Ferroptosis, a Regulated Form of Cell Death, as a Target for the Development of Novel Drugs Preventing Ischemia/Reperfusion of Cardiac Injury, Cardiomyopathy and Stress-Induced Cardiac Injury
Abstract
1. Introduction
2. The Main Manifestation of Ferroptosis
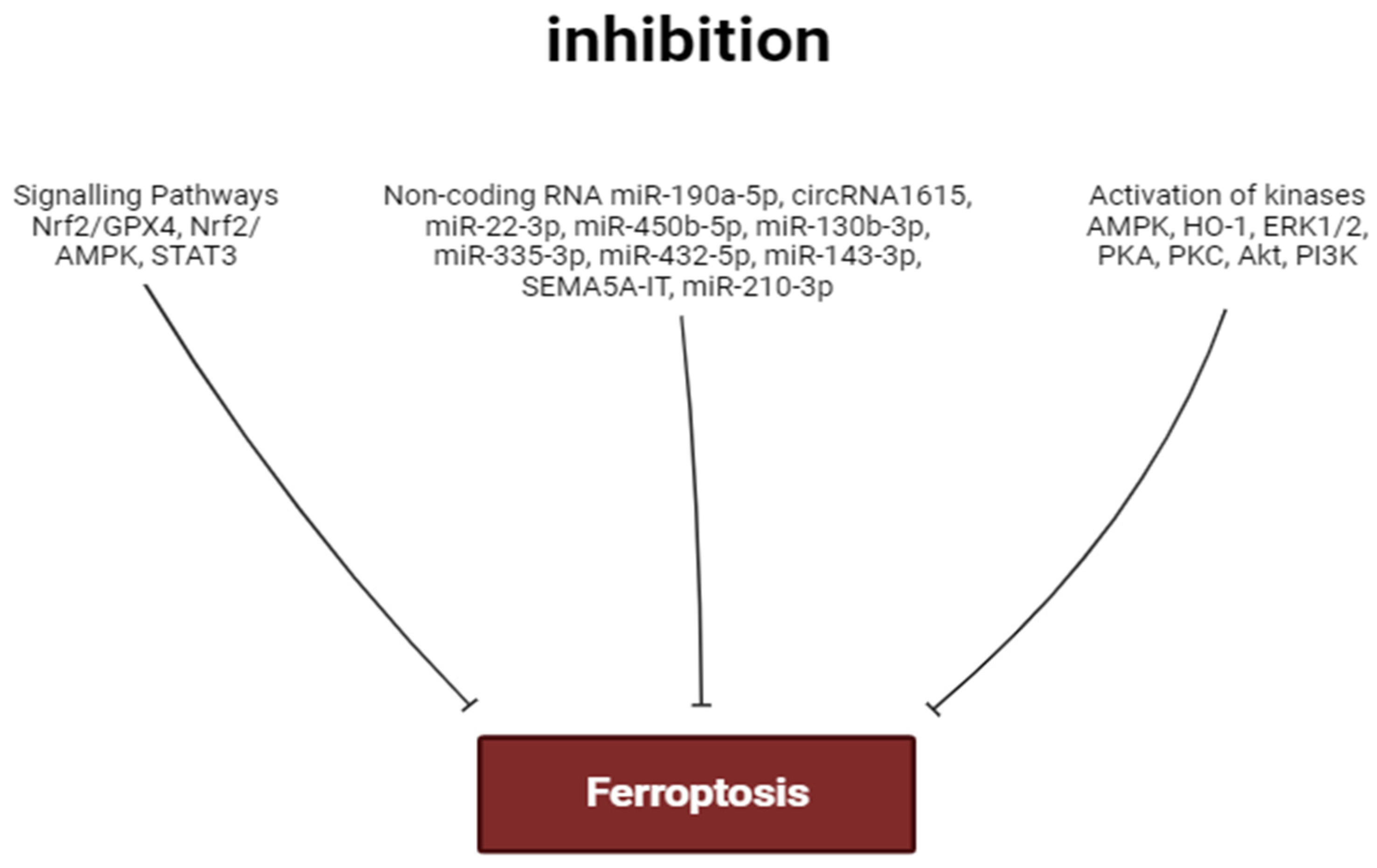
3. Inhibitors of Ferroptosis
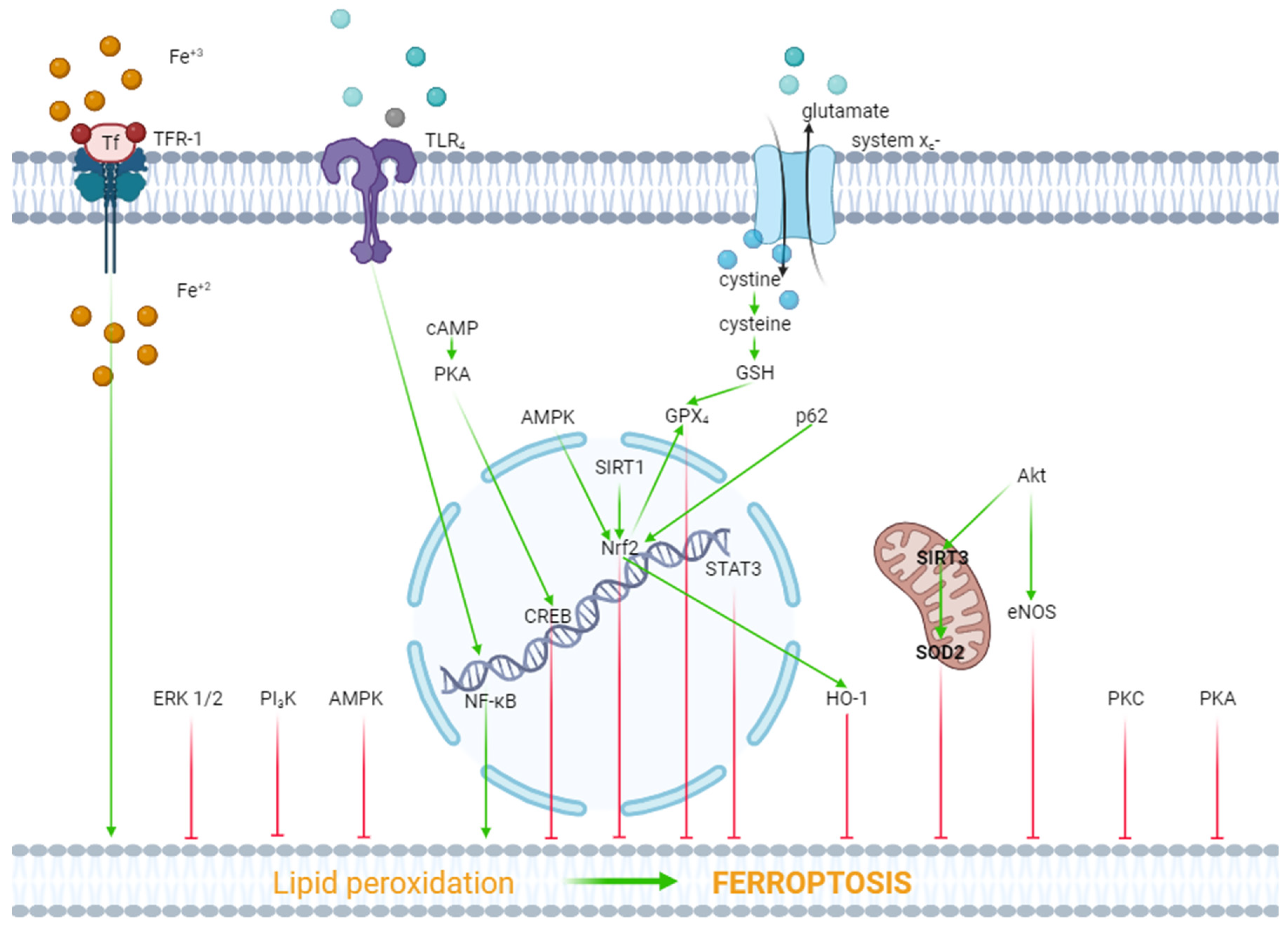
4. The Role of Kinases in the Regulation of Ferroptosis
5. The Role of Non-Coding RNA in the Regulation of Ferroptosis in the Heart
6. The Role of Transcription Factors in the Regulation of Ferroptosis
7. Inflammation
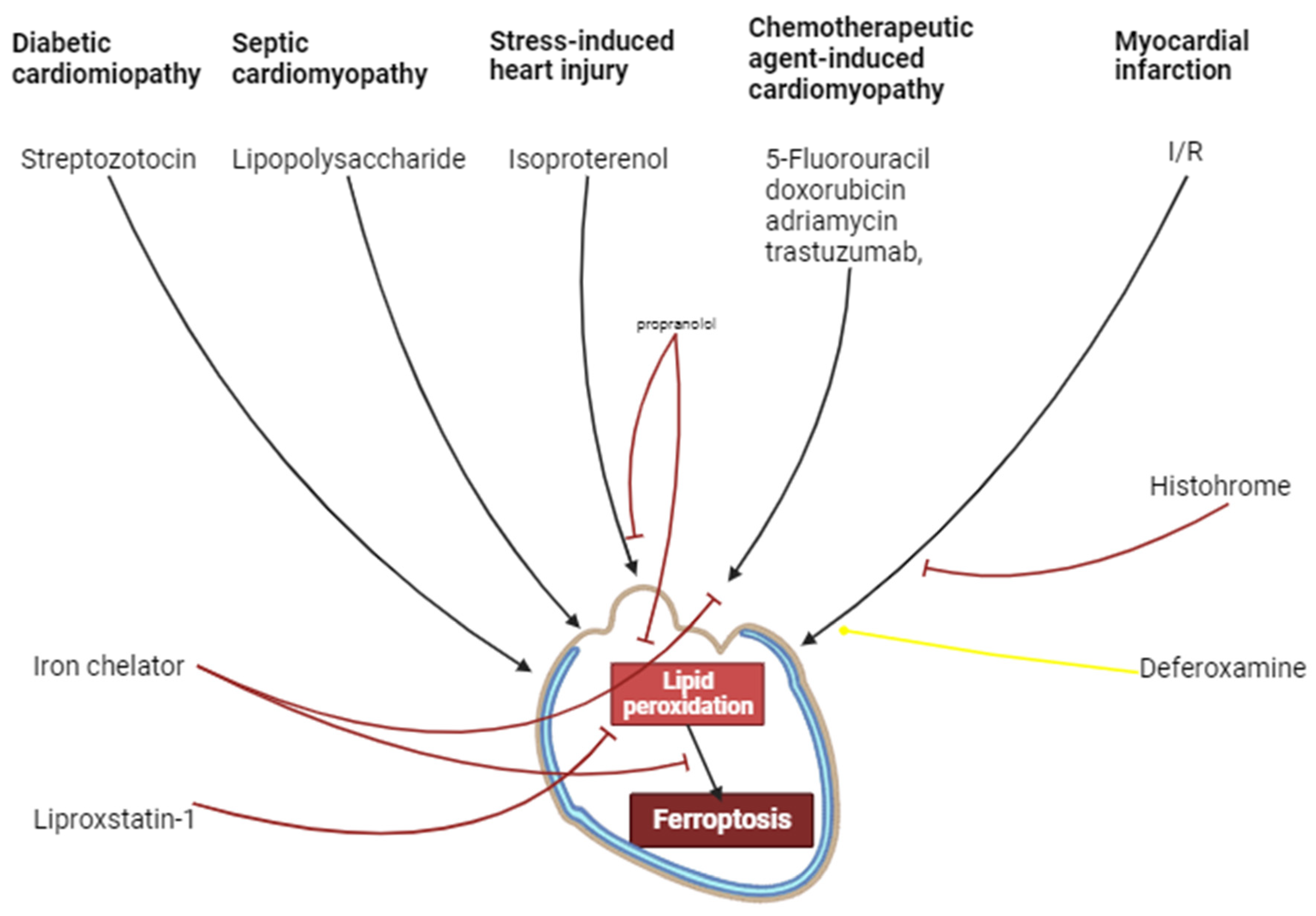
8. Ischemia/Reperfusion of Cardiac Injury
9. Chemotherapeutic Agent-Induced Cardiomyopathy
10. Septic Cardiomyopathy
11. Diabetic Cardiomyopathy
12. Stress-Induced Cardiac Injury
13. Unresolved Issues and Prospects for the Use of Ferroptosis Inhibitors for the Treatment of Cardiovascular Diseases
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menees, D.S.; Peterson, E.D.; Wang, Y.; Curtis, J.P.; Messenger, J.C.; Rumsfeld, J.S.; Gurm, H.S. Door-to-Balloon Time and Mortality among Patients Undergoing Primary PCI. N. Engl. J. Med. 2013, 369, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Fabris, E.; Kilic, S.; Schellings, D.A.A.M.; Ten Berg, J.M.; Kennedy, M.W.; van Houwelingen, K.G.; Giannitsis, E.; Kolkman, E.; Ottervanger, J.P.; Hamm, C.; et al. Long-Term Mortality and Prehospital Tirofiban Treatment in Patients with ST Elevation Myocardial Infarction. Heart 2017, 103, 1515–1520. [Google Scholar] [CrossRef]
- Vaidya, S.R.; Devarapally, S.R.; Arora, S. Infarct Related Artery Only versus Complete Revascularization in ST-Segment Elevation Myocardial Infarction and Multi Vessel Disease: A Meta-Analysis. Cardiovasc. Diagn. Ther. 2017, 7, 16. [Google Scholar] [CrossRef]
- Olier, I.; Sirker, A.; Hildick-Smith, D.J.R.; Kinnaird, T.; Ludman, P.; De Belder, M.A.; Baumbach, A.; Byrne, J.; Rashid, M.; Curzen, N.; et al. Association of Different Antiplatelet Therapies with Mortality after Primary Percutaneous Coronary Intervention. Heart 2018, 104, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Megaly, M.; Pershad, A.; Glogoza, M.; Elbadawi, A.; Omer, M.; Saad, M.; Mentias, A.; Elgendy, I.; Burke, M.N.; Capodanno, D. Use of Intravascular Imaging in Patients with ST-Segment Elevation Acute Myocardial Infarction. Cardiovasc. Revasc. Med. 2021, 30, 59–64. [Google Scholar] [CrossRef]
- Panteleev, O.O.; Ryabov, V.V. Cardiogenic shock: What’s new? Sib. J. Clin. Exp. Med. 2021, 36, 45–51. [Google Scholar] [CrossRef]
- Vyshlov, E.V.; Alexeeva, Y.A.; Ussov, W.Y.; Mochula, O.V.; Ryabov, V.V. Phenomena of microvascular myocardial injury in patients with primary ST-segment elevation myocardial infarction: Prevalence and association with clinical characteristics. Sib. J. Clin. Exp. Med. 2022, 37, 36–46. [Google Scholar] [CrossRef]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A Basic Biological Phenomenon with Wideranging Implications in Tissue Kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Vladimirov, Y.A.; Archakov, A.I. Lipid Peroxidation in Biological Membranes; Nauka: Moscow, Russia, 1972. [Google Scholar]
- Meerson, F.Z. Pathogenesis and Prevention of Stress and Ischemic Injures of Heart; Meditsina-Moscow: Moscow, Russia, 1984; 270p. [Google Scholar]
- Bilenko, M.V. Ischemic and Reperfusion Injuries of Organs; Meditsina-Moscow: Moscow, Russia, 1989. [Google Scholar]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Ma, X.; Gao, H.; Yang, B.; Zhao, H.; Zhu, Z. Huaier Polysaccharide Attenuates Doxorubicin-Induced Acute Cardiotoxicity by Regulating Ferroptosis. Bull. Exp. Biol. Med. 2022, 174, 37–42. [Google Scholar] [CrossRef]
- Maslov, L.N.; Popov, S.V.; Naryzhnaya, N.V.; Mukhomedzyanov, A.V.; Kurbatov, B.K.; Derkachev, I.A.; Boshchenko, A.A.; Khaliulin, I.; Prasad, N.R.; Singh, N.; et al. The Regulation of Necroptosis and Perspectives for the Development of New Drugs Preventing Ischemic/Reperfusion of Cardiac Injury. Apoptosis 2022, 27, 697–719. [Google Scholar] [CrossRef] [PubMed]
- Maslov, L.N.; Popov, S.V.; Mukhomedzyanov, A.V.; Naryzhnaya, N.V.; Voronkov, N.S.; Ryabov, V.V.; Boshchenko, A.A.; Khaliulin, I.; Prasad, N.R.; Fu, F.; et al. Reperfusion Cardiac Injury: Receptors and the Signaling Mechanisms. Curr. Cardiol. Rev. 2022, 18, 63–79. [Google Scholar] [CrossRef]
