Abstract
Anemonopsis Siebold et Zucc. is an unstudied single-species genus belonging to the tribe Cimicifugeae (Ranunculaceae). The only species of this genus—Anemonopsis macrophylla Siebold and Zucc.—is endemic to Japan. There are no data on its chemical composition. This work is the first to determine (with liquid chromatography–high-resolution mass spectrometry, LC-HRMS) the chemical composition of methanol extracts of leaves and flowers of A. macrophylla. More than 100 compounds were identified. In this plant, the classes of substances are coumarins (13 compounds), furocoumarins (3), furochromones (2), phenolic acids (21), flavonoids (27), and fatty acids and their derivatives (15 compounds). Isoferulic acid (detected in extracts from this plant) brings this species closer to plants of the genus Cimicifuga, one of the few genera containing this acid and ferulic acid at the same time. Isoferulic acid is regarded as a reference component of a quality indicator of Cimicifuga raw materials. The determined profiles of substances are identical between the leaf and flower methanol extracts. Differences in levels of some identified substances were revealed between the leaf and flower extracts of A. macrophylla; these differences may have a substantial impact on the manifestation of the biological and pharmacological effects of the extracts in question.
1. Introduction
The tribe Cimicifugeae Torrey and Gray is included in the family Ranunculaceae Juss., represented by four genera and more than 49 plant species [1,2,3,4]. These genera include Anemonopsis Siebold et Zucc. (one species), Actaea L. (32 species), Eranthis Salisb. (14 species), and Beesia Balf. f. et W. W. Sm. (two species), which have a circumboreal distribution. The larger genera occupy wide ranges in temperate forests or plains throughout Eurasia (Eranthis) or Eurasia and North America (Actaea). Both Anemonopsis macrophylla Siebold and Zucc., and the two species of Beesia occupy restricted forest habitats: Anemonopsis represents an island community of Honshu, Japan, and the two Beesia species inhabit the Sino-Himalayan mountains of East Asia [5].
Anemonopsis is a monotypic and endemic genus of Japan and grows in temperate deciduous forests in central Honshu [6]. A. macrophylla is a perennial plant characterized by 2–4-ternate basal and lower cauline leaves, irregularly incised-dentate leaflets, a loosely racemiform 3–8-flowered definite inflorescence, nutant flowers with slender pedicels and with 7–10 reddish purple sepals; 10 or more erect, concolorous with sepals, without a nectary, petals anchor-shaped with 2–4 follicles (fruits), bending down fruiting pedicels, follicles rising up with elongate stalks and squamate seeds (Figure 1). This genus and Actaea differ from Eranthis and Beesia by compound, not undivided simple leaves but are similar to Eranthis in terms of a nonrecemiform or laxly recemiform inflorescence (with at most several flowers or a single flower) or a nondensely racemiform inflorescence (with many flowers) as in Actaea [7]. According to morphological and molecular phylogenetic data, Anemonopsis and Beesia form a separate clade that is sister to the Eranthis + Actaea clade. [8]. A morphological analysis of Loconte et al. [9] was influenced by a reduction in leaf complexity in Beesia and Eranthis as compared with Actaea and Anemonopsis [1]. Nonetheless, leaf, floral, and fruiting characteristics in both Beesia and Eranthis provide a new basis for tribal redefinition inferred from a unique combination of traits, which are as follows: a rhizomatous or tuberous type of plant; actinomorphic flowers that are stand-alone or borne by racemes or by racemose panicles; free, follicular, or baccate fruits with many seeds; one to several follicles with transverse external venation; and simple or ternately compound leaves [5].
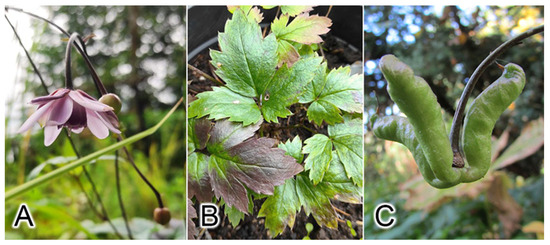
Figure 1.
A. macrophylla: (A) flowers, (B) leaves, and (C) a fruit. Photo by Andrey S. Erst.
In addition to morphological characteristics, chemosystematic traits could clarify the position of A. macrophylla in the tribe Cimicifugeae; however, research on the chemical composition of A. macrophylla has not been conducted to date. Studying the chemical composition of A. macrophylla is of interest not only because the obtained information (along with morphological, anatomical, and other characteristics) can be used in plant taxonomy but also due to practical interest: this knowledge makes it possible to search for plants that are promising producers of biologically active compounds. LC-HRMS (liquid chromatography–high-resolution mass spectrometry) is one of the most effective and fastest methods for analyzing metabolites in a multicomponent mixture (such as plant extracts) [10,11].
The aim of this study was to analyze methanol extracts from leaves and flowers of A. macrophylla for metabolites by LC-HRMS.
2. Results and Discussion
2.1. Analysis of the Set of Bioactive Compounds in the A. macrophylla Extracts by LC-HRMS
Methanol extracts from leaves and flowers of A. macrophylla were found to contain a large number of biologically active components (Table 1). Using LC-HRMS and several databases, it was possible to identify more than 100 compounds in extracts from these organs of the species under study. Among them, we identified 27 flavonoids, 21 phenolic acids, 16 coumarins, 15 fatty acids and derivatives, 10 organic acids, eight amino acids, five triterpenoids, two furochromones, and one sugar. The profiles of identified substances do not differ between the leaves and flowers of this plant.

Table 1.
Chemical constituents tentatively identified in methanol extracts from A. macrophylla leaves and flowers by LC-HRMS using databases mzCloud, mzVault, and ChemSpider.
2.1.1. Coumarins
At least 16 coumarins were found in the extracts from A. macrophylla. They are mainly represented by simple coumarins, and only three of them are furocoumarins.
Coumarins are unsaturated aromatic lactones based on a benzene cycle fused to an α-pyrone ring [12]. The simplest compound is coumarin (7) (2H-1-benzopyran-2-one), first isolated in 1820 independently by A. Vogel and by N. Guibourt from Coumarouna odorata Aubl. [Dipteryx odorata (Aubl.) Willd.].
