Morphological and Genetic Aspects for Post-Mortem Diagnosis of Hypertrophic Cardiomyopathy: A Systematic Review
Abstract
1. Introduction
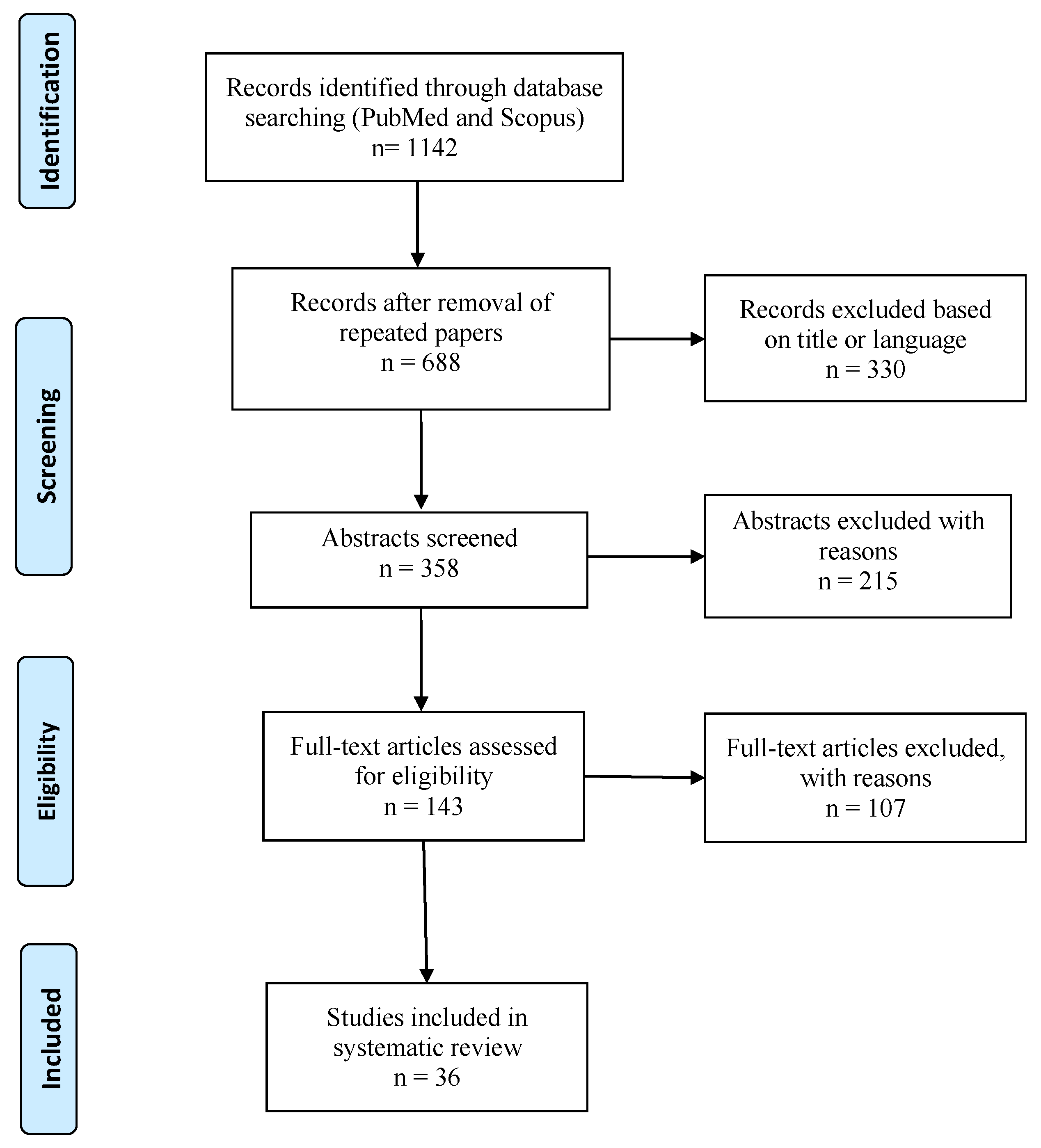
2. Materials and Methods
3. Results
3.1. Risk of Bias
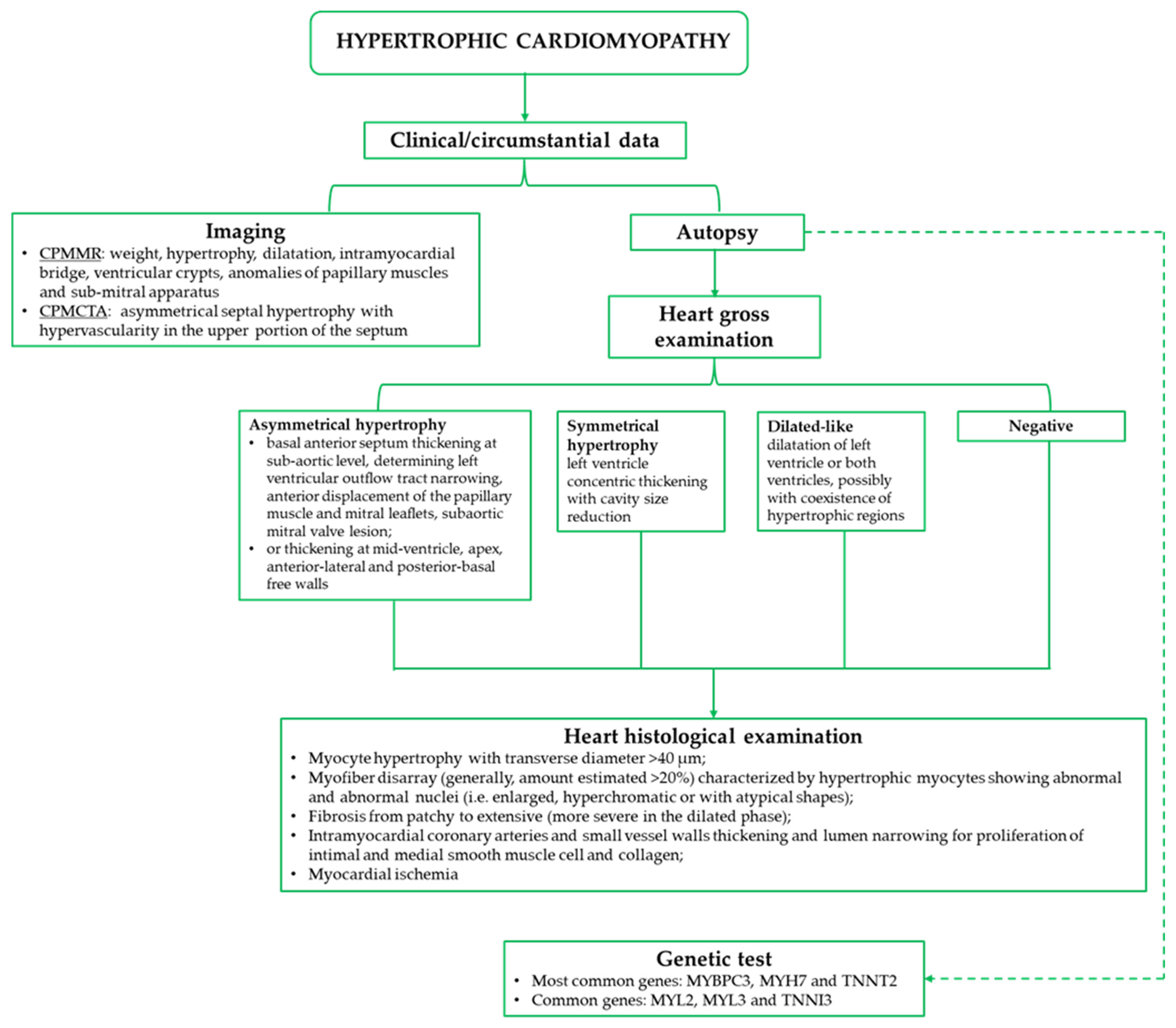
3.2. Gross Findings
3.3. Microscopic Findings
| Authors | Sample (n.) | Heart Weight | HCM Type | Thicknesses | Gross Findings | Microscopic Findings |
|---|---|---|---|---|---|---|
| Roberts et al. [18] | 37 (mean 44 years) | dnr | Asymmetric (32 cases) | dnr | Clear LV outflow obstruction associated with hypertrophy of LVW with similar degree (17 cases); reduction in both ventricular cavities (36); thickness of mitral valve leaflets (26 cases); dilatation of both atria (36 cases); endocardial scars; foci of myocardial necrosis (1 case) | Interstitial fibrosis of varying degrees in LVW and ventricular septum; varying degrees of disorientation of myocardial fibers in the ventricular septum; increased number in the ventricular septum; increased thickness of the walls of intramural coronary arteries (1 case) |
| Davies et al. [19] | 47 (13.87 years) | 180–740 g | Asymmetric (30); symmetric (17 cases) | dnr | Small LV cavity; band-like endocardial fibrous thickening in interventricular septum extending for 2 to 4 cm below the aortic valve with well-defined lower edge having the configuration of a mirror image of the mitral aortic surface; flat fibrous thickening of the aortic surface of the anterior mitral cusp; bullet-like shape of papillary muscle; RV mass varied from normal to grossly increased | Thick and abnormally branched myofibers, disorganized architecture, fibrosis, and whorl formation; endocardial superficial and dense collagenous and elastic fibrotic areas at both subaortic interventricular septum and aortic surface of anterior mitral cusp |
| Pomerance et al. [20] | 15 (>60 years) | mean 506 g | Asymmetric (5 cases); symmetric (10 cases) | dnr | Hypertrophy of the LVW and interventricular septum; band of fibrous endocardial thickening over the upper part of the interventricular septum | Areas of disorganized myofiber architecture with short broad abnormally branched fibers; fibrosis; endocardial ‘pockets’, jet lesions, and endocardial friction lesions |
| Edwards et al. [21] | 1 (13 years) | 690 g | Asymmetric | Septum 37 mm; LVW at mitral papillary muscle 26 mm; LVW at base 20 mm; RV 5 mm | Small LV cavity; hypertrophied mitral papillary muscle; endocardial fibrosis at subaortic outflow tract; mitral valve anterior leaflets firm and thickened | Abnormally oriented myocytes in interventricular septum, frequent in the LVW, infrequent in RV free wall; variable size of myofibers with bizarre triradiate and stellate forms, atypical hyperchromatic macronuclei; diffuse interstitial fibrosis in ventricles |
| Basso et al. [22] | 19 (mean 23 years) | mean 457 ± 202 g | Asymmetric (14 cases); symmetric (5 cases) | Septum mean 21.8 ± 6.7 mm | Mirror image septal subaortic plaque with anterior mitral valve leaflet thickening (6 cases); fibrotic scars within the ventricular septum (11 cases); LAD myocardial bridge (4 cases) | Ventricular septum disarray (mean percentage area 30 ± 16%); tiny interstitial myocardial fibrosis; variable degrees of medial hypertrophy–dysplasia and intimal hyperplasia of small intramural coronary arteries; multifocal, patchy signs of acute–subacute myocardial ischemia (coagulative necrosis, neutrophilic infiltrate, myocytolisis, and granulation tissue healing) in septal myocardium (14 cases) |
| Phadke et al. [23] | 14 (all ages) | mean 514 g | Asymmetric (7 cases); symmetric (7 cases) | Septum mean 1.7 cm; free wall 1.2 cm | Variable localization and degree of the asymmetric hypertrophy (dnr); mild to moderate RV hypertrophy (10 cases); mild to moderate chamber dilatation (7 cases); streaky fibrosis (4 cases); anterior mitral leaflet mirror image with leaflet thickening (3 cases) | Striking myofiber hypertrophy with nuclear enlargement and hyperchromasia (14 cases); box and cigar shaped nuclei with perinuclear clearing; myofiber disarray (>5%); obliterative small vessel disease (7 cases); myofibers waviness, smudging and vacuolation; interstitial, interfibrillar and less frequent perivascular fibrosis. |
| Ahmad et al. [24] | 15 (mean 26.6 years) | mean 440 g | Asymmetric (9 cases); symmetric (6 cases) | Septum mean 23.2 mm; LV wall 22.5 mm | Thickening of LV outflow tract (4 cases); obliterative small vessel disease with perivascular fibrosis (5 cases) | Hypertrophy of myofiber with nuclear enlargement and hyperchromasia; nuclei with a cigar or typical box shape; significant myofiber disarray in both septum and free left ventricular wall; patchy interstitial fibrosis. |
| Kundu et al. [25] | 2 (11 and 13 years) | 340 g; 450 g | Asymmetric (case 1); symmetric (case 2) | Case 1: LVW 15 mm—septum 18 mm—RV 8 mm; Case 2: Septum 21 mm—RV 6 mm | Case 1: narrowing of LV outflow tract; patchy areas of fibrosis in interventricular septum. Case 2: LV chamber reduced | Hypertrophied myocardium with myofiber disarray and a whirling pattern around areas of fibrosis in LV and interventricular septum; short myofibers, with anisonucleosis and perinuclear vacuolation in some; thickened walls of intramyocardial blood vessels; focal areas with replacement fibrosis in endocardium. |
