Rats Selected for Different Nervous Excitability: Long-Term Emotional–Painful Stress Affects the Dynamics of DNA Damage in Cells of Several Brain Areas
Abstract
1. Introduction
2. Results
2.1. DNA Instability Dynamics in PFC Cells
2.2. DNA Instability Dynamics in Hippocampal Cells
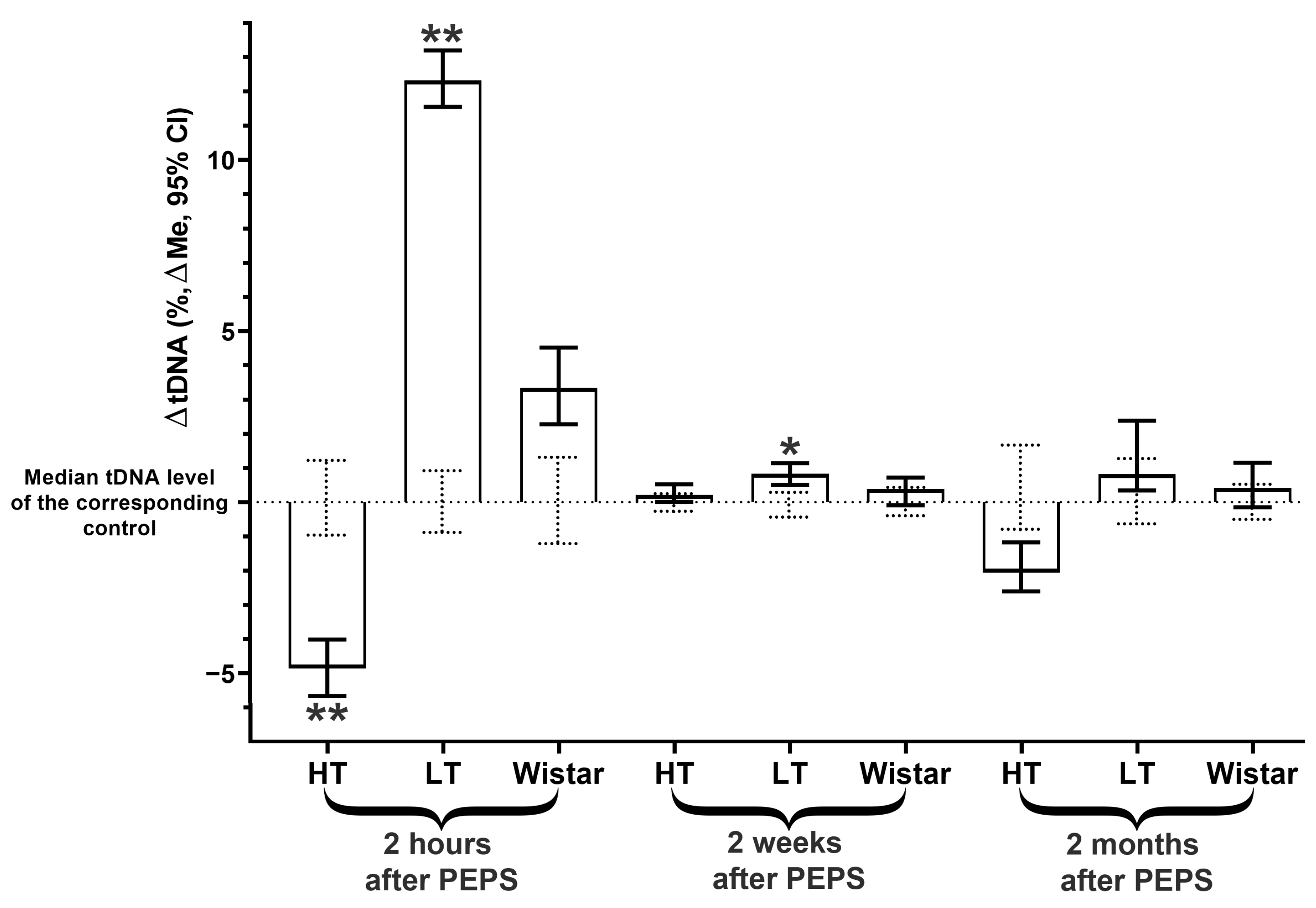
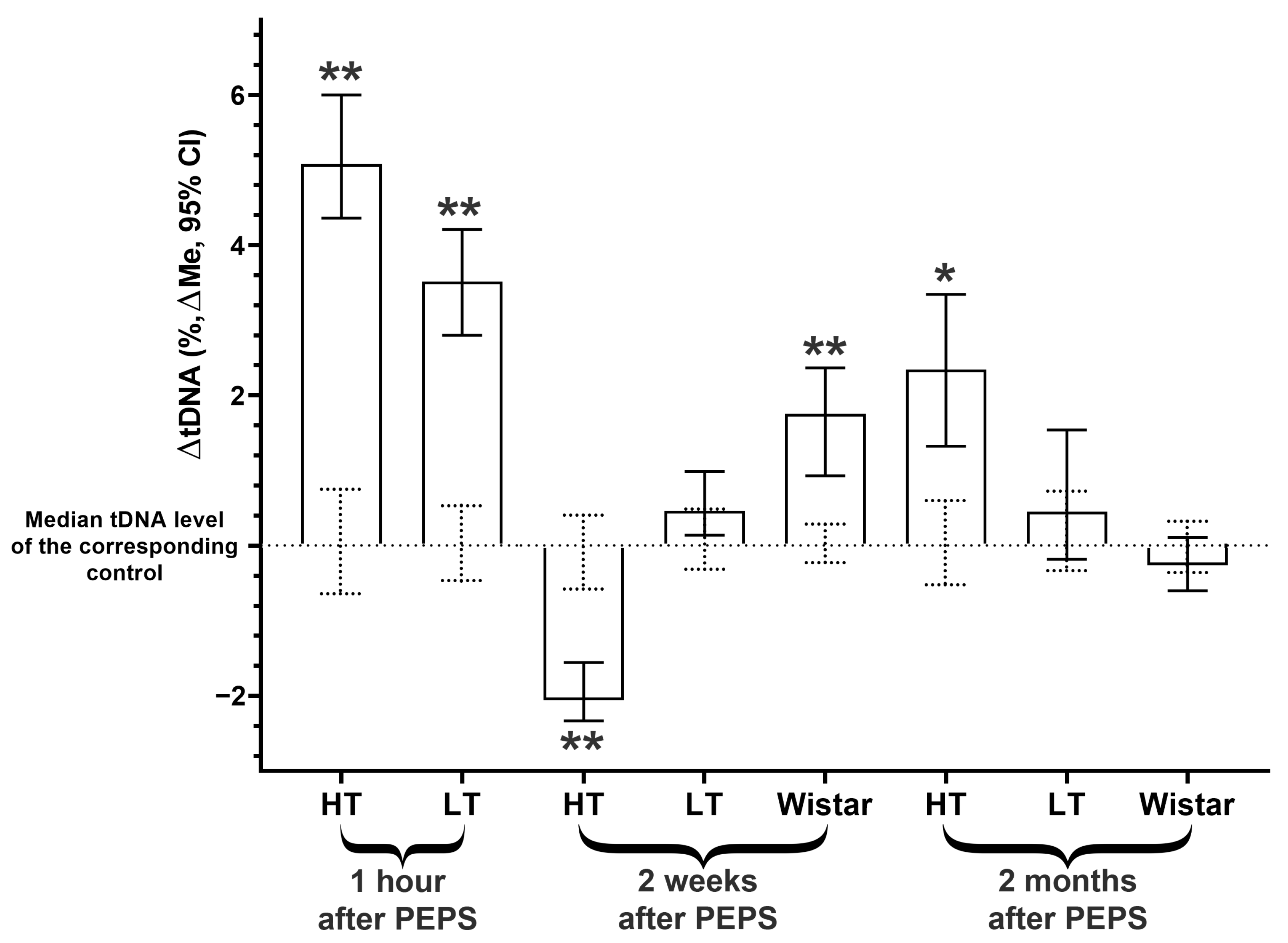
2.3. DNA Instability Dynamics in Amygdala Cells
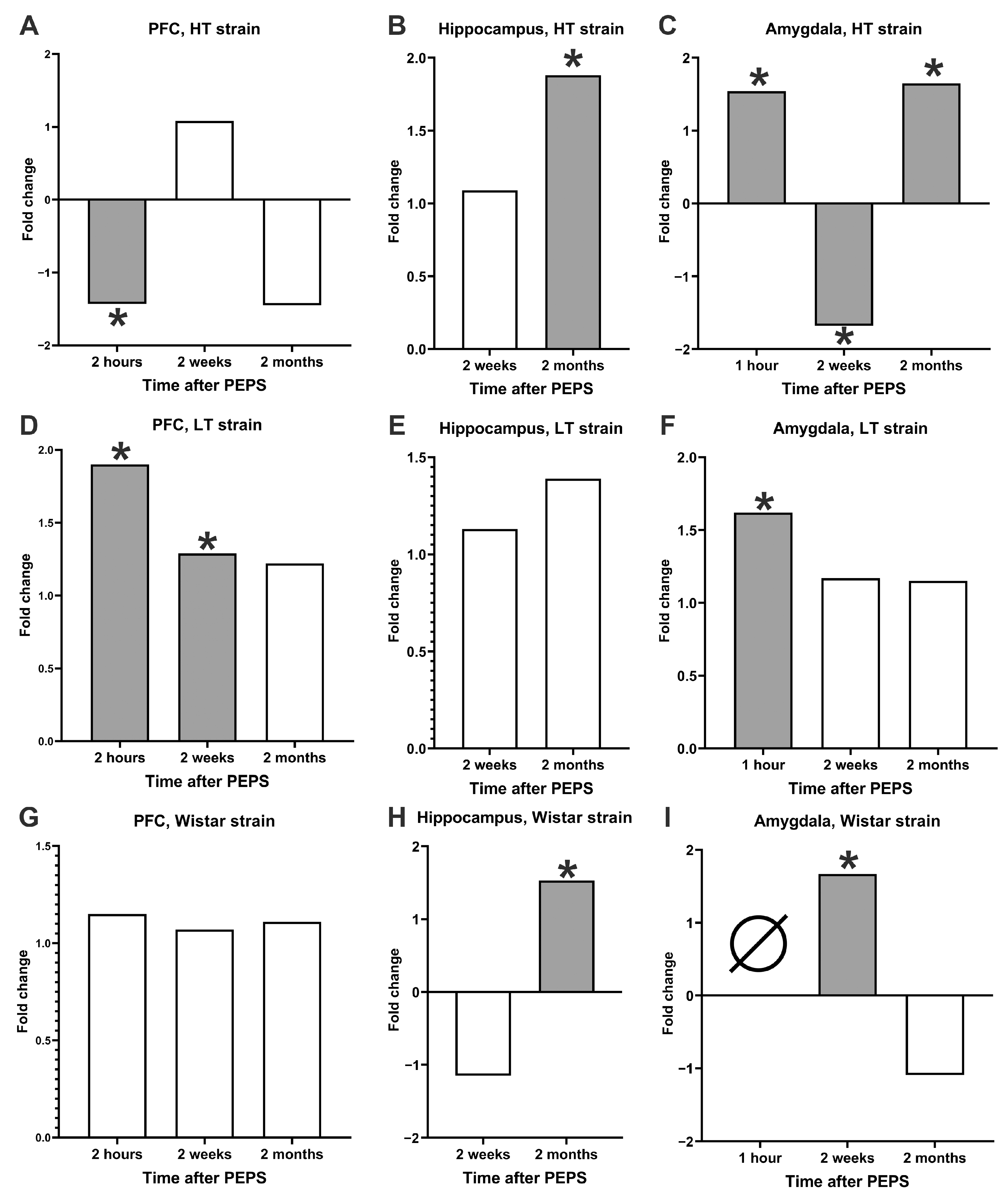
2.4. DNA Dynamics Summary
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Exposure to Stressor
4.3. Comet Assay
4.4. Statistics
5. Conclusions
- (1)
- In the low-excitable HT strain model of PTSD, we found the following:
- The level of DNA instability increased immediately after stress in the hippocampus and amygdala and then decreased to the control (in the hippocampus) or even lower (in the amygdala) in 2 weeks, but 1,5 months after, it increased again for both brain areas;
- In PFC, only 2 h after stress, significant changes (decrease in DNA damage level) were found;
- (2)
- In the high-excitable LT strain model of CD, we found the following:
- The level of genome instability increased in all three brain regions immediately after PEPS;
- Delayed changes in the DNA damage level were found only in PFC 2 weeks after the end of the stress procedure;
- (3)
