The Pupa Stage Is the Most Sensitive to Hypoxia in Drosophila melanogaster
Abstract
1. Introduction
2. Results
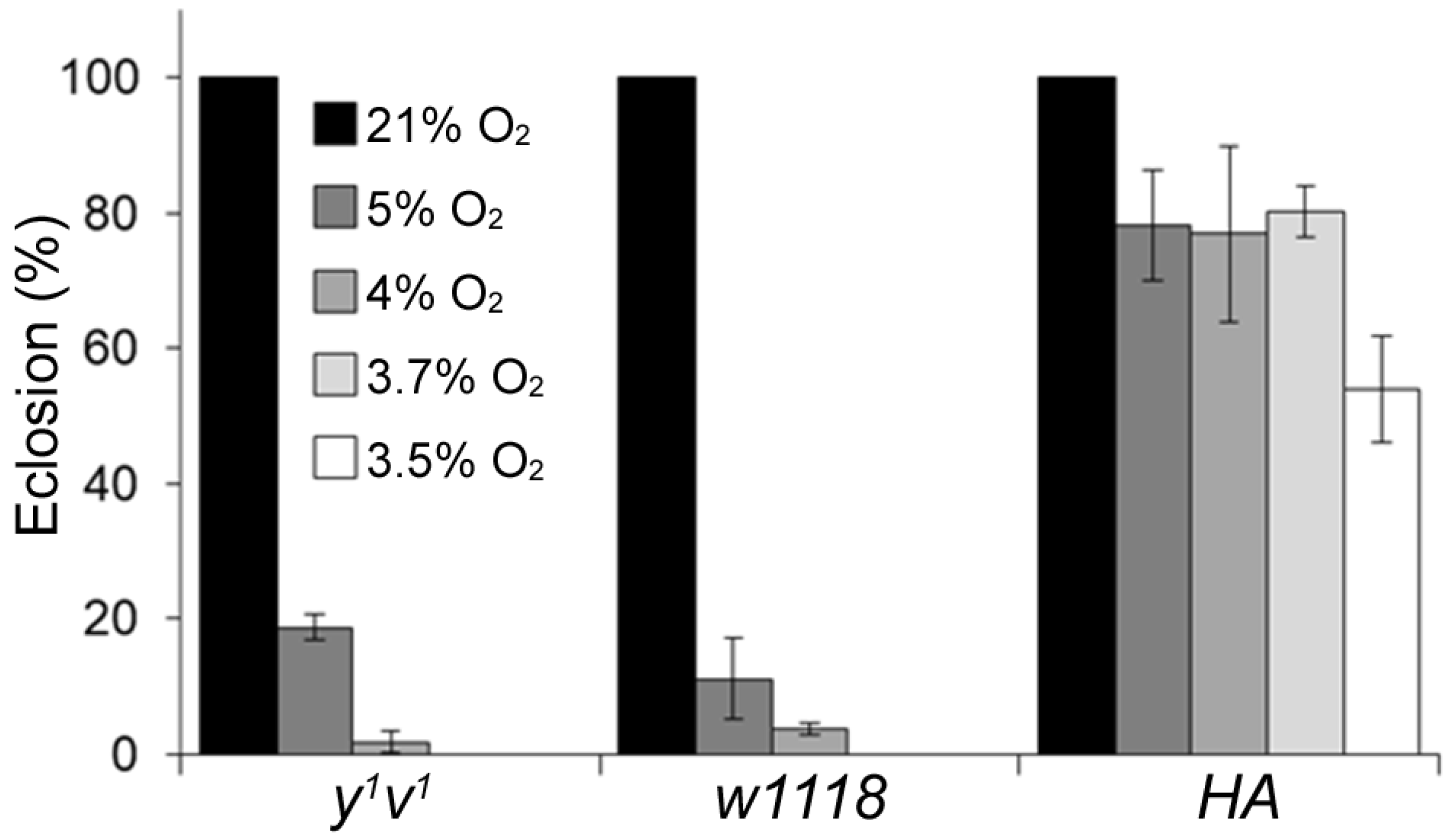
2.1. Determination of the Critical O2 Level for Hypoxia Sensitivity