| Williams et al. [26] | 4 (38, 38, 57, 67 years) | 890, 980, 450, 600 g | Asymmetric | Case 1: LVW 24 mm—RV 6 mm—Septum 36 mm; Case 2: LVW 20 mm—RV 8 mm—septum 33 mm; Case 3: LVW 13 mm—RV 3 mm septum 13 mm with anteroseptal region 30 mm; Case 4: LVW 11 mm—RV 2 mm—19 mm | Case 1: no other data; Case 2: heart dilated and asymmetrically hypertrophied; Case 3: no other data; Case 4: interventricular free wall asymmetric thickening, patchy subendocardial fibrosis at subaortic region of the outflow tract | Case 1: perivascular fibrosis; thickening of epicardial and intramyocardial blood vessels; septal myocyte hypertrophy with disarray. Case 2: myocyte hypertrophy, disarray, and interstitial fibrosis. Case 3: myocyte disarray. Case 4: myocyte disarray; perivascular and interstitial fibrosis. |
| Takei et al. [27] | 1 (20 years) | dnr | Asymmetric | dnr | LV hypertrophy with asymmetric hypertrophy of the interventricular septum | Marked hypertrophy of the myocytes, disorderly pattern of myocardial bundles and high degree of interstitial fibrosis in the ventricular septum and the posterior wall of the LV, focal area of myocardial fibrosis |
| Yamadori et al. [28] | 1 (60 years) | 450 g | DCM-like | AVS 9 mm; PVS 13 mm; AVW 20 mm; LVW 8 mm; PVW 10 mm | Dilatation of both ventricles; several fibrotic areas in septum and LV and RV walls; myocardial disappearance in AVW; dilatation of both atria | Massive disappearance of myofibers and scar-like fibrosis in posterior interventricular septum and LVW; small patches of fibrosis in both ventricles and papillary muscles; diffuse myocyte hypertrophy; disarray at interventricular septum, posterior wall of both ventricles, LV antero-lateral wall. |
| Horita et al. [29] | 1 (33 years) | 610 g | DCM-like | AVW 8 mm; PVW 22 mm; RV 7 mm | Hypertrophy of the posterior and interventricular septal wall; significant decrease in LV apical wall thickness; dilatation of LV cavity; significant fibrosis of LV and RV walls | Massive transmural fibrosis of the AVW; bizarre myocardial hypertrophy with disorganization in PVW and interventricular septum; intramural small artery intimal thickening with severe narrowing |
| Dettmeyer et al. [30] | 1 (8 years) | 270 g | DCM-like | LVW (basal region) 9 mm; septum 13 mm | Dilated ventricles with diffuse endocardial fibrosis; endocardial thickening of the ventricular septum below the aortic valve; slight thickness of mitral valve | Extensive fibrous scarring and microfocal myocytolysis in LV subendocardium and papillary muscles; scarring suggestive of ischemia associated with irregular arrangement of fibers and dysplastic vessels with wall increased thickness; extensive and partly bizarre hyperchromatic nuclei; extremely hypertrophic cardiomyocyte groups |
| Maron et al. [31] | 54 (mean 33 years; range 11–70 years) | mean 535 g | dnr | Septum mean 26 mm | dnr | Fifty-one cases with myocytes disorganization in more than 5% of the tissue section: areas of myocardium generally having small size in which adjacent cardiac muscle cells were aligned perpendicularly or obliquely to each other (type I-A); and relatively broad bundles of muscle cells (the cells were normally arranged) oriented at oblique or perpendicular angles to each other (type I-B) |
| Rose et al. [32] | 27 (dnr) | 551 ± 117 g | Asymmetric in 69% | Septum 23.9 ± 5.6 mm | dnr | All cases: disarray greater than 5% in ventricular septal section and a mean disarray value equal to 52% |
| Maron et al. [33] | 31 (mean 34 years; range 11–61 years) | adults 592 ± 171 g; children 270 to 530 g | Asymmetrical | Septum mean 22 mm | dnr | Septum: disorganization from 5 to 24% in 26% of the cases, 25 to 49% in 19%, and ≥50% in 42%. AVW (in 25 cases): disorganization from 5 to 24% in 29%, 25 to 49% in 6%, and was particularly extensive, involving ≥50% in 36%. PVW (in 23 cases): disorganization from 5 to 24% in 19%, 25 to 49% in 19%, and ≥50% in 16% |
3.4. Imaging
3.5. Molecular Autopsy
| Authors | Sample (n) | Gross/Microscopic Data | Gene/Nucleotide or Amino Acid Change | Mutation Type | Phen. |
|---|---|---|---|---|---|
| Loporcaro et al. [38] | 1 SUDS | - | MYH7 (R249Q) | MM | P |
| Maeda et al. [39] | 11 HCM (6) | + | Case 1: MYBPC3 (IVS3 + 41 G/C; Gln998Glu) | SM/MM | nr |
| + | Case 2: MYH7 (Arg1974Gln); MYBPC3 (IVS2 + 55 T/C); MYBPC3 (Ser25Ser) | MM/SM/SiM | nr | ||
| + | Case 3: MYBPC3 (Thr1046Met) | MM | nr | ||
| + | Case 4: MYH7 (Ile989Ile); TNNT2 (IVS15 + 61 A/G); TNNT2 (Thr284Thr) | SiM/SM/SiM | nr | ||
| + | Case 5: MYBPC3 (IVS3 + 41 G/C); MYBPC3 (Ser593fs:1) | SM/FM | nr | ||
| + | Case 6: MYBPC3 (Arg1073Pro); MYBPC3 (IVS3490 G/A) | MM/SM | nr | ||
| Allegue et al. [40] | 37 SCD of which 23 with autopsy cardiac anomalies (2) | + | Case 1: MYH7 (c.2945T>C) | nr | nr |
| + | Case 2: MYBPC3 (c.2440_2442delAAG) | nr | nr | ||
| Larsen et al. [41] | 12 HCM (1)—8 HCM/DCM (2) | + | Case 1 (HCM): MYBPC3 (c.1564G>A) | nr | B |
| + | Case 2 (HCM/DCM): MYH7 (c.1325G>A) | nr | LP | ||
| + | Case 3 (HCM/DCM): MYBPC3 (c.495G>C) | nr | B | ||
| Kane et al. [42] | 1 HCM | + | TNNI3 (Arg204His) | nr | nr |
