Abstract
With the explosion research on the gut microbiome in the recent years, much insight has been accumulated in comprehending the crosstalk between the gut microbiota community and host health. Acute pancreatitis (AP) is one of the gastrointestinal diseases associated with significant morbidity and subsequent mortality. Studies have elucidated that gut microbiota are engaged in the pathological process of AP. Herein, we summarize the major roles of the gut microbiome in the development of AP. We then portray the association between dysbiosis of the gut microbiota and the severity of AP. Finally, we illustrate the promises and challenges that arise when seeking to incorporate the microbiome in acute pancreatitis treatment.
1. Introduction
Acute pancreatitis (AP) is a type of inflammatory response in which pancreatic enzymes are activated in the pancreas due to multiple etiologies, causing self-digestion, edema, hemorrhage and even necrosis in the pancreatic tissue [1,2]. AP occurs due to various causative factors, such as alcohol, infection, obstruction and intestinal microecological factors. The intestines are connected to the outside world and need to fight against the invasion of outside bacteria and other pathogens, and at the same time, the intestines contain many colonizing bacteria. The immune system monitors and responds to the intestinal microbial consortium with both tolerance and intolerance. The pancreas maintains close communication with the digestive tract through the pancreatic ducts. Intestinal dysfunction is a common complication of severe acute pancreatitis (SAP) and an important factor contributing to the development of the disease and even inducing multi-organ dysfunction [3,4]. Dysbiosis of the intestinal flora may play an important role in the pathogenesis of AP and affect the prognosis, including structural disorders of the intestinal flora and bacterial translocation [5]. It may also affect host metabolism, increase the production of toxic metabolites and affect the treatment of AP. Intestinal dysfunction includes intestinal mucosal barrier damage and intestinal dysmotility. In recent years, there has been increasing evidence that the intestinal flora plays an important role in the progression of AP and that the balance of intestinal microecology can reduce bacterial overgrowth and the occurrence of intestinal-derived infections by regulating the immune system and metabolic pathways and forming a biological barrier in the intestinal mucosa. Moreover, the resumption of feeding in AP patients remains controversy. Some doctors believe AP should be managed with aggressive hydration with intravenous fluids and fasting, while other doctors believe oral feeding should be recommenced in mild pancreatitis once pain and nausea and vomiting have resolved. This controversy is focused on the relationship between gut microbes and AP. The doctors insisting on fasting believe the occurrence of AP is closely correlated with the disruption of commensal microbes in the gut and colonization of pathogens. The doctors advocating for early oral feeding believe the recovery of intestine function will aid the reconstruction of gut microbiota and thus promote the recovery of health. Thus, understanding the complexity and molecular aspects of the link between gut microbes and health will help lay the groundwork for new therapies that have been developed [6].
2. Dysbiosis of Intestinal Flora in Acute Pancreatitis
The occurrence of AP is inextricably linked to intestinal dysbiosis [6]. There are more than 1500 species of bacteria in the human intestinal tract, and the intestinal flora is characterized by bacteria of varying dynamics, number, variety and complexity. Intestinal microbiota include beneficial bacteria, intermediate or conditionally pathogenic bacteria and harmful bacteria. The interdependence and confrontation among these three types of bacteria maintain the stability of the intestinal microenvironment. During the development and progression of AP, abnormal secretion of trypsin and destruction of the pancreatic structure lead to abnormal pancreatic secretion, which in turn leads to an imbalance of homeostasis in the body and changes in the intestinal flora. It has been shown that intestinal flora can regulate intestinal mucosal barrier function by affecting intestinal mucosal epithelial cell renewal, intestinal permeability, release of intestinal antimicrobial peptides and the intestinal mucus layer. Zhu et al. found that the rate of bacterial detection was proportional to the severity of the patients’ disease [7]. It was shown that Escherichia coli, Enterococcus, Enterobacter, Immunobacterium, Shigella fowleri and Bacillus coagulans were the main intestinal pathogenic bacteria detected in peripheral blood. Escherichia coli, Enterococcus and Enterobacteriaceae are documented as the main pathogens of secondary intestinal infections caused by AP [8]. It was reported that in AP, an imbalance exists in the flora ratio [9,10]. Intestinal aerobic bacteria showed a significant increase in their proportion, represented by Escherichia coli, Enterococcus, Enterobacter and Streptococcus, while anaerobic bacteria showed inhibition in growth, represented by Bifidobacterium, Bacteroidetes and Prevotella [6]. The increase in pathogenic bacteria could disrupt the intestinal barrier and increase the intestinal permeability and bacterial translocation. Increased intestinal permeability leads to the occurrence of pathogen-associated molecular patterns (PAMPs) in the blood circulation, activating the innate immune response. The trigger of inflammation further promotes the development of AP. These studies suggest that the disturbance of the intestinal flora is closely associated with AP.
