Proteomic Profiling Identifies Candidate Diagnostic Biomarkers of Hydrosalpinx in Endometrial Fluid: A Pilot Study
Abstract
1. Introduction
2. Results
2.1. Demographic Characteristics
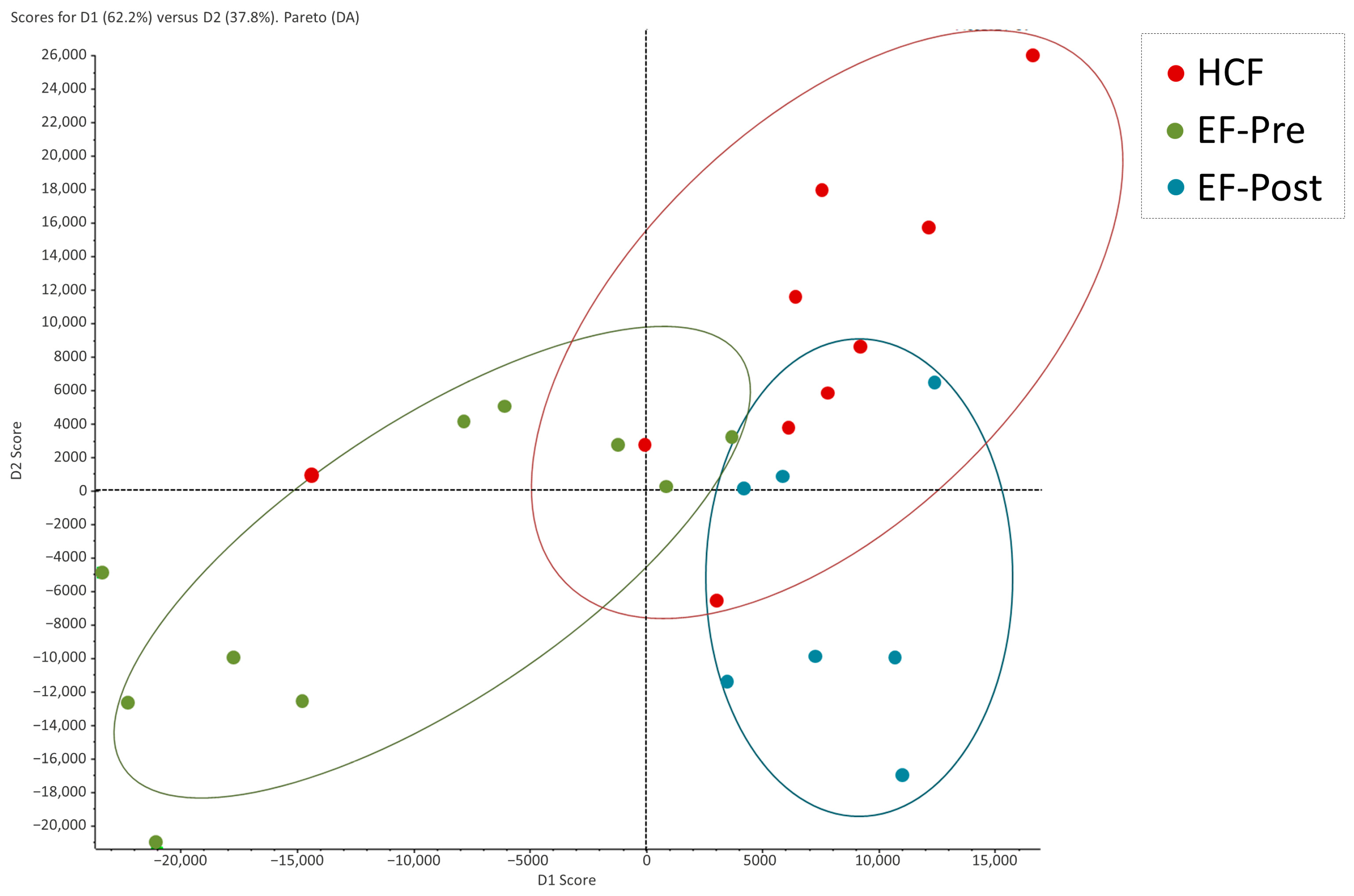
2.2. Proteomic Profiling of Hydrosalpinx Cystic Fluid and Endometrial Fluid
2.3. Comparison of Protein Abundance between Hydrosalpinx Cyst Fluid, Pre- and Post-Salpingectomy Endometrial Fluid
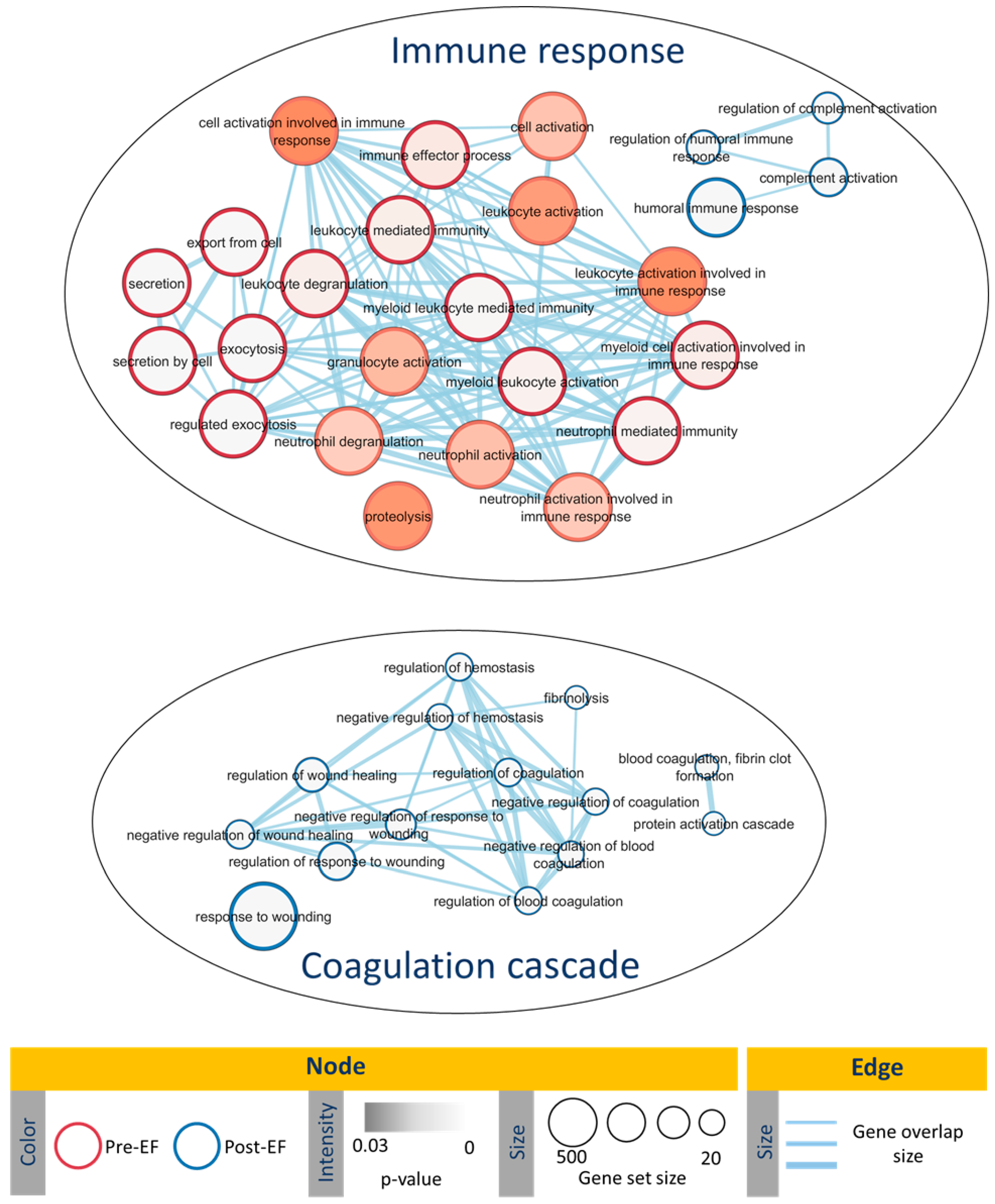
3. Discussion
4. Materials and Methods
4.1. Ethical Approval and Study Design
4.2. Hydrosalpinx Cyst Fluid and Endometrial Fluid Collection and Processing
4.3. Spectral Library Construction for LC-MS/MS
4.3.1. In-Gel Protein Digestion
4.3.2. LC-MS/MS
4.3.3. Peptide Query Parameters and Peptide-Centric Scoring
4.4. Proteomic Profiling of Hydrosalpinx Cyst Fluid, Pre- and Post- Salpingectomy Endometrial Fluid
4.4.1. In-Gel Protein Digestion and Peptide Purification of Individual Samples
4.4.2. SWATH-MS
4.4.3. SWATH-MS Data Analysis
4.5. Statistical Analyses
4.6. Functional Enrichment Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, K.Y.B.; Cheong, Y. Hydrosalpinx–Salpingostomy, salpingectomy or tubal occlusion. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 59, 41–47. [Google Scholar] [CrossRef]
- Yohannes, E.; Kazanjian, A.A.; Lindsay, M.E.; Fujii, D.T.; Ieronimakis, N.; Chow, G.E.; Beesley, R.D.; Heitmann, R.J.; Burney, R.O. The human tubal lavage proteome reveals biological processes that may govern the pathology of hydrosalpinx. Sci. Rep. 2019, 9, 8980. [Google Scholar] [CrossRef] [PubMed]
- Palagiano, A.; Cozzolino, M.; Ubaldi, F.M.; Palagiano, C.; Coccia, M.E. Effects of Hydrosalpinx on Endometrial Implantation Failures: Evaluating Salpingectomy in Women Undergoing in vitro fertilization. Rev. Bras. Ginecol. Obstet. 2021, 43, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Morell, A.; Nieto-Tous, M.; Andrada-Ripollés, C.; Pascual, M.Á.; Ajossa, S.; Guerriero, S.; Alcázar, J.L. Transvaginal Ultrasound Accuracy in the Hydrosalpinx Diagnosis: A Systematic Review and Meta-Analysis. Diagnostics 2023, 13, 948. [Google Scholar] [CrossRef] [PubMed]