- Popov, S.V.; Maslov, L.N.; Naryzhnaya, N.V.; Mukhomezyanov, A.V.; Krylatov, A.V.; Tsibulnikov, S.Y.; Ryabov, V.V.; Cohen, M.V.; Downey, J.M. The Role of Pyroptosis in Ischemic and Reperfusion Injury of the Heart. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Ni, X.; Wang, Y.; Zhang, A.; Zhang, Y.; Lin, F.; Li, S.; Lv, Y.; Zhu, J.; Yao, X.; et al. ICA69 Aggravates Ferroptosis Causing Septic Cardiac Dysfunction via STING Trafficking. Cell Death Discov. 2022, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; He, L.-L.; Zhang, G.-R.; Zuo, Q.-J.; Wang, Z.-L.; Zhai, J.-L.; Zhang, T.-T.; Wang, Y.; Ma, H.-J.; Guo, Y.-F. Canagliflozin Mitigates Ferroptosis and Ameliorates Heart Failure in Rats with Preserved Ejection Fraction. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 945–962. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhang, J.; Chen, Y.; Liu, Y.; Tang, X.; Xia, P.; Yu, P.; Yu, S. Puerarin Protects against Sepsis-Induced Myocardial Injury through AMPK-Mediated Ferroptosis Signaling. Aging 2022, 14, 3617. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-H.; Yang, K.-T.; Lee, W.-S.; Ting, P.-C.; Luo, Y.-P.; Lin, D.-J.; Wang, Y.-S.; Chang, J.-C. Xanthohumol Protects the Rat Myocardium against Ischemia/Reperfusion Injury-Induced Ferroptosis. Oxidative Med. Cell. Longev. 2022, 2022, 9523491. [Google Scholar] [CrossRef] [PubMed]
- Lei, D.; Li, B.; Isa, Z.; Ma, X.; Zhang, B. Hypoxia-Elicited Cardiac Microvascular Endothelial Cell-Derived Exosomal MiR-210–3p Alleviate Hypoxia/Reoxygenation-Induced Myocardial Cell Injury through Inhibiting Transferrin Receptor 1-Mediated Ferroptosis. Tissue Cell 2022, 79, 101956. [Google Scholar] [CrossRef]
- Liu, X.; Li, D.; Pi, W.; Wang, B.; Xu, S.; Yu, L.; Yao, L.; Sun, Z.; Jiang, J.; Mi, Y.; et al. LCZ696 Protects against Doxorubicin-Induced Cardiotoxicity by Inhibiting Ferroptosis via AKT/SIRT3/SOD2 Signaling Pathway Activation. Int. Immunopharmacol. 2022, 113, 109379. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Xu, D.; Yu, S.; Zhang, L.; Li, X. Lapatinib Induces Mitochondrial Dysfunction to Enhance Oxidative Stress and Ferroptosis in Doxorubicin-Induced Cardiomyocytes via Inhibition of PI3K/AKT Signaling Pathway. Bioengineered 2022, 13, 48–60. [Google Scholar] [CrossRef]
- Xiao, Z.; Kong, B.; Fang, J.; Qin, T.; Dai, C.; Shuai, W.; Huang, H. Ferrostatin-1 Alleviates Lipopolysaccharide-Induced Cardiac Dysfunction. Bioengineered 2021, 12, 9367–9376. [Google Scholar] [CrossRef]
- Li, T.; Tan, Y.; Ouyang, S.; He, J.; Liu, L. Resveratrol Protects against Myocardial Ischemia-Reperfusion Injury via Attenuating Ferroptosis. Gene 2022, 808, 145968. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-H.; Yang, K.-T.; Ting, P.-C.; Luo, Y.-P.; Lin, D.-J.; Wang, Y.-S.; Chang, J.-C. Gossypol Acetic Acid Attenuates Cardiac Ischemia/Reperfusion Injury in Rats via an Antiferroptotic Mechanism. Biomolecules 2021, 11, 1667. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and Function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Ardehali, H.; Min, J.; Wang, F. The Molecular and Metabolic Landscape of Iron and Ferroptosis in Cardiovascular Disease. Nat. Rev. Cardiol. 2023, 20, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Louandre, C.; Ezzoukhry, Z.; Godin, C.; Barbare, J.; Mazière, J.; Chauffert, B.; Galmiche, A. Iron-dependent Cell Death of Hepatocellular Carcinoma Cells Exposed to Sorafenib. Int. J. Cancer 2013, 133, 1732–1742. [Google Scholar] [CrossRef]
- Torii, S.; Shintoku, R.; Kubota, C.; Yaegashi, M.; Torii, R.; Sasaki, M.; Suzuki, T.; Mori, M.; Yoshimoto, Y.; Takeuchi, T.; et al. An Essential Role for Functional Lysosomes in Ferroptosis of Cancer Cells. Biochem. J. 2016, 473, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Wills, E.D. Lipid Peroxide Formation in Microsomes. The Role of Non-Haem Iron. Biochem. J. 1969, 113, 325–332. [Google Scholar] [CrossRef]
- Slater, T.F.; Sawyer, B.C. The Stimulatory Effects of Carbon Tetrachloride on Peroxidative Reactions in Rat Liver Fractions in vitro. Inhibitory Effects of Free-Radical Scavengers and Other Agents. Biochem. J. 1971, 123, 823–828. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the Ferroptosis Regulator Gpx4 Triggers Acute Renal Failure in Mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef]
- Devisscher, L.; Van Coillie, S.; Hofmans, S.; Van Rompaey, D.; Goossens, K.; Meul, E.; Maes, L.; De Winter, H.; Van Der Veken, P.; Vandenabeele, P.; et al. Discovery of Novel, Drug-like Ferroptosis Inhibitors with in vivo Efficacy. J. Med. Chem. 2018, 61, 10126–10140. [Google Scholar] [CrossRef] [PubMed]
