A Systematic Review of the Metabolism of High-Grade Gliomas: Current Targeted Therapies and Future Perspectives
Abstract
1. Introduction
2. Results
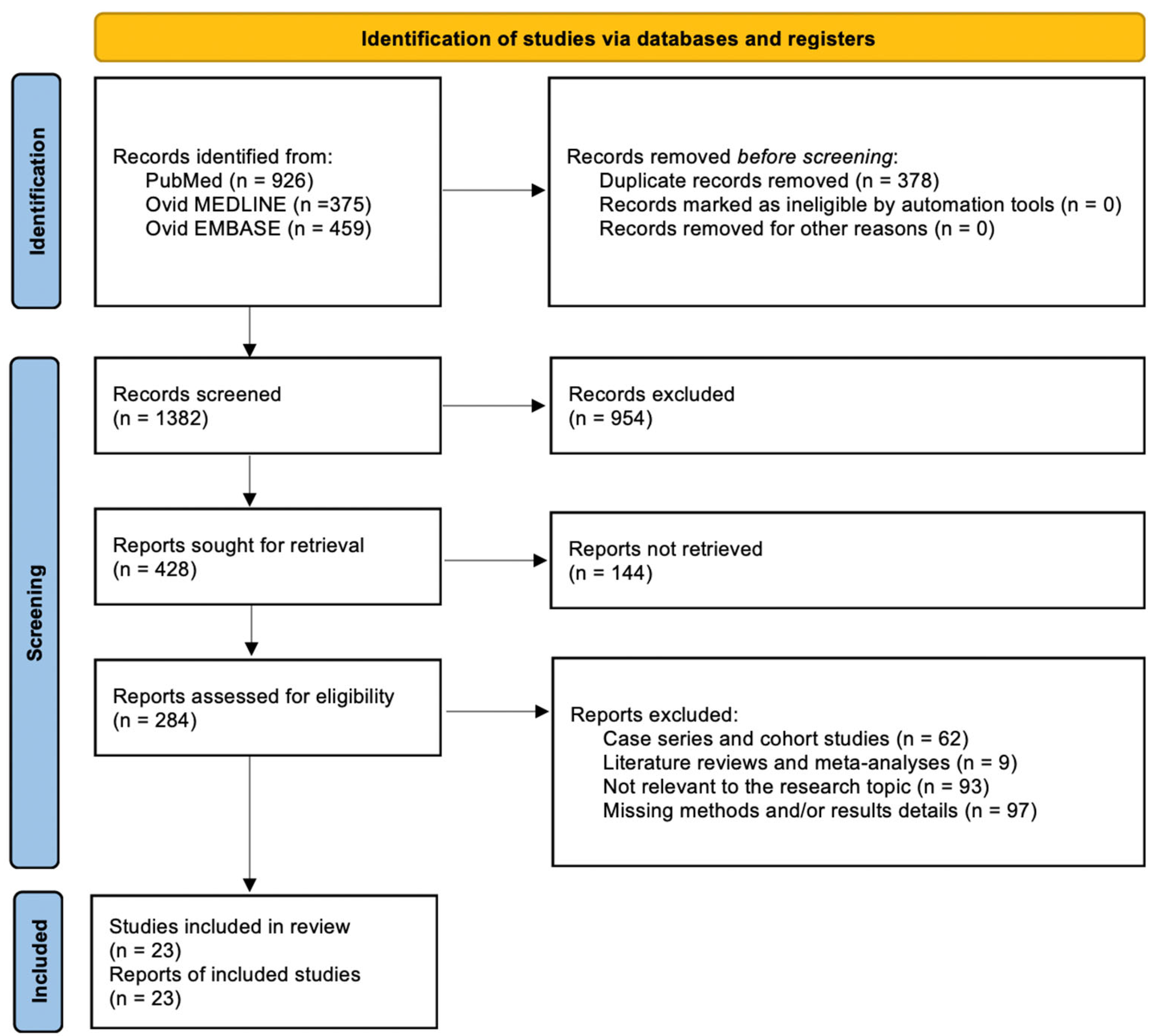
2.1. Literature Review
2.2. Extracted Data
3. Discussion
3.1. Overview of Included Studies
3.2. Targeted Pathways and Therapeutic Classes
3.3. Individual Targeted Pathways
3.4. The Role of Tumor Microenvironment and Immunotherapy
3.5. Challenges and Future Directions
4. Methods
4.1. Review of the Literature
4.2. Data Collection
4.3. Objectives
4.4. Evaluation of the Potential for Bias
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro Oncol. 2018, 20, iv1–iv86. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Pavlidis, N.; Jelic, S. ESMO Guidelines Task Force ESMO Minimum Clinical Recommendations for Diagnosis, Treatment and Follow-up of Malignant Glioma. Ann. Oncol. 2005, 16 (Suppl. S1), i64–i65. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Uhrbom, L. On the Origin of Glioma. Ups. J. Med. Sci. 2012, 117, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Tilak, M.; Holborn, J.; New, L.A.; Lalonde, J.; Jones, N. Receptor Tyrosine Kinase Signaling and Targeting in Glioblastoma Multiforme. Int. J. Mol. Sci. 2021, 22, 1831. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhu, M.; Chen, C.Y.; Luo, Y.; Wen, J. Diffusion Heterogeneity and Vascular Perfusion in Tumor and Peritumoral Areas for Prediction of Overall Survival in Patients with High-Grade Glioma. Magn. Reson. Imaging 2023, 104, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive Genomic Characterization Defines Human Glioblastoma Genes and Core Pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef]
- Baldewpersad Tewarie, N.M.S.; Burgers, I.A.V.; Dawood, Y.; den Boon, H.C.; den Brok, M.G.H.E.; Klunder, J.H.; Koopmans, K.B.; Rademaker, E.; van den Broek, H.B.; van den Bersselaar, S.M.; et al. NADP+ -Dependent IDH1 R132 Mutation and Its Relevance for Glioma Patient Survival. Med. Hypotheses 2013, 80, 728–731. [Google Scholar] [CrossRef]
- Chen, X.; Yan, Y.; Zhou, J.; Huo, L.; Qian, L.; Zeng, S.; Li, Z.; Wei, J.; Xu, Z.; Gong, Z. Clinical Prognostic Value of Isocitrate Dehydrogenase Mutation, O-6-Methylguanine-DNA Methyltransferase Promoter Methylation, and 1p19q Co-Deletion in Glioma Patients. Ann. Transl. Med. 2019, 7, 541. [Google Scholar] [CrossRef]
- Stichel, D.; Ebrahimi, A.; Reuss, D.; Schrimpf, D.; Ono, T.; Shirahata, M.; Reifenberger, G.; Weller, M.; Hänggi, D.; Wick, W.; et al. Distribution of EGFR Amplification, Combined Chromosome 7 Gain and Chromosome 10 Loss, and TERT Promoter Mutation in Brain Tumors and Their Potential for the Reclassification of IDHwt Astrocytoma to Glioblastoma. Acta Neuropathol. 2018, 136, 793–803. [Google Scholar] [CrossRef]
- Olympios, N.; Gilard, V.; Marguet, F.; Clatot, F.; Di Fiore, F.; Fontanilles, M. TERT Promoter Alterations in Glioblastoma: A Systematic Review. Cancers 2021, 13, 1147. [Google Scholar] [CrossRef]
- Board, W.C.T.E. Central Nervous System Tumours; IARC Publications: Lyon, France, 2022; ISBN 978-92-832-4508-7. [Google Scholar]
- Neftel, C.; Laffy, J.; Filbin, M.G.; Hara, T.; Shore, M.E.; Rahme, G.J.; Richman, A.R.; Silverbush, D.; Shaw, M.L.; Hebert, C.M.; et al. An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell 2019, 178, 835–849.e21. [Google Scholar] [CrossRef] [PubMed]
