Hemodiafiltration May Be Associated with Senescence-Related Phenotypic Alterations of Lymphocytes, Which May Predict Mortality in Patients Undergoing Dialysis
Abstract
:1. Introduction
2. Results
2.1. Patients’ Characteristics
2.2. Comparison of Immune Phenotypes between Patients Undergoing Dialysis and Healthy Controls
2.3. Comparison of Immune Phenotypes between Patients Undergoing HD and HDF
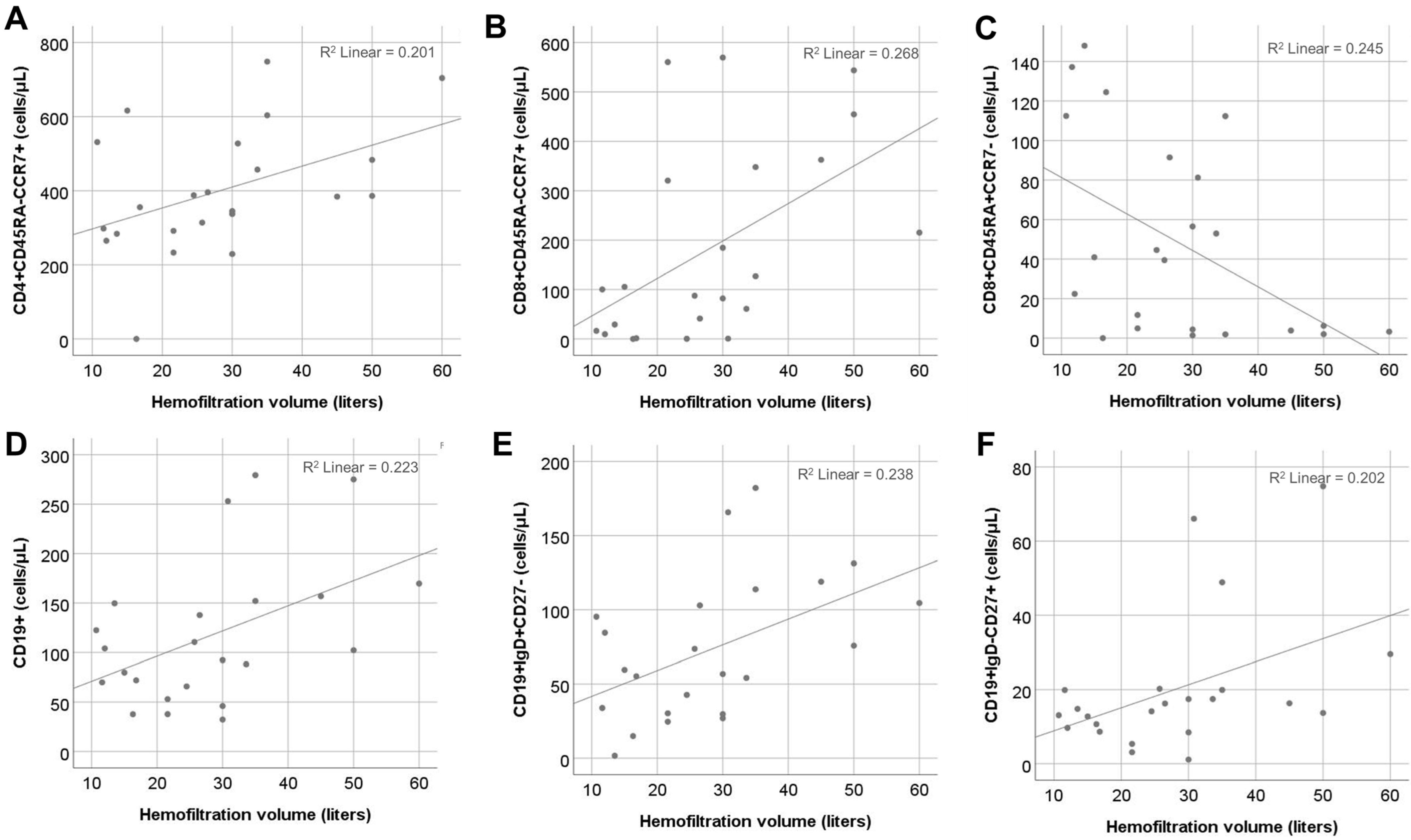
2.4. Effect of HFV
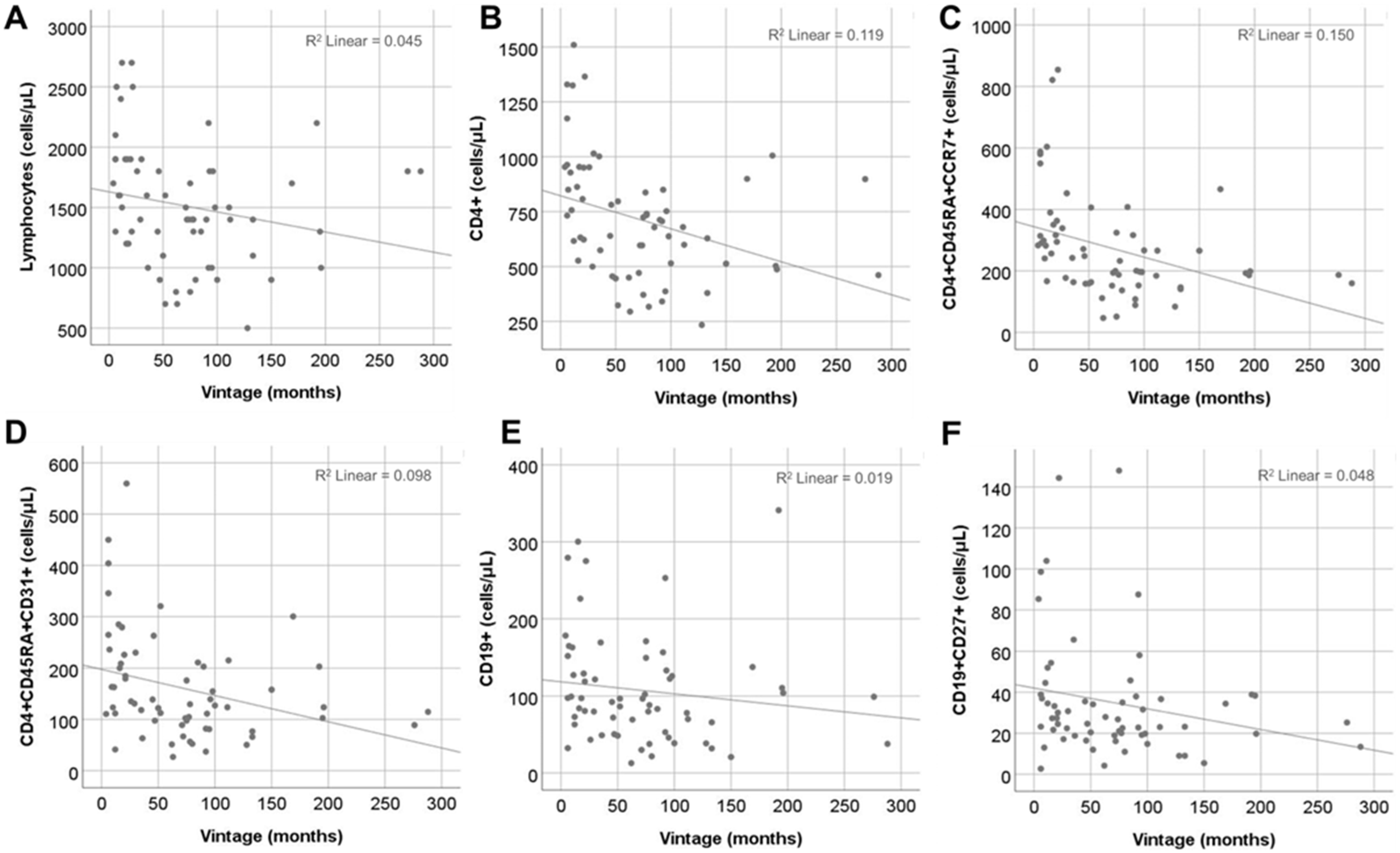
2.5. Effect of Vintage
2.6. Immune Phenotype May Be Related to Increased Mortality
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Flow Cytometry
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohen, G. Immune Dysfunction in Uremia 2020. Toxins 2020, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Pippias, M.; Noordzij, M.; Stel, V.S.; Andrusev, A.M.; Aparicio-Madre, M.I.; Arribas, M.; Arribas Monzón, F.E.; Åsberg, A.; Barbullushi, M.; et al. The European Renal Association - European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2016: A summary. Clin. Kidney J. 2019, 12, 702–720. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.-Y. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Vogelzang, J.L.; van Stralen, K.J.; Noordzij, M.; Diez, J.A.; Carrero, J.J.; Couchoud, C.; Dekker, F.W.; Finne, P.; Fouque, D.; Heaf, J.G.; et al. Mortality from infections and malignancies in patients treated with renal replacement therapy: Data from the ERA-EDTA registry. Nephrol. Dial. Transplant. 2015, 30, 1028–1037. [Google Scholar] [CrossRef]
- Lioulios, G.; Fylaktou, A.; Asouchidou, D.; Xochelli, A.; Nikolaidou, V.; Stai, S.; Christodoulou, M.; Giamalis, P.; Tsouchnikas, I.; Papagianni, A.; et al. Effect of Lymphocyte Phenotypic Alterations on the Humoral Response to Vaccination Against SARS CoV-2 in Dialysis Patients. Ann. Lab. Med. 2023, 43, 451–460. [Google Scholar] [CrossRef]
- Van Laecke, S.; Van Damme, K.; Dendooven, A. Immunosenescence: An unexplored role in glomerulonephritis. Clin. Transl. Immunol. 2022, 11, e1427. [Google Scholar] [CrossRef]
- Lioulios, G.; Fylaktou, A.; Papagianni, A.; Stangou, M. T cell markers recount the course of immunosenescence in healthy indi-viduals and chronic kidney disease. Clin. Immunol. 2021, 225, 108685. [Google Scholar] [CrossRef] [PubMed]