- Šimůnek, T.; Štěrba, M.; Popelová, O.; Adamcová, M.; Hrdina, R.; Geršl, V. Anthracycline-Induced Cardiotoxicity: Overview of Studies Examining the Roles of Oxidative Stress and Free Cellular Iron. Pharmacol. Rep. 2009, 61, 154–171. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a Target for Protection against Cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, D.C.; de Oliveira Faria, G.; Hermidorff, M.M.; dos Santos Silva, F.C.; de Assis, L.V.M.; Isoldi, M.C. Pre-and Post-Conditioning of the Heart: An Overview of Cardioprotective Signaling Pathways. Curr. Vasc. Pharmacol. 2021, 19, 499–524. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.; Paillard, M.; Thibault, H.; Derumeaux, G.; Ovize, M. Inhibition of GSK3β by Postconditioning Is Required to Prevent Opening of the Mitochondrial Permeability Transition Pore during Reperfusion. Circulation 2008, 117, 2761–2768. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qi, K.; Gong, Y.; Long, X.; Zhu, S.; Lu, F.; Lin, K.; Xu, J. Ferulic Acid Alleviates Myocardial Ischemia Reperfusion Injury via Upregulating AMPKα2 Expression-Mediated Ferroptosis Depression. J. Cardiovasc. Pharmacol. 2022, 79, 489. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Zhou, W.; Men, H.; Bao, T.; Sun, Y.; Wang, Q.; Tan, Y.; Keller, B.B.; Tong, Q.; et al. Ferroptosis Is Essential for Diabetic Cardiomyopathy and Is Prevented by Sulforaphane via AMPK/NRF2 Pathways. Acta Pharm. Sin. B 2022, 12, 708–722. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, M.; Jiang, L.; Wang, L.; Yang, Y.; Wang, Q.; Qian, X.; Zhao, Y.; Qian, J. Dexmedetomidine Attenuates Myocardial Ischemia/Reperfusion-Induced Ferroptosis via AMPK/GSK-3β/Nrf2 Axis. Biomed. Pharmacother. 2022, 154, 113572. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, J.; Wang, Y.; Sun, P.; Gao, T.; Xu, N.; Zhang, Y.; Xie, W. Canagliflozin Attenuates Lipotoxicity in Cardiomyocytes by Inhibiting Inflammation and Ferroptosis through Activating AMPK Pathway. Int. J. Mol. Sci. 2023, 24, 858. [Google Scholar] [CrossRef]
- Li, D.; Zhang, G.; Wang, Z.; Guo, J.; Liu, Y.; Lu, Y.; Qin, Z.; Xu, Y.; Cao, C.; Wang, B.; et al. Idebenone Attenuates Ferroptosis by Inhibiting Excessive Autophagy via the ROS-AMPK-MTOR Pathway to Preserve Cardiac Function after Myocardial Infarction. Eur. J. Pharmacol. 2023, 943, 175569. [Google Scholar] [CrossRef]
- Li, H.; Ding, J.; Liu, W.; Wang, X.; Feng, Y.; Guan, H.; Chen, Z. Plasma Exosomes from Patients with Acute Myocardial Infarction Alleviate Myocardial Injury by Inhibiting Ferroptosis through MiR-26b-5p/SLC7A11 Axis. Life Sci. 2023, 322, 121649. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xu, J.; Gao, N.; Tian, J.; Song, T. Dexmedetomidine Attenuates Myocardial Ischemia-Reperfusion Injury via Inhibiting Ferroptosis by the CAMP/PKA/CREB Pathway. Mol. Cell. Probes 2023, 68, 101899. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jin, Y.; Liu, J.; Liu, Q.; Shen, Y.; Zuo, S.; Yu, Y. EP1 Activation Inhibits Doxorubicin-Cardiomyocyte Ferroptosis via Nrf2. Redox Biol. 2023, 65, 102825. [Google Scholar] [CrossRef] [PubMed]
- Meggio, F.; Deana, A.D.; Ruzzene, M.; Brunati, A.M.; Cesaro, L.; Guerra, B.; Meyer, T.; Mett, H.; Fabbro, D.; Furet, P.; et al. Different Susceptibility of Protein Kinases to Staurosporine Inhibition: Kinetic Studies and Molecular Bases for the Resistance of Protein Kinase CK2. Eur. J. Biochem. 1995, 234, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, S.; Fujiki, H.; Suguri, H.; Suganuma, M.; Nakayasu, M.; Matsushima, R.; Sugimura, T. Tumor-Promoting Activity of Staurosporine, a Protein Kinase Inhibitor on Mouse Skin. Cancer Res. 1990, 50, 4974–4978. [Google Scholar] [PubMed]
- Zhang, G.Y.; Gao, Y.; Guo, X.Y.; Wang, G.; Guo, C.X. MiR-199a-5p Promotes Ferroptosis-induced Cardiomyocyte Death Responding to Oxygen–Glucose Deprivation/Reperfusion Injury via Inhibiting Akt/ENOS Signaling Pathway. Kaohsiung J. Med. Sci. 2022, 38, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Yang, W.; Gao, T.; Yu, X.; Chen, T.; Yang, Y.; Guo, J.; Li, Q.; Li, H.; Yang, L. Trastuzumab-Induced Cardiomyopathy via Ferroptosis-Mediated Mitochondrial Dysfunction. Free Radic. Biol. Med. 2023, 206, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Jabeen, F.; Kahwa, I.; Omara, T. Suberosin Alleviates Thiazolidinedione-Induced Cardiomyopathy in Diabetic Rats by Inhibiting Ferroptosis via Modulation of ACSL4-LPCAT3 and PI3K-AKT Signaling Pathways. Cardiovasc. Toxicol. 2023, 23, 295–304. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, W.; Hu, A.; Lin, J.; Xia, Z.; Yang, C.F.; Li, Y.; Zhang, Z. Dexmedetomidine Alleviated Sepsis-induced Myocardial Ferroptosis and Septic Heart Injury. Mol. Med. Rep. 2020, 22, 175–184. [Google Scholar] [CrossRef]
- Menon, A.V.; Liu, J.; Tsai, H.P.; Zeng, L.; Yang, S.; Asnani, A.; Kim, J. Excess Heme Upregulates Heme Oxygenase 1 and Promotes Cardiac Ferroptosis in Mice with Sickle Cell Disease. Blood J. Am. Soc. Hematol. 2022, 139, 936–941. [Google Scholar] [CrossRef]