- Lauko, A.; Lo, A.; Ahluwalia, M.S.; Lathia, J.D. Cancer Cell Heterogeneity & Plasticity in Glioblastoma and Brain Tumors. Semin. Cancer Biol. 2022, 82, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Han, Z. Immunotherapy: Advancing Glioblastoma Treatment-A Narrative Review of Scientific Studies. Cancer Rep. 2023, e1947. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Liu, Y.; Cai, S.J.; Qian, M.; Ding, J.; Larion, M.; Gilbert, M.R.; Yang, C. IDH Mutation in Glioma: Molecular Mechanisms and Potential Therapeutic Targets. Br. J. Cancer 2020, 122, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Garnier, D.; Renoult, O.; Alves-Guerra, M.-C.; Paris, F.; Pecqueur, C. Glioblastoma Stem-Like Cells, Metabolic Strategy to Kill a Challenging Target. Front. Oncol. 2019, 9, 118. [Google Scholar] [CrossRef]
- Zhou, W.; Wahl, D.R. Metabolic Abnormalities in Glioblastoma and Metabolic Strategies to Overcome Treatment Resistance. Cancers 2019, 11, 1231. [Google Scholar] [CrossRef]
- Galli, R.; Binda, E.; Orfanelli, U.; Cipelletti, B.; Gritti, A.; De Vitis, S.; Fiocco, R.; Foroni, C.; Dimeco, F.; Vescovi, A. Isolation and Characterization of Tumorigenic, Stem-like Neural Precursors from Human Glioblastoma. Cancer Res. 2004, 64, 7011–7021. [Google Scholar] [CrossRef]
- Berezovsky, A.D.; Poisson, L.M.; Cherba, D.; Webb, C.P.; Transou, A.D.; Lemke, N.W.; Hong, X.; Hasselbach, L.A.; Irtenkauf, S.M.; Mikkelsen, T.; et al. Sox2 Promotes Malignancy in Glioblastoma by Regulating Plasticity and Astrocytic Differentiation. Neoplasia 2014, 16, 193–206.e25. [Google Scholar] [CrossRef][Green Version]
- Heddleston, J.M.; Li, Z.; McLendon, R.E.; Hjelmeland, A.B.; Rich, J.N. The Hypoxic Microenvironment Maintains Glioblastoma Stem Cells and Promotes Reprogramming towards a Cancer Stem Cell Phenotype. Cell Cycle 2009, 8, 3274–3284. [Google Scholar] [CrossRef]
- Li, Y.; Li, A.; Glas, M.; Lal, B.; Ying, M.; Sang, Y.; Xia, S.; Trageser, D.; Guerrero-Cázares, H.; Eberhart, C.G.; et al. C-Met Signaling Induces a Reprogramming Network and Supports the Glioblastoma Stem-like Phenotype. Proc. Natl. Acad. Sci. USA 2011, 108, 9951–9956. [Google Scholar] [CrossRef] [PubMed]
- Dahan, P.; Martinez Gala, J.; Delmas, C.; Monferran, S.; Malric, L.; Zentkowski, D.; Lubrano, V.; Toulas, C.; Cohen-Jonathan Moyal, E.; Lemarie, A. Ionizing Radiations Sustain Glioblastoma Cell Dedifferentiation to a Stem-like Phenotype through Survivin: Possible Involvement in Radioresistance. Cell Death Dis. 2014, 5, e1543. [Google Scholar] [CrossRef] [PubMed]
- Auffinger, B.; Tobias, A.L.; Han, Y.; Lee, G.; Guo, D.; Dey, M.; Lesniak, M.S.; Ahmed, A.U. Conversion of Differentiated Cancer Cells into Cancer Stem-like Cells in a Glioblastoma Model after Primary Chemotherapy. Cell Death Differ. 2014, 21, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Kasenda, B.; König, D.; Manni, M.; Ritschard, R.; Duthaler, U.; Bartoszek, E.; Bärenwaldt, A.; Deuster, S.; Hutter, G.; Cordier, D.; et al. Targeting Immunoliposomes to EGFR-Positive Glioblastoma. ESMO Open 2022, 7, 100365. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.E.; Lewis, R.; Syed, N.; Shaffer, R.; Evanson, J.; Ellis, S.; Williams, M.; Feng, X.; Johnston, A.; Thomson, J.A.; et al. A Phase I Study of Pegylated Arginine Deiminase (Pegargiminase), Cisplatin, and Pemetrexed in Argininosuccinate Synthetase 1-Deficient Recurrent High-Grade Glioma. Clin. Cancer Res. 2019, 25, 2708–2716. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Q.; Zhang, P.; Wen, P.Y.; Gerstner, E.R.; Reardon, D.A.; Aldape, K.D.; deGroot, J.F.; Pan, E.; Raizer, J.J.; Kim, L.J.; et al. NRG/RTOG 1122: A Phase 2, Double-Blinded, Placebo-Controlled Study of Bevacizumab with and without Trebananib in Patients with Recurrent Glioblastoma or Gliosarcoma. Cancer 2020, 126, 2821–2828. [Google Scholar] [CrossRef] [PubMed]
- Hottinger, A.F.; Ben Aissa, A.; Espeli, V.; Squiban, D.; Dunkel, N.; Vargas, M.I.; Hundsberger, T.; Mach, N.; Schaller, K.; Weber, D.C.; et al. Phase I Study of Sorafenib Combined with Radiation Therapy and Temozolomide as First-Line Treatment of High-Grade Glioma. Br. J. Cancer 2014, 110, 2655–2661. [Google Scholar] [CrossRef]