The disturbance of the intestinal flora is associated with the disruption of the intestinal mucosal barrier. The disruption of the intestinal barrier contributes to the development of AP. A normal intestinal mucosal barrier consists of mechanical, chemical, immune and biological barriers. Studies have shown that the intestinal microbiota can regulate the intestinal mucosal barrier function by affecting intestinal epithelial cell regeneration, intestinal permeability, intestinal antimicrobial peptide release and intestinal mucosal layers [11,12,13,14]. There are many reasons for the disruption of the intestinal mucosal barrier. Fasting, gastrointestinal decompression and other therapeutic measures commonly used in the treatment of AP can cause intestinal flora disruption [15,16]. Additionally, microcirculatory disorders caused by reduced blood volume can lead to intestinal ischemia and hypoxia, thus weakening intestinal dynamics [17]. All the above can affect the normal excretion of harmful substances and thus leads to dysbiosis of the intestinal flora. The simultaneous use of a large amount of fluids during treatment can lead to intestinal reperfusion, resulting in intestinal wall cell dysfunction, increased intestinal ischemia–reperfusion injury, reduced intestinal barrier function and disruption of the intestinal microecological balance [18]. During the treatment of pancreatitis, broad-spectrum antibiotics are often used to control the infection, resulting in the suppression of normal intestinal flora [19]. In addition to these aforementioned factors affecting the intestinal mucosa, the role played by intestinal dysbiosis should not be underestimated. Intestinal commensal microbes themselves form a direct intestinal barrier by competing for space and nutrition, resisting colonization by pathogens [20]. Moreover, commensal microbes also secrete short-chain fatty acids, which modulate gut inflammation. When the intestinal mucosal barrier is disrupted, even health-promoting bacteria can weaken host health and promote pancreatitis. Due to the lack of inhibition of the normal flora, fungi and drug-resistant pathogens in the intestine are more likely to multiply [21]. The imbalance of flora species and ratios disturbs the environmental microecological balance of the intestine [22]. All of the above therapeutic measures lead to further dysbiosis and translocation of intestinal ecology, the increased release of endotoxins and increased inflammatory response in patients with AP. These studies suggest that the disturbance of the intestinal flora is closely correlated with AP.
3. Effect of Intestinal Flora on Acute Pancreatitis
An increase in pathogenic bacteria and a decrease in beneficial bacteria have been observed in AP patients after gut microbiota dysbiosis [23]. The beneficial bacteria that have been reported include Bacteroidetes, Lactobacillus and Bifidobacterium. These beneficial bacteria contribute substantially to the health status of the host by inhibiting the growth of harmful bacteria and secreting substances essential for gut health [24,25,26]. The pathogenic bacteria that have been reported include Enterobacteriaceae, Clostridia and Bacilli. These pathogenic floras also participate in relevant pathways in the development of AP. The ratio of beneficial bacteria to pathogenic bacteria maintains the normal functioning of the intestine. This ratio is significantly altered in pancreatitis. It was reported that the number of Escherichia/Shigella increased in rats with pancreatitis, and further research revealed the bacteria aggravated pancreatitis through targeting intestinal epithelial cells and being translocated by activated regulatory T cells, which causes necrosis of the pancreas [27]. Moreover, the elevation of Escherichia/Shigella increases the abundance of Toll-like receptor 4 (TLR4)/MyD88/p38 mitogen-activated protein (MAPK) and leads to endoplasmic reticulum stress (ERS) signaling-induced intestinal epithelial injury [28]. The TLR4/MyD88/p38 MAPK pathway is associated with tight-junction proteins. ERS signaling is not only associated with tight-junction proteins but also inflammation. Among these molecules, TLR4 plays a role in the pathogenesis of AP [29]. In a nutshell, the destruction of intestinal epithelial cells further causes necrosis of the pancreas.