Coumarin is well known not only for serving as the basis for the synthesis of a whole class of compounds but also for the pleasant smell of freshly cut hay [13]. It is believed that coumarin is absent in undamaged plant tissues but is formed from melilotoside when cells are damaged [14]. In contrast to coumarin itself (7), which has no substituents, other coumarins identified in A. macrophylla have substituents at the C-6, C-7, or C-8 positions (Figure 2).
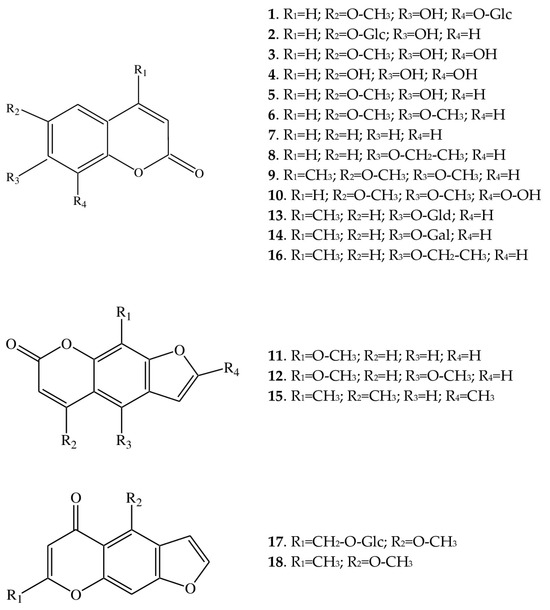
Figure 2.
Structures of coumarins (1–10, 13, 14, and 16), furocoumarins (11, 12, and 15), and furochromones (17 and 18) from A. macrophylla.
Such substituents are one or several hydroxyl groups [in fraxetin (3), fraxin (1), esculin (2), scopoletin (5), and esculetin (4)], an ethoxy group [in 7-ethoxycoumarin (8) and maraniol (16)], or a methyl- and/or methoxy group [in fraxetin (3), fraxin (1), scoparone (6), maraniol (16), and 6,7-dimethoxy-4-methylcoumarin (9)]. 8-Acetyl-6,7-dimethoxycoumarin (10), besides two methoxy groups, contains an acetyl group. In addition, two coumarins have glucuronic acid [4-methylumbelliferyl-β-glucuronide (13)] or galactopyranose [4-methylumbelliferyl-β-galactopyranoside (14)] as substituents at the C-7 position. Thus, the diversity of coumarin compounds in A. macrophylla is achieved via the addition of the above-mentioned functional groups. Simple coumarins are coumarins in which hydrogens at the C-6, C-7, or C-8 position only on the benzene ring are replaced by hydroxyl, methoxyl, isopentenyl, or some other groups without the formation of furan or pyran rings.
Furocoumarins are formed by the addition of furan to a simple coumarin either at the C-6 and C-7 or at the C-7 and C-8 positions, whereas pyranocoumarins are formed via the attachment of pyran. We did not find pyranocoumarins in A. macrophylla, but from the subclass of furocoumarins, we were able to identify methoxsalen (11), trioxsalen (15), and isopimpinellin (12). All three compounds are based on 7-H-furo [3,2-g]chromen-7-one; although methoxsalen carries an additional methoxy group at the C-8 position, isopimpinellin has two methoxy groups (at C-5 and C-8 positions), whereas trioxsalen contains methyl groups at positions C-4, C-8, and C-5’. Furocoumarins can be linear or angular compounds [15]; only linear furocoumarins were present in the analyzed extracts.
Furthermore, we detected 4-hydroxycoumarin in the extracts of A. macrophylla. This compound contains a hydroxyl group as a substituent, but it is located at the C-4 position, which is rare because substituents are most often present at positions C-6, C-7, and/or C-8. Additionally, in the literature, it was found that 4-hydroxycoumarin derives not from coumarin but directly from melilotoside as a result of metabolic processes involving Penicillium and some Aspergillus species [16]. For this reason, we did not include this compound in the list of substances (Table 1) identified in the extracts of A. macrophylla.
The coumarins found in A. macrophylla are quite widespread in the plant kingdom, and, in general, this class of secondary metabolites has now been detected in almost 30 families and more than 150 species. Representatives of such families as Rutaceae Juss., Clusiaceae Lindl., Apiaceae Lindl., and some others are rich in coumarins [17]. Coumarins have also been registered as representatives of the family Ranunculaceae Juss. [18]. As for the occurrence of coumarins in the genera of the tribe Cimicifugeae, which includes Anemonopsis, it is difficult to say anything because these plants have rarely been investigated by phytochemists. Continued research involving more genera closely related to Anemonopsis will allow for a more definitive assessment of the taxonomic significance of these compounds. Nonetheless, coumarins have been found in some species of Actaea L., Cimicifuga Wernisch., and Eranthis Salisb. [4,10,19,20]. The search for new plants containing coumarins is important because, in many natural coumarins, including those found in A. macrophylla (scopoletin and fraxetin), an anti-inflammatory activity has been detected [21,22,23,24]. A tight structure–activity relationship has also been documented for coumarins, thus making it possible to use coumarin molecules as the basis for the development of various pharmaceuticals [25,26].
2.1.2. Furochromones
From the furochromones class, khelloside (17) and visnagin (18) were identified in the extract. Both substances are based on furochromone structure with a methoxy group at position C-4, but in visnagin, a methyl group is located at position C-7, whereas in khelloside, there is a glucopyranoside at this position. In the last decade, this subclass of compounds has attracted research interest: e.g., reviews have been published on the natural diversity of furochromones [27], their biological activity [28], and their use in medicinal chemistry as fluorescent probes [29].
2.1.3. Phenolic Acids
In the extracts of A. macrophylla, the hydroxycinnamic-acid subclass proved to be highly diverse (Figure 3). Cinnamic acid (25) is an aromatic carboxylic acid and is a key compound present in many medicinal plants [30]. In nature, it occurs in cis- and trans-forms, the latter being the most common. Cinnamic acid has a variety of biological properties, allowing the creation of effective CNS-stimulatory, immunostimulatory, and antimicrobial drugs on its basis. There is evidence that the cinnamoyl moiety is crucial for the manifestation of antiradical activity, whereas the nature and position of substituents on the aromatic ring give only an increase or decrease in activity [31]. Nevertheless, it is more often reported that the introduction of additional hydroxyl substituents into this acid enhances the reducing properties; this is because the mobile proton of the OH group is the primary center of inhibition of a radical [32,33]. Even the presence of a methoxy group can enhance the antiradical properties [34,35].