- The Wistar strain that was not selected for excitability showed differences from both selected strains’ dynamics of genome instability.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McConnell, M.J.; Moran, J.V.; Abyzov, A.; Akbarian, S.; Bae, T.; Cortes-Ciriano, I.; Erwin, J.A.; Fasching, L.; Flasch, D.A.; Freed, D.; et al. Intersection of diverse neuronal genomes and neuropsychiatric disease: The Brain Somatic Mosaicism Network. Science 2017, 356, eaal1641. [Google Scholar] [CrossRef] [PubMed]
- Leija-Salazar, M.; Piette, C.; Proukakis, C. Review: Somatic mutations in neurodegeneration. Neuropathol. Appl. Neurobiol. 2018, 44, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Reiche, E.M.V.; Nunes, S.O.V.; Morimoto, H.K. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004, 5, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Villanueva, M.; Bürkle, A. Stress Hormone-Mediated DNA Damage Response—Implications for Cellular Senescence and Tumour Progression. Curr. Drug Targets 2016, 17, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Flint, M.S.; Bovbjerg, D.H. DNA damage as a result of psychological stress: Implications for breast cancer. Breast Cancer Res. 2012, 14, 320. [Google Scholar] [CrossRef] [PubMed]
- Walter, D.; Lier, A.; Geiselhart, A.; Thalheimer, F.B.; Huntscha, S.; Sobotta, M.C.; Moehrle, B.; Brocks, D.; Bayindir, I.; Kaschutnig, P.; et al. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature 2015, 520, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Dyrvig, M.; Gøtzsche, C.R.; Woldbye, D.P.D.; Lichota, J. Epigenetic regulation of Dnmt3a and Arc gene expression after electroconvulsive stimulation in the rat. Mol. Cell Neurosci. 