- Wang, J.-X.; Zhao, Y.; Chen, M.-S.; Zhang, H.; Cui, J.-G.; Li, J.-L. Heme-Oxygenase-1 as a Target for Phthalate-Induced Cardiomyocytes Ferroptosis. Environ. Pollut. 2023, 317, 120717. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Wan, W.; Fan, M. HMOX1 Silencing Prevents Doxorubicin-Induced Cardiomyocyte Injury, Mitochondrial Dysfunction, and Ferroptosis by Downregulating CTGF. Gen. Thorac. Cardiovasc. Surg. 2023, 71, 280–290. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, X.; Zeng, Y.; Mo, X.; Hong, S.; He, H.; Li, J.; Steinmetz, R.; Liu, Q. Ferroptosis Contributes to Catecholamine-Induced Cardiotoxicity and Pathological Remodeling. Free Radic. Biol. Med. 2023, 207, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Geng, W.; Yan, S.; Li, X.; Liu, Q.; Zhang, X.; Gu, X.; Tian, X.; Jiang, Y. miR-432-5p Inhibits the Ferroptosis in Cardiomyocytes Induced by Hypoxia/Reoxygenation via Activating Nrf2/SLC7A11 Axis by Degrading Keap1. Anal. Cell. Pathol. 2023, 2023, 1293200. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Xiao, H.; Dai, M.; Xue, Y.; Zhao, R. Britanin Relieves Ferroptosis-Mediated Myocardial Ischaemia/Reperfusion Damage by Upregulating GPX4 through Activation of AMPK/GSK3β/Nrf2 Signalling. Pharm. Biol. 2022, 60, 38–45. [Google Scholar] [CrossRef]
- Bian, J.; Ding, Y.; Wang, S.; Jiang, Y.; Wang, M.; Wei, K.; Si, L.; Zhao, X.; Shao, Y. Celastrol Confers Ferroptosis Resistance via AKT/GSK3β Signaling in High-Fat Diet-Induced Cardiac Injury. Free Radic. Biol. Med. 2023, 200, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, W.; Wang, J.; Ao, X.; Xue, J. Non-Coding RNA-Mediated Modulation of Ferroptosis in Cardiovascular Diseases. Biomed. Pharmacother. 2023, 164, 114993. [Google Scholar] [CrossRef]
- Ye, H.; Li, Y.; Li, L.; Huang, Y.; Wang, J.; Gao, Q. Construction of a ceRNA network of regulated ferroptosis in doxorubicin-induced myocardial injury. PeerJ 2023, 11, e14767. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, C.; Zhang, M.; Liu, Y.; Wang, L.; Xie, Y.; Qi, H.; Ba, L.; Shi, P.; Cao, Y.; et al. Super-enhancer-driven lncRNA Snhg7 aggravates cardiac hypertrophy via Tbx5/GLS2/ferroptosis axis. Eur. J. Pharmacol. 2023, 953, 175822. [Google Scholar] [CrossRef]
- Wu, T.; Shi, G.; Ji, Z.; Wang, S.; Geng, L.; Guo, Z. Circulating small extracellular vesicle-encapsulated SEMA5A-IT1 attenuates myocardial ischemia-reperfusion injury after cardiac surgery with cardiopulmonary bypass. Cell. Mol. Biol. Lett. 2022, 27, 95. [Google Scholar] [CrossRef]
- Zhou, X.; Zhuo, M.; Zhang, Y.; Shi, E.; Ma, X.; Li, H. MiR-190a-5p Regulates Cardiomyocytes Response to Ferroptosis via Directly Targeting GLS2. Biochem. Biophys. Res. Commun. 2021, 566, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Huang, W.; Qi, H.; Song, C.; He, C.; Liu, Y.; Zhang, Q.; Wang, L.; Sun, H. The Egr-1/MiR-15a-5p/GPX4 Axis Regulates Ferroptosis in Acute Myocardial Infarction. Eur. J. Pharmacol. 2021, 909, 174403. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Fan, C.; Gong, S.; Kang, S. Effect and Mechanism of LRP6 on Cardiac Myocyte Ferroptosis in Myocardial Infarction. Oxidative Med. Cell. Longev. 2021, 2021, 8963987. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yang, D.; Shi, S.; Wang, L.; Yu, M.; Meng, X.; Fan, Y.; Zhou, R.; Wang, F. MiR-375-3p Promotes Cardiac Fibrosis by Regulating the Ferroptosis Mediated by GPX4. Comput. Intell. Neurosci. 2022, 2022, 9629158. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Bie, Z.; Li, X. Hypoxic Cardiomyocyte-Derived Exosomes Regulate Cardiac Fibroblast Activation, Apoptosis, Migration and Ferroptosis through MiR-208a/B. Gen. Physiol. Biophys. 2023, 42, 149–158. [Google Scholar] [CrossRef]
- Yuan, Y.; Mei, Z.; Qu, Z.; Li, G.; Yu, S.; Liu, Y.; Liu, K.; Shen, Z.; Pu, J.; Wang, Y. Exosomes Secreted from Cardiomyocytes Suppress the Sensitivity of Tumor Ferroptosis in Ischemic Heart Failure. Signal Transduct. Target. Ther. 2023, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Jiang, L.; Zhang, Y.; Xu, S.; Liu, S.; Ye, J.; Liu, P. Inhibition of MiR-214-3p Attenuates Ferroptosis in Myocardial Infarction via Regulating ME2. Biochem. Biophys. Res. Commun. 2023, 661, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Liu, R.; Ju, H.; Huang, M.; Li, Z.; Li, W.; Wang, Y. MicroRNA-130b-3p Attenuates Septic Cardiomyopathy by Regulating the AMPK/MTOR Signaling Pathways and Directly Targeting ACSL4 against Ferroptosis. Int. J. Biol. Sci. 2023, 19, 4223. [Google Scholar] [CrossRef]