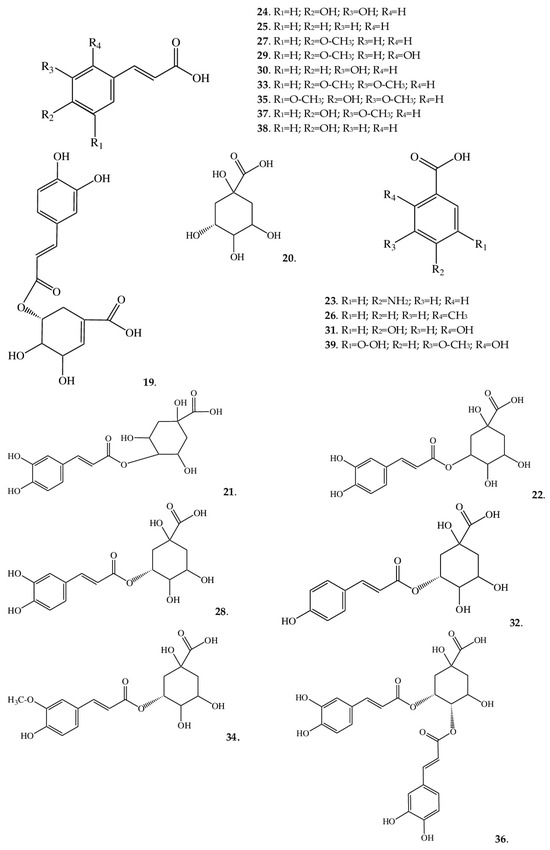
Figure 3.
Structures of phenolic acids from A. macrophylla.
In the A. macrophylla extracts, the diversity of cinnamic acid derivatives has several reasons: additional substituents in the form of hydroxyls [at the C-4 position in p-coumaric acid (38) or at positions C-3 and C-4 as in caffeic acid (24)] or methoxy groups [at position C-3 and position C-4 in 3,4-dimethoxycinnamic acid (33)]. Both hydroxy and methoxy groups can be attached simultaneously [as in 2-hydroxy-4-methoxycinnamic acid (29), sinapinic acid (35), ferulic acid (37), and isoferulic acid (27)]. Furthermore, when combined with quinic acid, cinnamic acid gives rise to a number of acids: neochlorogenic (22), cryptochlorogenic (21), and accordingly chlorogenic (28) acids. Chlorogenic acid is the most common in nature, but the combination of two different acids in its chemical structure [ester of caffeic acid and (–)-quinic acid] explains the high antioxidant activity [36]. Further, when combined with quinic acid, cinnamic acid gives such derivatives as 3-O-feruloylquinic (34), 5-coumaroylquinic (32), and 4,5-dicaffeoylquinic acids (36).
Aside from the fact that cinnamic acid itself has various types of activity, it has many derivatives: no less valuable substances that also have a wide range of pharmacological activities, such as antibacterial [37], antifungal [38], neuroprotective [39], and anticancer effects [40]. For this reason, they have been separated into an independent class of biologically active substances and have affected the chemical classification of medicinal plants. Furthermore, derivatives of cinnamic acid are intermediates in the synthesis of such compounds as stilbenes and styrenes and participate in the biosynthesis of lignin and other compounds. The structural features of derivatives of cinnamic acid (unsaturation and several hydroxylic and/or carboxylic groups) have even enabled their use in the synthesis of polymers: polyesters, polyamides, and polyanhydride esters, which in turn have found applications in industrial engineering and medical fields [41].
In A. macrophylla, aside from derivatives of cinnamic acid, we detected acids based on benzoic acid. The latter is a monobasic aromatic carboxylic acid that possesses high reactivity, resulting in a wide variety of compounds [30]. Depending on the characteristics of chemical structure, such substances have found applications in the food, perfume, and pharmaceutical industries. Numerous pharmacological studies have shown that benzoic acid derivatives that contain, for example, a carboxyl group is promising as potential diuretics, and carbamide derivatives hold promise as analgesics [42]. In the extract analyzed here, benzoic acid derivatives are represented by 2-methylbenzoic acid (26), 2,4-dihydroxybenzoic acid (31), 4-aminobenzoic acid (23), and 5-carboxyvanillic acid (39).
Most of the acids, among those detected in the extracts of A. macrophylla are widespread among plants, but, for example, isoferulic acid is much less common. For instance, the genus Cimicifuga (closely related to Anemonopsis) is one of the few genera containing both ferulic and isoferulic acids. At least three species of this genus (C. dahurica, C. foetida, and C. heracleifolia) are listed in the Pharmacopoeia of the People’s Republic of China, and their pharmacological properties are attributed to the presence of isoferulic acid in extracts. Additionally, this compound is regarded as a reference component of a quality indicator of Cimicifuga raw materials [43]. The actions of isoferulic acid as an anti-inflammatory and antiviral ingredient have been confirmed [44,45], but its antidiabetic effects are the most interesting [46]. Further, in Cimicifuga species, a number of phenolic acids have been registered: cimicifugic acids A–N, cimiracemates A–D, and others, which are exclusive chemical constituents of the genus Cimicifuga [47]. These phenolic compounds are formed via the condensation of piscidic acid or fukiic acid with isoferulic, ferulic, caffeic, 3,4-dihydroxybenzoic, or some other acids [48]. Further research is needed to verify whether A. macrophylla synthesizes such acids.
2.1.4. Flavonoids
One of the major classes of secondary metabolites produced by A. macrophylla is flavonoids (Table 1). We revealed and identified 27 compounds belonging to this class, with flavonols being the most diverse (Figure 4). The flavonols found in A. macrophylla are based on quercetin (40), kaempferol (46), and their derivatives. The following quercetin glycosides were identified in the assayed samples: quercetin-3β-glucoside (45), quercetin-6-O-β-xylopyranosyl-β-glucopyranoside (43), rutin (47), patulitrin (51), narcissin (56), rhamnetin-3-O-xylopyranosyl-glucopyranoside (48), and dihydroquercetin-3-rhamnoside, better known as astilbin (57). Additionally, a flavonoid (40) was found whose aglycone is quercetin that has substituents at positions C-3 and C-7 in the form of hexose sugars, which we were unable to identify. Among derivatives of kaempferol, the following glycosides were noted: trifolin (50), kaempferol-7-O-glucoside (53), dihydrokaempferol-7-O-glucoside (41), nicotiflorin (52), and tiliroside (65).
