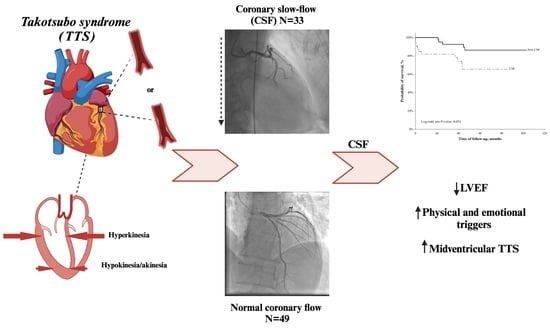
Coronary Slow-Flow Phenomenon in Takotsubo Syndrome: The Prevalence, Clinical Determinants, and Long-Term Prognostic Impact
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics
2.2. Angiographic Characteristics
2.3. Long-Term Mortality and Its Determinants
3. Discussion
4. Materials and Methods
4.1. Echocardiography
4.2. Angiography and Ventriculography
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medina de Chazal, H.; Del Buono, M.G.; Keyser-Marcus, L.; Ma, L.; Moeller, F.G.; Berrocal, D.; Abbate, A. Stress Cardiomyopathy Diagnosis and Treatment: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 1955–1971. [Google Scholar] [CrossRef] [PubMed]
- Akashi, Y.J.; Nef, H.M.; Lyon, A.R. Epidemiology and pathophysiology of Takotsubo syndrome. Nat. Rev. Cardiol. 2015, 12, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Quevedo, P.; Alonso-Martin, C.; Campuzano Ruiz, R.; Guzmán-Martinez, G.; Pedreira Perez, M.; Sambola, A. Cardiovascular disease in women: Do we need new diagnostic and therapeutic strategies? Kardiol Pol. 2023, 81, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Ojrzyńska-Witek, N.; Marczak, M.; Mazurkiewicz, Ł.; Petryka-Mazurkiewicz, J.; Miłosz-Wieczorek, B.; Grzybowski, J.; Śpiewak, M. Role of cardiac magnetic resonance in heart failure of initially unknown etiology: A 10-year observational study. Kardiol Pol. 2022, 80, 278–285. [Google Scholar] [CrossRef]
- Chalikias, G.; Tziakas, D. Slow Coronary Flow: Pathophysiology, Clinical Implications, and Therapeutic Management. Angiology 2021, 72, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, J.F. Defining the coronary slow flow phenomenon. Circ J. 2012, 76, 818–820. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Nie, S.-P. The coronary slow flow phenomenon: Characteristics, mechanisms and implications. Cardiovasc. Diagn. Ther. 2011, 1, 37–43. [Google Scholar] [CrossRef]
- Sezgin, A.T.; Sigirci, A.; Barutcu, I.; Topal, E.; Sezgin, N.; Ozdemir, R.; Yetkin, E.; Tandogan, I.; Kosar, F.; Ermis, N.; et al. Vascular endothelial function in patients with slow coronary flow. Coron. Artery Dis. 2003, 14, 155–161. [Google Scholar] [CrossRef]
- Camsarl, A.; Pekdemir, H.; Cicek, D.; Polat, G.; Akkus, M.N.; Döven, O.; Cin, V.G.; Katlrclbasl, T.; Parmakslz, T. Endothelin-1 and nitric oxide concentrations and their response to exercise in patients with slow coronary flow. Circ J. 2003, 67, 1022–1028. [Google Scholar] [CrossRef]
- Selcuk, M.T.; Selcuk, H.; Temizhan, A.; Maden, O.; Ulupinar, H.; Baysal, E.; Ozeke, O.; Sasmaz, A. Asymmetric dimethylarginine plasma concentrations and L-arginine/asymmetric dimethylarginine ratio in patients with slow coronary flow. Coron Artery Dis. 2007, 18, 545–551. [Google Scholar] [CrossRef]
- Loffi, M.; Santangelo, A.; Kozel, M.; Kocka, V.; Budesinsky, T.; Lisa, L.; Tousek, P. Takotsubo Cardiomyopathy: One More Angiographic Evidence of Microvascular Dysfunction. Biomed. Res. Int. 2018, 2018, 5281485. [Google Scholar] [CrossRef]
- Khalid, N.; Iqbal, I.; Coram, R.; Raza, T.; Fahsah, I.; Ikram, S. Thrombolysis In Myocardial Infarction Frame Count in Takotsubo Cardiomyopathy. Int. J. Cardiol. 2015, 191, 107–108. [Google Scholar] [CrossRef]