- Sampani, E.; Stangou, M.; Daikidou, D.; Nikolaidou, V.; Asouchidou, D.; Dimitriadis, C.; Lioulios, G.; Xochelli, A.; Fylaktou, A.; Papagianni, A. Influence of end stage renal disease on CD28 expression and T-cell immunity. Nephrology 2021, 26, 185–196. [Google Scholar] [CrossRef]
- Roe, K. NK-cell exhaustion, B-cell exhaustion and T-cell exhaustion—The differences and similarities. Immunology 2022, 166, 155–168. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Litjens, N.H.; van Druningen, C.J.; Betjes, M.G. Progressive loss of renal function is associated with activation and depletion of naive T lymphocytes. Clin. Immunol. 2006, 118, 83–91. [Google Scholar] [CrossRef]
- Yadav, A.K.; Lal, A.; Jha, V. Cytotoxic CD4+CD28null T lymphocytes, systemic inflammation and atherosclerotic risk in patients with chronic kidney disease. Nephron Clin. Pract. 2012, 120, c185–c193. [Google Scholar] [CrossRef]
- Ducloux, D.; Legendre, M.; Bamoulid, J.; Saas, P.; Courivaud, C.; Crepin, T. End-Stage Renal Disease-Related Accelerated Immune Senescence: Is Rejuvenation of the Immune System a Therapeutic Goal? Front. Med. 2021, 8, 720402. [Google Scholar] [CrossRef]
- Losappio, V.; Franzin, R.; Infante, B.; Godeas, G.; Gesualdo, L.; Fersini, A.; Castellano, G.; Stallone, G. Molecular mechanisms of premature aging in hemodialysis: The complex interplay between innate and adaptive immune dysfunction. Int. J. Mol. Sci. 2020, 21, 3422. [Google Scholar] [CrossRef]
- Glorieux, G.; Gryp, T.; Perna, A. Gut-derived metabolites and their role in immune dysfunction in chronic kidney disease. Toxins 2020, 12, 245. [Google Scholar] [CrossRef]
- Morena, M.; Creput, C.; Bouzernidj, M.; Rodriguez, A.; Chalabi, L.; Seigneuric, B.; Lauret, C.; Bargnoux, A.-S.; Dupuy, A.-M.; Cristol, J.-P. Randomised trial on clinical performances and biocompatibility of four high-flux hemodialyzers in two mode treatments: Hemodialysis vs. post dilution hemodiafiltration. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Leypoldt, J.K.; Storr, M.; Agar, B.U.; Boschetti-De-Fierro, A.; A Bernardo, A.; Kirsch, A.H.; Rosenkranz, A.R.; Krieter, D.H.; Krause, B. Intradialytic kinetics of middle molecules during hemodialysis and hemodiafiltration. Nephrol. Dial. Transplant. 2019, 34, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Roumelioti, M.-E.; Trietley, G.; Nolin, T.D.; Ng, Y.-H.; Xu, Z.; Alaini, A.; Figueroa, R.; Unruh, M.L.; Argyropoulos, C.P. Beta-2 microglobulin clearance in high-flux dialysis and convective dialysis modalities: A meta-analysis of published studies. Nephrol. Dial. Transplant. 