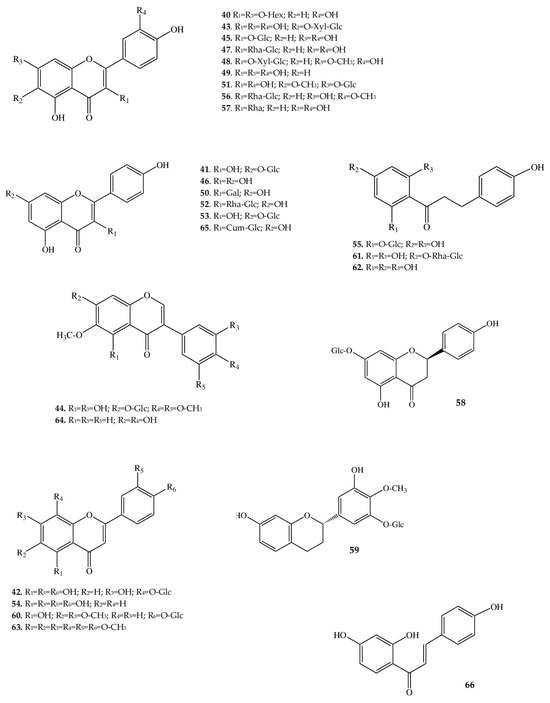
Figure 4.
Structures of flavonoids from A. macrophylla.
Analysis of our data allowed us to identify isoflavones and flavans in the extract, for which the following structural interpretation was proposed: iridin (44), glycitein (64), and auriculoside (59). Flavanones are represented by prunin (58), flavones by luteolin (54) and nobiletin (63), and glucosides by cirsimarin (60) and gossypin (42), which contain glucopyranose as a sugar residue.
Dihydrochalcones are also phenolic compounds with a flavonoid backbone but are characterized by the absence of heterocyclic ring C. The small set of dihydrochalcones here consists of four compounds: naringin dihydrochalcone (61), phloretin (62), and its glycosides phlorizin (55) and isoliquiritigenin (66). The latter compound is most often registered in underground parts of members of the family Fabaceae [49], but there is evidence of its identification in Ranunculaceae, too [50]. Isoliquiritigenin possesses a chalcone skeleton with three hydroxyl groups (at positions C-4, C-2’, and C-4’): such a hydrophobic compound is almost insoluble in water. Isoliquiritigenin is of particular interest not only as a compound from the class of chalcones (precursors of many flavanones [51]) but also as a substance that manifests antidiabetic [52], spasmogenic [53], anticancer [54], vasorelaxant [55], and other types of activity. Phloretin (62), just like isoliquiritigenin, contains two aromatic phenolic rings and a carbonyl group, but in addition to the three hydroxyl groups, there is another one at the C-6’ position. Phloretin has a wide range of pharmacological effects on the human body and is devoid of toxicity [56].
2.1.5. Other Classes of Compounds
In addition, amino acids (87–94), sugars (95), and organic acids (96–105) were detected in the extracts of A. macrophylla. Five compounds were identified as triterpenoids and matched ursolic acid (67), lupenone (68), oleanolic acid (69), cucurbitacin I (70), and cucurbitacin S (71). Several free saturated and unsaturated fatty acids and their derivatives were found, with chain lengths ranging from six carbon atoms (adipic acid) to 22 carbon atoms (docosahexaenoic acid) (72–86). The most numerous here are fatty acids with a chain length of 18 carbons.
2.2. A Comparative Analysis of Concentrations of the Identified Compounds between Leaf and Flower Methanol Extracts from A. macrophylla
Plant extracts having identical metabolite profiles may possess different pharmacological and biological activities. The manifestation of one or another activity is influenced not only by the profile of biologically active substances but also by their levels [57,58]. It was determined that the profiles of the identified substances are identical between the leaves and flowers of A. macrophylla. The comparative analysis of peak areas revealed differences in relative concentrations of some substances between the leaves and flowers of this species at a significance level of p ≤ 0.05. Levels of the following flavonoids turned out to be higher in the leaves: flavonol rutin (substance peak area ratio leaves/flowers = 1.46), chalcones [phloretin (ratio = 52.30) and isoliquiritigenin (23.32)], fatty acids [phloionolic acid (7.19), linolenelaidic acid (3.23), and 9,10-dihydroxystearic acid (2.92)], and a phenolcarboxylic acid [3-O-feruloylquinic acid (13.89)] as compared to concentration of these compounds in methanolic extracts from the plant’s flowers (Figure 5). Flowers of A. macrophylla are distinguished by higher concentrations of the following flavonols: quercetin (substance peak area ratio flowers/leaves = 1.67) and its derivatives [quercetin-6-O-β-xylopyranosyl-β-glucopyranoside (ratio = 30), rhamnetin-3-O-xylopyranosyl-glucopyranoside (8.30), and astilbin (2.85)], kaempferol derivatives [trifolin (11.24) and kaempferol-7-O-glucoside (16.86)], a flavanone [prunin (8.13)], a flavone [gossypin (17.76)], phenylcarboxylic acids [2-hydroxy-4-methoxycinnamic acid (7.68), 5-p-coumaroylquinic acid (2.69), and 4,5-dicaffeoylquinic acid (9.40)], a coumarin [fraxetin (13.24)], and a fatty acid [12-oxo-phytodienoic acid (4.45)] (Figure 5). The observed differences in concentrations of substances between the leaves and flowers may considerably affect the biological and pharmacological activities of the extracts.
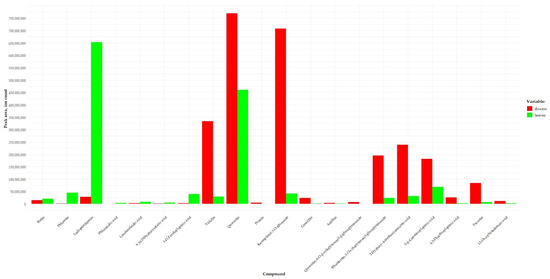
Figure 5.