Gut microbiota not only directly promote AP but also indirectly trigger relevant inflammation and are associated with the severity of AP. The diaminopimelic acid (DAP)-containing bacteria Romboutsia and Allobaculum are increased in SAP rat models. The increasing abundance of DAP activates the NOD1/RIP2 inflammatory signaling pathway and affects the systemic inflammatory response [30]. Gut-microbiota-derived nicotinamide mononucleotide alleviates AP by being transported to pancreas and converted into NAD+, which further activates pancreatic SIRT3 signaling through the SIRT3-PRDX5 pathway. SIRT3 improves the oxidative damage and inflammation caused by AP and then acetylates PRDX5, which aggravates the cell injury of pancreatic acinar cells [31]. Bifidobacterium spp. and their metabolite lactate can protect against multiple-organ dysfunction syndrome in AP patients via inhibition of pancreatic and systemic inflammatory responses [32]. Higher abundances of Proteobacteria phylum, Enterobacteriaceae family, Escherichia-Shigella genus and Klebsiella pneumoniae but lower abundances of Bifidobacterium genus were found in AP patients with acute respiratory distress syndrome [33].This association between gut microbiota and acute respiratory distress syndrome may be related to the lung microenvironment [33]. There are other reports demonstrating an association between specific intestinal floras and the development of acute pancreatitis, as achieved through specialized molecular mechanisms. According to Yue’s research, the decreasing abundance of Lactobacillus is associated with the downregulation of Paneth cells, which are essential components of the intestinal epithelium. Interestingly, the upregulation of Paneth cells could alleviate AP, thus indicating that the loss of Lactobacillus can indirectly exacerbate pancreatic damage [34]. This research also indicated the essential regulatory role of gut microbiota in intestinal epithelial cells and the development of AP. More research indicated the crosstalk between gut microbiota and intestinal epithelial cell receptor activation in the development of AP. It was identified that there existed a bidirectional modulation between the gut microbiota and NLRP3 in the progression of AP, which suggests the interplay of the host and microbiome during AP [35]. The researchers observed synchronous changes in gut microbiota restoration and intestinal NLRP3 inflammasome inactivation during AP recovery.
4. Molecular Actors in Acute Pancreatitis
Besides the intestinal flora itself, its metabolites have an effect on intestinal barrier function in AP. SCFAs play an important role in intestinal barrier function. SCFAs are metabolites produced by gut microbiota. SCFAs are a fermentative product of gut bacteria [6]. There are many bacteria that produce SCFAs, including Blautia hydrotrophica, Akkermansia muciniphila, Bacteroides vulgatus, Coprococcus catus, Megasphaera elsdenii, Ruminococcus bromii, Clostridium butyricum, Roseburia inulinivorans and so on. According to some research, SCFAs have been shown to maintain the mechanical barrier, enhance the immune barrier and regulate the biological barrier of the intestinal mucosa [36,37,38]. Mechanically speaking, SCFAs play crucial roles in the growth of intestinal epithelial cells and the expression of Zo-1 and Occuludin, which are tight-junction proteins in the intestinal epithelium. Intestinal epithelial cells can produce antimicrobial peptides, which participate in the immunity of the intestine. The pH of the gut can also be reduced by SCFAs, leading to an increase in beneficial bacteria. Recent studies have also shown that SCFAs play vital roles in SAP-associated lung injury. The possible mechanisms underlying this include inhibition in the proliferation of pathogens, anti-inflammatory effects, enhancement of intestinal epithelial barrier, a reduction in bacterial translocation and immunomodulatory effects [6].