2018, 33, 542, Erratum in Nephrol Dial Transplant. 2018, 33, 1025–1039. https://doi.org/10.1093/ndt/gfx311. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.D.; Guedes, M.; Rodrigues, S.D.; Flórido, A.C.S.; Moreno-Amaral, A.N.; Barra, A.B.; Canziani, M.E.; Cuvello-Neto, A.; Poli-De-Figueiredo, C.E.; Pecoits-Filho, R.; et al. High-volume hemodiafiltration decreases the pre-dialysis concentrations of indoxyl sulfate and p-cresyl sulfate compared to hemodialysis: A post-hoc analysis from the HDFit randomized controlled trial. J. Nephrol. 2022, 35, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Blankestijn, P.J.; Fischer, K.I.; Barth, C.; Cromm, K.; Canaud, B.; Davenport, A.; Grobbee, D.E.; Hegbrant, J.; Roes, K.C.; Rose, M.; et al. Benefits and harms of high-dose haemodiafiltration versus high-flux haemodialysis: The comparison of high-dose haemodiafiltration with high-flux haemodialysis (CONVINCE) trial protocol. BMJ Open 2020, 10, e033228. [Google Scholar] [CrossRef]
- Tattersall, J.E.; Ward, R.A.; EUDIAL group. Online haemodiafiltration: Definition, dose quantification and safety revisited. Nephrol. Dial. Transplant. 2013, 28, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Zawada, A.M.; Theis, L.; Braun, J.; Ottillinger, B.; Kopperschmidt, P.; Gagel, A.; Kotanko, P.; Stauss-Grabo, M.; Kennedy, J.P.; et al. Hemodiafiltration: Technical and Medical Insights. Bioengineering 2023, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Susantitaphong, P.; Siribamrungwong, M.; Jaber, B.L. Convective therapies versus low-flux hemodialysis for chronic kidney failure: A meta-analysis of randomized controlled trials. Nephrol. Dial. Transplant. 2013, 28, 2859–2874. [Google Scholar] [CrossRef] [PubMed]
- Guth, H.-J.; Gruska, S.; Kraatz, G. On-line production of ultrapure substitution fluid reduces TNF-alpha- and IL-6 release in patients on hemodiafiltration therapy. Int. J. Artif. Organs 2003, 26, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, A.N.; Bryl, E.; Klarskov, K.; Naylor, S.; Weyand, C.M.; Goronzy, J.J. Molecular basis for the loss of CD28 expression in senescent T cells. J. Biol. Chem. 2002, 277, 46940–46949. [Google Scholar] [CrossRef]
- Chiu, W.K.; Fann, M.; Weng, N.-P. Generation and growth of CD28nullCD8+ memory T cells mediated by IL-15 and its induced cytokines. J. Immunol. 2006, 177, 7802–7810. [Google Scholar] [CrossRef]
