Enhanced Stability and Improved Oral Absorption of Enzalutamide with Self-Nanoemulsifying Drug Delivery System
Abstract
1. Introduction
2. Results
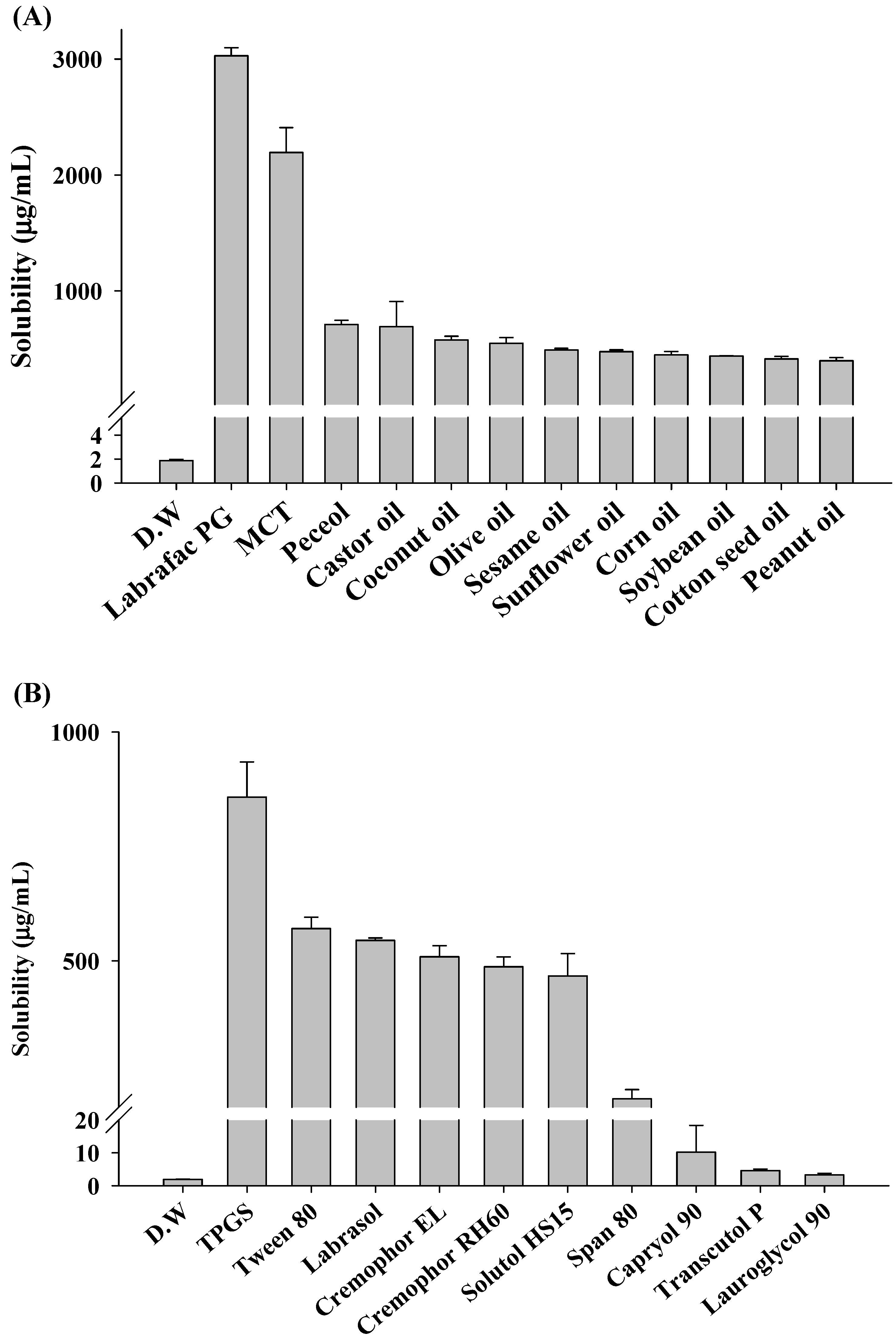
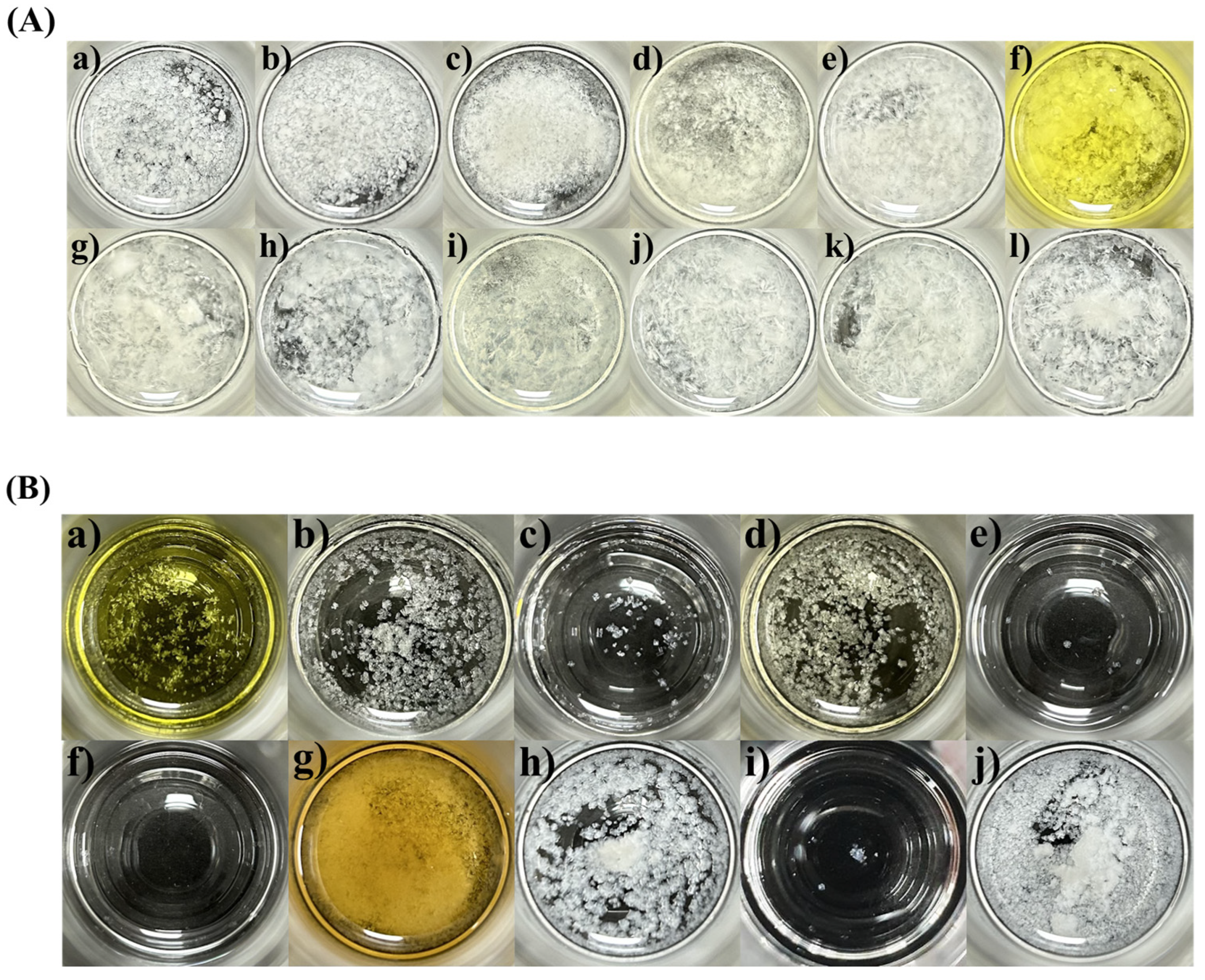
2.1. Solubility and Crystallization Test
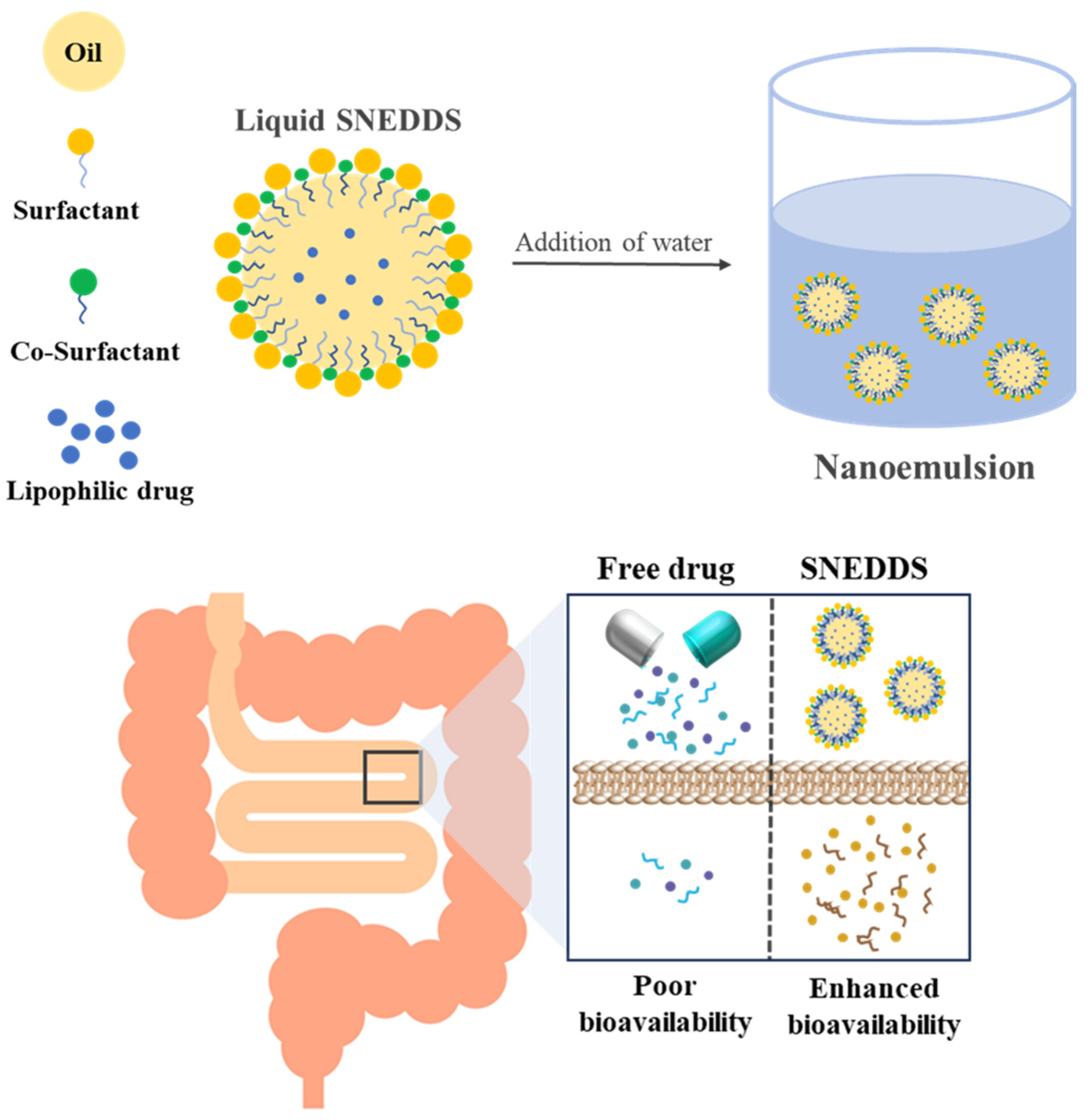
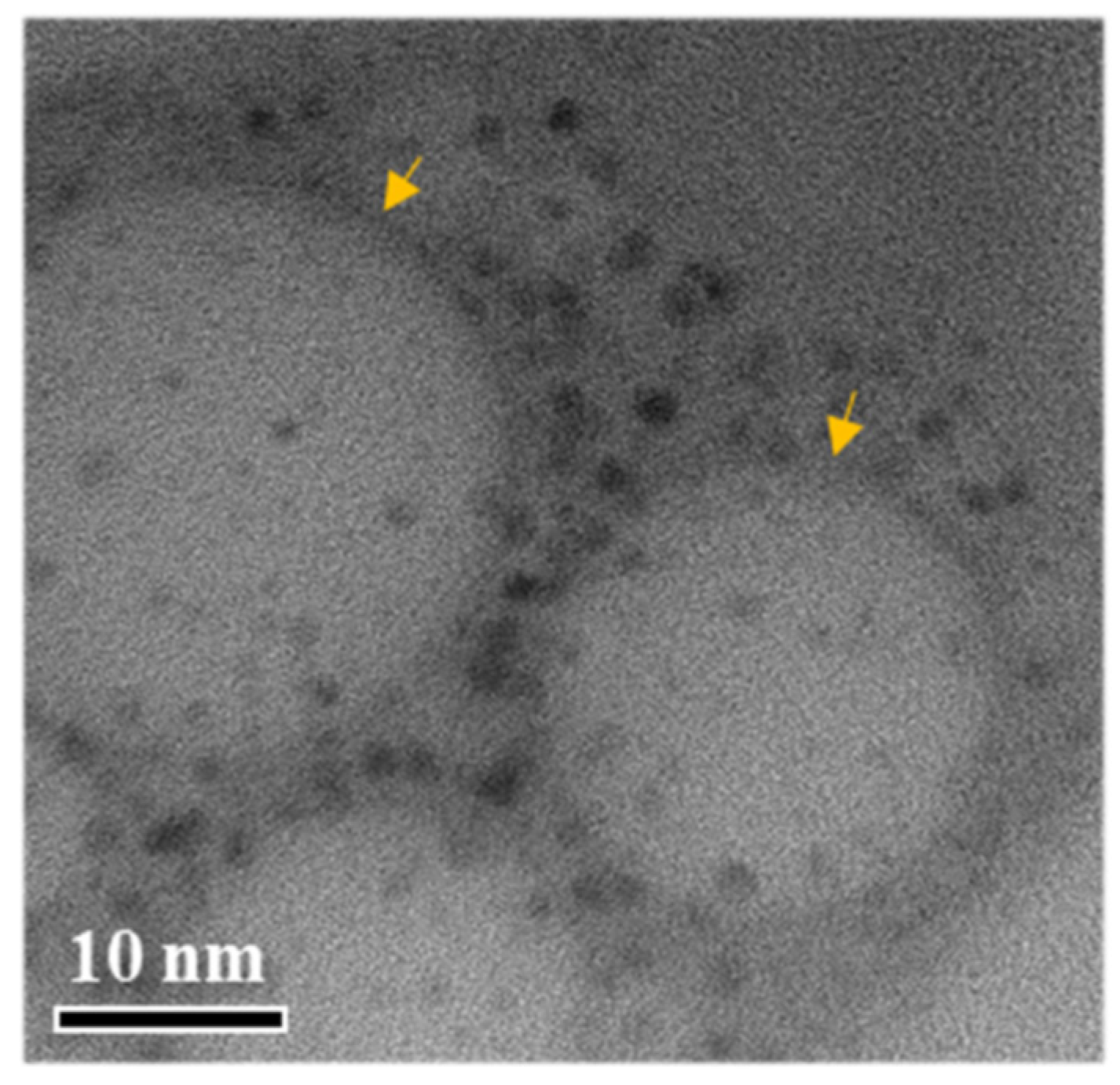
2.2. Characterization of SNEDDS
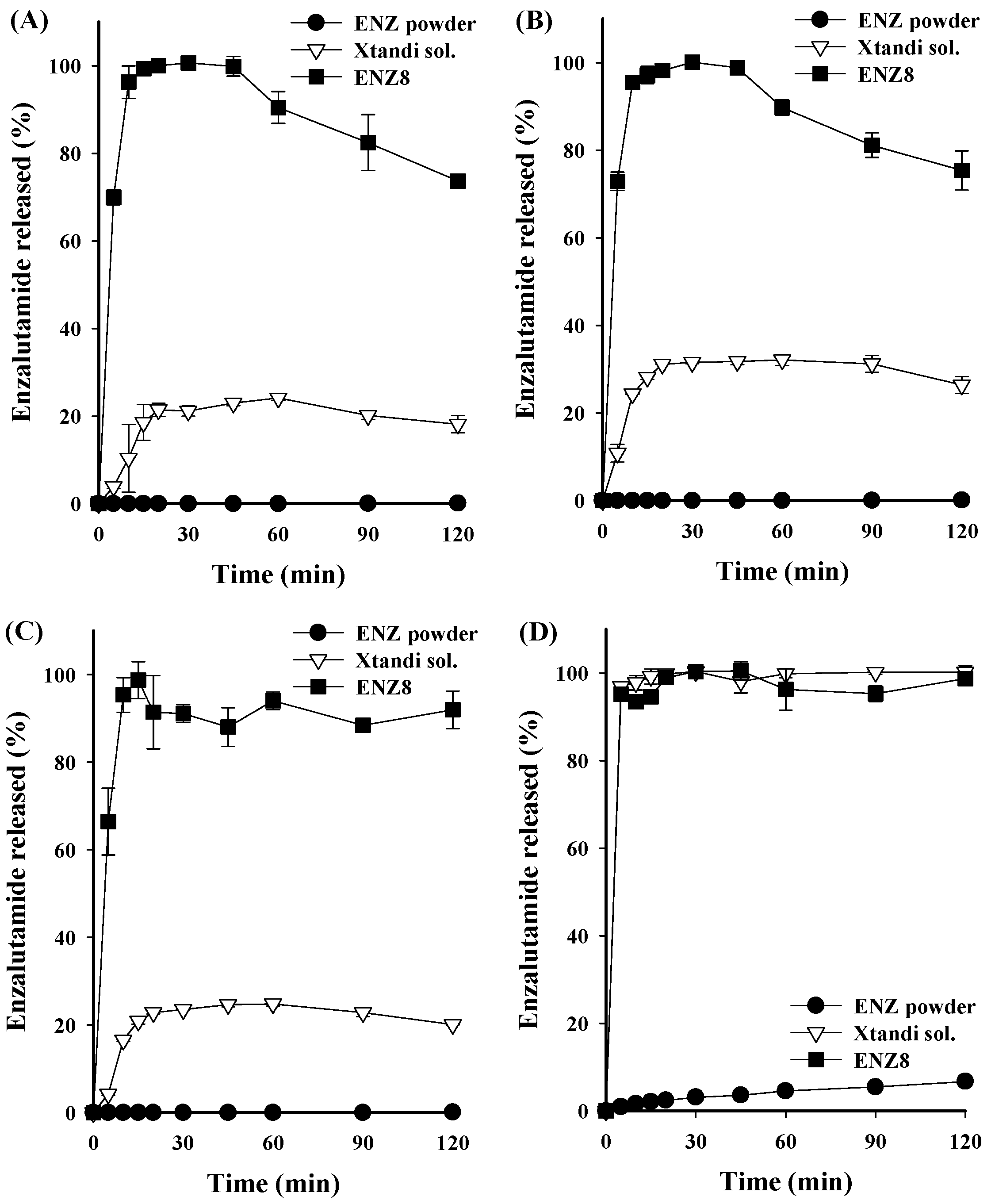
2.3. Dissolution Tests
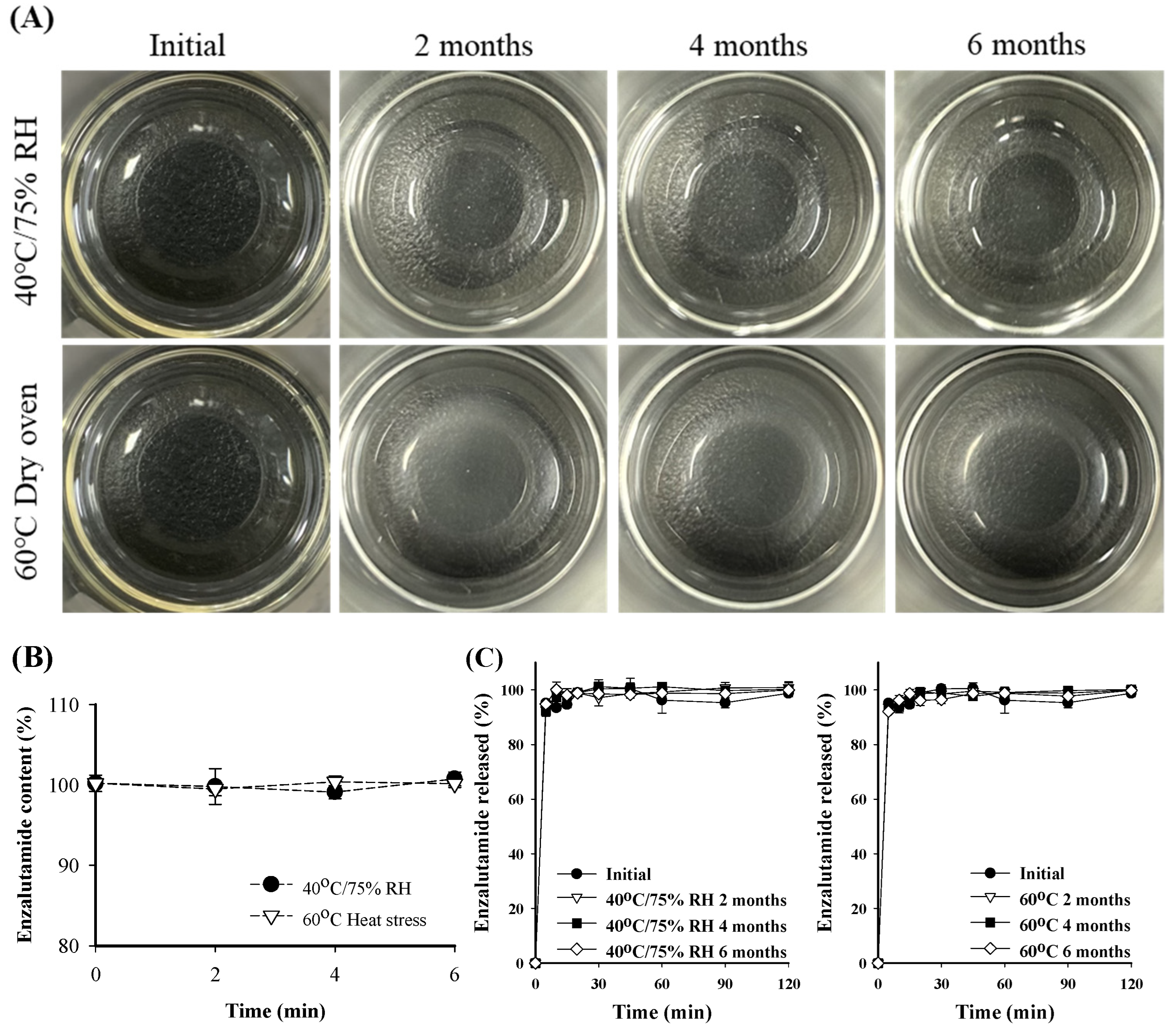
2.4. Stability Test
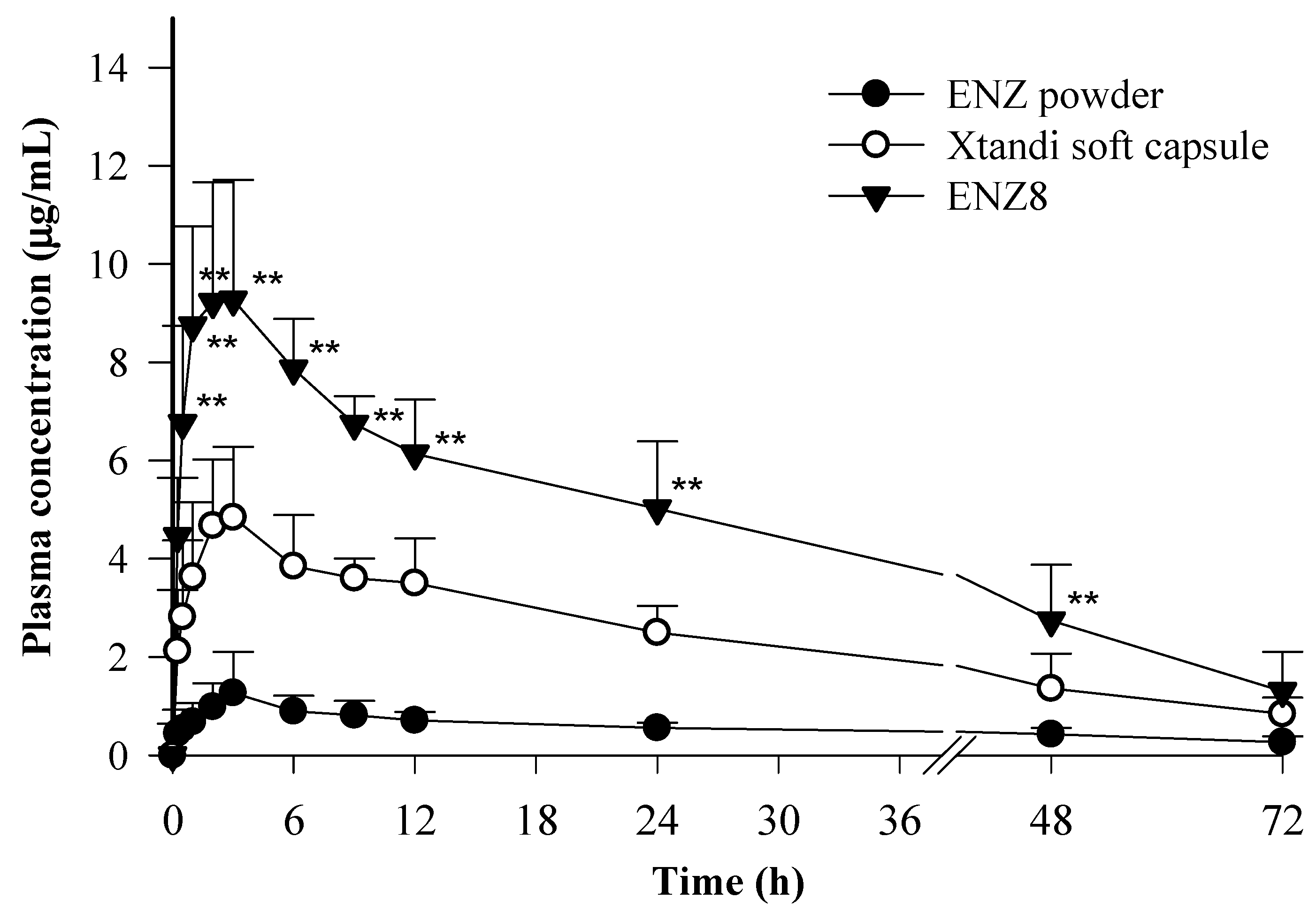
2.5. In Vivo Pharmacokinetic Study
3. Materials and Methods
3.1. Materials
3.2. HPLC Conditions
3.3. Solubility Test
3.4. Crystallization Test
3.5. Preparation of SNEDDS
3.6. Particle Size Analysis
3.7. Transmission Electron Microscopy (TEM) Analysis
3.8. Dissolution Test
3.9. Stability Test
3.10. In Vivo Pharmacokinetic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009, 324, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Schalken, J.; Fitzpatrick, J.M. Enzalutamide: Targeting the androgen signalling pathway in metastatic castration-resistant prostate cancer. BJU Int. 2016, 117, 215–225. [Google Scholar] [CrossRef]