Peak area of substances identified in A. macrophylla leaves and flowers.
3. Materials and Methods
3.1. Plant Material and Preparation of the Extract
Live material of A. macrophylla was collected in Japan: Saitama Prefecture, Chichibu Shi City, Shiroku, near a village, 340 m above sea level, 35.956556° N, 138.987667° E, by A.S. Erst, T.V. Erst, and H. Ikeda, 2 April 2019 (NS, s.n.). Leaves and flowers were collected from plants cultivated in the Central Siberian botanical garden RAS in 2023. Original live materials were collected from the natural population of Japan in 2019. The plant under study was collected and identified by an expert on the Ranunculaceae family: A.S. Erst, Ph.D. (a senior researcher at the Central Siberian Botanical Garden SB RAS) using the phenotype and morphological traits (voucher specimens No. AM-J-109). The material was collected from 5–10 typical specimens of A. macrophylla. The collected material was dried in silica gel.
Air-dried plant material was mechanically ground up to obtain a homogeneous powder with particle size as small as 0.5 mm. The methanol extract was prepared as follows: 40 mg of crushed leaves or flowers were placed in a 2 mL Eppendorf tube, then 800 μL of 96% methanol was added and mixed for 30 s on an IKA vortex 3 (IKA-Werke GmbH and Co. KG, Staufen, Germany). Next, the extracts were sonicated for 30 min in a UM-2 ultrasonic bath (Unitra-Unima Olsztyn, Poland) and then agitated for 30 min on a TS-100C thermal shaker (Biosan, Riga, Latvia). The resulting eluates were centrifuged for 1 min on a MiniSpin® plus centrifuge (Eppendorf, Hamburg, Germany) at 14,000× g. An aliquot of the supernatant was transferred to a clean, dry 2 mL Eppendorf tube. The remaining material was again covered with 800 μL of 96% methanol, and the procedure was repeated. Before the assay, the combined extract was centrifuged in an Eppendorf 5425 centrifuge (Eppendorf, Hamburg, Germany) at 15,000× g and passed through a membrane filter with a pore diameter of 0.45 μm.
3.2. LC-HRMS Analysis of Metabolites in the A. macrophylla Extracts
LC-HRMS was conducted at the Core Facility of Mass Spectrometric Analysis at the Institute of Chemical Biology and Fundamental Medicine SB RAS (Novosibirsk, Russia).
An Ultimate 3000 liquid chromatograph (Thermo Fisher Scientific, San Jose, CA, USA) coupled with a Q Exactive HF mass spectrometer (Thermo Fisher Scientific, San Jose, CA, USA) was utilized to determine metabolomic profiles of the A. macrophylla extracts. The chromatographic separation was carried out at a 0.4 mL/min flow rate on a Zorbax Eclipse XDB-C8 reversed-phase column (150 mm × 3.0 mm, 5 μm, Agilent Technologies, Santa Clara, CA, USA) thermostatted at 40 °C. The mobile phase was composed of 0.1% aqueous formic acid (eluent A) and 0.1% formic acid in acetonitrile (eluent B). The elution gradient was implemented as follows: from minute 0 to minute 1, 5% B; then 40 min from 5% to 70% B; followed by an increase to 100% B for 10 min; 100% B for 8 min; a decrease to 5% B for 2 min; and re-equilibration under the initial conditions for 10 min.
The settings of the electrospray ionization (ESI) source were as follows: electrospray voltage: 3.2 kV in the negative mode and 4.2 kV in the positive mode; capillary temperature: 350 °C; and the S lens RF level: 50. Data were obtained using two methods: the full-scan was used for compound detection, and full-scan data-dependent acquisition (FS-dd-MS2) was applied for the compounds’ identification. The scan was performed in positive and negative modes at a resolving power of 120,000 full-width at half maximum (FWHM) for m/z 200. The following settings of the mass spectrometer were employed: scan range: m/z 67–900; automatic gain control (AGC): 1e6; and injection time: 100 ms. A targeted tandem mass spectrometry (MS/MS, i.e., dd-MS2) analysis was performed in both positive and negative modes at 15,000 FWHM (m/z 200), and the isolation window was m/z 2.0. Normalized collision energy for the fragmentation of molecular ions was set to 20, 50, and 100 eV. AGC for dd-MS2 was set to 1 × 105, with an injection time of 50 ms and a loop count of 5. In the dd settings section, the AGC target was programmed at 5 × 103, and the maximum injection time was set to 50 ms. The data were analyzed using Xcalibur 4.0 and Compound Discoverer 3.1 software (Thermo Fisher Scientific, San Jose, CA, USA). All the samples, including blank samples, were assayed in triplicate. All the samples were processed in Compound Discoverer 3.1 via a common workflow called “Environmental Unknown ID w Online and Local Database Searches”. A mass tolerance of 10 ppm was applied to all nodes. Several databases, i.e., KEGG (https://www.genome.jp/kegg/; last accessed 5 November 2023), MassBank (https://massbank.eu/MassBank/; last accessed 5 November 2023), PlantCyc (https://plantcyc.org/; last accessed 5 November 2023), Planta Piloto de Quimica Fina Universidad de Alcala (http://www.cqab.eu/index.php/en/; last accessed 5 November 2023), AraCyc (https://www.arabidopsis.org/biocyc/; last accessed 5 November 2023), Extrasynthese (https://www.extrasynthese.com/; last accessed 5 November 2023), Golm Metabolome Database (last accessed 5 November 2023), Indofine (https://www.indofinechemical.com/; last accessed 5 November 2023), and Sequoia Research Products (http://www.chemcd.com/supplier/sequoia.html; last accessed 5 November 2023) were chosen in ChemSpider. A more detailed procedure for identifying substances is described in Ref. [10].
Several mzVaults were used in the mzVault Search node: Creation of a Plant Metabolite Spectral Library for Untargeted and targeted Metabolomics.db [59], Negative ion mode_Jan2021.db, Positive ion mode_Jan2021.db (https://more.bham.ac.uk/bamcg/resources/; last accessed 5 November 2023), and MS2_library.db (https://doi.org/10.1016/j.jchromb.2020.122105, last accessed 27 November 2023).