| Hertz et al. [43] | 5 HCM (2)—52 with non-diagnostic structural findings (5 with hypertrophy or hypertrophy/fibrosis) | +HCM | Case 1 (HCM): HCN4 (c.1403C>T) *; MYBPC3 (c.649A>G; c.150C>A) | MM/MM/MM | LP |
| Case 2 (HCM): TTN (c.48163C>T) | MM | LP | |||
| Other cases: MYH7 (c.5120 T>C); DSP (c.8033G>A); KCNA5 (c.98A>T); MYH6 (c.4193G>A); AKAP9 (c.2146G>C) | MM/MM/MM/MM/MM | LP | |||
| Corbett et al. [44] | 15 HCM (4) | + | Case 1: MYBPC3 (p.Gly1093Cys) | nr | NV |
| Case 2: MYBPC3 (p.Arg668His) | nr | LP | |||
| Case 3: MYBPC3 (p.Arg502Trp) | nr | LP | |||
| Case 4: MYH7 (p.Arg1045Leu) | nr | LP | |||
| Williams et al. [26] | 4 HCM | + | Case 1: MYBPC3 (p.Gly278GlufsTer22) | FM | P |
| Case 2: MYBPC3 (p.Phe295SerfsTer5) | FM | P | |||
| Case 3: MYBPC3 (p.Gln969Ter) | NSM | P | |||
| Case 4: MYBPC3 (c.3491-2A>T) | SM | P | |||
| Gaertner-Rommel et al. [45] | 1 HCM | + | MYBPC3 (c.442G>A); FHL1 (c.267C>A) | MM/DNM | LP/P |
| Liu et al. [46] | 18 HCM (9) | + | Cases 1 to 8: MYH7 (Thr446Pro) | MM | P |
| Case 9: MYH7 (Phe468Leu) | MM | P | |||
| Hata et al. [47] | 15 HCM (8) | + | Case 1: MYBPC3 (p.T1046M) | MM | LP-P |
| Case 2: MYBPC3 (p.R470Q) | MM | LP-P | |||
| Case 3: MYH6 (p.D629N) | MM | VUS | |||
| Case 4: PRKAG2 (p.G75A) | MM | VUS | |||
| Case 5: MYBPC3 (p.T1046M); MYBPC3 (p.R1138C) | MM/MM | LP-P/VUS | |||
| Case 6: CAV3 (p.R148Q) | MM | VUS | |||
| Case 7: MYBPC3 (p.E334K) | MM | VUS | |||
| Case 8: MYH7 (p.941H) | MM | VUS | |||
| Ripoll-Vera et al. [48] | 5 HCM (4), 30 SUDS (4) | +(HCM) | Case 1 (HCM): MYH7 (p.Arg652Lys) | nr | P |
| Case 2 (HCM): LMNA (p.Arg439Cys) ^ | nr | LP | |||
| Case 3 (HCM): JUP (p.Asp723_Tyr724del) 1 | nr | VUS | |||
| Case 4 (HCM): FLNC (p.Ala688Thr) 2 | nr | VUS | |||
| Case 5 (SUDS): MYBPC3 (p.Arg726Cys) 3 | nr | LP | |||
| Case 6 (SUDS): TTN (p.Thr34393Pro) 4 | nr | VUS | |||
| Case 7 (SUDS): MYBPC3 (p.Arg891 Alafs*160) | nr | P | |||
| Case 8 (SUDS): MYPN (p.Trp7Glyfs*26) 5 | nr | VUS | |||
| Marey et al. [49] | 11 HCM (4) | + | Case 1: MYH7 (c.2105T>A) | nr | LP |
| Girolami et al. [50] | 14 SCD (6) | - | Case 1: MYBPC3 (c.2441_2443del) | nr | LP |
| Case 2: TNNT2 (c.517_519del) | nr | LP | |||
| Case 3: MYH7 (c.2890G>C) 6 | nr | VUS | |||
| Case 4: ACTN2 (c. 1823G>A) | nr | VUS | |||
| Case 5: TNNT2 (c.113C>T) 7 | nr | VUS | |||
| Case 6: TTN (c.57515_57517del) 8 | nr | VUS | |||
| Kraoua et al. [51] | 1 HCM/DCM | + | ACTN2 (c.355G>A) | nr | P |
| Fadoni et al. [52] | 16 SCD (9) | +(2 HCM) | Case 1: MYBPC3 (c.2221delG); NEXN (c.1201delA) | FM/FM | P/VUS-NV |
| Case 2: MYBPC3 (c.2221delG); DSG2 (c.370delT); NME1 (c.413delA) | FM/FM/FM | P/VUS-NV/VUS-NV | |||
| Case 3: MYBPC3 (c.2221delG); MYH6 (c.5803delA) | FM/FM | P/VUS-NV | |||
| Case 4: MYBPC3 (c.2221delG); ACTN2 (c.842delG); MYO6 (c.973delA) | FM/FM/FM | P/VUS-NV/VUS-NV | |||
| Case 5: MYBPC3 (c.2221delG); MYH7 (c.2304delG); | FM/FM | P/VUS-NV | |||
| Case 6: MYH7 (c.G2348T); NEXN (c.1201delA) | MM/FM | LP/VUS-NV | |||
| Case 7: MYLK2 (c.C808T) | MM | VUS | |||
| Case 8: ACAD9 (c.G976A) | MM | VUS | |||
| Case 9: ACTN2 (c.C1330T); KCNE2 (c.T170C) | MM/MM | VUS/VUS | |||
| Tsaturyan et al. [53] | 1 SCD | - | TPM1 (c.656A>T) | nr | DN-LP |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zipes, D.P.; Wellens, H.J. Sudden cardiac death. Circulation 1998, 98, 2334–2351. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Aguilera, B.; Banner, J.; Cohle, S.; d’Amati, G.; de Gouveia, R.H.; di Gioia, C.; Fabre, A.; Gallagher, P.J.; Leone, O.; et al. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Arch. 2017, 471, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Chugh, S.S.; Jui, J.; Gunson, K.; Stecker, E.C.; John, B.T.; Thompson, B.; Ilias, N.; Vickers, C.; Dogra, V.; Daya, M.; et al. Current burden of sudden cardiac death: Multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J. Am. Coll. Cardiol. 2004, 44, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- de Vreede-Swagemakers, J.J.; Gorgels, A.P.; Dubois-Arbouw, W.I.; van Ree, J.W.; Daemen, M.J.; Houben, L.G.; Wellens, H.J. Out-ofhospital cardiac arrest in the 1990’s: A population-based study in the Maastricht area on incidence, characteristics and survival. J. Am. Coll. Cardiol. 1997, 3, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, C.; Stiell, I.G.; Canadian Cardiovascular Outcomes Research Team. Cardiac arrest care and emergency medical services in Canada. Can. J. Cardiol. 2004, 20, 1081–1090. [Google Scholar] [PubMed]
- Murakoshi, N.; Aonuma, K. Epidemiology of arrhythmias and sudden cardiac death in Asia. Circ. J. 2013, 77, 2419–2431. [Google Scholar] [CrossRef]
- Mondello, C.; Cardia, L.; Ventura-Spagnolo, E. Immunohistochemical detection of early myocardial infarction: A systematic review. Int. J. Leg. Med. 2017, 131, 411–421. [Google Scholar] [CrossRef]
- Semsarian, C.; Ingles, J.; Wilde, A.A. Sudden cardiac death in the young: The molecular autopsy and a practical approach to surviving relatives. Eur. Heart J. 2015, 36, 1290–1296. [Google Scholar] [CrossRef]
- Maron, B.J. Sudden death in young athletes. N. Engl. J. Med. 2003, 349, 1064–1075. [Google Scholar] [CrossRef]