- Watson, P.A.; Chen, Y.F.; Balbas, M.D.; Wongvipat, J.; Socci, N.D.; Viale, A.; Kim, K.; Sawyers, C.L. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc. Natl. Acad. Sci. USA 2010, 107, 16759–16765. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Miller, J.M.; Amidon, G.L. Prediction of solubility and permeability class membership: Provisional BCS classification of the world’s top oral drugs. AAPS J. 2009, 11, 740–746. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef]
- Dahan, A.; Miller, J.M. The solubility–permeability interplay and its implications in formulation design and development for poorly soluble drugs. AAPS J. 2012, 14, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Wilson, V.; Lou, X.; Osterling, D.J.; Stolarik, D.F.; Jenkins, G.; Gao, W.; Zhang, G.G.; Taylor, L.S. Relationship between amorphous solid dispersion in vivo absorption and in vitro dissolution: Phase behavior during dissolution, speciation, and membrane mass transport. J. Control. Release 2018, 292, 172–182. [Google Scholar] [CrossRef]
- Alam, M.A.; Ali, R.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Solid dispersions: A strategy for poorly aqueous soluble drugs and technology updates. Expert Opin. Drug Deliv. 2012, 9, 1419–1440. [Google Scholar] [CrossRef]
- Shukla, V.; Scholar, R. Techniques for solubility enhancement of poorly soluble drugs: An overview. J. Med. Pharm. Allied Sci. 2012, 1, 18–38. [Google Scholar]
- Fahr, A.; Liu, X. Drug delivery strategies for poorly water-soluble drugs. Expert Opin. Drug Deliv. 2007, 4, 403–416. [Google Scholar] [CrossRef]