Metabolites were identified on the basis of both accurate mass and fragment mass “fingerprint” spectra via searches against the spectra of compounds available in the mzCloud database (https://www.mzcloud.org; last accessed 5 November 2023). If compounds were absent in mzCloud, they were tentatively identified using a ChemSpider search. According to the workflow, the masses extracted from the chromatograms were aligned and filtered to remove (i) background compounds present in the blank sample and (ii) compounds’ masses that were absent in the databases.
3.3. Statistical Analysis
This analysis was carried out in R. All samples, including blank samples, which consisted of the pure solvent, were analyzed as two biological replicates with three technical replicates per treatment group.
4. Conclusions
This study shows that the only representative of the genus Anemonopsis (A. macrophylla) synthesizes and accumulates a number of secondary metabolites of a phenolic and nonphenolic nature. Metabolites of this endemic plant of Japan were analyzed for the first time using the LC-HRMS method. In methanol extracts from flowers and leaves of A. macrophylla, 105 metabolites of various chemical classes were identified. Structural diversity was noted for phenolic acids and flavonoids. Further, simple coumarins and furocoumarins, as well as furochromones, were found in the chemical profile of A. macrophylla. The data presented in this work indicate that A. macrophylla is a rich source of secondary metabolites and that this plant can be used for research on (and production of) new biologically active fractions and individual compounds. Furthermore, it was established that the phytochemical uniqueness of individual organs is primarily determined using the quantitative characteristics of metabolites, not by their qualitative profile. Differences in concentrations of some identified substances between the extract from leaves and the extract from flowers of A. macrophylla may have a substantial impact on the manifestation of the biological activity of the extracts in question.
Author Contributions
Conceptualization, A.S.E., V.A.K., A.A.C. and N.V.P.; methodology, A.A.C. and V.A.K.; software, A.A.C.; validation, A.A.C. and V.A.K.; formal analysis, A.A.C., V.A.K., E.R.K. and N.V.P.; investigation, A.A.C., V.A.K., N.V.P. and E.R.K.; resources, A.S.E.; data curation, A.A.C. and V.V.K.; Writing—Original draft preparation, N.V.P., V.A.K., W.W., A.S.E. and A.A.C.; Writing—Review and editing, N.V.P., V.A.K., A.A.C., V.V.K. and A.S.E.; visualization, A.S.E., N.V.P. and V.A.K.; supervision, A.A.C., W.W. and V.V.K.; project administration, A.S.E. and V.A.K.; funding acquisition, A.S.E. and V.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the Russian Science Foundation, grant No. 23-14-00230, https://rscf.ru/project/23-14-00230/ (accessed on 5 November 2023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw data are available upon request.
Acknowledgments
The authors thank Nikolai A. Shevchuk for comments and proofreading.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Compton, J.; Culham, A.; Gibbings, J.; Jury, S. Phylogeny of Actaea including Cimicifuga (Ranunculaceae) inferred from nrDNA ITS sequence variation. Biochem. Syst. Ecol. 1998, 26, 185–197. [Google Scholar] [CrossRef]
- Wang, W.; Li, R.Q.; Chen, Z.D. Systematic position of Asteropyrum (Ranunculaceae) inferred from chloroplast and nuclear sequences. Plant Syst. Evol. 2005, 255, 41–54. [Google Scholar] [CrossRef]
- Yuan, Q.; Yang, Q.E. Tribal relationships of Beesia, Eranthis and seven other genera of Ranunculaceae: Evidence from cytological characters. Bot. J. Linn. Soc. 2006, 150, 267–289. [Google Scholar] [CrossRef][Green Version]
- Erst, A.S.; Petrova, N.V.; Kaidash, O.A.; Wang, W.; Kostikova, V.A. The Genus Eranthis: Prospects of Research on Its Phytochemistry, Pharmacology, and Biotechnology. Plants 2023, 12, 3795. [Google Scholar] [CrossRef] [PubMed]
- Compton, J.A.; Culham, A. Phylogeny and circumscription of tribe Actaeeae (Ranunculaceae). Syst. Bot. 2002, 27, 502–511. [Google Scholar]
- Tamura, M. Eranthis. In Die Natürlichen Pflanzenfamilien; Duncker und Humblot: Berlin, Germany, 1995; Volume 17, pp. 253–255. [Google Scholar]
- Tamura, M. Ranunculaceae. In Flowering Plants Dicotyledons; Springer: Cham, Switzerland, 1993; pp. 563–583. [Google Scholar]
- Ling, Y.Y.; Xiang, K.L.; Peng, H.W.; Erst, A.S.; Lian, L.; Zhao, L.; Jabbour, F.; Wang, W. Biogeographic diversification of Actaea (Ranunculaceae): Insights into the historical assembly of deciduous broad-leaved forests in the Northern Hemisphere. Mol. Phylogenet. Evol. 2023, 186, 107870. [Google Scholar] [CrossRef]
- Loconte, H.L.; Cambell, M.; Stevenson, D.W. Ordinal and familial relationships of Ranunculid genera. Plant Syst. Evol. Suppl. 1995, 9, 99–118. [Google Scholar]
- Erst, A.S.; Chernonosov, A.A.; Petrova, N.V.; Kulikovskiy, M.S.; Maltseva, S.Y.; Wang, W.; Kostikova, V.A. Investigation of chemical constituents of Eranthis longistipitata (Ranunculaceae): Coumarins and furochromones. Int. J. Mol. Sci. 2022, 23, 406. [Google Scholar] [CrossRef]
- Abuzaid, H.; Amin, E.; Moawad, A.; Abdelmohsen, U.R.; Hetta, M.; Mohammed, R. Liquid Chromatography High Resolution Mass Spectrometry Analysis, Phytochemical and Biological Study of Two Aizoaceae Plants: A New Kaempferol Derivative from Trianthema portulacastrum L. Pharmacogn. Res. 2020, 12, 212. [Google Scholar]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef]