Bile acids (BAs) and lipopolysaccharides (LPSs) are two more common metabolites closely associated with AP. BAs are also important microbiota metabolites that affect the intestinal barrier and important cholesterol metabolites that can solubilize dietary lipids. Through their antimicrobial activity and by activating host signaling pathways that maintain gut homeostasis, BAs can shape the microbiota composition [39]. The dysbiosis of gut microbiota caused by bile acids leads to the dysregulation of epithelial transport and barrier function, which interacts with the pathogenesis of pancreatitis [40]. BAs are the leading cause of AP, and research has been carried out exclusively on the retrograde infusion of bile acids into the pancreatitis duct. However, systemic bile acids affect the severity of acute pancreatitis under different disease conditions. Research has found that hydrophobic BAs aggravate AP when AP is independent of serum cholecystokinin (CCK). However, BAs reverse CCK-induced injury based on an interaction with the CCK receptor on acinar cells [41,42]. Another metabolite worth mentioning is lipopolysaccharide (LPS). LPSs are an important component of the outer membrane of gram-negative bacteria. According to Vonlaufen’s research, it is believed that LPSs activate the Toll-like receptor 4 (TLR4) and CD 14 pathways of innate immunity, leading to pancreatic damage [43].
Some molecular actors affect the occurrence and development of AP by affecting the gut microbiota. The endogenous cannabinoid system (ECS) is widely expressed in the human body and plays important roles in gastrointestinal functions. Interaction between the ECS and intestinal flora regulates the permeability of the intestinal epithelial barrier [44]. It is reported that the increasing number of ligands and receptors in the ECS in the pancreas is associated with AP. The underlying mechanism is the ECS’s association with the brain–gut–microbiota axis and effect on systemic inflammation [45]. Cannabinoids (CBs) exert their activities of regulating inflammation and gut–adipose tissue signaling through binding to the CB1 and CB2 receptors and non-CB1/non-CB2 receptors such as G-protein-coupled receptor 55 (GPR55) or the transient receptor potential channels [46]. These receptors inhibit adenylate cyclase and the production of cAMP, attenuating the protein kinase A pathway, and thus regulate the inflammation of pancreas.
These newly discovered molecules provide potential therapy targets (Table 1). TLR4 mediates the recognition of bacterial LPSs and is thought to be highly relevant to systemic inflammatory response syndrome. TLR4 has been widely used as a potential mechanistic target for the treatment of AP [34]. SAP stimulated by caerulein (HY-A0190) and LPSs can be reduced by Sitagliptin [47]. Biliary pancreatitis is also one of the main causes of AP. The proposed mechanism is the reflux of bile acids into the pancreatic duct. The increase in bile acid concentration causes an increase in cytoplasmic calcium and further causes damage to pancreatic acinar cells, so targeting bile acid is also a potential treatment method necessitating further research [48]. In addition, NLRP3 was reported to play an essential role in promoting inflammation in AP. NLRP3 is one of the NLR proteins, mediating caspase-1 activation and the secretion of the pro-inflammatory cytokine IL-1β in response to microbial infection and cellular damage [35]. NLR3 also showed a bridging role in gut microbiota and AP. Recently, it has been reported that gut microbiota can secret nicotinamide mononucleotide, which alleviates AP by activating pancreatic SIRT3 signaling [31]. Further studies revealed that during the development of acute pancreatitis, microbiota dysbiosis is promoted by inflammatory factors. Gut microbiota dysbiosis in turn ameliorates the severity of AP, including mitochondrial dysfunction, oxidative damage and inflammation. It was proven that nicotinamide mononucleotide mitigates AP-mediated mitochondrial dysfunction, oxidative damage and inflammation by increasing pancreatic NAD+ levels. Molecular lab tests indicated that gut-microbiota-derived nicotinamide mononucleotide alleviates the severity of AP by activating the SIRT3-PRDX5 pathway. In conclusion, the gut microbiome influences AP in multiple ways (Figure 1).