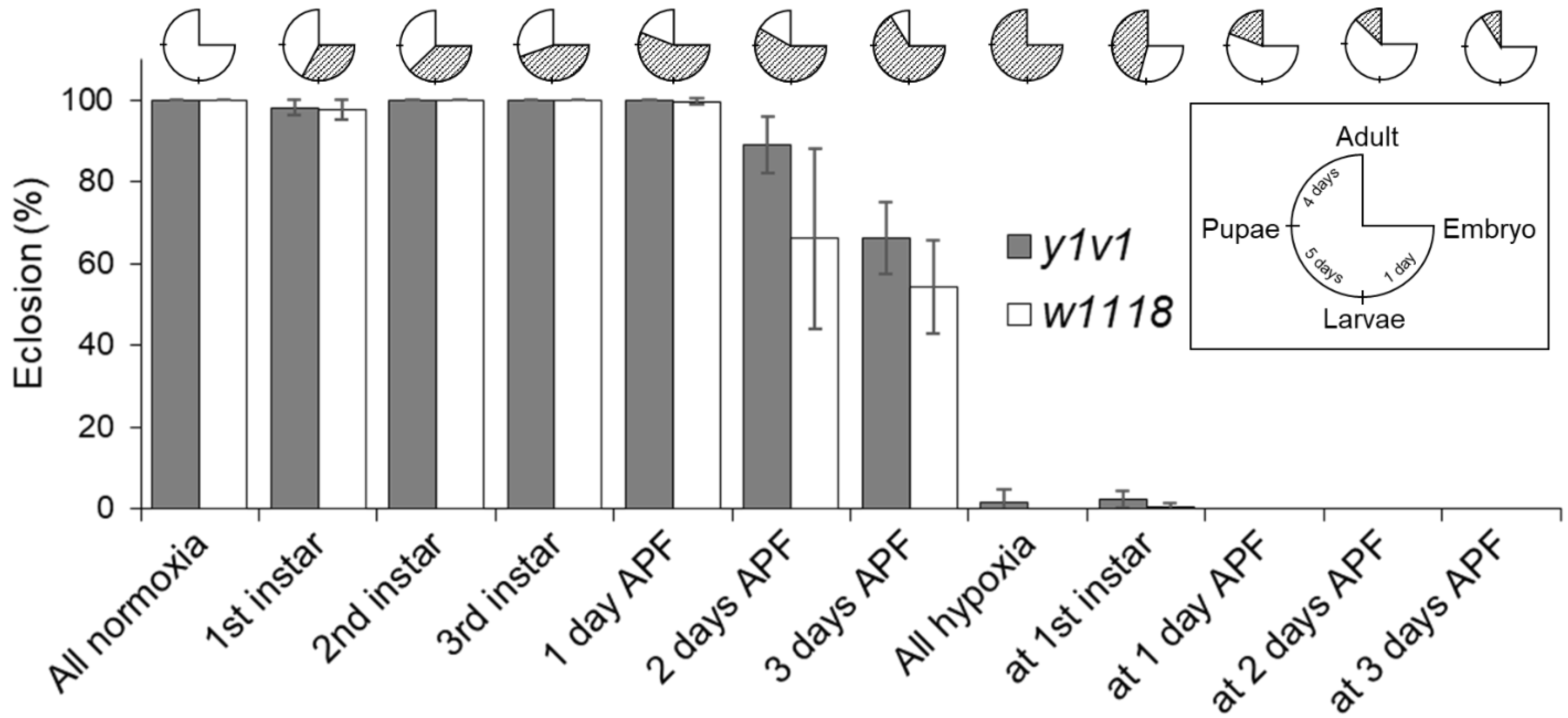
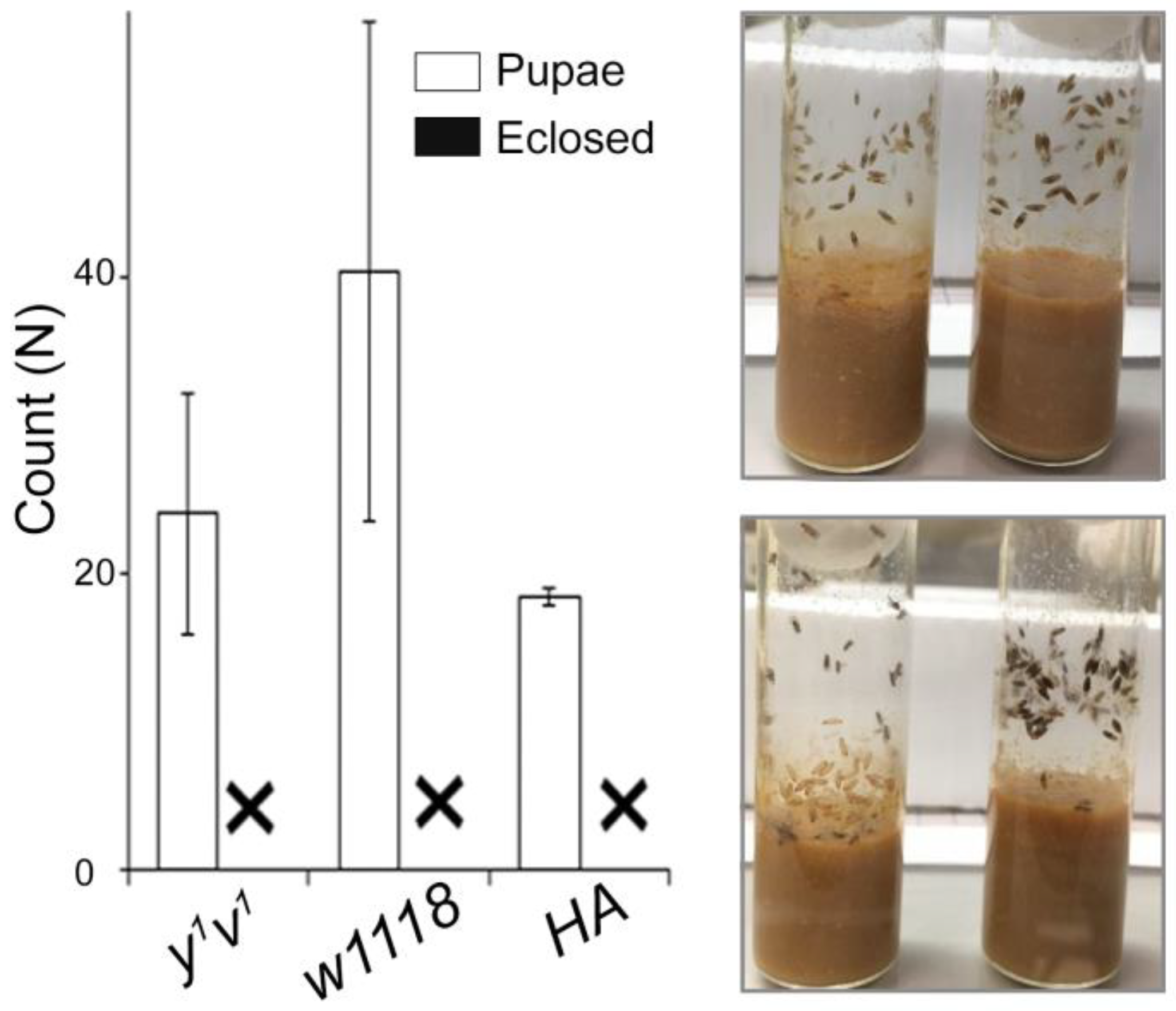
2.2. Identification of the Critical Hypoxia-Sensitive Stage
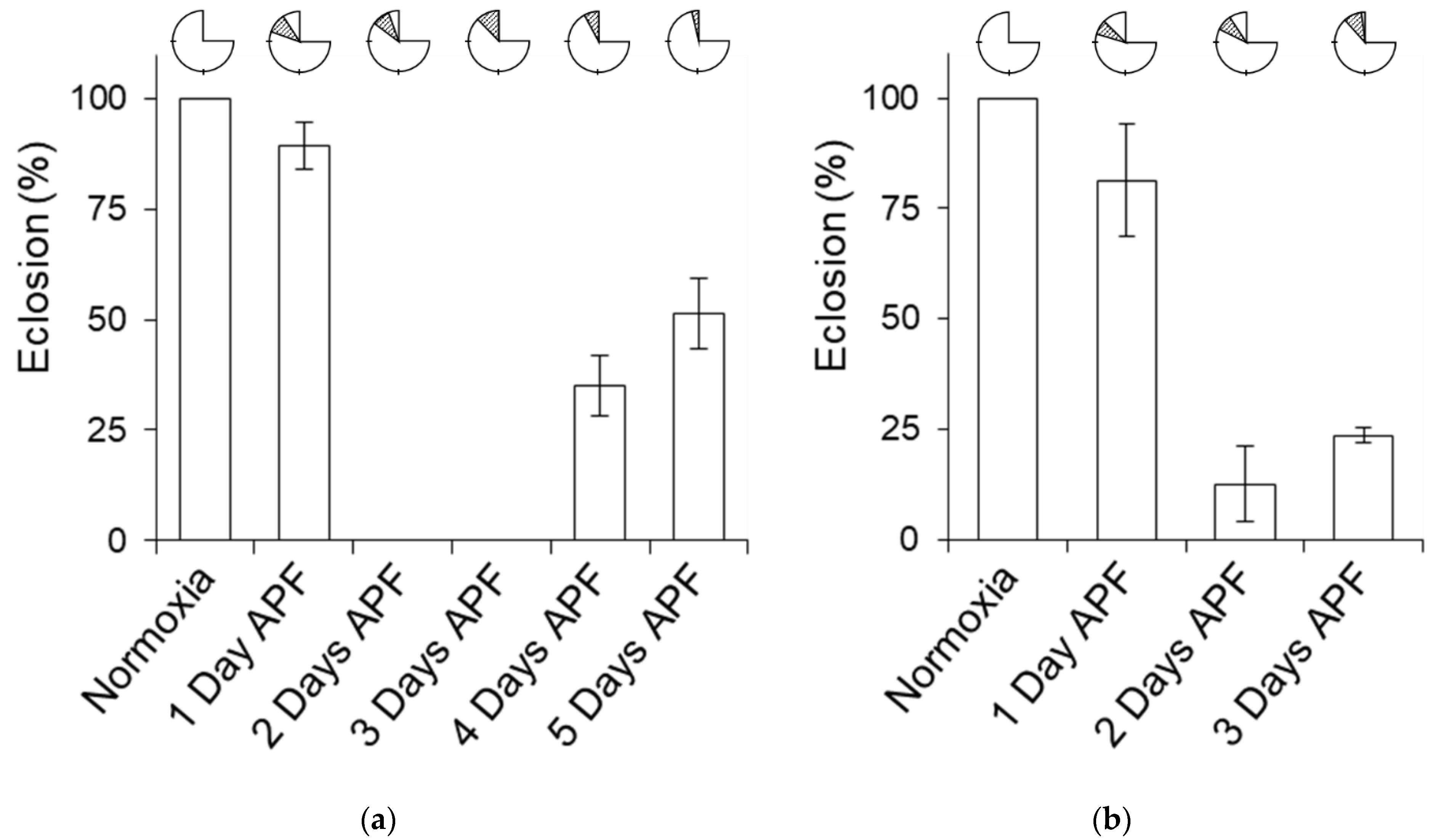
2.3. Critical Time Period for Pupa Hypoxia Exposure
3. Discussion
4. Materials and Methods
4.1. Fly Rearing and Collection
4.2. Critical O2 Level Determination
4.3. Hypoxia-Sensitive Stage Screening
4.4. Pupa Timeline Screening
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, F.B.; Anderson, R.N. The Leading Causes of Death in the US for 2020. JAMA 2021, 325, 1829–1830. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Top 10 Causes of Death. WHO Report. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 28 November 2023).