- Wetmore, C.; Daryani, V.M.; Billups, C.A.; Boyett, J.M.; Leary, S.; Tanos, R.; Goldsmith, K.C.; Stewart, C.F.; Blaney, S.M.; Gajjar, A. Phase II Evaluation of Sunitinib in the Treatment of Recurrent or Refractory High-Grade Glioma or Ependymoma in Children: A Children’s Oncology Group Study ACNS1021. Cancer Med. 2016, 5, 1416–1424. [Google Scholar] [CrossRef]
- Ronellenfitsch, M.W.; Zeiner, P.S.; Mittelbronn, M.; Urban, H.; Pietsch, T.; Reuter, D.; Senft, C.; Steinbach, J.P.; Westphal, M.; Harter, P.N. Akt and mTORC1 Signaling as Predictive Biomarkers for the EGFR Antibody Nimotuzumab in Glioblastoma. Acta Neuropathol. Commun. 2018, 6, 81. [Google Scholar] [CrossRef]
- Kalpathy-Cramer, J.; Chandra, V.; Da, X.; Ou, Y.; Emblem, K.E.; Muzikansky, A.; Cai, X.; Douw, L.; Evans, J.G.; Dietrich, J.; et al. Phase II Study of Tivozanib, an Oral VEGFR Inhibitor, in Patients with Recurrent Glioblastoma. J. Neurooncol. 2017, 131, 603–610. [Google Scholar] [CrossRef]
- Broniscer, A.; Baker, S.D.; Wetmore, C.; Pai Panandiker, A.S.; Huang, J.; Davidoff, A.M.; Onar-Thomas, A.; Panetta, J.C.; Chin, T.K.; Merchant, T.E.; et al. Phase I Trial, Pharmacokinetics, and Pharmacodynamics of Vandetanib and Dasatinib in Children with Newly Diagnosed Diffuse Intrinsic Pontine Glioma. Clin. Cancer Res. 2013, 19, 3050–3058. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; Gururangan, S.; Friedman, A.H.; Herndon, J.E.; Marcello, J.; Norfleet, J.A.; McLendon, R.E.; Sampson, J.H.; et al. Phase 2 Trial of Erlotinib plus Sirolimus in Adults with Recurrent Glioblastoma. J. Neurooncol. 2010, 96, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Gorlia, T.; Erridge, S.C.; Perry, J.; Hong, Y.-K.; Aldape, K.D.; Lhermitte, B.; Pietsch, T.; Grujicic, D.; et al. Cilengitide Combined with Standard Treatment for Patients with Newly Diagnosed Glioblastoma with Methylated MGMT Promoter (CENTRIC EORTC 26071-22072 Study): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2014, 15, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Galanis, E.; Jaeckle, K.A.; Maurer, M.J.; Reid, J.M.; Ames, M.M.; Hardwick, J.S.; Reilly, J.F.; Loboda, A.; Nebozhyn, M.; Fantin, V.R.; et al. Phase II Trial of Vorinostat in Recurrent Glioblastoma Multiforme: A North Central Cancer Treatment Group Study. J. Clin. Oncol. 2009, 27, 2052–2058. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.R.; Pugh, S.L.; Aldape, K.; Sorensen, A.G.; Mikkelsen, T.; Penas-Prado, M.; Bokstein, F.; Kwok, Y.; Lee, R.J.; Mehta, M. NRG Oncology RTOG 0625: A Randomized Phase II Trial of Bevacizumab with Either Irinotecan or Dose-Dense Temozolomide in Recurrent Glioblastoma. J. Neurooncol. 2017, 131, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.J.; Galanis, E.; Anderson, S.K.; Schiff, D.; Kaufmann, T.J.; Peller, P.J.; Giannini, C.; Brown, P.D.; Uhm, J.H.; McGraw, S.; et al. A Phase II Trial of Everolimus, Temozolomide, and Radiotherapy in Patients with Newly Diagnosed Glioblastoma: NCCTG N057K. Neuro Oncol. 2015, 17, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.; McBain, C.; Nash, S.; Hopkins, K.; Sanghera, P.; Saran, F.; Phillips, M.; Dungey, F.; Clifton-Hadley, L.; Wanek, K.; et al. Multi-Center Randomized Phase II Study Comparing Cediranib plus Gefitinib with Cediranib plus Placebo in Subjects with Recurrent/Progressive Glioblastoma. PLoS ONE 2016, 11, e0156369. [Google Scholar] [CrossRef]
- Lv, S.; Teugels, E.; Sadones, J.; De Brakeleer, S.; Duerinck, J.; Du Four, S.; Michotte, A.; De Grève, J.; Neyns, B. Correlation of EGFR, IDH1 and PTEN Status with the Outcome of Patients with Recurrent Glioblastoma Treated in a Phase II Clinical Trial with the EGFR-Blocking Monoclonal Antibody Cetuximab. Int. J. Oncol. 2012, 41, 1029–1035. [Google Scholar] [CrossRef]