- Mu, H.; Holm, R.; Müllertz, A. Lipid-based formulations for oral administration of poorly water-soluble drugs. Int. J. Pharm. 2013, 453, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Feeney, O.M.; Crum, M.F.; McEvoy, C.L.; Trevaskis, N.L.; Williams, H.D.; Pouton, C.W.; Charman, W.N.; Bergström, C.A.; Porter, C.J. 50 years of oral lipid-based formulations: Provenance, progress and future perspectives. Adv. Drug Deliv. Rev. 2016, 101, 167–194. [Google Scholar] [CrossRef] [PubMed]
- Hauss, D.J. Oral lipid-based formulations. Adv. Drug Deliv. Rev. 2007, 59, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Buya, A.B.; Beloqui, A.; Memvanga, P.B.; Préat, V. Self-nano-emulsifying drug-delivery systems: From the development to the current applications and challenges in oral drug delivery. Pharmaceutics 2020, 12, 1194. [Google Scholar] [CrossRef]
- Rani, S.; Rana, R.; Saraogi, G.K.; Kumar, V.; Gupta, U. Self-emulsifying oral lipid drug delivery systems: Advances and challenges. AAPS Pharmscitech 2019, 20, 129. [Google Scholar] [CrossRef]
- Mehanna, M.M.; Mneimneh, A.T. Formulation and applications of lipid-based nanovehicles: Spotlight on self-emulsifying systems. Adv. Pharm. Bull. 2021, 11, 56. [Google Scholar] [CrossRef]