- Borthwick, N.J.; Lowdell, M.; Salmon, M.; Akbar, A.N. Loss of CD28 expression on CD8(+) T cells is induced by IL-2 receptor gamma chain signalling cytokines and type I IFN, and increases susceptibility to activation-induced apoptosis. Int. Immunol. 2000, 12, 1005–1013. [Google Scholar] [CrossRef]
- Surh, C.D.; Sprent, J. Homeostasis of naive and memory T cells. Immunity 2008, 29, 848–862. [Google Scholar] [CrossRef]
- Meijers, R.W.; Litjens, N.H.; A de Wit, E.; Langerak, A.W.; van der Spek, A.; Baan, C.C.; Weimar, W.; Betjes, M.G. Uremia causes premature ageing of the T cell compartment in end-stage renal disease patients. Immun. Ageing 2012, 9, 19. [Google Scholar] [CrossRef]
- Crépin, T.; Legendre, M.; Carron, C.; Vachey, C.; Courivaud, C.; Rebibou, J.-M.; Ferrand, C.; Laheurte, C.; Vauchy, C.; Gaiffe, E.; et al. Uraemia-induced immune senescence and clinical outcomes in chronic kidney disease patients. Nephrol. Dial. Transplant. 2020, 35, 624–632. [Google Scholar] [CrossRef]
- Zal, B.; Kaski, J.C.; Arno, G.; Akiyu, J.P.; Xu, Q.; Cole, D.; Whelan, M.; Russell, N.; Madrigal, J.A.; Dodi, I.A.; et al. Heat-Shock Protein 60-Reactive CD4+CD28null T Cells in Patients with Acute Coronary Syndromes. Circulation 2004, 109, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Liuzzo, G.; Goronzy, J.J.; Yang, H.; Kopecky, S.L.; Holmes, D.R.; Frye, R.L.; Weyand, C.M. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation 2000, 101, 2883–2888. [Google Scholar] [CrossRef]
- Liuzzo, G.; Kopecky, S.L.; Frye, R.L.; Fallon, W.M.O.; Maseri, A.; Goronzy, J.J.; Weyand, C.M. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation 1999, 100, 2135–2139. [Google Scholar] [CrossRef] [PubMed]
- Liuzzo, G.; Biasucci, L.M.; Trotta, G.; Brugaletta, S.; Pinnelli, M.; Digianuario, G.; Rizzello, V.; Rebuzzi, A.G.; Rumi, C.; Maseri, A.; et al. Unusual CD4+CD28nullT Lymphocytes and Recurrence of Acute Coronary Events. J. Am. Coll. Cardiol. 2007, 50, 1450–1458. [Google Scholar] [CrossRef]
- Nowik, M.; Nowacki, P.; Grabarek, J.; Drechsler, H.; Białecka, M.; Widecka, K.; Stankiewicz, J.; Safranow, K. Can we talk about CD4+CD28- Lymphocytes as a risk factor for ischemic stroke? Eur. Neurol. 