- Reardon, D.A.; Egorin, M.J.; Desjardins, A.; Vredenburgh, J.J.; Beumer, J.H.; Lagattuta, T.F.; Gururangan, S.; Herndon, J.E.; Salvado, A.J.; Friedman, H.S. Phase I Pharmacokinetic Study of the Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitor Vatalanib (PTK787) plus Imatinib and Hydroxyurea for Malignant Glioma. Cancer 2009, 115, 2188–2198. [Google Scholar] [CrossRef]
- Galanis, E.; Buckner, J.C.; Maurer, M.J.; Kreisberg, J.I.; Ballman, K.; Boni, J.; Peralba, J.M.; Jenkins, R.B.; Dakhil, S.R.; Morton, R.F.; et al. Phase II Trial of Temsirolimus (CCI-779) in Recurrent Glioblastoma Multiforme: A North Central Cancer Treatment Group Study. J. Clin. Oncol. 2005, 23, 5294–5304. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Kovanlikaya, I.; Boockvar, J.A.; Mao, X.; Shin, B.; Burkhardt, J.K.; Kesavabhotla, K.; Christos, P.; Riina, H.; Shungu, D.C.; et al. Metabolic Response of Glioblastoma to Superselective Intra-Arterial Cerebral Infusion of Bevacizumab: A Proton MR Spectroscopic Imaging Study. AJNR Am. J. Neuroradiol. 2012, 33, 2095–2102. [Google Scholar] [CrossRef]
- Babak, S.; Mason, W.P. mTOR Inhibition in Glioblastoma: Requiem for a Dream? Neuro Oncol. 2018, 20, 584–585. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Conrad, C.A.; Cloughesy, T.; Prados, M.D.; Friedman, H.S.; Aldape, K.D.; Mischel, P.; Xia, J.; DiLea, C.; Huang, J.; et al. Phase I Study of AEE788, a Novel Multitarget Inhibitor of ErbB- and VEGF-Receptor-Family Tyrosine Kinases, in Recurrent Glioblastoma Patients. Cancer Chemother. Pharmacol. 2012, 69, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Pollack, I.F.; Stewart, C.F.; Kocak, M.; Poussaint, T.Y.; Broniscer, A.; Banerjee, A.; Douglas, J.G.; Kun, L.E.; Boyett, J.M.; Geyer, J.R. A Phase II Study of Gefitinib and Irradiation in Children with Newly Diagnosed Brainstem Gliomas: A Report from the Pediatric Brain Tumor Consortium. Neuro Oncol. 2011, 13, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.D.; Soo, Y.W.J.; Rieunier, G.; Aleksic, T.; Ansorge, O.; Jones, C.; Macaulay, V.M. Type 1 IGF Receptor Associates with Adverse Outcome and Cellular Radioresistance in Paediatric High-Grade Glioma. Br. J. Cancer 2020, 122, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Touat, M.; Alexander, B.M.; Mellinghoff, I.K.; Ramkissoon, S.; McCluskey, C.S.; Pelton, K.; Haidar, S.; Basu, S.S.; Gaffey, S.C.; et al. Buparlisib in Patients With Recurrent Glioblastoma Harboring Phosphatidylinositol 3-Kinase Pathway Activation: An Open-Label, Multicenter, Multi-Arm, Phase II Trial. J. Clin. Oncol. 2019, 37, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Hottinger, A.F.; Stupp, R.; Homicsko, K. Standards of Care and Novel Approaches in the Management of Glioblastoma Multiforme. Chin. J. Cancer 2014, 33, 32–39. [Google Scholar] [CrossRef]
- Agosti, E.; Zeppieri, M.; De Maria, L.; Tedeschi, C.; Fontanella, M.M.; Panciani, P.P.; Ius, T. Glioblastoma Immunotherapy: A Systematic Review of the Present Strategies and Prospects for Advancements. Int. J. Mol. Sci. 2023, 24, 15037. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-Specific Chimeric Antigen Receptor-Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2017, 3, 1094–1101. [Google Scholar] [CrossRef]
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.-C.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C.; et al. Bioactivity and Safety of IL13Rα2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2015, 21, 4062–4072. [Google Scholar] [CrossRef]