- Darville, T. Pelvic Inflammatory Disease. Sex. Transm. Dis. 2013, 40, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Azkargorta, M.; Escobes, I.; Iloro, I.; Osinalde, N.; Corral, B.; Ibañez-Perez, J.; Exposito, A.; Prieto, B.; Elortza, F.; Matorras, R. Differential proteomic analysis of endometrial fluid suggests increased inflammation and impaired glucose metabolism in non-implantative IVF cycles and pinpoints PYGB as a putative implantation marker. Hum. Reprod. 2018, 33, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Casado-Vela, J.; Rodriguez-Suarez, E.; Iloro, I.; Ametzazurra, A.; Alkorta, N.; García-Velasco, J.A.; Matorras, R.; Prieto, B.; González, S.; Nagore, D.; et al. Comprehensive proteomic analysis of human endometrial fluid aspirate. J. Proteome Res. 2009, 8, 4622–4632. [Google Scholar] [CrossRef]
- Ametzazurra, A.; Matorras, R.; García-Velasco, J.A.; Prieto, B.; Simón, L.; Martínez, A.; Nagore, D. Endometrial fluid is a specific and non-invasive biological sample for protein biomarker identification in endometriosis. Hum. Reprod. 2009, 24, 954–965. [Google Scholar] [CrossRef]
- Savaris, R.F.; Giudice, L.C. The influence of hydrosalpinx on markers of endometrial receptivity. Semin. Reprod. Med. 2007, 25, 476–482. [Google Scholar] [CrossRef]
- Ng, E.H.Y.; Chan, C.C.W.; Tang, O.S.; Yeung, W.S.B.; Ho, P.C. Comparison of endometrial and subendometrial blood flow measured by three-dimensional power Doppler ultrasound between stimulated and natural cycles in the same patients. Hum. Reprod. 2004, 19, 2385–2390. [Google Scholar] [CrossRef]
- Vandromme, J.; Chasse, E.; Lejeune, B.; Van Rysselberge, M.; Delvigne, A.; Leroy, F. Infertility: Hydrosalpinges in in-vitro fertilization: An unfavourable prognostic feature. Hum. Reprod. 1995, 10, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Zeyneloglu, H.B.; Arici, A.; Olive, D.L. Adverse effects of hydrosalpinx on pregnancy rates after in vitro fertilization-embryo transfer. Fertil. Steril. 1998, 70, 492–499. [Google Scholar] [CrossRef]