2007, 58, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Niessner, A.; Kopecky, S.L.; Frye, R.L.; Goronzy, J.J.; Weyand, C.M. TRAIL-expressing T cells induce apoptosis of vascular smooth muscle cells in the atherosclerotic plaque. J. Exp. Med. 2006, 203, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Callender, L.A.; Carroll, E.C.; Beal, R.W.J.; Chambers, E.S.; Nourshargh, S.; Akbar, A.N.; Henson, S.M. Human CD8(+) EMRA T cells display a senescence-associated secretory phenotype regulated by p38 MAPK. Aging Cell. 2018, 17, e12675. [Google Scholar] [CrossRef]
- Lorenz, G.; Shen, Y.; Hausinger, R.I.; Scheid, C.; Eckermann, M.; Hornung, S.; Cardoso, J.; Lech, M.; Ribeiro, A.; Haller, B.; et al. A randomized prospective cross over study on the effects of medium cut-off membranes on T cellular and serologic immune phenotypes in hemodialysis. Sci. Rep. 2022, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-L.; Shu, K.-H.; Yang, F.-J.; Chou, T.-Y.; Chen, P.-M.; Lay, F.-Y.; Pan, S.-Y.; Lin, C.-J.; Litjens, N.H.R.; Betjes, M.G.H.; et al. A comprehensive characterization of aggravated aging-related changes in T lymphocytes and monocytes in end-stage renal disease: The iESRD study. Immun. Ageing 2018, 15, 27. [Google Scholar] [CrossRef]
- Borges, A.; Borges, M.; Fernandes, J.; Nascimento, H.; Sameiro-Faria, M.; Miranda, V.; Reis, F.; Belo, L.; Costa, E.; Santos-Silva, A. Apoptosis of peripheral CD4+T-lymphocytes in end-stage renal disease patients under hemodialysis and rhEPO therapies. Ren. Fail. 2011, 33, 138–143. [Google Scholar] [CrossRef]
- Stefanidis, I.; Voliotis, G.; Papanikolaou, V.; Chronopoulou, I.; Eleftheriadis, T.; Kowald, A.; Zintzaras, E.; Tsezou, A. Telomere Length in Peripheral Blood Mononuclear Cells of Patients on Chronic Hemodialysis Is Related With Telomerase Activity and Treatment Duration. Artif. Organs 2015, 39, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.; Chen, R.; Cao, X.; Shen, B.; Chen, X.; Ding, X.; Zou, J. Premature aging of circulating T cells predicts all-cause mortality in hemodialysis patients. BMC Nephrol. 2020, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.; Allende, L.M.; Ramos, L.E.; Gutiérrez, E.; Pleguezuelo, D.E.; Hernández, E.R.; Ríos, F.; Fernández, C.; Praga, M.; Morales, E. CD19+ B-cells, a new biomarker of mortality in hemodialysis patients. Front. Immunol. 2018, 9, 1221. [Google Scholar] [CrossRef] [PubMed]