- Goff, S.L.; Morgan, R.A.; Yang, J.C.; Sherry, R.M.; Robbins, P.F.; Restifo, N.P.; Feldman, S.A.; Lu, Y.-C.; Lu, L.; Zheng, Z.; et al. Pilot Trial of Adoptive Transfer of Chimeric Antigen Receptor-Transduced T Cells Targeting EGFRvIII in Patients with Glioblastoma. J. Immunother. 2019, 42, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Benmebarek, M.-R.; Karches, C.H.; Cadilha, B.L.; Lesch, S.; Endres, S.; Kobold, S. Killing Mechanisms of Chimeric Antigen Receptor (CAR) T Cells. Int. J. Mol. Sci. 2019, 20, 1283. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.C.; Maus, M.V. Recent Advances and Discoveries in the Mechanisms and Functions of CAR T Cells. Nat. Rev. Cancer 2021, 21, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.A.; Finney, O.; Annesley, C.; Brakke, H.; Summers, C.; Leger, K.; Bleakley, M.; Brown, C.; Mgebroff, S.; Kelly-Spratt, K.S.; et al. Intent-to-Treat Leukemia Remission by CD19 CAR T Cells of Defined Formulation and Dose in Children and Young Adults. Blood 2017, 129, 3322–3331. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lu, X.; Liu, R.; Zhang, F.; Zhao, H. HLA Tetramer Based Artificial Antigen-Presenting Cells Efficiently Stimulate CTLs Specific for Malignant Glioma. Clin. Cancer Res. 2007, 13, 7329–7334. [Google Scholar] [CrossRef]
- Thaci, B.; Brown, C.E.; Binello, E.; Werbaneth, K.; Sampath, P.; Sengupta, S. Significance of Interleukin-13 Receptor Alpha 2-Targeted Glioblastoma Therapy. Neuro Oncol. 2014, 16, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Wange, W.; Cai, L.; Zhu, P.; Gao, Z.; Zheng, W. IL-13 Receptor A2 Stimulates Human Glioma Cell Growth and Metastasis through the Src/PI3K/Akt/mTOR Signaling Pathway. Tumour Biol. 2016, 37, 14701–14709. [Google Scholar] [CrossRef]
- Brown, C.E.; Warden, C.D.; Starr, R.; Deng, X.; Badie, B.; Yuan, Y.-C.; Forman, S.J.; Barish, M.E. Glioma IL13Rα2 Is Associated with Mesenchymal Signature Gene Expression and Poor Patient Prognosis. PLoS ONE 2013, 8, e77769. [Google Scholar] [CrossRef]
- Kong, S.; Sengupta, S.; Tyler, B.; Bais, A.J.; Ma, Q.; Doucette, S.; Zhou, J.; Sahin, A.; Carter, B.S.; Brem, H.; et al. Suppression of Human Glioma Xenografts with Second-Generation IL13R-Specific Chimeric Antigen Receptor-Modified T Cells. Clin. Cancer Res. 2012, 18, 5949–5960. [Google Scholar] [CrossRef]
- Felsberg, J.; Hentschel, B.; Kaulich, K.; Gramatzki, D.; Zacher, A.; Malzkorn, B.; Kamp, M.; Sabel, M.; Simon, M.; Westphal, M.; et al. Epidermal Growth Factor Receptor Variant III (EGFRvIII) Positivity in EGFR-Amplified Glioblastomas: Prognostic Role and Comparison between Primary and Recurrent Tumors. Clin. Cancer Res. 2017, 23, 6846–6855. [Google Scholar] [CrossRef]
- Yang, S.; Wei, W.; Zhao, Q. B7-H3, a Checkpoint Molecule, as a Target for Cancer Immunotherapy. Int. J. Biol. Sci. 2020, 16, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Majzner, R.G.; Theruvath, J.L.; Nellan, A.; Heitzeneder, S.; Cui, Y.; Mount, C.W.; Rietberg, S.P.; Linde, M.H.; Xu, P.; Rota, C.; et al. CAR T Cells Targeting B7-H3, a Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity against Pediatric Solid Tumors and Brain Tumors. Clin. Cancer Res. 2019, 25, 2560–2574. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wang, Y.; Huang, J.; Zhang, Z.; Liu, F.; Xu, J.; Guo, G.; Wang, W.; Tong, A.; Zhou, L. Administration of B7-H3 Targeted Chimeric Antigen Receptor-T Cells Induce Regression of Glioblastoma. Signal Transduct. Target. Ther. 2021, 6, 125. [Google Scholar] [CrossRef] [PubMed]