- Semenza, G.L.; Nejfelt, M.K.; Chi, S.M.; Antonarakis, S.E. Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc. Natl. Acad. Sci. USA 1991, 88, 5680–5684. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Wang, G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell Biol. 1992, 12, 5447–5454. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Barretto, E.C.; Grewal, S.S. TORC1 modulation in adipose tissue is required for organismal adaptation to hypoxia in Drosophila. Nat. Commun. 2019, 10, 1878. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Xue, J.; Chen, J.; Morcillo, P.; Lambert, J.D.; White, K.P.; Haddad, G.G. Experimental selection for Drosophila survival in extremely low O2 environment. PLoS ONE 2007, 2, e490. [Google Scholar] [CrossRef] [PubMed]
- Ayelén Valko, A.; Perez-Pandolfo, S.; Sorianello, E.; Brech, A.; Wappner, P.; Melani, M. Adaptation to hypoxia in Drosophila melanogaster requires autophagy. Autophagy 2022, 18, 909–920. [Google Scholar] [CrossRef] [PubMed]
- DiGregorio, P.J.; Ubersax, J.A.; O’Farrell, P.H. Hypoxia and nitric oxide induce a rapid, reversible cell cycle arrest of the Drosophila syncytial divisions. J. Biol. Chem. 2001, 276, 1930–1937. [Google Scholar] [CrossRef]
- Douglas, R.M.; Xu, T.; Haddad, G.G. Cell cycle progression and cell division are sensitive to hypoxia in Drosophila melanogaster embryos. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R1555–R1563. [Google Scholar] [CrossRef]
- Fischer, M.G.; Heeger, S.; Häcker, U.; Lehner, C.F. The mitotic arrest in response to hypoxia and of polar bodies during early embryogenesis requires Drosophila Mps1. Curr. Biol. 2004, 14, 2019–2024. [Google Scholar] [CrossRef]
- Wingrove, J.A.; O’Farrell, P.H. Nitric oxide contributes to behavioral, cellular, and developmental responses to low oxygen in Drosophila. Cell 1999, 98, 105–114. [Google Scholar] [CrossRef]
- Viviane Callier, V.; Hand, S.C.; Campbell, J.B.; Biddulph, T.; Harrison, J.F. Developmental changes in hypoxic exposure and responses to anoxia in Drosophila melanogaster. J. Exp. Biol. 2015, 218, 2927–2934. [Google Scholar] [CrossRef]
- Haddad, G.G.; Wyman, R.J.; Mohsenin, A.; Sun, Y.; Krishnan, S.N. Behavioral and Electrophysiologic Responses of Drosophila melanogaster to Prolonged Periods of Anoxia. J. Insect Physiol. 1997, 43, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, E.C.; Farzin, M.; Klok, C.J.; Harrison, J.F. The effect of developmental stage on the sensitivity of cell and body size to hypoxia in Drosophila melanogaster. J. Exp. Biol. 2011, 214, 1419–1427. [Google Scholar] [CrossRef][Green Version]
- Heyland, A.; Moroz, L.L. Signaling mechanisms underlying metamorphic transitions in animals. Integr. Comp. Biol. 2006, 46, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Rascon, B.; Harrison, J.F. Lifespan and oxidative stress show a non-linear response to atmospheric oxygen in Drosophila. J. Exp. Biol. 2010, 213, 3441–3448. [Google Scholar] [CrossRef] [PubMed]
- Polan, D.M.; Alansari, M.; Lee, B.; Grewal, S.S. Early-life hypoxia alters adult physiology and reduces stress resistance and lifespan in Drosophila. J. Exp. Biol. 2020, 223, jeb226027. [Google Scholar] [CrossRef]
- Peck, L.S.; Maddrell, S.H. Limitation of size by hypoxia in the fruit fly Drosophila melanogaster. J. Exp. Zool. A Comp. Exp. Biol. 2005, 303, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Mutamiswa, R.; Tarusikirwa, V.L.; Nyamukondiwa, C.; Cuthbert, R.N.; Chidawanyika, F. Thermal stress exposure of pupal oriental fruit fly has strong and trait-specific consequences in adult flies. Physiol. Entomol. 2023, 48, 35–44. [Google Scholar] [CrossRef]