- Weerapol, Y.; Limmatvapirat, S.; Nunthanid, J.; Sriamornsak, P. Self-nanoemulsifying drug delivery system of nifedipine: Impact of hydrophilic–lipophilic balance and molecular structure of mixed surfactants. AAPS Pharmscitech 2014, 15, 456–464. [Google Scholar] [CrossRef]
- Patil, S.C.; Tagalpallewar, A.A.; Kokare, C.R. Natural anti-proliferative agent loaded self-microemulsifying nanoparticles for potential therapy in oral squamous carcinoma. J. Pharm. Investig. 2019, 49, 527–541. [Google Scholar] [CrossRef]
- Wilson, V.R.; Lou, X.; Osterling, D.J.; Stolarik, D.F.; Jenkins, G.J.; Nichols, B.L.; Dong, Y.; Edgar, K.J.; Zhang, G.G.; Taylor, L.S. Amorphous solid dispersions of enzalutamide and novel polysaccharide derivatives: Investigation of relationships between polymer structure and performance. Sci. Rep. 2020, 10, 18535. [Google Scholar] [CrossRef]
- Ilevbare, G.A.; Liu, H.; Pereira, J.; Edgar, K.J.; Taylor, L.S. Influence of additives on the properties of nanodroplets formed in highly supersaturated aqueous solutions of ritonavir. Mol. Pharm. 2013, 10, 3392–3403. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A formulation approach for poorly water-soluble compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Vazifehasl, Z.; Salatin, S.; Adibkia, K.; Javadzadeh, Y. Nanosizing of drugs: Effect on dissolution rate. Res. Pharm. Sci. 2015, 10, 95. [Google Scholar]