2015, 67, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Vaido, A.I.; Shiryaeva, N.V.; Pavlova, M.B.; Levina, A.S.; Khlebaeva, D.A.-A.; Lyubashina, O.A.; Dyuzhikova, N.A. Selected rat strains HT, LT as a model for the study of dysadaptation states dependent on the level of excitability of the nervous system. Lab. Zhivotnye Dlia Nauchnykh Issled. 2018, 3, 205. [Google Scholar] [CrossRef]
- Diaz, V.; Lin, D. Neural circuits for coping with social defeat. Curr. Opin. Neurobiol. 2020, 60, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Atrooz, F.; Alkadhi, K.A.; Salim, S. Understanding stress: Insights from rodent models. Curr. Res. Neurobiol. 2021, 2, 100013. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Gianaros, P.J. Stress- and allostasis-induced brain plasticity. Annu. Rev. Med. 2011, 62, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Czéh, B.; Vardya, I.; Varga, Z.; Febbraro, F.; Csabai, D.; Martis, L.S.; Højgaard, K.; Henningsen, K.; Bouzinova, E.V.; Miseta, A.; et al. Long-Term Stress Disrupts the Structural and Functional Integrity of GABAergic Neuronal Networks in the Medial Prefrontal Cortex of Rats. Front. Cell Neurosci. 2018, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Shcherbinina, V.; Vaido, A.; Khlebaeva, D.; Dyuzhikova, N.; Daev, E. Genome response of hippocampal cells to stress in male rats with different excitability of the nervous system. Biol. Commun. 2022, 67, 12–18. [Google Scholar] [CrossRef]