- De Caterina, A.R.; Leone, A.M.; Galiuto, L.; Basile, E.; Fedele, E.; Paraggio, L.; De Maria, G.L.; Porto, I.; Niccoli, G.; Burzotta, F.; et al. Angiographic assessment of myocardial perfusion in Tako-Tsubo syndrome. Int. J. Cardiol. 2013, 168, 4717–4722. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Galiuto, L.; Meucci, M.C.; Del Buono, M.G.; Vergni, F.; Camilli, M.; Sanna, T.; Pedicino, D.; Buffon, A.; D’Amario, D.; et al. Coronary slow flow is associated with a worse clinical outcome in patients with Takotsubo syndrome. Heart 2020, 106, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Bybee, K.A.; Prasad, A.; Barsness, G.W.; Lerman, A.; Jaffe, A.S.; Murphy, J.G.; Wright, R.S.; Rihal, C.S. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am. J. Cardiol. 2004, 94, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Kurisu, S.; Inoue, I.; Kawagoe, T.; Ishihara, M.; Shimatani, Y.; Nishioka, K.; Umemura, T.; Nakamura, S.; Yoshida, M.; Sato, H. Myocardial perfusion and fatty acid metabolism in patients with tako-tsubo-like left ventricular dysfunction. J. Am. Coll. Cardiol. 2003, 41, 743–748. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction Across the Spectrum of Cardiovascular Diseases: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef]
- Pelliccia, F.; Kaski, J.C.; Crea, F.; Camici, P.G. Pathophysiology of Takotsubo Syndrome. Circulation 2017, 135, 2426–2441. [Google Scholar] [CrossRef]
- Ekenbäck, C.; Nickander, J.; Jokhaji, F.; Tornvall, P.; Engblom, H.; Spaak, J.; Persson, J. Coronary microvascular dysfunction in Takotsubo syndrome and associations with left ventricular function. ESC Heart Fail. 2023, 10, 2395–2405. [Google Scholar] [CrossRef]
- Gao, Y.F.; Chen, Y.; Wang, C.J.; Du, Y.; Ding, Y.H. Atrial fibrillation episode status and incidence of coronary slow flow: A propensity score-matched analysis. Front. Cardiovasc. Med. 2023, 10, 1047748. [Google Scholar] [CrossRef]
- Hang, C.L.; Wang, C.P.; Yip, H.K.; Yang, C.H.; Guo, G.B.; Wu, C.J.; Chen, S.M. Early administration of intracoronary verapamil improves myocardial perfusion during percutaneous coronary interventions for acute myocardial infarction. Chest 2005, 128, 2593–2598. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Ke, Q.; Li, W.; Jin, M.; Luo, Y.; Zhang, L.; Yang, D.; Zhang, X. Effect of inflammatory factor-induced cyclo-oxygenase expression on the development of reperfusion-related no-reflow phenomenon in acute myocardial infarction. Clin. Exp. Pharmacol. Physiol. 2015, 42, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.M.; Lennon, R.J.; Prasad, A. Regional wall motion abnormality in apical ballooning syndrome (Takotsubo/stress cardiomyopathy): Importance of biplane left ventriculography for differentiating from spontaneously aborted anterior myocardial infarction. Int. J. Cardiovasc. Imaging 2012, 28, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Alashi, A.; Isaza, N.; Faulx, J.; Popovic, Z.B.; Menon, V.; Ellis, S.G.; Faulx, M.; Kapadia, S.R.; Griffin, B.P.; Desai, M.Y. Characteristics and Outcomes of Patients With Takotsubo Syndrome: Incremental Prognostic Value of Baseline Left Ventricular Systolic Function. J. Am. Heart Assoc. 2020, 18, e016537. [Google Scholar] [CrossRef] [PubMed]