- Richardson, P.; McKenna, W.; Bristow, M.; Maisch, B.; Mautner, B.; O’Connell, J.; Olsen, E.; Thiene, G.; Goodwin, J. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation 1996, 93, 841–842. [Google Scholar]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Gardin, J.M.; Flack, J.M.; Gidding, S.S.; Kurosaki, T.T.; Bild, D.E. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation 1995, 92, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Roberts, W.C. Cardiomyopathies in the first two decades of life. Cardiovasc. Clin. 1981, 11, 35–78. [Google Scholar] [PubMed]
- Charron, P.; Carrier, L.; Dubourg, O.; Tesson, F.; Desnos, M.; Richard, P.; Bonne, G.; Guicheney, P.; Hainque, B.; Bouhour, J.B.; et al. Penetrance of familial hypertrophic cardiomyopathy. Genet. Couns. 1997, 8, 107–114. [Google Scholar] [PubMed]
- Cross, B.J.; Estes, N.A., 3rd; Link, M.S. Sudden cardiac death in young athletes and nonathletes. Curr. Opin. Crit. Care 2011, 17, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.E. The pathology of hypertrophic cardiomyopathy. Histopathology 2004, 44, 412–427. [Google Scholar] [CrossRef]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC guidelines for the Management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar]
- Roberts, W.C.; Ferrans, U.J.; Buja, L.M. Pathologic aspects of the idiopathic cardiomyopathies. Adv. Cardiol. 1974, 13, 349–367. [Google Scholar]
- Davies, M.J.; Pomerance, A.; Teare, R.D. Pathological features of hypertrophic obstructive cardiomyopathy. J. Clin. Pathol. 1974, 27, 529–535. [Google Scholar] [CrossRef]
- Pomerance, A.; Davies, M.J. Pathological features of hypertrophic obstructive cardiomyopathy (HOCM) in the elderly. Br. Heart J. 1975, 37, 305–312. [Google Scholar] [CrossRef][Green Version]
- Edwards, W.D.; Zakheim, R.; Mattioli, L. Asymmetric septal hypertrophy in childhood. Unreliability of histologic criteria for differentiation of obstructive and nonobstructive forms. Hum. Pathol. 1977, 8, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Thiene, G.; Corrado, D.; Buja, G.; Melacini, P.; Nava, A. Hypertrophic cardiomyopathy and sudden death in the young: Pathologic evidence of myocardial ischemia. Hum. Pathol. 2000, 31, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Phadke, R.S.; Vaideeswar, P.; Mittal, B.; Deshpande, J. Hypertrophic cardiomyopathy: An autopsy analysis of 14 cases. J. Postgrad. Med. 2001, 47, 165–170. [Google Scholar] [PubMed]
- Ahmad, M.; Afzal, S.; Malik, I.A.; Mushtaq, S.; Mubarik, A. An autopsy study of hypertrophic cardiomyopathy. J. Pak. Med. Assoc. 2003, 53, 459–462. [Google Scholar] [PubMed]
- Kundu, R.; Punia, R.S.; Handa, U.; Singh, A.; Mohan, H. Sudden cardiac death in 2 young siblings. Am. J. Forensic Med. Pathol. 2014, 35, 246–248. [Google Scholar] [CrossRef]
- Williams, N.; Marion, R.; McDonald, T.V.; Wang, D.; Zhou, B.; Eng, L.S.; Um, S.Y.; Lin, Y.; Ruiter, K.; Rojas, L.; et al. Phenotypic variations in carriers of predicted protein-truncating genetic variants in MYBPC3: An autopsy-based case series. Cardiovasc. Pathol. 2018, 37, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Takei, H.; Sano, R.; Takahashi, Y.; Takahashi, K.; Kominato, Y.; Tokue, H.; Shimada, T.; Awata, S.; Hirasawa, S.; Ohta, N. Usefulness of coronary postmortem computed tomography angiography to detect lesions in the coronary artery and myocardium in cases of sudden death. Leg. Med. 2018, 30, 46–51. [Google Scholar] [CrossRef]
- Yamadori, I.; Murakami, M. An autopsy case of hypertrophic cardiomyopathy showing clinical features of dilated cardiomyopathy. Acta Med. 1985, 39, 481–488. [Google Scholar]
- Horita, Y.; Shimizu, M.; Sugihara, N.; Suematsu, T.; Shibayama, S.; Itoh, H.; Takeda, R.; Terada, T. An autopsy case of hypertrophic cardiomyopathy showing dilated cardiomyopathy-like features by serial ventriculography. Jpn. J. Med. 1990, 29, 448–453. [Google Scholar] [CrossRef][Green Version]
- Dettmeyer, R.; Schmidt, P.; Kandolf, R.; Madea, B. Evolution of dilated cardiomyopathy (DCM) from idiopathic hypertrophic cardiomyopathy (IHCM) vs. inflammatory dilated cardiomyopathy (DCMi): A rare case of sudden death in an 8-year-old boy. Pathol. Res. Pract. 2004, 200, 411–415; discussion 417–421. [Google Scholar] [CrossRef]
- Maron, B.J.; Roberts, W.C. Quantitative analysis of cardiac muscle cell disorganization in the ventricular septum of patients with hypertrophic cardiomyopathy. Circulation 1979, 59, 689–706. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.G. Evaluation of pathological criteria for diagnosis of hypertrophic cardiomyopathy. Histopathology 1984, 8, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Wolfson, J.K.; Roberts, W.C. Relation between extent of cardiac muscle cell disorganization and left ventricular wall thickness in hypertrophic cardiomyopathy. Am. J. Cardiol. 1992, 70, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Jackowski, C.; Schweitzer, W.; Thali, M.; Yen, K.; Aghayev, E.; Sonnenschein, M.; Vock, P.; Dirnhofer, R. Virtopsy: Postmortem Imaging of the Human Heart in situ Using MSCT and MRI. Forensic Sci. Int. 2005, 149, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Guidi, B.; Aquaro, G.D.; Gesi, M.; Emdin, M.; Di Paolo, M. Postmortem Cardiac Magnetic Resonance in Sudden Cardiac Death. Heart Fail. Rev. 2018, 23, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; Guidi, B.; Biondi, F.; Chiti, E.; Santurro, A.; Scopetti, M.; Turillazzi, E.; Di Paolo, M. Post-Mortem Cardiac Magnetic Resonance for the Diagnosis of Hypertrophic Cardiomyopathy. Diagnostics 2020, 10, 981. [Google Scholar] [CrossRef]