- Hartzell, S.; Bin, S.; Cantarelli, C.; Haverly, M.; Manrique, J.; Angeletti, A.; La Manna, G.; Murphy, B.; Zhang, W.; Levitsky, J.; et al. Kidney Failure Associates With T Cell Exhaustion and Imbalanced Follicular Helper T Cells. Front. Immunol. 2020, 11, 583702. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fribourg, M.; Anderson, L.; Fischman, C.; Cantarelli, C.; Perin, L.; La Manna, G.; Rahman, A.; Burrell, B.E.; Heeger, P.S.; Cravedi, P. T-cell exhaustion correlates with improved outcomes in kidney transplant recipients. Kidney Int. 2019, 96, 436–449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sampani, E.; Daikidou, D.-V.; Lioulios, G.; Xochelli, A.; Mitsoglou, Z.; Nikolaidou, V.; Dimitriadis, C.; Fylaktou, A.; Papagianni, A.; Stangou, M. CD28null and Regulatory T Cells Are Substantially Disrupted in Patients with End-Stage Renal Disease Due to Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 2975. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lioulios, G.; Fylaktou, A.; Xochelli, A.; Sampani, E.; Tsouchnikas, I.; Giamalis, P.; Daikidou, D.-V.; Nikolaidou, V.; Papagianni, A.; Theodorou, I.; et al. Clustering of End Stage Renal Disease Patients by Dimensionality Reduction Algorithms According to Lymphocyte Senescence Markers. Front. Immunol. 2022, 13, 841031. [Google Scholar] [CrossRef]



| Age Group | 20–40 | 41–60 | 61–80 | Total |
|---|---|---|---|---|
| Ν | 21 | 21 | 20 | 62 |
| Mean age ± sd, years | 30.5 ± 6.3 | 52.2 ± 5.6 | 71.2 ± 6.4 | 51.3 ± 17.7 |
| Sex, male/female | 14/7 | 14/7 | 9/11 | 37/25 |
| Mean weight ± sd, kg | 61 ± 11.5 | 81.3 ± 19.2 | 71.1 ± 12.5 | 71.3 ± 17 |
| Mean BMI ± sd, kg/m2 | 21 ± 3.2 | 25.9 ± 3.9 | 25.5 ± 3.6 | 24.5 ± 4.1 |
| Cause of ESKD | ||||
| Primary glomerulonephritis (%) | 3 (14.3) | 10 (47.6) | 4 (20) | 17 (27.4) |
| Hypertension | 1 (4.8) | 2 (9.5) | 0 (0) | 3 (4.8) |
| Obstructive nephropathy | 5 (23.8) | 1 (4.8) | 2 (10) | 8 (12.9) |
| Polycystic kidney disease | 1 (4.8) | 2 (9.5) | 4 (20) | 7 (11.2) |
| Other | 6 (28.6) | 1 (4.8) | 1 (5) | 7 (11.2) |
| Unknown | 5 (23.8) | 5 (23.8) | 9 (45) | 19 (30.6) |
| Dialysis characteristics | ||||
| Classic hemodialysis | 11 | 9 | 16 | 36 |
| Online hemodiafiltration | 10 | 12 | 4 | 26 |
| Mean vintage ± sd, months | 45 ± 48 | 101 ± 81 | 67 ± 44 | 72 ± 64 |
| Kt/V ± sd | 1.29 ± 0.05 | 1.27 ± 0.06 | 1.23 ± 0.04 | 1.27 ± 0.07 |
| Mean dialysis duration ± sd, Min | 240 ± 14 | 246 ± 11 | 234 ± 13 | 240 ± 14 |
| Comorbidities | ||||
| Hypertension | 11 | 11 | 10 | 32 |
| Cardiovascular disease | 2 | 11 | 8 | 21 |
| Secondary hyperparathyroidism | 11 | 12 | 11 | 34 |
