Scholar] [CrossRef]
- Yamasaki, S.; Shirai, O.; Kano, K.; Kozai, N.; Sakamoto, F.; Ohnuki, T. Adsorption behavior of lanthanoids ions on nonbiological phospholipid membranes: A model study using liposome. Chem. Lett. 2013, 42, 819–821. [Google Scholar] [CrossRef]
- Nikolova, V.; Kircheva, N.; Dobrev, S.; Angelova, S.; Dudev, T. Lanthanoidss as Calcium Mimetic Species in Calcium-Signaling/ Buffering Proteins: The Effect of Lanthanoids Type on the Ca2+/Ln3+ Competition. Int. J. Mol. Sci. 2023, 24, 6297. [Google Scholar] [CrossRef]
- Lock, J.T.; Parker, I.; Smith, I.F. Communication of Ca2+ signals via tunneling membrane nanotubes is mediated by transmission of inositol trisphosphate through gap junctions. Cell Calcium 2016, 60, 266–272. [Google Scholar] [CrossRef]
- Komiyama, M. Ce-based solid-phase catalysts for phosphate hydrolysis as new tools for next-generation nanoarchitectonics. Sci. Technol. Adv. Mater. 2023, 24, 2250705. [Google Scholar] [CrossRef]
- Kassai, M.; Teopipithaporn, R.; Grant, K.B. Hydrolysis of phosphatidylcholine by cerium (IV) releases significant amounts of choline and inorganic phosphate at lysosomal pH. J. Inorg. Biochem. 2011, 105, 215–223. [Google Scholar] [CrossRef]
- Huang, C.; Cai, S.; Zou, L.; Feng, J.; Xie, J.; Xie, B. Kinetic Study of the Hydrolysis of BNPP by the Cerium (III) Complex. J. Dispers. Sci. Technol. 2012, 33, 1292–1296. [Google Scholar] [CrossRef]
- Mirza, M.R.; Rainer, M.; Güzel, Y.; Choudhary, I.M.; Bonn, G.K. A novel strategy for phosphopeptide enrichment using lanthanoids phosphate co-precipitation. Anal. Bioanal. Chem. 2012, 404, 853–862. [Google Scholar] [CrossRef]
- Luedtke, N.W.; Schepartz, A. Lanthanoids-mediated phosphoester hydrolysis and phosphate elimination from phosphopeptides. Chem. Commun. 2005, 43, 5426–5428. [Google Scholar] [CrossRef] [PubMed]
- Ro, H.A.; Carson, J.H. pH microdomains in oligodendrocytes. J. Biol. Chem. 2004, 279, 37115–37123. [Google Scholar] [CrossRef] [PubMed]
- Angelova, M.I.; Bitbol, A.F.; Seigneuret, M.; Staneva, G.; Kodama, A.; Sakuma, Y.; Puff, N. pH sensing by lipids in membranes: The fundamentals of pH-driven migration, polarization and deformations of lipid bilayer assemblies. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 2042–2063. [Google Scholar] [CrossRef]
- Sánchez-Armáss, S.; Sennoune, S.R.; Maiti, D.; Ortega, F.; Martínez-Zaguilán, R. Spectral imaging microscopy demonstrates cytoplasmic pH oscillations in glial cells. Am. J. Physiol.-Cell Physiol. 2006, 290, C524–C538. [Google Scholar] [CrossRef]
- Bon, N.; Couasnay, G.; Bourgine, A.; Sourice, S.; Beck-Cormier, S.; Guicheux, J.; Beck, L. Phosphate (Pi)-regulated heterodimerization of the high-affinity sodium-dependent Pi transporters PiT1/Slc20a1 and PiT2/Slc20a2 underlies extracellular Pi sensing independently of Pi uptake. J. Biol. Chem. 2018, 293, 2102–2114. [Google Scholar] [CrossRef] [PubMed]
- Michigami, T.; Kawai, M.; Yamazaki, M.; Ozono, K. Phosphate as a Signaling Molecule and Its Sensing Mechanism. Physiol. Rev. 2018, 98, 2317–2348. [Google Scholar] [CrossRef]