- Pieńkowski, T.; Zielinski, C.C. Trastuzumab Treatment in Patients with Breast Cancer and Metastatic CNS Disease. Ann. Oncol. 2010, 21, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Bielamowicz, K.; Fousek, K.; Byrd, T.T.; Samaha, H.; Mukherjee, M.; Aware, N.; Wu, M.-F.; Orange, J.S.; Sumazin, P.; Man, T.-K.; et al. Trivalent CAR T Cells Overcome Interpatient Antigenic Variability in Glioblastoma. Neuro Oncol. 2018, 20, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Kreisl, T.N.; Kim, L.; Moore, K.; Duic, P.; Royce, C.; Stroud, I.; Garren, N.; Mackey, M.; Butman, J.A.; Camphausen, K.; et al. Phase II Trial of Single-Agent Bevacizumab Followed by Bevacizumab plus Irinotecan at Tumor Progression in Recurrent Glioblastoma. J. Clin. Oncol. 2009, 27, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Momcilovic, M.; Bailey, S.T.; Lee, J.T.; Fishbein, M.C.; Braas, D.; Go, J.; Graeber, T.G.; Parlati, F.; Demo, S.; Li, R.; et al. The GSK3 Signaling Axis Regulates Adaptive Glutamine Metabolism in Lung Squamous Cell Carcinoma. Cancer Cell 2018, 33, 905–921.e5. [Google Scholar] [CrossRef]
- Tanaka, K.; Sasayama, T.; Irino, Y.; Takata, K.; Nagashima, H.; Satoh, N.; Kyotani, K.; Mizowaki, T.; Imahori, T.; Ejima, Y.; et al. Compensatory Glutamine Metabolism Promotes Glioblastoma Resistance to mTOR Inhibitor Treatment. J. Clin. Invest. 2015, 125, 1591–1602. [Google Scholar] [CrossRef]
- Keunen, O.; Johansson, M.; Oudin, A.; Sanzey, M.; Rahim, S.A.A.; Fack, F.; Thorsen, F.; Taxt, T.; Bartos, M.; Jirik, R.; et al. Anti-VEGF Treatment Reduces Blood Supply and Increases Tumor Cell Invasion in Glioblastoma. Proc. Natl. Acad. Sci. USA 2011, 108, 3749–3754. [Google Scholar] [CrossRef]
- Fack, F.; Espedal, H.; Keunen, O.; Golebiewska, A.; Obad, N.; Harter, P.N.; Mittelbronn, M.; Bähr, O.; Weyerbrock, A.; Stuhr, L.; et al. Bevacizumab Treatment Induces Metabolic Adaptation toward Anaerobic Metabolism in Glioblastomas. Acta Neuropathol. 2015, 129, 115–131. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]

| Author | Journal | Year | Phase | Patients (No.) | WHO Grade | Recurrent or Newly Diagnosed | Median OS (Months) | Median PFS (Months) | Treatment | Combination with CMT or RT or Both | Target |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kasenda B et al. [25] | ESMO Open | 2022 | I | 9 | IV | NA | 8 | 1.5 | Anti-EGFR immunoliposomes loaded with Doxorubicin | No | Telomerase II |
| Hall PE et al. [26] | Clin Cancer Res | 2019 | I | 10 | III–IV | Recurrent | 6.3 | 5.2 | Pegargiminase, Cisplatin, and Pemetrexed | CMT | Arginine |
| Lee EQ et al. [27] | Cancer | 2020 | II | 57 | IV | NA | 7.5 | 4.2 | Bevacizumab | Alone and with CMT | VEGF |
| Hottinger AF et al. [28] | Br J Cancer | 2014 | I | 17 | III–IV | Newly diagnosed | 17.8 | 7.9 | Sorafenib | Both | VEGFR, Flt3, c-RAF, wild type and V599E mutant B-RAF, PDGFRβ, c-KIT, FGFR1, p38α and RET |
| Wetmore C et al. [29] | Cancer Med | 2016 | II | 30 | III–IV | NA | 8 | 4 | Sunitinib | No | VEGFR, PDGFR, KIT |
| Michael W Ronellenfitsch et al. [30] | Acta Neuropathol Commun | 2018 | III | 149 | IV | Newly diagnosed | 17 | NA | Nimotuzumab | No | EGFR |
| Kalpathy-Cramer J et al. [31] | J Neurooncol | 2017 | II | 10 | IV | Recurrent | 8.1 | 2.3 | Tivozanib | No | VEGFR |
| Broniscer A et al. [32] | Clin Cancer Res | 2013 | I | 25 | IV | Newly diagnosed | 15 | NA | Vandetanib and Dasatinib | No | Dasatinib: c-Kit, Src, and PDGFRA and B; Vandetanib: VEGF receptor 2, EGFR and RET |
| Reardon DA et al. [33] | J Neurooncol | 2010 | II | 32 | IV | Recurrent | 8.5 | 1.7 | Erlotinib | No | mTOR |
| Roger Stupp et al. [34] | The Lancet Oncol | 2016 | III | 3471 | IV | Newly diagnosed | 26.3 | NA | Cilengitide | CMT | αvβ3 and αvβ5 integrin |
| Galanis E et al. [35] | J Clin Oncol | 2009 | II | 66 | IV | Recurrent | 5.7 | NA | Varinostat | No | Histone deacetylase |