- Bourgaud, F.; Hehn, A.; Larbat, R.; Doerper, S.; Gontier, E.; Kellner, S.; Matern, U. Biosynthesis of coumarins in plants: A major pathway still to be unraveled for cytochrome P450 enzymes. Phytochem. Rev. 2006, 5, 293–308. [Google Scholar] [CrossRef]
- Matern, U.; Lüer, P.; Kreusch, D. Biosynthesis of coumarins. In Comprehensive Natural Products Chemistry: Polyketides and Other Secondary Metabolites Including Fatty Acids and Their Derivatives; Sankawa, U., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 1, pp. 623–637. [Google Scholar]
- Zhu, J.J.; Jiang, J.G. Pharmacological and nutritional effects of natural coumarins and their structure-activity relationships. Mol. Nutr. Food Res. 2018, 62, 1701073. [Google Scholar] [CrossRef]
- Liu, B.; Raeth, T.; Beuerle, T. A novel 4-hydroxycoumarin biosynthesic pathway. Plant Mol. Biol. 2010, 72, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. Biomed. Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef] [PubMed]
- Budantsev, A.L. (Ed.) Plant Resources of Russia: Wild Flowering Plants, Their Component Composition and Biological Activity. T. 1: Families Magnoliaceae—Juglandaceae, Ulmaceae, Moraceae, Cannabaceae, Urticaceae; KMK: Saint Petersburg, Russia; Moscow, Russia, 2008; 421p. (In Russian) [Google Scholar]
- Zhao, X.H.; Chen, D.H.; Si, J.Y.; Pan, R.I.; Shen, L.G. Studies on the phenolic acid constituents from Chinese medicine “sheng-ma”, rhizome of Cimicifuga foetida L. Yao Xue Xue Bao = Acta Pharm. Sinica 2002, 37, 535–538. [Google Scholar]
- Niu, X.; Qin, R.; Zhao, Y.; Han, L.; Lu, J.; Lv, C. Simultaneous determination of 19 constituents in Cimicifugae rhizome by HPLC-DAD and screening for antioxidants through DPPH free radical scavenging assay. Biomed. Chromatogr. 2019, 33, 4624. [Google Scholar] [CrossRef]
- Silvan, A.M.; Abad, M.J.; Bermejo, P.; Sollhuber, M.; Villar, A. Antiinflammatory activity of coumarins from Santolina oblongifolia. J. Nat. Prod. 1996, 59, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, X.; Xu, W.; Farzaneh, F.; Xu, R. The structure and pharmacological functions of coumarins and their derivatives. Curr. Med. Chem. 2009, 16, 4236–4260. [Google Scholar] [CrossRef]
- Pan, R.; Gao, X.; Lu, D.; Xu, X.; Xia, Y.; Dai, Y. Prevention of FGF-2-angiogenesis by scopoletin, a coumarin compound isolated from Erycibe obtusifolia Benth. and its mechanism of action. Int. Immunopharmacol. 2011, 11, 2007–2016. [Google Scholar] [CrossRef]
- Witaicenis, A.; Seito, L.N.; Chagas, A.S.; de Almeda, L.; Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; Di Stasi, L.C. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine 2014, 21, 240–246. [Google Scholar] [CrossRef]
- Grover, J.; Jachak, S.M. Coumarins as privileged scaffold for anti-inflammatory drug development. RSC Adv. 2015, 5, 38892–38905. [Google Scholar] [CrossRef]
- Wei, Y.; Peng, W.; Wang, D.; Hao, S.H.; Li, W.W.; Ding, F. Design, synthesis, antifungal activity, and 3D-QSAR of coumarin derivatives. J. Pest. Sci. 2018, 43, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Kumar, S.; Chand, K.; Kathuria, A.; Gupta, A.; Jain, R. An update on natural occurrence and biological activity of chromones. Curr. Med. Chem. 2011, 18, 3825–3852. [Google Scholar] [CrossRef] [PubMed]
- Khadem, S.; Marles, R.J. Chromone and flavonoid alkaloids: Occurrence and bioactivity. Molecules 2012, 17, 191–206. [Google Scholar] [CrossRef]
- Klymchenko, A.S.; Pivovarenko, V.G.; Demchenko, A.P. Perturbation of planarity as the possible mechanism of solvent-dependent variations of fluorescence quantum yield in 2-aryl-3-hydroxychromones. Mol. Biomol. Spectros. 2003, 59, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Avanesyan, A.A.; Pashkov, A.N.; Simonyan, N.A.; Simonyan, A.V.; Myachina, O.V. Anti-radical activity of cinnamic acid derivatives. Pharm. Chem. J. 2009, 43, 18–19. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, S.; Hao, S.; Zhang, Z.; Wang, W.; Yao, S. Double roles of hydroxycinnamic acid derivatives in protection against lysozyme oxidation. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 1810–1818. [Google Scholar] [CrossRef]
- Urbaniak, A.; Molski, M.; Szelag, M. Quantum-chemical calculations of the antioxidant properties of trans-p-coumaric acid and trans-sinapinic acid. Comp. Methods Sci. Technol. 2012, 18, 117–128. [Google Scholar] [CrossRef]
- Cheng, J.C.; Dai, F.; Zhou, B.; Yang, L.; Liu, Z.L. Antioxidant activity of hydroxycinnamic acid derivatives in human low density lipoprotein: Mechanism and structure-activity relationship. Food Chem. 2007, 104, 132–139. [Google Scholar] [CrossRef]
- Moazzen, A.; Öztinen, N.; Ak-Sakalli, E.; Koşar, M. Structure-antiradical activity relationships of 25 natural antioxidant phenolic compounds from different classes. Heliyon 2022, 8, e10467. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, M.; He, L.; Song, X.; Cao, T. Chlorogenic acid: A review on its mechanisms of anti-inflammation, disease treatment, and related delivery systems. Front. Pharmacol. 2023, 14, 1218015. [Google Scholar] [CrossRef]