- Camus, E.; Poncelet, C.; Goffinet, F.; Wainer, B.; Merlet, F.; Nisand, I.; Philippe, H.J. Pregnancy rates after in-vitro fertilization in cases of tubal infertility with and without hydrosalpinx: A meta-analysis of published comparative studies. Hum. Reprod. 1999, 14, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.N.; Yue, Z.; Meng, F.J.; Petersen, K. Implantation: Low implantation rate after in-vitro fertilization in patients with hydrosalpinges diagnosed by ultrasonography. Hum. Reprod. 1994, 9, 1935–1938. [Google Scholar] [CrossRef] [PubMed]
- Strandell, A. The influence of hydrosalpinx on IVF and embryo transfer: A review. Hum. Reprod. Update 2000, 6, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Tsiami, A.; Chaimani, A.; Mavridis, D.; Siskou, M.; Assimakopoulos, E.; Sotiriadis, A. Surgical treatment for hydrosalpinx prior to in-vitro fertilization embryo transfer: A network meta-analysis. Ultrasound Obstet. Gynecol. 2016, 48, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Van Den Broek, I.; Sparidans, R.W.; Van Winden, A.W.J.; Gast, M.C.W.; Van Dulken, E.J.; Schellens, J.H.M.; Beijnen, J.H. The absolute quantification of eight inter-α-trypsin inhibitor heavy chain 4 (ITIH4)-derived peptides in serum from breast cancer patients. Proteomics-Clin. Appl. 2010, 4, 931–939. [Google Scholar] [CrossRef]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef]
- Gaipl, U.S.; Kuenkele, S.; Voll, R.E.; Beyer, T.D.; Kolowos, W.; Heyder, P.; Kalden, J.R.; Herrmann, M. Complement binding is an early feature of necrotic and a rather late event during apoptotic cell death. Cell Death Differ. 2001, 8, 327–334. [Google Scholar] [CrossRef]
- Koski, C.L.; Ramm, L.E.; Hammer, C.H.; Mayer, M.M.; Shin, M.L. Cytolysis of nucleated cells by complement: Cell death displays multi-hit characteristics. Proc. Natl. Acad. Sci. USA 1983, 80, 3816–3820. [Google Scholar] [CrossRef]
- Hill, C.J.; Fakhreldin, M.; Maclean, A.; Dobson, L.; Nancarrow, L.; Bradfield, A.; Choi, F.; Daley, D.; Tempest, N.; Hapangama, D.K. Endometriosis and the fallopian tubes: Theories of origin and clinical implications. J. Clin. Med. 2020, 9, 1905. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Surti, N.; Kesavan, S.; Agarwal, A. Significance of oxidative stress in human reproduction. Arch. Med. Sci. 2009, 5, S28–S42. [Google Scholar]