| Mark R Gilbert et al. [36] | J Neurooncol | 2017 | II | 60 | IV | Recurrent | 9.4 (temozolomide arm); 7.7 (ironotecan arm) | 4.7 (temozolomide arm); 4.1 (irinotecan arm) | Bevacizumab | CMT | VEGF |
| Ma DJ et al. [37] | Neuro Oncol | 2015 | II | 100 | IV | Newly diagnosed | 21 | 13 | Everolimus | Both | mTOR |
| Brown N et al. [38] | PLoS One | 2016 | II | 38 | IV | Recurrent | 5.5 (placebo); 7.2 (combined with gefitinib) | 2.8 (placebo); 3.6 (combined with gefitinib) | Cediranib | No | VEGFR |
| Lv S et al. [39] | Int J Oncol | 2012 | II | 35 | IV | Recurrent | 5.6 (EGFR amplification and EGFRvIII negative glioblastoma); 4 (EGFR amplification and EGFRvIII positive glioblastoma) | 3 (EGFR amplification and EGFRvIII negative glioblastoma); 1.6 (EGFR amplification and EGFRvIII positive glioblastoma) | Cetuximab | No | EGFR |
| Reardon DA et al. [40] | Cancer | 2009 | I | 37 | III–IV | Recurrent | 10 | NA | Vatalanib | CMT | VEGFR, PDGFR |
| Galanis E et al. [41] | J Clin Oncol | 2005 | II | 65 | IV | Recurrent | 4.4 | NA | Temsirolimus | No | mTOR |
| Jeon JY et al. [42] | Am J Neuroradiol | 2012 | NA | 18 | IV | Recurrent | NA | 4.2 | Bevacizumab | No | VEGF |
| Babak et al. [43] | Neuro Oncol | 2018 | II | 171 | IV | Newly diagnosed | NA | 8.2 | Everolimus | Both | mTORC |
| David A. Reardon [44] | Cancer Chemother Pharmacol | 2012 | I | 64 | IV | Recurrent | NA | 2.7 (arm 1); 1.6 (arm 2) | AEE788 | No | ERBB2, VEGFR |
| Ian F. Pollack et al. [45] | Neuro Oncol | 2011 | II | 43 | III–IV | Newly diagnosed | 12 | 9 | Gefitinib | RT | EGFR |
| Aaron D Simpson et al. [46] | Br J Cancer | 2020 | NA | 408 | III–IV | Newly diagnosed | 18 | NA | BMS-754807 | No | IGF1R |
| Patrick Y Wen et al. [47] | J Clin Oncol | 2019 | II | 65 | IV | Recurrent | 17.9 (eligible for re-operation); 9.8 (not eligible for re-operation) | 1.7 | Buparlisib | No | PI3K |
| Author | Journal | Year | Phase | Patients | Outcome | Treatment | Target |
|---|---|---|---|---|---|---|---|
| Lee EQ et al. [27] | Cancer | 2020 | II | 57 | OS, PFS | Bevacizumab | VEGF |
| Mark R Gilbert et al. [36] | J Neurooncol | 2017 | II | 60 | OS, PFS | Bevacizumab | VEGF |
| Jeon JY et al. [42] | Am J Neuroradiol | 2012 | II | 18 | PFS | Bevacizumab | VEGF |
| Brown N et al. [38] | PLoS One | 2016 | II | 38 | OS, PFS | Cediranib | VEGFR |
| Hottinger AF et al. [28] | Br J Cancer | 2014 | I | 17 | OS, PFS | Sorafenib | VEGFR |
| Wetmore C et al. [29] | Cancer Med | 2016 | II | 30 | OS, PFS | Sunitinib | VEGFR |
| Kalpathy-Cramer J et al. [31] | J Neurooncol | 2017 | II | 10 | OS, PFS | Tivozanib | VEGFR |
| Broniscer A et al. [32] | Clin Cancer Res | 2013 | I | 25 | OS | Dasatinib and Vandetanib | VEGFR |
| Reardon DA et al. [40] | Cancer | 2009 | I | 37 | OS | Vatalanib | VEGFR |
| David A. Reardon [44] | Cancer Chemother Pharmacol | 2012 | I | 64 | PFS | AEE788 | VEGFR |
| Author | Journal | Year | Phase | Patients | Outcome | Treatment | Target |
|---|---|---|---|---|---|---|---|
| Michael W Ronellenfitsch et al. [30] | Acta Neuropathol Commun | 2018 | III | 149 | OS, PFS | Nimotuzumab | EGFR |
| Lv S et al. [39] | Int J Oncol | 2012 | II | 35 | OS, PFS | Cetuximab | EGFR |
| Ian F. Pollack et al. [45] | Neuro Oncol | 2011 | II | 43 | OS, PFS | Gefitinib | EGFR |
| Broniscer A et al. [32] | Clin Cancer Res | 2013 | I | 25 | OS | Dasatinib and Vandetanib | EGFR |
| David A. Reardon [44] | Cancer Chemother Pharmacol | 2012 | I | 64 | PFS | AEE788 | ERBB2 |
| Author | Journal | Year | Phase | Patients | Outcome | Treatment | Target |
|---|---|---|---|---|---|---|---|
| Hottinger AF et al. [28] | Br J Cancer | 2014 | I | 17 | OS, PFS | Sorafenib | PDGFR |
| Wetmore C et al. [29] | Cancer Med | 2016 | II | 30 | OS, PFS | Sunitinib | PDGFR |