- De Lima, G.D.A.; Rodrigues, M.P.; de Mendes, T.A.O.; Moreira, G.A.; Siqueira, G.A.; da Silva, A.M.; Vaz, B.G.; Fietto, J.L.R.; Bressan, G.C.; Machado-Neves, M. Synthesis and antimetastatic activity evaluation of cinnamic acid derivatives containing 1,2,3-triazolic portions. Toxicol. In Vitro 2018, 53, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Korošec, B.; Sova, M.; Turk, S.; Kraševec, N.; Novak, M.; Lah, L.; Stojan, J.; Podobnik, B.; Berne, S.; Zupanec, N.; et al. Antifungal activity of cinnamic acid derivatives involves inhibition of benzoate 4-hydroxylase (CYP53). J. Appl. Microbiol. 2014, 116, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Chen, Z.M.; Zhou, Z.L.; Huang, G.L.; Zhou, Q.; Xi, Y.J. New cinnamic acid derivatives with potential neuroprotective activities from the stems of Melicope ptelefolia. Phytochem. Lett. 2023, 53, 161–165. [Google Scholar] [CrossRef]
- De, P.; Baltas, M.; Bedos-Belval, F. Cinnamic acid derivatives as anticancer agents—A review. Curr. Med. Chem. 2011, 18, 1672–1703. [Google Scholar] [CrossRef]
- Fonseca, A.C.; Lima, M.S.; Sousa, A.F.; Silvestre, A.J.; Coelho, J.F.J.; Serra, A.C. Cinnamic acid derivatives as promising building blocks for advanced polymers: Synthesis, properties and applications. Polym. Chem. 2019, 14, 1696–1723. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Petrushova, L.A.; Dzyubenko, S.P.; Grinevich, L.A.; Sim, G. Synthesis and biological properties of {[(4-hydroxy-1-methyl-2,2-di-oxido-1H-2,1-benzothiazin-3-yl]amino}-benzoic acid and their derivatives. Pharm. Chem. J. 2017, 51, 30–31. [Google Scholar] [CrossRef]
- Shen, B.J.; Qin, K.M.; Zhang, X.H.; Liu, Q.D.; Cai, H.; Liu, X.; Cai, B.C. Study on quality evaluation of cimicifugae rhizome from different producing areas by HPLC fingerprint. Chin. J. Chin. Mater. Med. 2013, 38, 2155–2158. [Google Scholar]
- Sakai, S.; Ochiai, H.; Mantani, N.; Kogure, T.; Shibahara, N.; Terasawa, K. Administration of isoferulic acid improved the survival rate of lethal influenza virus pneumonia in mice. Mediat. Inflamm. 2001, 10, 93–96. [Google Scholar] [CrossRef]
- Chen, C.; Liu, J.; Li, B.; Wang, T.; Wang, E.; Wang, G. Isoferulic acid affords the antiviral potential and restrains white spot syndrome virus proliferation in crayfish (Procambarus clarkia). Aquacult. Fish. 2022, in press. [Google Scholar] [CrossRef]
- Liu, I.M.; Hsu, F.L.; Chen, C.F.; Cheng, J.T. Antihyperglycemic action of isoferulic acid in streptozotocin-induced diabetic. Br. J. Pharmacol. 2000, 129, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yin, T.; Wang, X.; Zhang, F.; Pan, G.; Lv, H.; Wang, X.; Orgah, J.O.; Zhu, Y.; Wu, H. Traditional uses, phytochemistry, pharmacology and toxicology of the genus Cimicifuga: A review. J. Ethnopharmacol. 2017, 14, 264–282. [Google Scholar] [CrossRef]
- Jahn, A.; Petersen, M. Fukinolic acid and cimicifugic acids: A review. Phytochem. Rev. 2022, 21, 1247–1271. [Google Scholar] [CrossRef]
- Budantsev, A.L. (Ed.) Plant Resources of Russia: Wild Flowering Plants, Their Component Composition and Biological Activity: Families Fabaceae—Apiaceae; KMK: Saint Petersburg, Russia; Moscow, Russia, 2010; Volume 3, 601p. (In Russian) [Google Scholar]
- Dong, C.X.; Shi, S.P.; Wu, K.S.; Tu, P.F. Chemical constituents from the roots and rhizomes of Clematis hexapetala Pall. Z. Naturforsch. 2007, 62, 854–858. [Google Scholar] [CrossRef]
- Simmler, C.; Hajirahimkhan, A.; Lankin, D.C.; Bolton, J.L.; Jones, T.; Soejarto, D.D.; Chen, S.N.; Pauli, G.F. Dynamic residual complexity of the isoquiritigenin-liquiritigenin interconversion during bioassay. J. Agric. Food Chem. 2013, 61, 2146–2157. [Google Scholar] [CrossRef]
- Peng, F.; Du, Q.; Peng, C.; Wang, N.; Tang, H.; Xie, X.; Shen, J.; Chen, J. A review: The pharmacology of isoliquiritigenin. Phytother. Res. 2015, 29, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, J.; Wen, Q.; Li, Y. Isoquiritigenin, a flavonoid from licorice, relaxes guinea-pig tracheal smooth muscle in vitro and in vivo: Role of cGMP/PKG. Eur. J. Pharmacol. 2008, 587, 257–266. [Google Scholar] [CrossRef]
- Wu, M.; Wu, Y.; Deng, B.; Li, J.; Cao, H.; Qu, Y.; Qian, X.; Zhong, G. Isoliquiritigenin decreases the incidence of colitis-associated colorectal cancer by modulating the intestinal microbiota. Oncotarget 2016, 7, 85318–85331. [Google Scholar] [CrossRef]
- Yu, S.M.; Kuo, S.C. Vasorelaxant effect of isoliquiritigenin, a novel soluble guanylate cyclase activator, in rat aorta. Br. J. Pharmacol. 1995, 114, 1587–1594. [Google Scholar] [CrossRef]
- Behzad, S.; Sureda, A.; Barreca, D.; Nabavi, S.F.; Rastrelli, L.; Nabavi, S.M. Health effects of phloretin: From chemistry to medicine. Phytochem. Rev. 2017, 16, 527–533. [Google Scholar] [CrossRef]
- Kovalikova, Z.; Lnenicka, J.; Andrys, R. The Influence of Locality on Phenolic Profile and Antioxidant Capacity of Bud Extracts. Foods 2021, 10, 1608. [Google Scholar] [CrossRef]
- Hudec, J.; Burdová, M.; Kobida, L.U.; Komora, L.; Macho, V.; Kogan, G.; Turianica, I.; Kochanová, R.; Ložek, O.; Habán, M.; et al. Antioxidant capacity changes and phenolic profile of Echinacea purpurea, nettle (Urtica dioica L.), and dandelion (Taraxacum officinale) after application of polyamine and phenolic biosynthesis regulators. J. Agric. Food Chem. 2007, 55, 5689–5696. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, W.; Xiang, Q.; Kim, J.; Dufresne, C.; Liu, Y.; Li, T.; Chen, S. Creation of a Plant Metabolite Spectral Library for Untargeted and Targeted Metabolomics. Int. J. Mol. Sci. 2023, 24, 2249. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).