- Kritmetapak, K.; Kumar, R. Phosphate as a Signaling Molecule. Calcif. Tissue Int. 2021, 108, 16–31. [Google Scholar] [CrossRef]
- Bradbury, D.A.; Simmons, T.D.; Slater, K.J.; Crouch, S.P.M. Measurement of the ADP:ATP ratio in human leukaemic cell lines can be used as an indicator of cell viability, necrosis and apoptosis. J. Immunol. Methods 2000, 240, 79–92. [Google Scholar] [CrossRef]
- Lagadic-Gossmann, D.; Huc, L.; Lecureur, V. Alterations of intracellular pH homeostasis in apoptosis: Origins and roles. Cell Death Differ. 2004, 11, 953–961. [Google Scholar] [CrossRef]
- Izard, T.; Brown, D.T. Mechanisms and Functions of Vinculin Interactions with Phospholipids at Cell Adhesion Sites. J. Biol. Chem. 2016, 291, 2548–2555. [Google Scholar] [CrossRef]
- Matsubayashi, H.T.; Mountain, J.; Takahashi, N.; Deb Roy, A.; Yao, T.; Peterson, A.F.; Saez Gonzalez, C.; Kawamata, I.; Inoue, T. Non-catalytic role of phosphoinositide 3-kinase in mesenchymal cell migration through non-canonical induction of p85β/AP2-mediated endocytosis. Nat. Commun. 2024, 15, 2612. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Jiang, X.; Yang, Q.; Zhao, J.; Zhou, Q.; Zhou, Y. The Functions, Methods, and Mobility of Mitochondrial Transfer Between Cells. Front. Oncol. 2021, 11, 672781. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, M.; Alikhani, Z.; Graves, D.T. Apoptotic effects of LPS on fibroblasts are indirectly mediated through TNFR1. J. Dent. Res. 2004, 83, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Usage Protocol «BioREE-A». Available online: https://bioree.ru/ru/produkty-serii-bioree/nabor-dlya-supravitalnogo-kontrastirovaniya-bioree/#4 (accessed on 7 September 2024).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kravchik, M.; Subbot, A.; Bilyalov, A.; Novikov, I.; Deviatiiarov, R.; Yusef, Y.; Gusev, O. Neodymium-Facilitated Visualization of Extreme Phosphate Accumulation in Fibroblast Filopodia: Implications for Intercellular and Cell–Matrix Interactions. Int. J. Mol. Sci. 2024, 25, 11076. https://doi.org/10.3390/ijms252011076
Kravchik M, Subbot A, Bilyalov A, Novikov I, Deviatiiarov R, Yusef Y, Gusev O. Neodymium-Facilitated Visualization of Extreme Phosphate Accumulation in Fibroblast Filopodia: Implications for Intercellular and Cell–Matrix Interactions. International Journal of Molecular Sciences. 2024; 25(20):11076. https://doi.org/10.3390/ijms252011076
Chicago/Turabian StyleKravchik, Marina, Anastasia Subbot, Airat Bilyalov, Ivan Novikov, Ruslan Deviatiiarov, Yusef Yusef, and Oleg Gusev. 2024. "Neodymium-Facilitated Visualization of Extreme Phosphate Accumulation in Fibroblast Filopodia: Implications for Intercellular and Cell–Matrix Interactions" International Journal of Molecular Sciences 25, no. 20: 11076. https://doi.org/10.3390/ijms252011076
APA StyleKravchik, M., Subbot, A., Bilyalov, A., Novikov, I., Deviatiiarov, R., Yusef, Y., & Gusev, O. (2024). Neodymium-Facilitated Visualization of Extreme Phosphate Accumulation in Fibroblast Filopodia: Implications for Intercellular and Cell–Matrix Interactions. International Journal of Molecular Sciences, 25(20), 11076. https://doi.org/10.3390/ijms252011076