- Bignotti, E.; Tassi, R.A.; Calza, S.; Ravaggi, A.; Rossi, E.; Donzelli, C.; Todeschini, P.; Romani, C.; Bandiera, E.; Zanotti, L.; et al. Secretoglobin expression in ovarian carcinoma: Lipophilin B gene upregulation as an independent marker of better prognosis. J. Transl. Med. 2013, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Von Brünneck, A.C.; Hornung, D.; Denkert, C.; Ufer, C.; Schiebel, H.; Kuhn, H.; Borchert, A. Differential expression of secretoglobins in normal ovary and in ovarian carcinoma-Overexpression of mammaglobin-1 is linked to tumor progression. Arch. Biochem. Biophys. 2014, 547, 27–36. [Google Scholar] [CrossRef]
- Schoutrop, E.; El-Serafi, I.; Poiret, T.; Zhao, Y.; Gultekin, O.; He, R.; Moyano-Galceran, L.; Carlson, J.W.; Lehti, K.; Hassan, M.; et al. Mesothelin-specific CAR T cells target ovarian cancer. Cancer Res. 2021, 81, 3022–3055. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Pastan, I.; Willingham, M.C. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int. J. Cancer 1992, 50, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Udby, L.; Sørensen, O.E.; Pass, J.; Johnsen, A.H.; Behrendt, N.; Borregaard, N.; Kjeldsen, L. Cysteine-Rich Secretory Protein 3 Is a Ligand of α 1 B-Glycoprotein in Human Plasma. Biochemistry 2004, 43, 12877–12886. [Google Scholar] [CrossRef]
- Wang, Y.; Sheng, N.; Xie, Y.; Chen, S.; Lu, J.; Zhang, Z.; Shan, Q.; Wu, D.; Zheng, G.; Li, M.; et al. Low expression of CRISP3 predicts a favorable prognosis in patients with mammary carcinoma. J. Cell. Physiol. 2019, 234, 13629–13638. [Google Scholar] [CrossRef]
- Armstrong, G.M.; Maybin, J.A.; Murray, A.A.; Nicol, M.; Walker, C.; Saunders, P.T.K.; Rossi, A.G.; Critchley, H.O.D. Endometrial apoptosis and neutrophil infiltration during menstruation exhibits spatial and temporal dynamics that are recapitulated in a mouse model. Sci. Rep. 2017, 7, 17416. [Google Scholar] [CrossRef]