- Wei, Q.; Jiang, M.; Tang, B.; You, L.; Zhao, L. Downregulation of Circular RNA 00091761 Protects against Heart Failure after Myocardial Infarction via MicroRNA-335-3p/ASCL4 Axis. Acta Biochim. Pol. 2023, 70, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhang, N.; Gan, X.; Chen, L.; Wang, R.; Liang, R.; Jian, J. EGCG Attenuated Acute Myocardial Infarction by Inhibiting Ferroptosis via MiR-450b-5p/ACSL4 Axis. Phytomedicine 2023, 119, 154999. [Google Scholar] [CrossRef]
- Zenkov, N.K.; Kozhin, P.M.; Chechushkov, A.V.; Martinovich, G.G.; Kandalintseva, N.V.; Menshchikova, E.B. Mazes of Nrf2 Regulation. Biochemistry 2017, 82, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Xiong, Y.; Zhang, Y.; Leng, Y.; Tao, J.; Li, L.; Qiu, Z.; Xia, Z. Activation of NRF2/FPN1 Pathway Attenuates Myocardial Ischemia–Reperfusion Injury in Diabetic Rats by Regulating Iron Homeostasis and Ferroptosis. Cell Stress Chaperones 2022, 27, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.-F.; Guan, P.; Qin, L.-Y.; Wang, J.-X.; Wang, N.; Ji, E.-S. Astragaloside IV Inhibits Adriamycin-Induced Cardiac Ferroptosis by Enhancing Nrf2 Signaling. Mol. Cell. Biochem. 2021, 476, 2603–2611. [Google Scholar] [CrossRef]
- Hwang, J.-W.; Park, J.-H.; Park, B.-W.; Kim, H.; Kim, J.-J.; Sim, W.-S.; Mishchenko, N.P.; Fedoreyev, S.A.; Vasileva, E.A.; Ban, K.; et al. Histochrome Attenuates Myocardial Ischemia-Reperfusion Injury by Inhibiting Ferroptosis-Induced Cardiomyocyte Death. Antioxidants 2021, 10, 1624. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lv, Y.; Cui, W.; Li, Y.; Liu, Y.; Xue, Y.; Dong, F. Icariin Inhibits Hypoxia/Reoxygenation-induced Ferroptosis of Cardiomyocytes via Regulation of the Nrf2/HO-1 Signaling Pathway. FEBS Open Bio 2021, 11, 2966–2976. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wu, B.; Zhong, B.; Lin, L.; Ding, Y.; Jin, X.; Huang, Z.; Lin, M.; Wu, H.; Xu, D. Naringenin Alleviates Myocardial Ischemia/Reperfusion Injury by Regulating the Nuclear Factor-Erythroid Factor 2-Related Factor 2 (Nrf2)/System Xc-/Glutathione Peroxidase 4 (GPX4) Axis to Inhibit Ferroptosis. Bioengineered 2021, 12, 10924–10934. [Google Scholar] [CrossRef]
- Yu, W.; Chen, C.; Xu, C.; Xie, D.; Wang, Q.; Liu, W.; Zhao, H.; He, F.; Chen, B.; Xi, Y. Activation of P62-NRF2 Axis Protects against Doxorubicin-Induced Ferroptosis in Cardiomyocytes: A Novel Role and Molecular Mechanism of Resveratrol. Am. J. Chin. Med. 2022, 50, 2103–2123. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Simayi, A.; Fu, J.; Zhao, X.; Xu, G. Resveratrol Mediates the MiR-149/HMGB1 Axis and Regulates the Ferroptosis Pathway to Protect Myocardium in Endotoxemia Mice. Am. J. Physiol.-Endocrinol. Metab. 2022, 323, E21–E32. [Google Scholar] [CrossRef]
- Li, X.; Liang, J.; Qu, L.; Liu, S.; Qin, A.; Liu, H.; Wang, T.; Li, W.; Zou, W. Exploring the Role of Ferroptosis in the Doxorubicin-Induced Chronic Cardiotoxicity Using a Murine Model. Chem.-Biol. Interact. 2022, 363, 110008. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, S.; Liu, X.; Deng, F.; Wang, P.; Yang, L.; Hu, L.; Huang, K.; He, J. PRMT4 Promotes Ferroptosis to Aggravate Doxorubicin-Induced Cardiomyopathy via Inhibition of the Nrf2/GPX4 Pathway. Cell Death Differ. 2022, 29, 1982–1995. [Google Scholar] [CrossRef]
- Mei, S.; Xia, Z.; Qiu, Z.; Jia, Y.; Zhou, J.; Zhou, B. Shenmai Injection Attenuates Myocardial Ischemia/Reperfusion Injury by Targeting Nrf2/GPX4 Signalling-Mediated Ferroptosis. Chin. J. Integr. Med. 2022, 28, 983–991. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, J.; Wu, G.; Hu, Z.; Ying, P.; Bao, Z.; Ding, Z.; Tan, X. 6-Gingerol Alleviates Ferroptosis and Inflammation of Diabetic Cardiomyopathy via the Nrf2/HO-1 Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 3027514. [Google Scholar] [CrossRef]
- Li, D.; Song, C.; Zhang, J.; Zhao, X. Resveratrol Alleviated 5-FU-Induced Cardiotoxicity by Attenuating GPX4 Dependent Ferroptosis. J. Nutr. Biochem. 2023, 112, 109241. [Google Scholar] [CrossRef]
- Zeng, Y.; Cao, G.; Lin, L.; Zhang, Y.; Luo, X.; Ma, X.; Aiyisake, A.; Cheng, Q. Resveratrol Attenuates Sepsis-Induced Cardiomyopathy in Rats through Anti-Ferroptosis via the Sirt1/Nrf2 Pathway. J. Investig. Surg. 2023, 36, 2157521. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, X.; Zhu, B.; Ding, S.; Shi, H.; Yang, X. Disruption of Histamine/H1R-STAT3-SLC7A11 Axis Exacerbates Doxorubicin-Induced Cardiac Ferroptosis. Free Radic. Biol. Med. 2022, 192, 98–114. [Google Scholar] [CrossRef]
- Matsumori, A. Nuclear Factor-ΚB Is a Prime Candidate for the Diagnosis and Control of Inflammatory Cardiovascular Disease. Eur. Cardiol. Rev. 2023, 18, e40. [Google Scholar] [CrossRef]