- Pouton, C.W. Formulation of self-emulsifying drug delivery systems. Adv. Drug Deliv. Rev. 1997, 25, 47–58. [Google Scholar] [CrossRef]
- Cole, E.T.; Cadé, D.; Benameur, H. Challenges and opportunities in the encapsulation of liquid and semi-solid formulations into capsules for oral administration. Adv. Drug Deliv. Rev. 2008, 60, 747–756. [Google Scholar] [CrossRef]
- Puszkiel, A.; Plé, A.; Huillard, O.; Noé, G.; Thibault, C.; Oudard, S.; Goldwasser, F.; Vidal, M.; Alexandre, J.; Blanchet, B. A simple HPLC-UV method for quantification of enzalutamide and its active metabolite N-desmethyl enzalutamide in patients with metastatic castration-resistant prostate cancer. J. Chromatogr. B 2017, 1058, 102–107. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, K.S. Development of a novel ticagrelor solid dispersion-loaded tablet with enhanced solubility. Yakhak Hoeji 2020, 64, 21–27. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, K.S. Development of Solid Self-nanoemulsifying Drug Delivery Systems of Ticagrelor Using Porous Carriers. J. Life Sci. 2021, 31, 502–510. [Google Scholar]
- Choi, S.A.; Park, E.J.; Lee, J.H.; Min, K.A.; Kim, S.T.; Jang, D.-J.; Maeng, H.-J.; Jin, S.G.; Cho, K.H. Preparation and Characterization of Pazopanib Hydrochloride-Loaded Four-Component Self-Nanoemulsifying Drug Delivery Systems Preconcentrate for Enhanced Solubility and Dissolution. Pharmaceutics 2022, 14, 1875. [Google Scholar] [CrossRef] [PubMed]