| Anemia | 18 | 15 | 18 | 51 |
| History of kidney transplantation (%) | 4 (19) | 6 (28.6) | 0 (0) | 10 (16.1) |
| Prior immunosuppression (%) | 5 (23.8) | 11 (52.3) | 3 (15) | 19 (30) |
| Median time since immunosuppression cessation (IQR), months | 75 (43–148) | 45 (36–63) | 48 (-) | 48 (40–96) |
| Laboratory values, median (IQR) | ||||
| Hemoglobin, g/dL | 11.6 (10.8–11.9) | 11.2 (10.4–11.7) | 10.5 (10–11.1) | 11 (10.5–11.6) |
| Urea, mg/dL | 130 (96–149) | 145 (117–154) | 121 (112–140) | 127 (110–149) |
| Creatinine, mg/dL | 9.7 (7.8–11.8) | 9.1 (7.1–10.9) | 8.4 (5.8–9.5) | 9.1 (7.1–10.8) |
| Calcium, mg/dL | 9.1 (8.8–9.2) | 8.9 (8.8–9.5) | 9.2 (8.7–9.6) | 9.1 (8.8–9.3) |
| Phosphorus, mg/dL | 4.6 (4–5.5) | 4.6 (3.3–5) | 4 (3.8–4.5) | 4.4 (3.8–5.1) |
| Parathormone, pg/mL | 162 (89–391) | 268 (145–376) | 197 (98–244) | 206 (106–353) |
| Albumin, g/dL | 4.3 (3.9–4.4) | 4.2 (3.9–4.3) | 3.9 (3.8–4.1) | 4.1 (3.9–4.3) |
| C reactive protein (mg/L) | 1.7 (1.2–3.7) | 2.5 (1.4–7.4) | 3.7 (1.8–7.4) | 2.4 (1.4–4.7) |
| Lymphocyte Subsets (Cells/μL) | Univariate Linear Regression | Multivariate Linear Regression | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hemofiltration Volume | Hemofiltration Volume | Age (Years) | ||||||||
| B | R2 | 95% CI | p | PC | 95% CI | p | PC | 95% CI | p | |
| CD4+CD45RA-CCR7+ | 0.45 | 0.2 | 0.5, 10.8 | 0.03 | 0.33 | −1.1, 9.0 | 0.12 | −0.43 | −9.8, −0.2 | 0.04 |
| CD8+CD45RA-CCR7+ | 0.52 | 0.26 | 1.8, 13.1 | 0.01 | 0.48 | 1.1, 13.3 | 0.02 | −0.05 | −6, 5 | 0.81 |
| CD8+CD45RA+CCR7- | −0.49 | 0.24 | −3.2, −0.3 | 0.02 | −0.56 | −3.6, −0.6 | 0.006 | −0.32 | −2.4, 0.3 | 0.13 |
| CD19+ | 0.47 | 0.22 | 0.3, 4.5 | 0.02 | 0.36 | −0.3, 3.8 | 0.09 | −0.42 | −3.9, −0.03 | 0.04 |
| CD19+IgD+CD27- | 0.48 | 0.23 | 0.3, 3.2 | 0.02 | 0.38 | −0.2, 2.6 | 0.08 | 0.48 | −2.8, −0.2 | 0.02 |
| CD19+IgD-CD27+ | 0.45 | 0.20 | −0.05, 1.1 | 0.03 | 0.39 | 0.05, 1.1 | 0.07 | −0.16 | −0.7, 0.35 | 0.47 |
| CD19+IgD-CD27- | 0.12 | 0.01 | −0.3, 1.3 | 0.30 | −0.07 | −1.1, 0.8 | 0.75 | −0.11 | −1.2, 0.6 | 0.61 |
| Lymphocyte Subsets (Cells/μL) | Univariate Linear Regression | Multivariate Linear Regression | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vintage (Months) | Vintage (Months) | Age (Years) | ||||||||
| BC | R2 | 95% CI | p | PC | 95% CI | p | PC | 95% CI | p | |
| Total Lymphs | −0.21 | 0.04 | −3.6, 0.3 | 0.09 | −0.17 | −3.3, 0.6 | 0.18 | −0.16 | −12.0, 2.5 | 0.19 |