- Chen, D.; Geng, Y.; Deng, Z.; Li, P.; Xue, S.; Xu, T.; Li, G. Inhibition of TLR4 Alleviates Heat Stroke-Induced Cardiomyocyte Injury by Down-Regulating Inflammation and Ferroptosis. Molecules 2023, 28, 2297. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, Q.; Zhang, J.; Zha, Y.; Wang, J.; Li, Y.; Zhang, S. Platelet-Rich Plasma Ameliorates Lipopolysaccharide-Induced Cardiac Injury by Inflammation and Ferroptosis Regulation. Front. Pharmacol. 2022, 13, 1026641. [Google Scholar] [CrossRef]
- Feng, Y.; Madungwe, N.B.; Aliagan, A.D.I.; Tombo, N.; Bopassa, J.C. Ferroptosis Inhibitor, Liproxstatin-1, Protects the Myocardium against Ischemia/Reperfusion Injury by Decreasing VDAC1 Levels and Rescuing GPX4 Levels. Biochem. Biophys. Res. Commun. 2019, 520, 606. [Google Scholar] [CrossRef]
- Li, W.; Li, W.; Leng, Y.; Xiong, Y.; Xia, Z. Ferroptosis Is Involved in Diabetes Myocardial Ischemia/Reperfusion Injury through Endoplasmic Reticulum Stress. DNA Cell Biol. 2020, 39, 210–225. [Google Scholar] [CrossRef]
- Tang, L.-J.; Zhou, Y.-J.; Xiong, X.-M.; Li, N.-S.; Zhang, J.-J.; Luo, X.-J.; Peng, J. Ubiquitin-Specific Protease 7 Promotes Ferroptosis via Activation of the P53/TfR1 Pathway in the Rat Hearts after Ischemia/Reperfusion. Free Radic. Biol. Med. 2021, 162, 339–352. [Google Scholar] [CrossRef]
- Tang, L.-J.; Luo, X.-J.; Tu, H.; Chen, H.; Xiong, X.-M.; Li, N.-S.; Peng, J. Ferroptosis Occurs in Phase of Reperfusion but Not Ischemia in Rat Heart Following Ischemia or Ischemia/Reperfusion. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 401–410. [Google Scholar] [CrossRef]
- Shan, X.; Lv, Z.-Y.; Yin, M.-J.; Chen, J.; Wang, J.; Wu, Q.-N. The Protective Effect of Cyanidin-3-Glucoside on Myocardial Ischemia-Reperfusion Injury through Ferroptosis. Oxidative Med. Cell. Longev. 2021, 2021, 8880141. [Google Scholar] [CrossRef]
- Miyamoto, H.D.; Ikeda, M.; Ide, T.; Tadokoro, T.; Furusawa, S.; Abe, K.; Ishimaru, K.; Enzan, N.; Sada, M.; Yamamoto, T.; et al. Iron Overload via Heme Degradation in the Endoplasmic Reticulum Triggers Ferroptosis in Myocardial Ischemia-Reperfusion Injury. Basic Transl. Sci. 2022, 7, 800–819. [Google Scholar] [CrossRef]
- Dhaibar, H.A.; Kamberov, L.; Carroll, N.G.; Amatya, S.; Cosic, D.; Gomez-Torres, O.; Vital, S.; Sivandzade, F.; Bhalerao, A.; Mancuso, S.; et al. Exposure to Stress Alters Cardiac Gene Expression and Exacerbates Myocardial Ischemic Injury in the Female Murine Heart. Int. J. Mol. Sci. 2023, 24, 10994. [Google Scholar] [CrossRef]
- Jiang, Y.-Q.; Yang, X.-Y.; Duan, D.-Q.; Zhang, Y.-Y.; Li, N.-S.; Tang, L.-J.; Peng, J.; Luo, X.-J. Inhibition of MALT1 Reduces Ferroptosis in Rat Hearts Following Ischemia/Reperfusion via Enhancing the Nrf2/SLC7A11 Pathway. Eur. J. Pharmacol. 2023, 950, 175774. [Google Scholar] [CrossRef]
- Qiu, M.; Yan, W.; Liu, M. Klf6 Aggravates Myocardial Ischemia/Reperfusion Injury by Activating Acsl4-mediated Ferroptosis. Kaohsiung J. Med. Sci. 2023, 39, 989–1001. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, W.; Zhang, C.; Yin, Y.; Mu, N.; Wang, Y.; Yu, L.; Ma, H. Frataxin Inhibits the Sensitivity of the Myocardium to Ferroptosis by Regulating Iron Homeostasis. Free Radic. Biol. Med. 2023, 205, 305–317. [Google Scholar] [CrossRef]
- Sementsov, A.S.; Naryzhnaya, N.V.; Sirotina, M.A.; Maslov, L.N. The role of reactive oxygen species in the infarct-limiting effect of hypoxic preconditioning. Reg. Blood Circ. Microcirc. 2021, 20, 87–91. [Google Scholar] [CrossRef]
- Tu, H.; Zhou, Y.-J.; Tang, L.-J.; Xiong, X.-M.; Zhang, X.-J.; Sheikh, M.S.A.; Zhang, J.-J.; Luo, X.-J.; Yuan, C.; Peng, J. Combination of Ponatinib with Deferoxamine Synergistically Mitigates Ischemic Heart Injury via Simultaneous Prevention of Necroptosis and Ferroptosis. Eur. J. Pharmacol. 2021, 898, 173999. [Google Scholar] [CrossRef]
- Zhang, W.; Qiao, W.; Zuo, L. A1 and A2b Adenosine Receptors Regulate GPX4 against Ferroptosis of Cardiomyocytes in Myocardial Infarction Rat Model and in vitro. Tissue Cell 2022, 77, 101828. [Google Scholar] [CrossRef]
- Fu, C.; Yu, S.; Liu, Z.; Wang, J.; Liu, P.; Su, G. PFKFB2 Inhibits Ferroptosis in Myocardial Ischemia/Reperfusion Injury through Adenosine Monophosphate–Activated Protein Kinase Activation. J. Cardiovasc. Pharmacol. 2023, 82, 128–137. [Google Scholar] [CrossRef]
- Li, S.; Lei, Z.; Yang, X.; Zhao, M.; Hou, Y.; Wang, D.; Tang, S.; Li, J.; Yu, J. Propofol Protects Myocardium from Ischemia/Reperfusion Injury by Inhibiting Ferroptosis through the Akt/P53 Signaling Pathway. Front. Pharmacol. 2022, 13, 841410. [Google Scholar] [CrossRef]