- Puranik, R.; Gray, B.; Lackey, H.; Yeates, L.; Parker, G.; Duflou, J.; Semsarian, C. Comparison of conventional autopsy and magnetic resonance imaging in determining the cause of sudden death in the young. J. Cardiovasc. Magn. Reson. 2014, 16, 44. [Google Scholar] [CrossRef]
- Loporcaro, C.G.; Tester, D.J.; Maleszewski, J.J.; Kruisselbrink, T.; Ackerman, M.J. Confirmation of cause and manner of death via a comprehensive cardiac autopsy including whole exome next-generation sequencing. Arch. Pathol. Lab. Med. 2014, 138, 1083–1089. [Google Scholar] [CrossRef]
- Maeda, K.; Shigeki, N.; Chikako, M.; Masamune, K.; Wataru, I.; Bunta, W.; Maiko, H.; Chizuko, S.; Masataka, F.; Katsuyoshi, K. Analysis of three major sarcomeric genes (MYH7, TNNT2, MYBPC3) in cardiomyopathy. Forensic Sci. Int. Genet. Suppl. Ser. 2009, 2, 499–500. [Google Scholar] [CrossRef]
- Allegue, C.; Gil, R.; Blanco-Verea, A.; Santori, M.; Rodríguez-Calvo, M.; Concheiro, L.; Carracedo, A.; Brion, M. Prevalence of HCM and long QT syndrome mutations in young sudden cardiac death-related cases. Int. J. Leg. Med. 2011, 125, 565–572. [Google Scholar] [CrossRef]
- Larsen, M.K.; Nissen, P.H.; Berge, K.E.; Leren, T.P.; Kristensen, I.B.; Jensen, H.K.; Banner, J. Molecular autopsy in young sudden cardiac death victims with suspected cardiomyopathy. Forensic Sci. Int. 2012, 219, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Kane, D.A.; Triedman, J. Post-mortem genetic testing in a family with long-QT syndrome and hypertrophic cardiomyopathy. Cardiovasc. Pathol. 2014, 23, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Hertz, C.L.; Christiansen, S.L.; Ferrero-Miliani, L.; Dahl, M.; Weeke, P.E.; LuCamp; Ottesen, G.L.; Frank-Hansen, R.; Bundgaard, H.; Morling, N. Next-generation sequencing of 100 candidate genes in young victims of suspected sudden cardiac death with structural abnormalities of the heart. Int. J. Leg. Med. 2016, 130, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Cann, F.; Corbett, M.; O’Sullivan, D.; Tennant, S.; Hailey, H.; Grieve, J.H.; Broadhurst, P.; Rankin, R.; Dean, J.C. Phenotype-driven molecular autopsy for sudden cardiac death. Clin. Genet. 2017, 91, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Gaertner-Rommel, A.; Tiesmeier, J.; Jakob, T.; Strickmann, B.; Veit, G.; Bachmann-Mennenga, B.; Paluszkiewicz, L.; Klingel, K.; Schulz, U.; Laser, K.T.; et al. Molecular autopsy and family screening in a young case of sudden cardiac death reveals an unusually severe case of FHL1 related hypertrophic cardiomyopathy. Mol. Genet. Genom. Med. 2019, 7, e841. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Ji, F.F.; Wei, L.; Zuo, A.J.; Gao, Y.X.; Qi, L.; Jin, B.; Wang, J.G.; Zhao, P. Screening of MYH7 gene mutation sites in hypertrophic cardiomyopathy and its significance. Chin. Med. J. 2019, 132, 2835–2841. [Google Scholar] [CrossRef]
- Hata, Y.; Ichimata, S.; Yamaguchi, Y.; Hirono, K.; Oku, Y.; Ichida, F.; Nishida, N. Clinicopathological and Genetic Profiles of Cases with Myocytes Disarray-Investigation for Establishing the Autopsy Diagnostic Criteria for Hypertrophic Cardiomyopathy. J. Clin. Med. 2019, 8, 463. [Google Scholar] [CrossRef]
- Ripoll-Vera, T.; Pérez Luengo, C.; Borondo Alcázar, J.C.; García Ruiz, A.B.; Sánchez Del Valle, N.; Barceló Martín, B.; Poncela García, J.L.; Gutiérrez Buitrago, G.; Dasi Martínez, C.; Canós Villena, J.C.; et al. Sudden cardiac death in persons aged 50 years or younger: Diagnostic yield of a regional molecular autopsy program using massive sequencing. Rev. Esp. Cardiol. Engl. Ed. 2021, 74, 402–413. [Google Scholar] [CrossRef]
- Marey, I.; Fressart, V.; Rambaud, C.; Fornes, P.; Martin, L.; Grotto, S.; Alembik, Y.; Gorka, H.; Millat, G.; Gandjbakhch, E.; et al. Clinical impact of post-mortem genetic testing in cardiac death and cardiomyopathy. Open Med. 2020, 15, 435–446. [Google Scholar] [CrossRef]
- Girolami, F.; Spinelli, V.; Maurizi, N.; Focardi, M.; Nesi, G.; Maio, V.; Grifoni, R.; Albora, G.; Bertaccini, B.; Targetti, M.; et al. Genetic characterization of juvenile sudden cardiac arrest and death in Tuscany: The ToRSADE registry. Front. Cardiovasc. Med. 2022, 9, 1080608. [Google Scholar] [CrossRef]
- Kraoua, L.; Jaouadi, H.; Allouche, M.; Achour, A.; Kaouther, H.; Ahmed, H.B.; Chaker, L.; Maazoul, F.; Ouarda, F.; Zaffran, S.; et al. Molecular autopsy and clinical family screening in a case of sudden cardiac death reveals ACTN2 mutation related to hypertrophic/dilated cardiomyopathy and a novel LZTR1 variant associated with Noonan syndrome. Mol. Genet. Genom. Med. 2022, 10, e1954. [Google Scholar] [CrossRef] [PubMed]