Table 1.
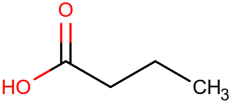
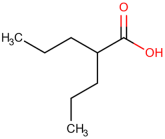
Newly discovered molecules associated with the gut microbiome and their mechanisms in AP. (The pictures of structural formulae are from Human Metabolome Database, https://hmdb.ca/, accessed on 1 November 2023).
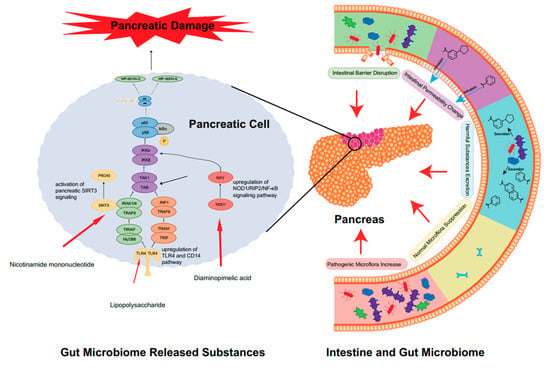
Figure 1.
The close relationship between gut microbiota and the activation of AP. In AP, significant disorganization of the intestinal flora occurs. The first manifestation is the abnormal ratio of intestinal flora species, which is mainly characterized by an increase in harmful bacteria and a decrease in probiotics, resulting in a serious imbalance in the ratio of harmful bacteria to probiotics. Secondly, the alteration in gut flora leads to the explosion of harmful microbiome-released substances. The metabolites of harmful bacteria increase, while the beneficial metabolites of probiotics (e.g., SCFAs) decrease. Altered intestinal permeability also accelerates the progression of AP. The intestinal mucosal barrier, which can be directly and indirectly disrupted, is also affected, leading to translocation and direct invasion of the flora. These changes will cause harmful substances to accumulate in the intestine, directly stimulate the TLR4 receptor of the epithelial cells in the intestine, activate the relevant inflammatory response pathways and ultimately accelerate the destruction of pancreatic cells and aggravate AP. Some harmful substances may even enter the blood circulation directly and activate the body’s immune response, thus causing the development of AP.
5. Future and Prospects
With an enhanced understanding of gut microbiota and pancreatitis, the treatment of pancreatitis has been constantly improving. The application of probiotics and the early recovery of diet are considered important in the recovery of AP. Probiotics are beneficial bacteria that can be consumed from various sources and can improve health in different ways. In inflammatory diseases, probiotics have many functions. First of all, probiotics compete with harmful bacterial communities to inhibit the growth of pathogenic bacteria [55]. Secondly, probiotics stimulate the intestinal mucosa to secrete mucin, inhibit the binding of pathogens to the intestinal epithelium and reduce their passage through the intestinal wall into peripheral organs [56]. Thirdly, probiotics promote the biosynthesis of glutathione, improve the dysfunction of the intestinal mucosal barrier, reduce oxidative stress, reduce the permeability and apoptosis level of the ileal mucosa and thereby inhibit the migration of intestinal bacteria [57]. Fourthly, probiotics affect the function of immune cells in various ways (for example, by regulating the production of pro-inflammatory and anti-inflammatory cytokines) [58]. Thus, the application of probiotics in AP can restore the intestinal microbiota balance and prevent bacterial translocation and infection. In pancreatitis, the most commonly used probiotics are Lactobacillus acidophilus, Lactobacillus bulgaricus and Bifidobacterium bifidum [59,60,61]. Studies have shown that probiotics may shorten the length of hospital stay and reduce the infection rate in mild AP [62]. Moreover, it has been reported that probiotic supplementation for experimental AP did not show an adverse effect on mortality but showed a positive effect of reduced histopathology of the pancreas and bacterial translocation to the pancreas and mesenteric lymph nodes [63]. In addition, a study by Hooijmans et al. also revealed that single-strain probiotic supplementation might be more effective in reducing the risk of bacterial translocation than multi-strain supplementation. The safety of probiotics is also monitored. The probiotics used in clinical trials are various strains of bacteria that are intended to modulate the gut microbiota and enhance the host immune system, such as Lactobacillus paracasei ssp. paracasei F19 and Bifidobacterium lactis Bb12. They have been tested for different conditions, such as infectious diseases and inflammatory bowel diseases [64]. The safety of probiotics clinical trials is generally good, but there are some potential risks and adverse effects that need to be monitored. These include bacteremia, fungemia, endocarditis, sepsis, organ failure, antibiotic resistance and immune dysregulation [65]. However, these adverse events are only documented in case reports of individuals. We lack specific statistics of the adverse effects of probiotics.