- Cao, G.; Yang, C.; Jin, Z.; Wei, H.; Xin, C.; Zheng, C.; Xu, J.; Huang, Q.; Zhang, Z.; Hu, T. FNDC5/Irisin Reduces Ferroptosis and Improves Mitochondrial Dysfunction in Hypoxic Cardiomyocytes by Nrf2/HO-1 Axis. Cell Biol. Int. 2022, 46, 723–736. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, J.; Ding, Y.; Chen, D.; Sun, H.; Yuan, F.; Li, S.; Li, X.; Yang, P.; Fu, L.; et al. Dexmedetomidine Post-Conditioning Alleviates Myocardial Ischemia–Reperfusion Injury in Rats by Ferroptosis Inhibition via SLC7A11/GPX4 Axis Activation. Hum. Cell 2022, 35, 836–848. [Google Scholar] [CrossRef]
- Yang, F.; Wang, W.; Zhang, Y.; Nong, J.; Zhang, L. Effects of Ferroptosis in Myocardial Ischemia/Reperfusion Model of Rat and Its Association with Sestrin 1. Adv. Clin. Exp. Med. 2023, 32, 219–231. [Google Scholar] [CrossRef]
- Liu, C.; Chen, H.; Guo, S.; Liu, Q.; Chen, Z.; Huang, H.; Zhao, Q.; Li, L.; Cen, H.; Jiang, Z.; et al. Anti-Breast Cancer-Induced Cardiomyopathy: Mechanisms and Future Directions. Biomed. Pharmacother. 2023, 166, 115373. [Google Scholar] [CrossRef]
- Sun, X.; Sun, P.; Zhen, D.; Xu, X.; Yang, L.; Fu, D.; Wei, C.; Niu, X.; Tian, J.; Li, H. Melatonin Alleviates Doxorubicin-Induced Mitochondrial Oxidative Damage and Ferroptosis in Cardiomyocytes by Regulating YAP Expression. Toxicol. Appl. Pharmacol. 2022, 437, 115902. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, Z.; Fang, B. Propofol Protects Cardiomyocytes from Doxorubicin-Induced Toxic Injury by Activating the Nuclear Factor Erythroid 2-Related Factor 2/Glutathione Peroxidase 4 Signaling Pathways. Bioengineered 2022, 13, 9145–9155. [Google Scholar] [CrossRef]
- Tadokoro, T.; Ikeda, M.; Abe, K.; Ide, T.; Miyamoto, H.D.; Furusawa, S.; Ishimaru, K.; Watanabe, M.; Ishikita, A.; Matsushima, S.; et al. Ethoxyquin Is a Competent Radical-Trapping Antioxidant for Preventing Ferroptosis in Doxorubicin Cardiotoxicity. J. Cardiovasc. Pharmacol. 2022, 80, 690–699. [Google Scholar] [CrossRef]
- Abe, K.; Ikeda, M.; Ide, T.; Tadokoro, T.; Miyamoto, H.D.; Furusawa, S.; Tsutsui, Y.; Miyake, R.; Ishimaru, K.; Watanabe, M.; et al. Doxorubicin Causes Ferroptosis and Cardiotoxicity by Intercalating into Mitochondrial DNA and Disrupting Alas1-Dependent Heme Synthesis. Sci. Signal. 2022, 15, eabn8017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Liu, Z.; Du, K.; Lu, X. Protective Effects of Dexazoxane on Rat Ferroptosis in Doxorubicin-Induced Cardiomyopathy through Regulating HMGB1. Front. Cardiovasc. Med. 2021, 8, 685434. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M.; Seddiek, H.; Aboulhoda, B.E.; Morcos, G.N.B.; Akabawy, A.; Elbaset, M.A.; Ibrahim, A.A.; Khalifa, M.M.; Khalifah, I.M.; Fadel, M.S.; et al. Synergistic Cardioprotective Effects of Melatonin and Deferoxamine through the Improvement of Ferritinophagy in Doxorubicin-Induced Acute Cardiotoxicity. Front. Physiol. 2022, 13, 1050598. [Google Scholar] [CrossRef]
- Huo, L.; Liu, C.; Yuan, Y.; Liu, X.; Cao, Q. Pharmacological Inhibition of Ferroptosis as a Therapeutic Target for Sepsis-Associated Organ Damage. Eur. J. Med. Chem. 2023, 257, 115438. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, W.; Zhou, H.; Wu, Q.; Duan, M.; Liu, C.; Wu, H.; Deng, W.; Shen, D.; Tang, Q. Ferritinophagy-Mediated Ferroptosis Is Involved in Sepsis-Induced Cardiac Injury. Free Radic. Biol. Med. 2020, 160, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zou, Q.; Tang, H.; Liu, J.; Zhang, S.; Fan, C.; Zhang, J.; Liu, R.; Liu, Y.; Liu, R.; et al. Melanin Nanoparticles Alleviate Sepsis-Induced Myocardial Injury by Suppressing Ferroptosis and Inflammation. Bioact. Mater. 2023, 24, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Zhang, Y.; Guo, J.; Gao, L.; Ding, Y.; Zhuo, X.; Lei, Q.; Bian, J.; Lei, R.; Gong, W.; et al. What Is the Impact of Ferroptosis on Diabetic Cardiomyopathy: A Systematic Review. Heart Fail. Rev. 2023. [Google Scholar] [CrossRef]
- Ge, Z.-D.; Lian, Q.; Mao, X.; Xia, Z. Current Status and Challenges of NRF2 as a Potential Therapeutic Target for Diabetic Cardiomyopathy. Int. Heart J. 2019, 60, 512–520. [Google Scholar] [CrossRef]
- Miao, W.; Chen, M.; Chen, M.; Cui, C.; Zhu, Y.; Luo, X.; Wu, B. Nr2f2 Overexpression Aggravates Ferroptosis and Mitochondrial Dysfunction by Regulating the PGC-1α Signaling in Diabetes-Induced Heart Failure Mice. Mediat. Inflamm. 2022, 2022, 8373389. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Dong, X.; Wu, Y.; Li, B.; Kuang, B.; Chen, G.; Zhang, L. Astragaloside IV Attenuates Myocardial Dysfunction in Diabetic Cardiomyopathy Rats through Downregulation of CD36-mediated Ferroptosis. Phytother. Res. 2023, 37, 3042–3056. [Google Scholar] [CrossRef]
- Abdul, Y.; Li, W.; Ward, R.; Abdelsaid, M.; Hafez, S.; Dong, G.; Jamil, S.; Wolf, V.; Johnson, M.H.; Fagan, S.C.; et al. Deferoxamine Treatment Prevents Post-Stroke Vasoregression and Neurovascular Unit Remodeling Leading to Improved Functional Outcomes in Type 2 Male Diabetic Rats: Role of Endothelial Ferroptosis. Transl. Stroke Res. 2021, 12, 615–630. [Google Scholar] [CrossRef]