- Hahn, S.; Giaglis, S.; Hoesli, I.; Hasler, P. Neutrophil NETs in reproduction: From infertility to preeclampsia and the possibility of fetal loss. Front. Immunol. 2012, 3, 362. [Google Scholar] [CrossRef]
- Shevchenko, A.; Jensen, O.N.; Podtelejnikov, A.V.; Sagliocco, F.; Wilm, M.; Vorm, O.; Mortensen, P.; Shevchenko, A.; Boucherie, H.; Mann, M. Linking genome and proteome by mass spectrometry: Large-scale identification of yeast proteins from two dimensional gels. Proc. Natl. Acad. Sci. USA 1996, 93, 14440–14445. [Google Scholar] [CrossRef] [PubMed]
- Shilov, I.V.; Seymour, S.L.; Patel, A.A.; Loboda, A.; Tang, W.H.; Keating, S.P.; Hunter, C.L.; Nuwaysir, L.M.; Schaeffer, D.A. The Paragon Algorithm, a Next Generation Search Engine That Uses Sequence Temperature Values and Feature Probabilities to Identify Peptides from Tandem Mass Spectra. Mol. Cell. Proteomics 2007, 6, 1638–1655. [Google Scholar] [CrossRef] [PubMed]
- Gillet, L.C.; Navarro, P.; Tate, S.; Röst, H.; Selevsek, N.; Reiter, L.; Bonner, R.; Aebersold, R. Targeted Data Extraction of the MS/MS Spectra Generated by Data-independent Acquisition: A New Concept for Consistent and Accurate Proteome Analysis. Mol. Cell. Proteomics 2012, 11, O111.016717. [Google Scholar] [CrossRef] [PubMed]
- Hopfgartner, G.; Tonoli, D.; Varesio, E. High-resolution mass spectrometry for integrated qualitative and quantitative analysis of pharmaceuticals in biological matrices. Anal. Bioanal. Chem. 2012, 402, 2587–2596. [Google Scholar] [CrossRef]
- Reimand, J.; Isserlin, R.; Voisin, V.; Kucera, M.; Tannus-Lopes, C.; Rostamianfar, A.; Wadi, L.; Meyer, M.; Wong, J.; Xu, C.; et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 2019, 14, 482–517. [Google Scholar] [CrossRef]
| Accession Code | Protein Name | Protein Description | p-Value | FC (HCF vs. Pre-Salpingectomy EF) |
|---|---|---|---|---|
| P18206 | VINC | Vinculin | 0.037 | 1.51 |
| P0DOX3 | IGD | Immunoglobulin delta heavy chain | 0.016 | 1.48 |
| P19823 | ITIH2 | Inter-alpha-trypsin inhibitor heavy chain H2 | 0.040 | 1.40 |
| P01031 | CO5 | Complement C5 | 0.042 | 1.27 |
| Q9NRX4 | PHP14 | 14 kDa phosphohistidine phosphatase | 0.038 | −2.27 |
| P0DMV9 | HS71B | Heat shock 70 kDa protein 1B | 0.014 | −2.33 |