Since gut microbiota are closely associated with the development of AP, gut microbiota may have predictive values in AP [66]. With the emergence of the concept of precision medicine, the study of biomarkers has become particularly important. Precision medicine is an emerging approach to disease prevention and management that takes into account differences in an individual’s genes, environment and lifestyle habits [67,68]. Precision medicine is a new medical concept and medical model based on individualized medicine and developed with the cross-application of bioinformatics and big data science [69,70]. The essence of precision medicine is to analyze, identify, validate and apply biomarkers to a large sample of people and specific disease types through analyzing the genome and proteome and other genomics technologies and cutting-edge medical technologies, so as to accurately search for the causes of diseases and the targets of treatments and accurately categorize the different states and processes of a disease, and ultimately to achieve the goal of personalized and precise treatments for diseases and specific patients and to improve the effectiveness of disease diagnosis and treatment as well as prevention. All in all, this improves the efficiency of disease diagnosis and treatment and prevention. AP is categorized as mild or severe, with or without necrosis. For different types of pancreatitis, the prognosis and treatment are very different. For instance, acute necrotizing pancreatitis has a higher mortality and worse outcome than non-necrotizing pancreatitis. Current research suggests that changes in the intestinal flora have a close relationship with the severity and prognosis of pancreatitis (Figure 2). Therefore, the intestinal flora has the potential to become a marker for measuring the prognosis of pancreatitis, thus realizing the precise treatment of pancreatitis. Studies show that the intestinal microbiota in necrotizing pancreatitis patients are distinct from those in non-necrotizing pancreatitis patients’ intestinal microbiota [66]. Necrotizing pancreatitis patients presented a lower abundance of Bifidobacterium and Blautia and higher abundance of Enterococcus and Escheichia/Shigella compared with non-necrotizing pancreatitis patients. It is worth mentioning that Bifidobacterium and Blautia are considered probiotic candidates, while Enterococcus and Escheichia/Shigella are regarded as possible pathogens. Surprisingly, both necrotizing pancreatitis and non-necrotizing pancreatitis are associated with a decrease in certain probiotic microbes and increase in opportunistic pathogens. This research also pointed out that the gut microbiota’s influence on the progression of the narcotizing of the pancreas may be associated with ketone body or benzoate metabolism [71]. Thus, Enterococcus faecium and Finegoldia magna are potential biomarkers for necrotizing pancreatitis [66]. Research by others showed that different types of pancreatitis are associated with different gut microbiota compositions. The gut microbiota class Melainabacteria was specifically mentioned due to its strong causality to acute necrotizing pancreatitis. This association between gut microbiota and pancreatitis may indicate biomarkers for the non-invasive identification of AP [72]. Moreover, researchers found that an alteration in gut microbiota may indicate the possibility of complications. One study showed that during the early stage of AP, higher abundances of the Proteobacteria phylum, Enterobacteriaceae family, Escherichia-Shigella genus and Klebsiella pneumoniae but lower abundances of the Bifidobacterium genus were strong indications for the occurrence of acute respiratory distress syndrome in AP patients [33]. Not only do gut microbiota show potential as biomarkers in AP; microbiome-associated substances also show potential in AP. It was reported in pancreatitis mouse models that an increased abundance of Streptomyces, Turicibacter and Methylobacterium was associated with pancreatic fibrosis [71]. Further experiments