- Gulyaeva, N.V. Biochemical mechanisms and translational relevance of hippocampal vulnerability to distant focal brain injury: The price of stress response. Biochemistry 2019, 84, 1622–1648. [Google Scholar] [CrossRef] [PubMed]
- Shalaginova, I.G.; Tuchina, O.P.; Sidorova, M.V.; Levina, A.S.; Khlebaeva, D.A.-A.; Vaido, A.I.; Dyuzhikova, N.A. Effects of psychogenic stress on some peripheral and central inflammatory markers in rats with the different level of excitability of the nervous system. PLoS ONE 2021, 16, e0255380. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, J.A.; Venheim, E.R.; Padival, M. Chronic Stress Causes Amygdala Hyperexcitability in Rodents. Biol. Psychiatry 2010, 67, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Dubé, C.M.; Molet, J.; Singh-Taylor, A.; Ivy, A.; Maras, P.M.; Baram, T.Z. Hyper-excitability and epilepsy generated by chronic early-life stress. Neurobiol. Stress 2015, 2, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Falkowska, M.; Ntamati, N.R.; Nevian, N.E.; Nevian, T.; Acuña, M.A. Environmental enrichment promotes resilience to neuropathic pain-induced depression and correlates with decreased excitability of the anterior cingulate cortex. Front. Behav. Neurosci. 2023, 17, 1139205. [Google Scholar] [CrossRef] [PubMed]