| Q08211 | DHX9 | ATP-dependent RNA helicase A | 0.024 | −2.33 |
| P00966 | ASSY | Argininosuccinate synthase | 0.038 | −2.63 |
| O14773 | TPP1 | Tripeptidyl-peptidase 1 | 0.020 | −2.63 |
| O94760 | DDAH1 | N(G),N(G)-dimethylarginine dimethylaminohydrolase 1 | 0.040 | −3.03 |
| Q96TA1 | NIBA2 | Protein Niban 2 | 0.011 | −3.03 |
| Q01995 | TAGL | Transgelin | 0.010 | −3.13 |
| P68371 | TBB4B | Tubulin beta-4B chain | 0.047 | −3.85 |
| Q9NPH2 | INO1 | Inositol-3-phosphate synthase 1 | 0.038 | −5.00 |
| Accession Code | Protein Name | Protein Description | p-Value | FC (HCF vs. Post-Salpingectomy EF) |
|---|---|---|---|---|
| O95969 | SG1D2 | Secretoglobin family 1D member 2 | 0.023 | 1.98 |
| P60891 | PRPS1 | Ribose-phosphate pyrophosphokinase 1 | 0.043 | 1.71 |
| Q92530 | PSMF1 | Proteasome inhibitor PI31 subunit | 0.040 | 1.38 |
| Q96G03 | PGM2 | Phosphoglucomutase-2 | 0.031 | −1.61 |
| P15169 | CBPN | Carboxypeptidase N catalytic chain | 0.025 | −1.79 |
| P30085 | KCY | UMP-CMP kinase | 0.037 | −2.00 |
| Q08211 | DHX9 | ATP-dependent RNA helicase A | 0.038 | −2.08 |
| Q86Z20 | CC125 | Coiled-coil domain-containing protein 125 | 0.040 | −2.08 |
| Q99536 | VAT1 | Synaptic vesicle membrane protein VAT-1 homolog | 0.026 | −2.22 |
| Q15582 | BGH3 | Transforming growth factor-beta-induced protein ig-h3 | 0.050 | −2.38 |
| Q15113 | PCOC1 | Procollagen C-endopeptidase enhancer 1 | 0.032 | −2.63 |
| O14773 | TPP1 | Tripeptidyl-peptidase 1 | 0.042 | −2.70 |
| Q01995 | TAGL | Transgelin | 0.045 | −4.00 |
| Accession Code | Protein Name | Protein Description | p-Value | FC (Pre- vs. Post-Salpingectomy EF) |
|---|---|---|---|---|
| Q7L266 | ASGL1 | Isoaspartyl peptidase/L-asparaginase | 0.021 | 14.57 |
| Q13421 | MSLN | Mesothelin | 0.044 | 12.22 |
| P54108 | CRIS3 | Cysteine-rich secretory protein 3 | 0.017 | 10.29 |
| P10909 | CLUS | Clusterin | 0.013 | 9.70 |
| Q53GD3 | CTL4 | Choline transporter-like protein 4 | 0.010 | 6.57 |
| Q08380 | LG3BP | Galectin-3-binding protein | 0.034 | 6.42 |
| Q9NPH2 | INO1 | Inositol-3-phosphate synthase 1 | 0.031 | 5.83 |
| Q13938 | CAYP1 | Calcyphosin | 0.037 | 5.71 |