revealed that dysbiosis of the gut microbiota may increase CD4+ T cells and macrophage infiltration in pancreas tissues and modulate fibrotic progression. Since gut microbiota play a key role in AP, we can determine the severity of pancreatitis based on changes in the intestinal flora, which will determine the appropriate time to start eating and restore intestinal function as early as possible without aggravating the intestinal infection. Considering these studies, interventions based on probiotic intake could be designed to ameliorate the conditions of AP patients. Based on the relationship between the gut and AP, we can not only intervene in pancreatitis by directly interfering with the gut flora but also alleviate the inflammatory response associated with pancreatitis by influencing the pathways associated with the gut flora. AP-associated dysbiosis is often associated with activation of intracellular inflammatory pathways. The most common pathways are the TLR4-related pathway and CD14 pathway, as well as the NOD1/RIP2/NF-κB-related pathway. Inhibiting these inflammatory pathways activated by aberrant floras by designing relevant drug molecules is not a new idea for the treatment of AP.
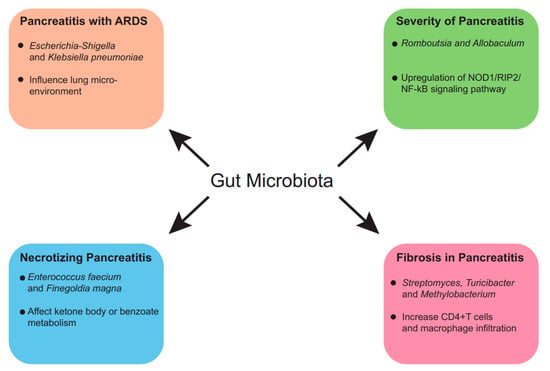
Figure 2.
Predictive value of gut microbiota in AP. The gut microbiome is not only predictive of AP but also the severity, prognosis and comorbidities of AP, and the associations between alterations in gut microbiome and disease prognosis have underlying molecular biological mechanisms.
With the further study of AP and intestinal floras, researchers have realized that intestinal microbiota are important modulatory elements in the human body. The interaction of intestinal microbiota with other organs provides multiple promising therapeutic targets. New concepts have been introduced to represent these newly discovered interactions, such as lung–gut axis, brain–gut axis and liver–gut–heart axis. AP can cause various complications, the most serious of which is acute respiratory distress syndrome (ARDS), which is diffuse damage to the lung [73]. By regulating the intestinal microbiota, reducing bacterial translocation and pathogen-associated molecular pattern (PAMP) production, the intestinal permeability and inflammatory response can be reduced [74]. This is referred to as the lung–gut axis. The protection of lung tissue can be achieved through improving intestinal barrier function, preventing pancreatic enzymes and toxins from entering the lung, balancing the immune system, inhibiting excessive pro-inflammatory factors and oxidative stress and enhancing anti-inflammatory factors and antioxidant capacity [75]. Another gut–organ axis is the gut–brain axis, which can regulate the balance of gut microbiota that may affect the function of the pancreas and reduce inflammation reactions. Through the release of neurotransmitters and hormones, the axis can inhibit the stress response, which in a way reduces the damage of pancreatitis. The protection of the integrity of the intestinal barrier by neuroimmune pathways can prevent intestinal endotoxins from entering the bloodstream and cause systemic inflammatory reactions [76]. Last but not least, in the context of the liver–gut–heart axis, the interconnectedness of different organs plays a crucial role in health. An unhealthy liver can influence the gut microbiota, which in turn can impact the cardiovascular system. Within the liver–gut axis, reducing the production of intestinal endotoxins can lower the risk of a systemic inflammatory response and multiple-organ failure caused by acute pancreatitis (AP) [77]. In the gut–heart axis, regulation of the cardiovascular system can enhance the circulatory condition of AP patients, thereby reducing the risk of stroke and heart failure associated with AP. Lastly, within the liver–heart axis, regulation of metabolic pathways can improve the nutritional status of AP patients, which in turn can reduce the risk of complications such as consumptive disorders and infections associated with AP [78].