- Fadoni, J.; Santos, A.; Cainé, L. Post-mortem genetic investigation in sudden cardiac death victims: Complete exon sequencing of forty genes using next-generation sequencing. Int. J. Leg. Med. 2022, 136, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Tsaturyan, A.K.; Zaklyazminskaya, E.V.; Polyak, M.E.; Kopylova, G.V.; Shchepkin, D.V.; Kochurova, A.M.; Gonchar, A.D.; Kleymenov, S.Y.; Koubasova, N.A.; Bershitsky, S.Y.; et al. De Novo Asp219Val Mutation in Cardiac Tropomyosin Associated with Hypertrophic Cardiomyopathy. Int. J. Mol. Sci. 2022, 24, 18. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J. Hypertrophic cardiomyopathy: A systematic review. JAMA 2002, 287, 1308–1320. [Google Scholar] [CrossRef] [PubMed]
- Kocovski, L.; Fernandes, J. Sudden Cardiac Death. A Modern Pathology Approach to Hypertrophic Cardiomyopathy. Arch. Pathol. Lab. Med. 2015, 139, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Teare, D. Asymmetrical hypertrophy of the heart in young adults. Br. Heart J. 1958, 20, 1–8. [Google Scholar] [CrossRef]
- Wigle, E.D. Cardiomyopathy: The diagnosis of hypertrophic cardiomyopathy. Heart 2001, 86, 709–714. [Google Scholar] [CrossRef]
- Krasnow, N. Subaortic septal bulge simulates hypertrophic cardiomyopathy by angulation of the septum with age, independent of focal hypertrophy. An echocardiographic study. J. Am. Soc. Echocardiogr. 1997, 10, 545–555. [Google Scholar] [CrossRef]
- Waller, B.F. The old-age heart: Normal aging changes which can produce or mimic cardiac disease. Clin. Cardiol. 1988, 11, 513–517. [Google Scholar] [CrossRef]
- Jain, C.C.; Newman, D.B.; Geske, J.B. Mitral Valve Disease in Hypertrophic Cardiomyopathy: Evaluation and Management. Curr. Cardiol. Rep. 2019, 21, 136. [Google Scholar] [CrossRef]
- Hong, J.H.; Schaff, H.V.; Nishimura, R.A.; Abel, M.D.; Dearani, J.A.; Li, Z.; Ommen, S.R. Mitral Regurgitation in Patients With Hypertrophic Obstructive Cardiomyopathy: Implications for Concomitant Valve Procedures. J. Am. Coll. Cardiol. 2016, 68, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Sherrid, M.V.; Balaram, S.; Kim, B.; Axel, L.; Swistel, D.G. The Mitral Valve in Obstructive Hypertrophic Cardiomyopathy: A Test in Context. J. Am. Coll. Cardiol. 2016, 67, 1846–1858. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.S.; Spears, D.A.; Care, M. Evaluation of cardiac hypertrophy in the setting of sudden cardiac death. Forensic Sci. Res. 2019, 4, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, S.; Kolm, P.; Ho, C.Y.; Kwong, R.Y.; Desai, M.Y.; Dolman, S.F.; Appelbaum, E.; Desvigne-Nickens, P.; DiMarco, J.P.; Friedrich, M.G.; et al. Distinct Subgroups in Hypertrophic Cardiomyopathy in the NHLBI HCM Registry. J. Am. Coll. Cardiol. 2019, 74, 2333–2345. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Z.J.; Chan, R.H.; Montazeri, M.; Hoss, S.; Adler, A.; Nguyen, E.T.; Rakowski, H. Left Ventricular Apical Aneurysms in Hypertrophic Cardiomyopathy: Equivalent Detection by Magnetic Resonance Imaging and Contrast Echocardiography. J. Am. Soc. Echocardiogr. 2021, 34, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Z.J.; Montazeri, M.; Bataiosu, R.; Hoss, S.; Adler, A.; Nguyen, E.T.; Rakowski, H.; Chan, R.H. Clinical Characteristics and Prognostic Importance of Left Ventricular Apical Aneurysms in Hypertrophic Cardiomyopathy. JACC Cardiovasc. Imaging 2022, 15, 1696–1711. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Song, Y.Y.; Chen, X.Y.; Wang, J.X.; Li, L.; Yin, G.; Zheng, Y.C.; Wei, M.D.; Lu, M.J.; Zhao, S.H. Apical hypertrophic cardiomyopathy with left ventricular apical aneurysm: Prevalence, cardiac magnetic resonance characteristics, and prognosis. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1341–1350. [Google Scholar] [CrossRef]
- Davies, M.J.; McKenna, W.J. Hypertrophic cardiomyopathy-pathology and pathogenesis. Histopathology 1995, 26, 493–500. [Google Scholar] [CrossRef]
- Hina, K.; Kusachi, S.; Iwasaki, K.; Nogami, K.; Moritani, H.; Kita, T.; Taniguchi, G.; Tsuji, T. Progression of left ventricular enlargement in patients with hypertrophic cardiomyopathy: Incidence and prognostic value. Clin. Cardiol. 1993, 16, 403–407. [Google Scholar] [CrossRef]
- Yutani, C.; Imakita, M.; Ishibashi-Ueda, H.; Hatanaka, K.; Nagata, S.; Sakakibara, H.; Nimura, Y. Three autopsy cases of progression to left ventricular dilatation in patients with hypertrophic cardiomyopathy. Am. Heart J. 1985, 109, 545–553. [Google Scholar] [CrossRef]
- Davies, M.J. The current status of myocardial disarray in hypertrophic cardiomyopathy. Br. Heart J. 1984, 51, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fujiwara, H.; Onodera, T.; Wu, D.J.; Hamashima, Y.; Kawai, C. Quantitative analysis of myocardial fibrosis in normals, hypertensive hearts, and hypertrophic cardiomyopathy. Br. Heart J. 1986, 55, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Unverferth, D.V.; Baker, P.B.; Pearce, L.I.; Lautman, J.; Roberts, W.C. Regional myocyte hypertrophy and increased interstitial myocardial fibrosis in hypertrophic cardiomyopathy. Am. J. Cardiol. 1987, 59, 932–936. [Google Scholar] [CrossRef]
- Maron, B.J.; Wolfson, J.K.; Epstein, S.E.; Roberts, W.C. Structural basis for myocardial ischemia in hypertrophic cardiomyopathy. In Nonatherosclerotic Ischemic Heart Disease; Virmani, R., Forman, M.B., Eds.; Raven Press: New York, NY, USA, 1989; pp. 305–324. [Google Scholar]