- Bang, K.-H.; Kim, K.S. Development of trans-cinnamaldehyde self-microemulsifying drug delivery system (SMEDDS) with superior stability. J. Korea Acad.-Ind. Coop. Soc. 2019, 20, 555–562. [Google Scholar]
- Kim, T.-H.; Jeong, J.-W.; Song, J.-H.; Lee, K.-R.; Ahn, S.; Ahn, S.-H.; Kim, S.; Koo, T.-S. Pharmacokinetics of enzalutamide, an anti-prostate cancer drug, in rats. Arch. Pharmacal Res. 2015, 38, 2076–2082. [Google Scholar] [CrossRef]
- Bibi, M.; ud Din, F.; Anwar, Y.; Alkenani, N.A.; Zari, A.T.; Mukhtiar, M.; Zeid, I.M.A.; Althubaiti, E.H.; Nazish, H.; Zeb, A. Cilostazol-loaded solid lipid nanoparticles: Bioavailability and safety evaluation in an animal model. J. Drug Deliv. Sci. Technol. 2022, 74, 103581. [Google Scholar] [CrossRef]
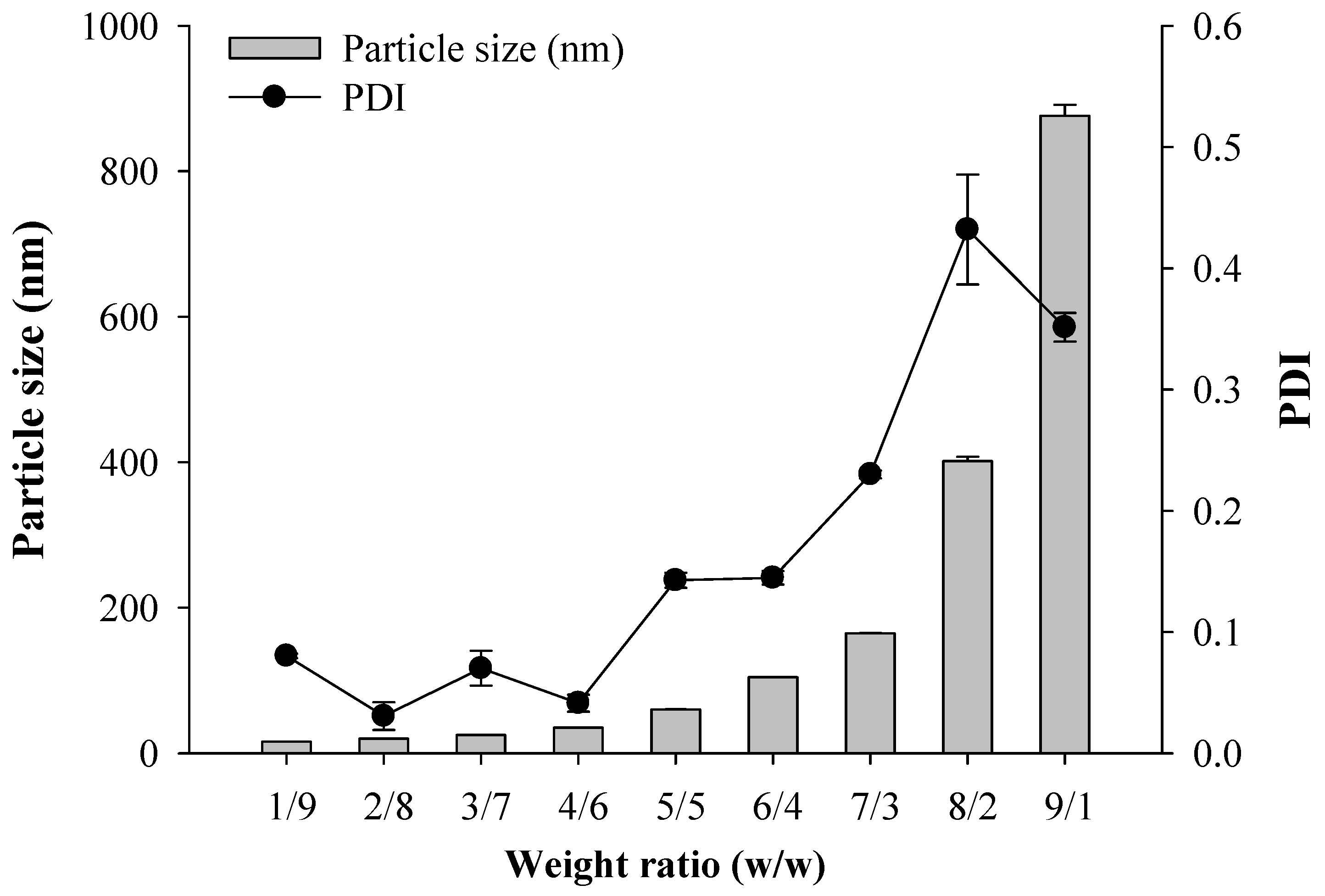
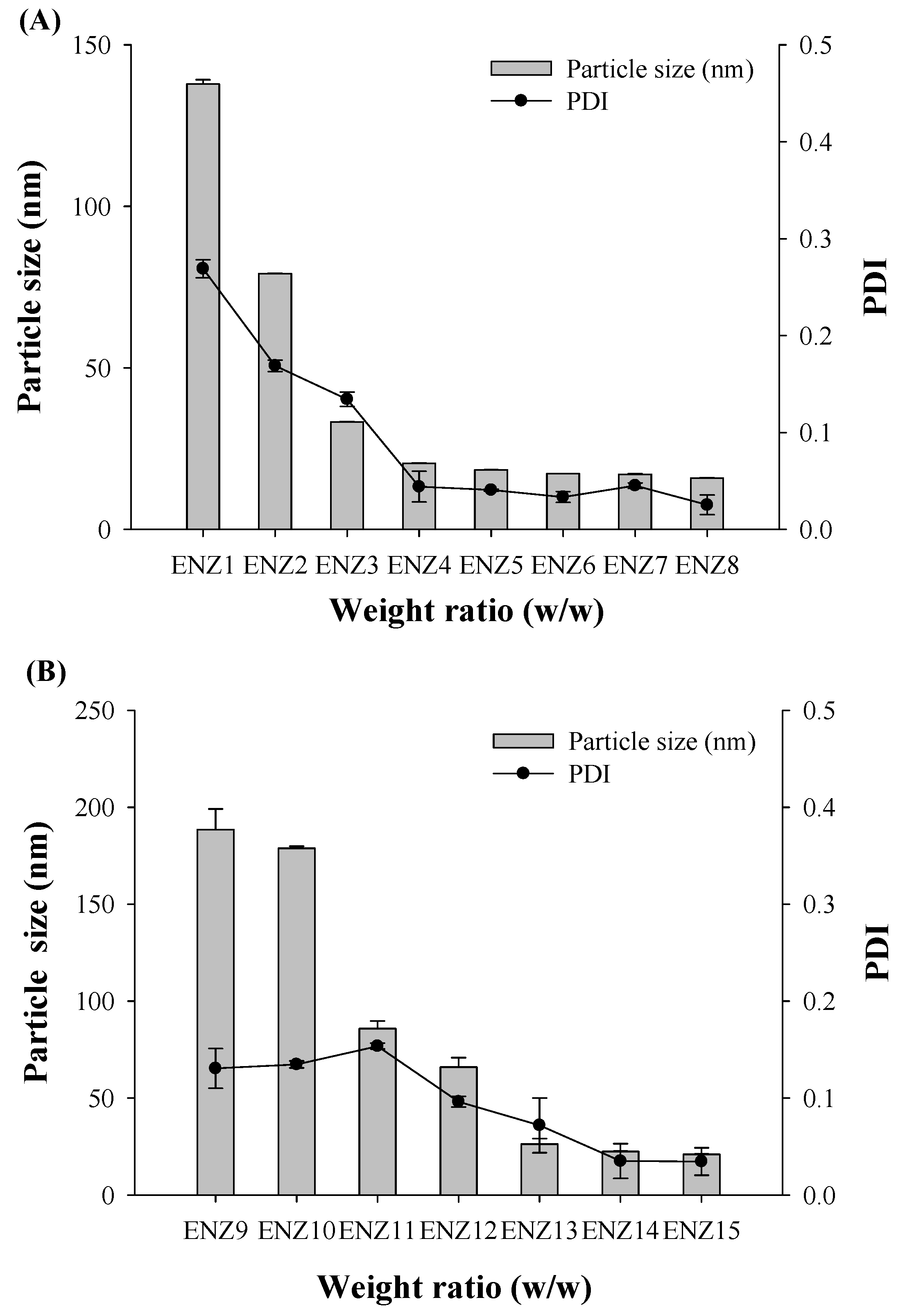
| Formulation No. | Vehicle Composition (Weight Ratio %) | Particle Size (nm) | PDI | ||
|---|---|---|---|---|---|
| Labrafac PG | Solutol HS15 | Transcutol P | |||
| ENZ1 | 10 | 10 | 80 | 137.87 ± 1.30 | 0.27 ± 0.01 |
| ENZ2 | 10 | 20 | 70 | 79.22 ± 0.15 | 0.17 ± 0.01 |
| ENZ3 | 10 | 30 | 60 | 33.29 ± 0.11 | 0.13 ± 0.01 |
| ENZ4 | 10 | 40 | 50 | 20.51 ± 0.09 | 0.04 ± 0.02 |
| ENZ5 | 10 | 50 | 40 | 18.45 ± 0.05 | 0.04 ± 0.00 |
| ENZ6 | 10 | 60 | 30 | 17.30 ± 0.00 | 0.03 ± 0.01 |