- Zhang, W.; Chang, X.Q.; Hoffmann, A.; Zhang, S.; Ma, C.S. Impact of hot events at different developmental stages of a moth: The closer to adult stage, the less reproductive output. Sci. Rep. 2015, 5, 10436. [Google Scholar] [CrossRef]
- Iranmehr, A.; Stobdan, T.; Zhou, D.; Zhao, H.; Kryazhimskiy, S.; Bafna, V.; Haddad, G.G. Multiple mechanisms drive genomic adaptation to extreme O2 levels in Drosophila melanogaster. Nat. Commun. 2021, 12, 997. [Google Scholar] [CrossRef]
- Seong, K.H.; Ly, N.H.; Katou, Y.; Yokota, N.; Nakato, R.; Murakami, S.; Hirayama, A.; Fukuda, S.; Kang, S.; Soga, T.; et al. Paternal restraint stress affects offspring metabolism via ATF-2 dependent mechanisms in Drosophila melanogaster germ cells. Commun. Biol. 2020, 3, 208. [Google Scholar] [CrossRef]
- Obata, F.; Fons, C.O.; Gould, A.P. Early-life exposure to low-dose oxidants can increase longevity via microbiome remodelling in Drosophila. Nat. Commun. 2018, 9, 975. [Google Scholar] [CrossRef] [PubMed]
- Aiello, G.; Sabino, C.; Pernici, D.; Audano, M.; Antonica, F.; Gianesello, M.; Ballabio, C.; Quattrone, A.; Mitro, N.; Romanel, A.; et al. Transient rapamycin treatment during developmental stage extends lifespan in Mus musculus and Drosophila melanogaster. EMBO Rep. 2022, 23, e55299. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Kim, K.; O’Brown, Z.K.; Levan, A.; Dodson, A.E.; Kennedy, S.G.; Chernoff, C.; Greer, E.L. Hypoxia induces transgenerational epigenetic inheritance of small RNAs. Cell Rep. 2022, 41, 111800. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Park, J.W. Hypoxia-driven epigenetic regulation in cancer progression: A focus on histone methylation and its modifying enzymes. Cancer Lett. 2020, 489, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Sun, H.; Chen, H.; Zavadil, J.; Kluz, T.; Arita, A.; Costa, M. Hypoxia induces trimethylated H3 lysine 4 by inhibition of JARID1A demethylase. Cancer Res. 2010, 70, 4214–4221. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.; Yi, S.J.; Kim, K. Gene regulation by histone-modifying enzymes under hypoxic conditions: A focus on histone methylation and acetylation. Exp. Mol. Med. 2022, 54, 878–889. [Google Scholar] [CrossRef]
- Li, T.; Mao, C.; Wang, X.; Shi, Y.; Tao, Y. Epigenetic crosstalk between hypoxia and tumor driven by HIF regulation. J. Exp. Clin. Cancer Res. 2020, 39, 224. [Google Scholar] [CrossRef]
- Camuzi, D.; de Amorim, I.S.S.; Ribeiro Pinto, L.F.; Oliveira Trivilin, L.; Mencalha, A.L.; Soares Lima, S.C. Regulation Is in the Air: The Relationship between Hypoxia and Epigenetics in Cancer. Cells 2019, 8, 300. [Google Scholar] [CrossRef]
- Taddei, M.L.; Giannoni, E.; Comito, G.; Chiarugi, P. Microenvironment and tumor cell plasticity: An easy way out. Cancer Lett. 2013, 341, 80–96. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stobdan, T.; Wen, N.J.; Lu-Bo, Y.; Zhou, D.; Haddad, G.G. The Pupa Stage Is the Most Sensitive to Hypoxia in Drosophila melanogaster. Int. J. Mol. Sci. 2024, 25, 710. https://doi.org/10.3390/ijms25020710
Stobdan T, Wen NJ, Lu-Bo Y, Zhou D, Haddad GG. The Pupa Stage Is the Most Sensitive to Hypoxia in Drosophila melanogaster. International Journal of Molecular Sciences. 2024; 25(2):710. https://doi.org/10.3390/ijms25020710
Chicago/Turabian StyleStobdan, Tsering, Nicholas J. Wen, Ying Lu-Bo, Dan Zhou, and Gabriel G. Haddad. 2024. "The Pupa Stage Is the Most Sensitive to Hypoxia in Drosophila melanogaster" International Journal of Molecular Sciences 25, no. 2: 710. https://doi.org/10.3390/ijms25020710
APA StyleStobdan, T., Wen, N. J., Lu-Bo, Y., Zhou, D., & Haddad, G. G. (2024). The Pupa Stage Is the Most Sensitive to Hypoxia in Drosophila melanogaster. International Journal of Molecular Sciences, 25(2), 710. https://doi.org/10.3390/ijms25020710