- Maron, B.J.; Wolfson, J.K.; Epstein, S.E.; Roberts, W.C. Intramural (‘small vessel’) coronary artery disease in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 1986, 8, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Yetman, A.T.; McCrindle, B.W.; MacDonald, C.; Freedom, R.M.; Gow, R. Myocardial bridging in children with hypertrophic cardiomyopathy—A risk factor for sudden death. N. Engl. J. Med. 1998, 339, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Epstein, S.E.; Roberts, W.C. Hypertrophic cardiomyopathy and transmural myocardial infarction without significant atherosclerosis of the extramural coronary arteries. Am. J. Cardiol. 1979, 43, 1086–1102. [Google Scholar] [CrossRef] [PubMed]
- Sepp, R.; Severs, N.J.; Gourdie, R.G. Altered patterns of cardiac intercellular junction distribution in hypertrophic cardiomyopathy. Heart 1996, 76, 412–417. [Google Scholar] [CrossRef]
- Varnava, A.M.; Elliott, P.M.; Baboonian, C.; Davison, F.; Davies, M.J.; McKenna, W.J. Hypertrophic cardiomyopathy. Histological features of sudden death in cardiac troponin T disease. Circulation 2001, 104, 1380–1384. [Google Scholar] [CrossRef]
- Kitazume, H.; Kramer, J.R.; Krauthamer, D.; El Tobgi, S.; Proudfit, W.I.; Sones, F.M. Myocardial bridges in obstructive hypertrophic cardiomyopathy. Am. Heart J. 1983, 106, 131–135. [Google Scholar] [CrossRef]
- Lee, A.H.S.; Gray, P.B.; Gallagher, P.J. Sudden death and regional left ventricular fibrosis with fibromuscular dysplasia of small intramyocardial coronary arteries. Heart 2000, 83, 101–102. [Google Scholar] [CrossRef][Green Version]
- Forzese, E.; Pitrone, C.; Cianci, V.; Sapienza, D.; Ieni, A.; Tornese, L.; Cianci, A.; Gualniera, P.; Asmundo, A.; Mondello, C. An Insight into Kounis Syndrome: Bridging Clinical Knowledge with Forensic Perspectives. Life 2024, 14, 91. [Google Scholar] [CrossRef]
- Tesic, M.; Beleslin, B.; Giga, V.; Jovanovic, I.; Marinkovic, J.; Trifunovic, D.; Petrovic, O.; Dobric, M.; Aleksandric, S.; Juricic, S.; et al. Prognostic Value of Transthoracic Doppler Echocardiography Coronary Flow Velocity Reserve in Patients with Asymmetric Hypertrophic Cardiomyopathy. J. Am. Heart Assoc. 2021, 10, e021936. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.S.; Olivotto, I.; Maron, B.J.; Prasad, S.K.; Cecchi, F.; Udelson, J.E.; Camici, P.G. The case for myocardial ischemia in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2009, 54, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Melacini, P.; Basso, C.; Angelini, A.; Calore, C.; Bobbo, F.; Tokajuk, B.; Bellini, N.; Smaniotto, G.; Zucchetto, M.; Iliceto, S.; et al. Clinicopathological profiles of progressive heart failure in hypertrophic cardiomyopathy. Eur. Heart J. 2010, 31, 2111–2123. [Google Scholar] [CrossRef] [PubMed]
- Stassi, C.; Mondello, C.; Baldino, G.; Cardia, L.; Gualniera, P.; Calapai, F.; Sapienza, D.; Asmundo, A.; Ventura Spagnolo, E. State of the Art on the Role of Postmortem Computed Tomography Angiography and Magnetic Resonance Imaging in the Diagnosis of Cardiac Causes of Death: A Narrative Review. Tomography 2022, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Bonzon, J.; Schön, C.A.; Schwendener, N.; Zech, W.D.; Kara, L.; Persson, A.; Jackowski, C. Rigor mortis at the myocardium investigated by post-mortem magnetic resonance imaging. Forensic Sci. Int. 2015, 257, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Richard, P.; Charron, P.; Carrier, L.; Ledeuil, C.; Cheav, T.; Pichereau, C.; Benaiche, A.; Isnard, R.; Dubourg, O.; Burban, M.; et al. Hypertrophic cardiomyopathy: Distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 2003, 107, 2227–2232. [Google Scholar] [CrossRef]
- Sabater-Molina, M.; Pérez-Sánchez, I.; Hernández Del Rincón, J.P.; Gimeno, J.R. Genetics of hypertrophic cardiomyopathy: A review of current state. Clin Genet. 2018, 93, 3–14. [Google Scholar] [CrossRef]
- Basso, C.; Michaud, K.; d’Amati, G.; Banner, J.; Lucena, J.; Cunningham, K.; Leone, O.; Vink, A.; van der Wal, A.C.; Sheppard, M.N. Association for European Cardiovascular Pathology. Cardiac hypertrophy at autopsy. Virchows Arch. 2021, 479, 79–94. [Google Scholar] [CrossRef]
- Hickey, E.J.; Mehta, R.; Elmi, M.; Asoh, K.; McCrindle, B.W.; Williams, W.G.; Manlhiot, C.; Benson, L. Survival implications: Hypertrophic cardiomyopathy in Noonan syndrome. Congenit. Heart Dis. 2011, 6, 41–47. [Google Scholar] [CrossRef]
- Daoud, E.; Zwick, D. Noonan Syndrome Case Report: PTPN11 and Other Potential Genetic Factors Contributing to Lethal Hypertrophic Right Ventricular Cardiomyopathy. Pediatr. Dev. Pathol. 2019, 22, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Weidemann, F.; Linhart, A.; Monserrat, L.; Strotmann, J. Cardiac challenges in patients with Fabry disease. Int. J. Cardiol. 2010, 141, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, L.; Wang, J.; Li, X.; Hu, S.; Fu, Y.; Wang, X.; Hao, S.; Hu, C. Differentiating between cardiac amyloidosis and hypertrophic cardiomyopathy on non-contrast cine-magnetic resonance images using machine learning-based radiomics. Front. Cardiovasc. Med. 2022, 9, 1001269. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef] [PubMed]
- ClinGen Clinical Genome Resource. Available online: https://search.clinicalgenome.org/kb/gene-validity (accessed on 30 November 2023).