- Armillotta, M.; Amicone, S.; Bergamaschi, L.; Angeli, F.; Rinaldi, A.; Paolisso, P.; Stefanizzi, A.; Sansonetti, A.; Impellizzeri, A.; Bodega, F.; et al. Predictive value of Killip classification in MINOCA patients. Eur. J. Intern. Med. 2023, 117, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wischnewsky, M.B.; Candreva, A.; Bacchi, B.; Cammann, V.L.; Kato, K.; Szawan, K.A.; Gili, S.; D’Ascenzo, F.; Dichtl, W.; Citro, R.; et al. Prediction of short- and long-term mortality in takotsubo syndrome: The InterTAK Prognostic Score. Eur. J. Heart Fail. 2019, 21, 1469–1472. [Google Scholar] [CrossRef]
- TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. N. Engl. J. Med. 1985, 312, 932–936. [Google Scholar] [CrossRef]
- Gibson, C.M.; Cannon, C.P.; Daley, W.L.; Dodge, J.T., Jr.; Alexander, B.J.; Marble, S.J.; McCabe, C.H.; Raymond, L.; Fortin, T.; Poole, W.K.; et al. TIMI frame count: A quantitative method of assessing coronary artery flow. Circulation 1996, 93, 879–888. [Google Scholar] [CrossRef]
- Appleby, M.A.; Angeja, B.G.; Dauterman, K.; Gibson, C.M. Angiographic assessment of myocardial perfusion: TIMI myocardial perfusion (TMP) grading system. Heart 2001, 86, 485–486. [Google Scholar] [CrossRef]
- Stępień, K.; Nowak, K.; Nessler, J.; Zalewski, J. Worse long term prognosis in myocardial infarction occurring at weekends or public holidays with insight into myocardial infarction with nonobstructive coronary arteries. Pol. Arch. Intern. Med. 2020, 130, 942–952. [Google Scholar] [CrossRef]
- Stepien, K.; Nowak, K.; Wypasek, E.; Zalewski, J.; Undas, A. High prevalence of inherited thrombophilia and antiphospholipid syndrome in myocardial infarction with non-obstructive coronary arteries: Comparison with cryptogenic stroke. Int. J. Cardiol. 2019, 290, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, K.; Stepien, K.; Nowak, K.; Nessler, J.; Zalewski, J. ST-segment re-elevation following primary angioplasty in acute myocardial infarction with patent infarct-related artery: Impact on left ventricular function recovery and remodeling. Adv. Interv. Cardiol./Postępy w Kardiologii Interwencyjnej 2019, 15, 412–421. [Google Scholar] [CrossRef]
- Stępień, K.; Siudut, J.; Konieczyńska, M.; Nowak, K.; Zalewski, J.; Undas, A. Effect of high-dose statin therapy on coagulation factors: Lowering of factor XI as a modifier of fibrin clot properties in coronary artery disease. Vascul. Pharmacol. 2023, 149, 107153. [Google Scholar] [CrossRef] [PubMed]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef] [PubMed]
- Stepien, K.; Nowak, K.; Kachnic, N.; Horosin, G.; Walczak, P.; Karcinska, A.; Schwarz, T.; Wojtas, M.; Zalewska, M.; Pastuszak, M.; et al. Statin Use in Cancer Patients with Acute Myocardial Infarction and Its Impact on Long-Term Mortality. Pharmaceuticals 2022, 15, 919. [Google Scholar] [CrossRef]
- Stepien, K.; Nowak, K.; Szlosarczyk, B.; Nessler, J.; Zalewski, J. Clinical Characteristics and Long-Term Outcomes of MINOCA Accompanied by Active Cancer: A Retrospective Insight Into a Cardio-Oncology Center Registry. Front. Cardiovasc. Med. 2022, 9, 785246. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef]
- Stępień, K.; Nowak, K.; Pasieka, P.; Warmuz, K.; Stępień, A.; Nessler, J.; Zalewski, J. Typical variant of takotsubo cardiomyopathy in oncological patients. Two case reports and review of the literature. Folia Med. Cracov. 2020, 60, 45–54. [Google Scholar] [CrossRef]



| Slow-Flow TTS n = 33 | Normal-Flow TTS n = 49 | p-Value | |