- Hecht, K.; Trepetov, K.; Choinovski, K.; Peschel, M. Die Raum-Zetliche Organization der Reiz-Reactios-Beziehungen Bedingtreflectorisher Prozesse; Fisher: Schaffhausen, Switzerland, 1972; 213p. [Google Scholar]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: San Diego, CA, USA, 2007; 456p. [Google Scholar]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shcherbinina, V.; Pavlova, M.; Daev, E.; Dyuzhikova, N. Rats Selected for Different Nervous Excitability: Long-Term Emotional–Painful Stress Affects the Dynamics of DNA Damage in Cells of Several Brain Areas. Int. J. Mol. Sci. 2024, 25, 994. https://doi.org/10.3390/ijms25020994
Shcherbinina V, Pavlova M, Daev E, Dyuzhikova N. Rats Selected for Different Nervous Excitability: Long-Term Emotional–Painful Stress Affects the Dynamics of DNA Damage in Cells of Several Brain Areas. International Journal of Molecular Sciences. 2024; 25(2):994. https://doi.org/10.3390/ijms25020994
Chicago/Turabian StyleShcherbinina, Veronika, Marina Pavlova, Eugene Daev, and Natalia Dyuzhikova. 2024. "Rats Selected for Different Nervous Excitability: Long-Term Emotional–Painful Stress Affects the Dynamics of DNA Damage in Cells of Several Brain Areas" International Journal of Molecular Sciences 25, no. 2: 994. https://doi.org/10.3390/ijms25020994
APA StyleShcherbinina, V., Pavlova, M., Daev, E., & Dyuzhikova, N. (2024). Rats Selected for Different Nervous Excitability: Long-Term Emotional–Painful Stress Affects the Dynamics of DNA Damage in Cells of Several Brain Areas. International Journal of Molecular Sciences, 25(2), 994. https://doi.org/10.3390/ijms25020994