- Sheppard, M.N.; van der Wal, A.C.; Banner, J.; d’Amati, G.; De Gaspari, M.; De Gouveia, R.; Di Gioia, C.; Giordano, C.; Larsen, M.K.; Lynch, M.J.; et al. Genetically determined cardiomyopathies at autopsy: The pivotal role of the pathologist in establishing the diagnosis and guiding family screening. Virchows Arch. 2023, 482, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Tower-Rader, A.; Desai, M.Y. Phenotype-Genotype Correlation in Hypertrophic Cardiomyopathy: Less Signal, More Noise? Circ. Cardiovasc. Imaging 2017, 10, e006066. [Google Scholar] [CrossRef] [PubMed]
- Niimura, H.; Bachinski, L.L.; Sangwatanaroj, S.; Watkins, H.; Chudley, A.E.; McKenna, W.; Kristinsson, A.; Roberts, R.; Sole, M.; Maron, B.J.; et al. Mutations in the gene for cardiac myosin-binding protein C and late-onset familial hypertrophic cardiomyopathy. N. Engl. J. Med. 1998, 338, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Bonne, G.; Carrier, L.; Richard, P.; Hainque, B.; Schwarz, K. Familial hypertrophic cardiomyopathy: From mutations to functional defects. Circ. Res. 1998, 83, 580–593. [Google Scholar] [CrossRef]
- Marian, A.J. Pathogenesis of diverse clinical and pathological phenotypes in hypertrophic cardiomyopathy. Lancet 2000, 355, 58–60. [Google Scholar] [CrossRef]
- Redwood, C.S.; Moolman-Smook, J.C.; Watkins, H. Properties of mutant contractile proteins that cause hypertrophic cardiomyopathy. Cardiovasc. Res. 1999, 44, 20–36. [Google Scholar] [CrossRef]
- Coppini, R.; Santini, L.; Olivotto, I.; Ackerman, M.J.; Cerbai, E. Abnormalities in sodium current and calcium homoeostasis as drivers of arrhythmogenesis in hypertrophic cardiomyopathy. Cardiovasc. Res. 2020, 116, 1585–1599. [Google Scholar] [CrossRef] [PubMed]
- Velicki, L.; Jakovljevic, D.G.; Preveden, A.; Golubovic, M.; Bjelobrk, M.; Ilic, A.; Stojsic, S.; Barlocco, F.; Tafelmeier, M.; Okwose, N.; et al. Genetic determinants of clinical phenotype in hypertrophic cardiomyopathy. BMC Cardiovasc. Disord. 2020, 20, 516. [Google Scholar] [CrossRef] [PubMed]
- Zahka, K.; Kalidas, K.; Simpson, M.A.; Cross, H.; Keller, B.B.; Galambos, C.; Gurtz, K.; Patton, M.A.; Crosby, A.H. Homozygous mutation of MYBPC3 associated with severe infantile hypertrophic cardiomyopathy at high frequency among the Amish. Heart 2008, 94, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.R.; Rahman, M.S.; Elliott, P.M. A systematic review and meta-analysis of genotype-phenotype associations in patients with hypertrophic cardiomyopathy caused by sarcomeric protein mutations. Heart 2013, 99, 1800–1811. [Google Scholar] [CrossRef]
- Weissler-Snir, A.; Hindieh, W.; Gruner, C.; Fourey, D.; Appelbaum, E.; Rowin, E.; Care, M.; Lesser, J.R.; Haas, T.S.; Udelson, J.E.; et al. Lack of phenotypic differences by cardiovascular magnetic resonance imaging in MYH7 (β-myosin heavy chain)—Versus MYBPC3 (myosin-binding protein C)-related hypertrophic cardiomyopathy. Circ. Cardiovasc. Imaging 2017, 10, e005311. [Google Scholar] [CrossRef]
- Coppini, R.; Ho, C.Y.; Ashley, E.; Day, S.; Ferrantini, C.; Girolami, F.; Tomberli, B.; Bardi, S.; Torricelli, F.; Cecchi, F.; et al. Clinical phenotype and outcome of hypertrophic cardiomyopathy associated with thin-filament gene mutations. J. Am. Coll. Cardiol. 2014, 64, 2589–2600. [Google Scholar] [CrossRef]
- Ko, Y.L.; Chen, J.J.; Tang, T.K.; Cheng, J.J.; Lin, S.Y.; Liou, Y.C.; Kuan, P.; Wu, C.W.; Lien, W.P.; Liew, C.C. Malignant familial hypertrophic cardiomyopathy in a family with a 453Arg–>Cys mutation in the beta-myosin heavy chain gene: Coexistence of sudden death and end-stage heart failure. Hum. Genet. 1996, 97, 585–590. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cianci, V.; Forzese, E.; Sapienza, D.; Cardia, L.; Cianci, A.; Germanà, A.; Tornese, L.; Ieni, A.; Gualniera, P.; Asmundo, A.; et al. Morphological and Genetic Aspects for Post-Mortem Diagnosis of Hypertrophic Cardiomyopathy: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 1275. https://doi.org/10.3390/ijms25021275
Cianci V, Forzese E, Sapienza D, Cardia L, Cianci A, Germanà A, Tornese L, Ieni A, Gualniera P, Asmundo A, et al. Morphological and Genetic Aspects for Post-Mortem Diagnosis of Hypertrophic Cardiomyopathy: A Systematic Review. International Journal of Molecular Sciences. 2024; 25(2):1275. https://doi.org/10.3390/ijms25021275
Chicago/Turabian StyleCianci, Vincenzo, Elena Forzese, Daniela Sapienza, Luigi Cardia, Alessio Cianci, Antonino Germanà, Lorenzo Tornese, Antonio Ieni, Patrizia Gualniera, Alessio Asmundo, and et al. 2024. "Morphological and Genetic Aspects for Post-Mortem Diagnosis of Hypertrophic Cardiomyopathy: A Systematic Review" International Journal of Molecular Sciences 25, no. 2: 1275. https://doi.org/10.3390/ijms25021275
APA StyleCianci, V., Forzese, E., Sapienza, D., Cardia, L., Cianci, A., Germanà, A., Tornese, L., Ieni, A., Gualniera, P., Asmundo, A., & Mondello, C. (2024). Morphological and Genetic Aspects for Post-Mortem Diagnosis of Hypertrophic Cardiomyopathy: A Systematic Review. International Journal of Molecular Sciences, 25(2), 1275. https://doi.org/10.3390/ijms25021275