|---|---|---|---|
| Female gender, % | 31 (93.9) | 44 (89.8) | 0.51 |
| Age, years | 68 (63–76) | 73 (65–80) | 0.56 |
| Body mass index, kg/m2 | 24.2 (23–31.1) | 25.7 (23.1–29.7) | 0.75 |
| Diabetes mellitus, % | 6 (18.2) | 11 (22.5) | 0.64 |
| Hypertension, % | 28 (84.9) | 38 (77.6) | 0.41 |
| Dyslipidemia, % | 20 (60.6) | 31 (63.3) | 0.81 |
| Renal failure, % | 8 (24.2) | 17 (34.7) | 0.31 |
| Active smoking, % | 5 (15.2) | 3 (6.1) | 0.18 |
| Atrial fibrillation, % | 8 (24.2) | 6 (12.2) | 0.16 |
| Anemia, % | 6 (18.2) | 5 (10.2) | 0.30 |
| Thrombocytopenia, % | 1 (3.0) | 2 (4.1) | 0.80 |
| Prior myocardial infarction, % | 2 (6.1) | 3 (6.1) | 0.99 |
| Prior PCI, % | 1 (3.0) | 1 (2.0) | 0.78 |
| Prior stroke, % | 0 | 1 (2.0) | 0.41 |
| Psychiatric disorder, % | 3 (9.1) | 10 (20.4) | 0.17 |
| Chronic obstructive pulmonary disease, % | 4 (12.1) | 2 (4.1) | 0.17 |
| Active or prior malignancy, % | 7 (21.2) | 4 (8.2) | 0.09 |
| Heart failure, % | 9 (27.3) | 8 (16.3) | 0.23 |
| Clinical presentation, % | |||
| NSTEMI | 17 (51.5) | 23 (46.9) | 0.68 |
| STEMI | 16 (48.5) | 26 (53.1) | |
| Left ventricular ejection fraction, % | |||
| On admission | 33.5 (25–40) | 40 (35–45) | 0.019 |
| On discharge | 45 (35–50) | 46 (40–55) | 0.13 |
| Ventriculography, % | 14 (42.4) | 31 (63.3) | 0.06 |
| Improvement during hospitalization | 12.5 (5–20) | 9 (0–15) | 0.23 |
| Killip class III/IV on admission, % | 4 (12.1) | 2 (4.1) | 0.17 |
| Length of hospitalization, days | 4 (2–5) | 4 (3–6) | 0.19 |
| Cardiogenic shock, % | 4 (12.1) | 2 (4.1) | 0.17 |
| In-hospital mortality, % | 2 (6.1) | 0 | 0.08 |
| Type of TTS, % | |||
| Apical | 24 (72.7) | 45 (91.8) | 0.020 |
| Midventricular | 9 (27.3) | 4 (8.2) | |
| Trigger, % | |||
| Physical | 7 (21.2) | 6 (12.2) | 0.28 |
| Emotional | 10 (30.3) | 18 (36.7) | 0.55 |
| Both types | 3 (9.1) | 0 | 0.032 |
| Undetermined | 19 (57.6) | 25 (51.0) | 0.56 |
| Drugs before admission, % | |||
| Aspirin | 3 (9.1) | 2 (4.1) | 0.35 |
| P2Y12 inhibitor | 0 (0.0) | 1 (2.0) | 0.41 |
| ACE-I/ARB | 21 (63.6) | 28 (57.1) | 0.56 |
| Beta-adrenolytic | 17 (51.5) | 17 (34.7) | 0.13 |
| Statin | 11 (33.3) | 12 (24.5) | 0.38 |
| Prescribed drugs on discharge, % | |||
| Aspirin | 26 (78.8) | 40 (81.6) | 0.75 |
| P2Y12 inhibitor | 10 (30.3) | 16 (32.7) | 0.82 |
| ACE-I/ARB | 30 (90.9) | 45 (91.8) | 0.88 |
| Beta-adrenolytic | 30 (90.9) | 44 (89.8) | 0.87 |
| Statin | 29 (87.9) | 40 (81.6) | 0.45 |
| Slow-Flow TTS n = 33 | Normal-FLOW TTS n = 49 | p-Value | |
|---|---|---|---|
| White blood cells, ×103/µL | 11.3 (9–14) | 10.4 (8.3–12) | 0.19 |
| Hemoglobin, g/dL | 13.6 (12.8–14.5) | 13.5 (12.6–14.3) | 0.59 |
| Platelet count, ×103/µL | 263 (221–297) | 243 (204–286) | 0.24 |
| Creatinine, µmol/L | 73 (65–84) | 75 (64–89) | 0.90 |
| Glomerular filtration rate, ml/min | 69.6 (51.2–99.2) | 66.2 (50.9–80.5) | 0.70 |
| C-reactive protein, mg/L | 4 (2–16) | 5 (3–12) | 0.60 |
| Myocardial necrosis markers—on admission: | |||
| Troponin, ng/ml | 0.481 (0.271–0.734) | 0.354 (0.196–0.622) | 0.23 |
| Creatine kinase, IU/L | 184 (117–301) | 172 (125–273) | 1.00 |
| Creatine kinase MB isoenzyme, IU/L | 29 (21–43.5) | 27 (19–40) | 0.39 |
| Myocardial necrosis markers—peak values: | |||
| Troponin, ng/ml | 0.554 (0.290–0.830) | 0.413 (0.198–0.785) | 0.35 |
| Creatine kinase, IU/L | 237 (133–332) | 213 (127–339) | 0.87 |