| ENZ7 | 10 | 70 | 20 | 17.10 ± 0.13 | 0.05 ± 0.00 |
| ENZ8 | 10 | 80 | 10 | 15.92 ± 0.13 | 0.03 ± 0.01 |
| ENZ9 | 20 | 10 | 70 | 188.50 ± 10.66 | 0.13 ± 0.02 |
| ENZ10 | 20 | 20 | 60 | 178.87 ± 1.01 | 0.13 ± 0.00 |
| ENZ11 | 20 | 30 | 50 | 85.87 ± 3.92 | 0.15 ± 0.00 |
| ENZ12 | 20 | 40 | 40 | 65.95 ± 4.89 | 0.10 ± 0.01 |
| ENZ13 | 20 | 50 | 30 | 26.25 ± 2.87 | 0.07 ± 0.03 |
| ENZ14 | 20 | 60 | 20 | 22.47 ± 0.13 | 0.04 ± 0.02 |
| ENZ15 | 20 | 70 | 10 | 20.99 ±0.12 | 0.03 ± 0.01 |
| Time | Stability Assessment | |||
|---|---|---|---|---|
| Particle Size (nm) | PDI | Dissolution Rate (%) | Drug Content (%) | |
| Initial | 15.92 ± 0.13 | 0.03 ± 0.01 | 98.77 ± 4.24 | 101.2 ± 1.0 |
| 40 °C/75% RH 2 months | 17.68 ± 0.10 | 0.18 ± 0.01 | 97.46 ± 3.06 | 100.9 ± 3.3 |
| 40 °C/75% RH 4 months | 17.93 ± 0.62 | 0.19 ± 0.03 | 97.76 ± 1.59 | 101.1 ± 0.8 |
| 40 °C/75% RH 6 months | 19.05 ± 4.75 | 0.14 ± 0.07 | 98.06 ± 0.74 | 100.8 ± 0.9 |
| 60 °C Heat stress 2 months | 16.15 ± 0.34 | 0.03 ± 0.01 | 98.00 ± 1.41 | 102.8 ± 0.8 |
| 60 °C Heat stress 4 months | 16.12 ± 0.16 | 0.07 ± 0.04 | 97.73 ± 0.53 | 100.4 ± 0.7 |
| 60 °C Heat stress 6 months | 18.66 ± 1.23 | 0.05 ± 0.01 | 98.67 ± 1.51 | 100.2 ± 0.5 |
| PK Parameters | ENZ | Xtandi® Soft Capsule | ENZ8 (SNEDDS) |
|---|---|---|---|
| AUC0–72 (μg∙h/mL) | 37.5 ± 6.4 | 151.4 ± 36.5 | 291.5 ± 43.4 |