- Pei, Z.; Liu, Y.; Liu, S.; Jin, W.; Luo, Y.; Sun, M.; Duan, Y.; Ajoolabady, A.; Sowers, J.R.; Fang, Y.; et al. FUNDC1 Insufficiency Sensitizes High Fat Diet Intake-Induced Cardiac Remodeling and Contractile Anomaly through ACSL4-Mediated Ferroptosis. Metabolism 2021, 122, 154840. [Google Scholar] [CrossRef]
- Ni, T.; Huang, X.; Pan, S.; Lu, Z. Inhibition of the Long Non-coding RNA ZFAS1 Attenuates Ferroptosis by Sponging MiR-150-5p and Activates CCND2 against Diabetic Cardiomyopathy. J. Cell. Mol. Med. 2021, 25, 9995–10007. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Liu, Y.; Lin, Y.; Wang, F.; Han, Y.; Zhang, S.; Gao, X.; Xu, C.; Yuan, H. Exogenous Spermidine Alleviates Diabetic Cardiomyopathy via Suppressing Pannexin-1-Mediated Ferroptosis in Db/Db Mice. Biomol. Biomed. 2023, 23, 825–837. [Google Scholar]
- Chen, L.; Yin, Z.; Qin, X.; Zhu, X.; Chen, X.; Ding, G.; Sun, D.; Wu, N.N.; Fei, J.; Bi, Y.; et al. CD74 Ablation Rescues Type 2 Diabetes Mellitus-Induced Cardiac Remodeling and Contractile Dysfunction through Pyroptosis-Evoked Regulation of Ferroptosis. Pharmacol. Res. 2022, 176, 106086. [Google Scholar] [CrossRef]
- Prokudina, E.S.; Kurbatov, B.K.; Zavadovsky, K.V.; Vrublevsky, A.V.; Naryzhnaya, N.V.; Lishmanov, Y.B.; Maslov, L.N.; Oeltgen, P.R. Takotsubo Syndrome: Clinical Manifestations, Etiology and Pathogenesis. Curr. Cardiol. Rev. 2021, 17, 188–203. [Google Scholar] [CrossRef]
- Kurbatov, B.K.; Prokudina, E.S.; Maslov, L.N.; Naryzhnaya, N.V.; Logvinov, S.V.; Gorbunov, A.S.; Mukhomedzyanov, A.V.; Krylatov, A.V.; Voronkov, N.S.; Sementsov, A.S.; et al. The Role of Adrenergic and Muscarinic Receptors in Stress-Induced Cardiac Injury. Pflügers Arch.-Eur. J. Physiol. 2021, 473, 1641–1655. [Google Scholar] [CrossRef] [PubMed]
- Ning, D.; Yang, X.; Wang, T.; Jiang, Q.; Yu, J.; Wang, D. Atorvastatin Treatment Ameliorates Cardiac Function and Remodeling Induced by Isoproterenol Attack through Mitigation of Ferroptosis. Biochem. Biophys. Res. Commun. 2021, 574, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Meerson, F.Z.; Vasil’ev, V.K. Prevention of Structural Damage to Myocardial DNA Caused by Emotionally Painful Stress by Means of Beta-Adrenoreceptor and Lipid Peroxidation Blockade. Vopr. Meditsinskoi Khimii 1982, 28, 115–118. [Google Scholar]
- Meerson, F.Z.; Kagan, V.E.; Prilipko, L.L.; Rozhitskaia, I.I.; Giber, L.M. Activation of Lipid Peroxidation in Emotional-Pain Stress. Biulleten’Eksperimental’noi Biol. I Meditsiny 1979, 88, 404–406. [Google Scholar]
- Meerson, F.Z.; Kagan, V.E.; Prilipko, L.L.; Rozhitskaia, I.I. Inhibition of Lipid Peroxidation during Emotional-Painful Stress by Ionol and Gamma-Hydroxybutyric Acid. Biulleten’ Eksperimental’noi Biol. I Meditsiny 1980, 90, 661–663. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryabov, V.V.; Maslov, L.N.; Vyshlov, E.V.; Mukhomedzyanov, A.V.; Kilin, M.; Gusakova, S.V.; Gombozhapova, A.E.; Panteleev, O.O. Ferroptosis, a Regulated Form of Cell Death, as a Target for the Development of Novel Drugs Preventing Ischemia/Reperfusion of Cardiac Injury, Cardiomyopathy and Stress-Induced Cardiac Injury. Int. J. Mol. Sci. 2024, 25, 897. https://doi.org/10.3390/ijms25020897
Ryabov VV, Maslov LN, Vyshlov EV, Mukhomedzyanov AV, Kilin M, Gusakova SV, Gombozhapova AE, Panteleev OO. Ferroptosis, a Regulated Form of Cell Death, as a Target for the Development of Novel Drugs Preventing Ischemia/Reperfusion of Cardiac Injury, Cardiomyopathy and Stress-Induced Cardiac Injury. International Journal of Molecular Sciences. 2024; 25(2):897. https://doi.org/10.3390/ijms25020897
Chicago/Turabian StyleRyabov, Vyacheslav V., Leonid N. Maslov, Evgeniy V. Vyshlov, Alexander V. Mukhomedzyanov, Mikhail Kilin, Svetlana V. Gusakova, Alexandra E. Gombozhapova, and Oleg O. Panteleev. 2024. "Ferroptosis, a Regulated Form of Cell Death, as a Target for the Development of Novel Drugs Preventing Ischemia/Reperfusion of Cardiac Injury, Cardiomyopathy and Stress-Induced Cardiac Injury" International Journal of Molecular Sciences 25, no. 2: 897. https://doi.org/10.3390/ijms25020897
APA StyleRyabov, V. V., Maslov, L. N., Vyshlov, E. V., Mukhomedzyanov, A. V., Kilin, M., Gusakova, S. V., Gombozhapova, A. E., & Panteleev, O. O. (2024). Ferroptosis, a Regulated Form of Cell Death, as a Target for the Development of Novel Drugs Preventing Ischemia/Reperfusion of Cardiac Injury, Cardiomyopathy and Stress-Induced Cardiac Injury. International Journal of Molecular Sciences, 25(2), 897. https://doi.org/10.3390/ijms25020897