| Creatine kinase MB isoenzyme, IU/L | 31 (25–47) | 31 (19–44) | 0.62 |
| Slow-Flow TTS n = 33 | Normal-Flow TTS n = 49 | p-Value | |
|---|---|---|---|
| >50% stenosis, % | 3 (9.1) | 12 (24.5) | 0.08 |
| 30–50% stenosis, % | 5 (15.2) | 13 (26.5) | 0.22 |
| <30% stenosis, % | 25 (75.8) | 24 (49.0) | 0.015 |
| Slow-flow phenomenon, % | |||
| Generalized (LAD + LCx + RCA) | 11 (33.3) | - | |
| Left artery (LAD + LCx) | 15 (45.5) | - | |
| Isolated LAD | 7 (21.2) | - | |
| TFC, frames | |||
| LAD | 47.6 (40.6–54.7) | 25.9 (21.2–28.2) | <0.001 |
| LCx | 54 (39–72) | 33 (24–42) | <0.001 |
| RCA | 27 (18–36) | 20 (12–21) | 0.008 |
| TMPG LAD, % | |||
| 3 | 11 (33.3) | 46 (93.9) | <0.001 |
| 2 | 15 (45.5) | 0 | |
| 1 | 5 (15.2) | 2 (4.1) | |
| 0 | 2 (6.1) | 1 (2.0) | |
| TMPG LCx, % | |||
| 3 | 11 (33.3) | 45 (91.8) | <0.001 |
| 2 | 14 (42.4) | 1 (2.0) | |
| 1 | 4 (12.1) | 1 (2.0) | |
| 0 | 4 (12.1) | 2 (4.1) | |
| TMPG RCA, % | |||
| 3 | 10 (30.3) | 43 (87.8) | <0.001 |
| 2 | 6 (18.2) | 3 (6.1) | |
| 1 | 13 (39.4) | 2 (4.1) | |
| 0 | 4 (12.1) | 1 (2.0) |
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| p-Value | HR | 95% CI for HR | p-Value | HR | 95% CI for HR | |||
| Lower | Upper | Lower | Upper | |||||
| Age, per 1 year | <0.001 | 1.13 | 1.06 | 1.20 | 0.154 | 1.09 | 0.97 | 1.22 |
| BMI, per 1 kg/m2 | 0.008 | 0.83 | 0.74 | 0.95 | 0.040 | 0.73 | 0.54 | 0.99 |
| LVEF on admission, per 1% | 0.003 | 0.89 | 0.83 | 0.96 | 0.008 | 0.82 | 0.70 | 0.95 |
| TIMI, 2 vs. 3 | 0.031 | 3.23 | 1.11 | 9.09 | 0.017 | 23.81 | 1.75 | 81.40 |
| TTS type, 1 vs. 2 | 0.676 | 0.73 | 0.16 | 3.23 | 0.071 | 0.07 | 0.01 | 1.27 |
| Creatinine, per 1 µmol/L | <0.001 | 1.03 | 1.01 | 1.05 | 0.715 | 0.99 | 0.96 | 1.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stępień, K.; Nowak, K.; Karcińska, A.; Horosin, G.; del Carmen Yika, A.; Lenart, J.; Górowska, A.; Iwańczyk, S.; Podolec, M.; Siniarski, A.; et al. Coronary Slow-Flow Phenomenon in Takotsubo Syndrome: The Prevalence, Clinical Determinants, and Long-Term Prognostic Impact. Int. J. Mol. Sci. 2024, 25, 1297. https://doi.org/10.3390/ijms25021297
Stępień K, Nowak K, Karcińska A, Horosin G, del Carmen Yika A, Lenart J, Górowska A, Iwańczyk S, Podolec M, Siniarski A, et al. Coronary Slow-Flow Phenomenon in Takotsubo Syndrome: The Prevalence, Clinical Determinants, and Long-Term Prognostic Impact. International Journal of Molecular Sciences. 2024; 25(2):1297. https://doi.org/10.3390/ijms25021297
Chicago/Turabian StyleStępień, Konrad, Karol Nowak, Aleksandra Karcińska, Grzegorz Horosin, Alicia del Carmen Yika, Julia Lenart, Anna Górowska, Sylwia Iwańczyk, Mateusz Podolec, Aleksander Siniarski, and et al. 2024. "Coronary Slow-Flow Phenomenon in Takotsubo Syndrome: The Prevalence, Clinical Determinants, and Long-Term Prognostic Impact" International Journal of Molecular Sciences 25, no. 2: 1297. https://doi.org/10.3390/ijms25021297
APA StyleStępień, K., Nowak, K., Karcińska, A., Horosin, G., del Carmen Yika, A., Lenart, J., Górowska, A., Iwańczyk, S., Podolec, M., Siniarski, A., Nessler, J., & Zalewski, J. (2024). Coronary Slow-Flow Phenomenon in Takotsubo Syndrome: The Prevalence, Clinical Determinants, and Long-Term Prognostic Impact. International Journal of Molecular Sciences, 25(2), 1297. https://doi.org/10.3390/ijms25021297