| CD4+ | −0.34 | 0.12 | −2.6, −0.4 | 0.006 | −0.30 | −2.3, −0.2 | 0.02 | −0.22 | −7.1, 0.4 | 0.08 |
| CD4+CD45RA+CD31+ | −0.31 | 0.98 | −0.9, −0.1 | 0.01 | −0.23 | −0.7, 0.02 | 0.06 | −0.46 | −3.9, −1.3 | <0.001 |
| CD4+CD45RA+CCR7+ | −0.38 | 0.15 | −1.6, −0.3 | 0.002 | −0.35 | −1.5, −0.2 | 0.006 | −0.17 | −3.8, 0.8 | 0.19 |
| CD4+CD45RA-CCR7+ | −0.17 | 0.03 | −1.2, 0.2 | 0.17 | −0.14 | −1.2, 0.3 | 0.26 | −0.11 | −4.0, 1.5 | 0.37 |
| CD8+CD45RA-CCR7+ | −0.13 | 0.02 | −1.2, −0.4 | 0.32 | −0.08 | −1.0, 0.5 | 0.53 | −0.20 | −5.3, 0.6 | 0.12 |
| CD19+ | −0.14 | 0.02 | −0.4, 0.1 | 0.28 | −0.01 | −0.2, 0.2 | 0.88 | −0.52 | −3.0, −1.2 | <0.001 |
| CD19+CD27+ | −0.22 | 0.05 | −0.2, 0.02 | 0.08 | −0.15 | −0.1, 0.04 | 0.22 | −0.29 | −0.8, −0.06 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lioulios, G.; Fylaktou, A.; Xochelli, A.; Tourountzis, T.; Christodoulou, M.; Moysidou, E.; Stai, S.; Vagiotas, L.; Stangou, M. Hemodiafiltration May Be Associated with Senescence-Related Phenotypic Alterations of Lymphocytes, Which May Predict Mortality in Patients Undergoing Dialysis. Int. J. Mol. Sci. 2024, 25, 10925. https://doi.org/10.3390/ijms252010925
Lioulios G, Fylaktou A, Xochelli A, Tourountzis T, Christodoulou M, Moysidou E, Stai S, Vagiotas L, Stangou M. Hemodiafiltration May Be Associated with Senescence-Related Phenotypic Alterations of Lymphocytes, Which May Predict Mortality in Patients Undergoing Dialysis. International Journal of Molecular Sciences. 2024; 25(20):10925. https://doi.org/10.3390/ijms252010925
Chicago/Turabian StyleLioulios, Georgios, Asimina Fylaktou, Aliki Xochelli, Theodoros Tourountzis, Michalis Christodoulou, Eleni Moysidou, Stamatia Stai, Lampros Vagiotas, and Maria Stangou. 2024. "Hemodiafiltration May Be Associated with Senescence-Related Phenotypic Alterations of Lymphocytes, Which May Predict Mortality in Patients Undergoing Dialysis" International Journal of Molecular Sciences 25, no. 20: 10925. https://doi.org/10.3390/ijms252010925
APA StyleLioulios, G., Fylaktou, A., Xochelli, A., Tourountzis, T., Christodoulou, M., Moysidou, E., Stai, S., Vagiotas, L., & Stangou, M. (2024). Hemodiafiltration May Be Associated with Senescence-Related Phenotypic Alterations of Lymphocytes, Which May Predict Mortality in Patients Undergoing Dialysis. International Journal of Molecular Sciences, 25(20), 10925. https://doi.org/10.3390/ijms252010925