| Cmax (μg/mL) | 1.4 ± 0.8 | 5.1 ± 1.2 | 9.5 ± 2.3 |
| Tmax (h) | 3.8 ± 1.7 | 3.5 ± 2.8 | 2.1 ± 1.1 |
| Kel | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| T1/2 | 47.3 ± 25.7 | 30.2 ± 8.5 | 27.7 ± 12.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-M.; Lee, J.-G.; Yun, T.-H.; Cho, J.-H.; Kim, K.-S. Enhanced Stability and Improved Oral Absorption of Enzalutamide with Self-Nanoemulsifying Drug Delivery System. Int. J. Mol. Sci. 2024, 25, 1197. https://doi.org/10.3390/ijms25021197
Lee S-M, Lee J-G, Yun T-H, Cho J-H, Kim K-S. Enhanced Stability and Improved Oral Absorption of Enzalutamide with Self-Nanoemulsifying Drug Delivery System. International Journal of Molecular Sciences. 2024; 25(2):1197. https://doi.org/10.3390/ijms25021197
Chicago/Turabian StyleLee, Su-Min, Jeong-Gyun Lee, Tae-Han Yun, Jung-Hyun Cho, and Kyeong-Soo Kim. 2024. "Enhanced Stability and Improved Oral Absorption of Enzalutamide with Self-Nanoemulsifying Drug Delivery System" International Journal of Molecular Sciences 25, no. 2: 1197. https://doi.org/10.3390/ijms25021197
APA StyleLee, S.-M., Lee, J.-G., Yun, T.-H., Cho, J.-H., & Kim, K.-S. (2024). Enhanced Stability and Improved Oral Absorption of Enzalutamide with Self-Nanoemulsifying Drug Delivery System. International Journal of Molecular Sciences, 25(2), 1197. https://doi.org/10.3390/ijms25021197