| Broniscer A et al. [32] | Clin Cancer Res | 2013 | I | 25 | OS | Dasatinib and Vandetanib | PDGFR |
| Reardon DA et al. [40] | Cancer | 2009 | I | 37 | OS | Vatalanib | PDGFR |
| Author | Journal | Year | Phase | Patients | Outcome | Treatment | Target |
|---|---|---|---|---|---|---|---|
| Reardon DA et al. [33] | J Neurooncol | 2010 | II | 32 | OS, PFS | Erlotinib | mTOR |
| Ma DJ et al. [37] | Neuro Oncol | 2015 | II | 100 | OS, PFS | Everolimus | mTOR |
| Galanis E et al. [41] | J Clin Oncol | 2005 | II | 65 | OS | Temsirolimus | mTOR |
| Babak et al. [43] | Neuro-Oncol | 2018 | II | 171 | PFS | Everolimus | mTOR |
| No. | Criterion | Decision Rule | Score (* = 1; no * = 0) |
|---|---|---|---|
| SELECTION | |||
| 1 | Representativeness of the exposed cohort | (a) Consecutive eligible participants were selected, participants were randomly selected, or all participants were invited to participate from the source population; * (b) Not satisfying requirements in part (a), or not stated. | |
| 2 | Selection of the non-exposed cohort | (a) Selected from the same source population; * (b) Selected from a different source population; (c) No description. | |
| 3 | Ascertainment of exposure | (a) Medical record; * (b) Structured interview; * (c) No description. | |
| 4 | Demonstration that outcome of interest was not present at the start of the study | (a) Yes; * (b) No or not explicitly stated. | |
| COMPARABILITY | |||
| 1 | Were there clearly defined inclusion and exclusion criteria? | (a) Yes; * (b) No or not explicitly stated. | |
| OUTCOME | |||
| 1 | Assessment of outcome | (a) Independent or blind assessment stated, or confirmation of the outcome by reference to secure records; * (b) Record linkage (e.g., identified through ICD codes on database records); * (c) Self-report with no reference to original structured injury data or imaging; (d) No description. | |
| 2 | Was follow-up long enough for outcomes to occur? | (a) Yes (≥12 months); * (b) No (<3 months). | |
| 3 | Adequacy of follow up of cohorts | (a) Complete follow up—all participants accounted for; * (b) Subjects lost to follow up unlikely to introduce bias (<20% lost to follow up or description provided of those lost); * (c) Follow up rate <85% and no description of those lost provided; (d) No statement. | |
| SCORE | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Maria, L.; Panciani, P.P.; Zeppieri, M.; Ius, T.; Serioli, S.; Piazza, A.; Di Giovanni, E.; Fontanella, M.M.; Agosti, E. A Systematic Review of the Metabolism of High-Grade Gliomas: Current Targeted Therapies and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 724. https://doi.org/10.3390/ijms25020724
De Maria L, Panciani PP, Zeppieri M, Ius T, Serioli S, Piazza A, Di Giovanni E, Fontanella MM, Agosti E. A Systematic Review of the Metabolism of High-Grade Gliomas: Current Targeted Therapies and Future Perspectives. International Journal of Molecular Sciences. 2024; 25(2):724. https://doi.org/10.3390/ijms25020724
Chicago/Turabian StyleDe Maria, Lucio, Pier Paolo Panciani, Marco Zeppieri, Tamara Ius, Simona Serioli, Amedeo Piazza, Emanuele Di Giovanni, Marco Maria Fontanella, and Edoardo Agosti. 2024. "A Systematic Review of the Metabolism of High-Grade Gliomas: Current Targeted Therapies and Future Perspectives" International Journal of Molecular Sciences 25, no. 2: 724. https://doi.org/10.3390/ijms25020724
APA StyleDe Maria, L., Panciani, P. P., Zeppieri, M., Ius, T., Serioli, S., Piazza, A., Di Giovanni, E., Fontanella, M. M., & Agosti, E. (2024). A Systematic Review of the Metabolism of High-Grade Gliomas: Current Targeted Therapies and Future Perspectives. International Journal of Molecular Sciences, 25(2), 724. https://doi.org/10.3390/ijms25020724