| Q9BW30 | TPPP3 | Tubulin polymerization-promoting protein family member 3 | 0.045 | 5.62 |
| P08294 | SODE | Extracelular superoxide dismutase [Cu-Zn] | 0.033 | 5.18 |
| P67936 | TPM4 | Tropomyosin alpha-4 chain | 0.033 | −2.33 |
| P02647 | APOA1 | Apolipoprotein A-I | 0.001 | −2.63 |
| P02671 | FIBA | Fibrinogen alpha chain | 0.007 | −2.70 |
| P02675 | FIBB | Fibrinogen beta chain | 0.038 | −2.70 |
| P00738 | HPT | Haptoglobin | 0.046 | −3.03 |
| P02679 | FIBG | Fibrinogen gamma chain | 0.043 | −3.13 |
| P02538 | K2C6A | Keratin, type II cytoskeletal 6A | 0.043 | −3.70 |
| Q9BRX8 | PXL2A | Peroxiredoxin-like 2A | 0.041 | −4.17 |
| P08572 | CO4A2 | Collagen alpha-2(IV) chain | 0.049 | −4.17 |
| Q13509 | TBB3 | Tubulin beta-3 chain | 0.043 | −6.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Martin, R.; de Castro, P.; Fernandez, C.; Quintana, F.; Quiñonero, A.; Ferrando, M.; Dominguez, F. Proteomic Profiling Identifies Candidate Diagnostic Biomarkers of Hydrosalpinx in Endometrial Fluid: A Pilot Study. Int. J. Mol. Sci. 2024, 25, 968. https://doi.org/10.3390/ijms25020968
Gonzalez-Martin R, de Castro P, Fernandez C, Quintana F, Quiñonero A, Ferrando M, Dominguez F. Proteomic Profiling Identifies Candidate Diagnostic Biomarkers of Hydrosalpinx in Endometrial Fluid: A Pilot Study. International Journal of Molecular Sciences. 2024; 25(2):968. https://doi.org/10.3390/ijms25020968
Chicago/Turabian StyleGonzalez-Martin, Roberto, Pedro de Castro, Carmen Fernandez, Fernando Quintana, Alicia Quiñonero, Marcos Ferrando, and Francisco Dominguez. 2024. "Proteomic Profiling Identifies Candidate Diagnostic Biomarkers of Hydrosalpinx in Endometrial Fluid: A Pilot Study" International Journal of Molecular Sciences 25, no. 2: 968. https://doi.org/10.3390/ijms25020968
APA StyleGonzalez-Martin, R., de Castro, P., Fernandez, C., Quintana, F., Quiñonero, A., Ferrando, M., & Dominguez, F. (2024). Proteomic Profiling Identifies Candidate Diagnostic Biomarkers of Hydrosalpinx in Endometrial Fluid: A Pilot Study. International Journal of Molecular Sciences, 25(2), 968. https://doi.org/10.3390/ijms25020968