6. Conclusions
In this review, we have discussed the essential role of gut microbiota in AP. We also illustrated how gut microbiota dysbiosis, bacterial translocation and molecular actors affect the pathogenesis and progression of AP and its complications. The potential therapeutic strategies that target the gut microbiota are highlighted, such as probiotics. Furthermore, we have explored the emerging concepts of gut–organ axes, providing new insights into the mechanisms and interventions of AP. However, more studies are needed to elucidate the causal relationship between gut microbiota and AP to identify the specific microbial strains and metabolites that are involved and to evaluate the safety and efficacy of microbiota-based therapies in clinical settings.
Author Contributions
Conceptualization, D.W. (Dong Wu); writing—original draft preparation, R.Z. and Q.W.; writing—review and editing Z.Y., Y.C. and D.W. (Duan Wang); visualization, R.Z.; supervision, D.W. (Dong Wu); project administration, D.W. (Dong Wu); funding acquisition, D.W. (Dong Wu). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant number 32170788), the National High Level Hospital Clinical Research Funding (grant number 2022-PUMCH-B-023), the National Key Clinical Specialty Construction Project (grant number ZK108000) and the Beijing Natural Science Foundation (grant number 7232123).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Boxhoorn, L.; Voermans, R.P.; Bouwense, S.A.; Bruno, M.J.; Verdonk, R.C.; Boermeester, M.A.; van Santvoort, H.C.; Besselink, M.G. Acute pancreatitis. Lancet 2020, 396, 726–734. [Google Scholar] [CrossRef]
- Mederos, M.A.; Reber, H.A.; Girgis, M.D. Acute Pancreatitis: A Review. JAMA 2021, 325, 382–390. [Google Scholar] [CrossRef]
- Lee, P.J.; Papachristou, G.I. New insights into acute pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.B. Acute Pancreatitis. Ann. Intern. Med. 2021, 174, ITC17–ITC32. [Google Scholar] [CrossRef]
- Hu, X.; Gong, L.; Zhou, R.; Han, Z.; Ji, L.; Zhang, Y.; Zhang, S.; Wu, D. Variations in Gut Microbiome are Associated with Prognosis of Hypertriglyceridemia-Associated Acute Pancreatitis. Biomolecules 2021, 11, 695. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Li, F.; Luo, Y.; Ge, P.; Zhang, Y.; Wen, H.; Yang, Q.; Ma, S.; Chen, H. The gut-lung axis in severe acute Pancreatitis-associated lung injury: The protection by the gut microbiota through short-chain fatty acids. Pharmacol. Res. 2022, 182, 106321. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; He, C.; Li, X.; Cai, Y.; Hu, J.; Liao, Y.; Zhao, J.; Xia, L.; He, W.; Liu, L.; et al. Gut microbiota dysbiosis worsens the severity of acute pancreatitis in patients and mice. J. Gastroenterol. 2019, 54, 347–358. [Google Scholar] [CrossRef]
- Zhu, Y.; Mei, Q.; Fu, Y.; Zeng, Y. Alteration of gut microbiota in acute pancreatitis and associated therapeutic strategies. Biomed. Pharmacother. 2021, 141, 111850. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, M.; Li, J.; Jiang, C.; Yang, S.; Zheng, S.; Li, M.; Ai, X.; Xu, X.; Zhang, W.; et al. Composition and functional profiles of gut microbiota reflect the treatment stage, severity, and etiology of acute pancreatitis. Microbiol. Spectr. 2023, 11, e0082923. [Google Scholar] [CrossRef]
- Liu, J.; Luo, M.; Qin, S.; Li, B.; Huang, L.; Xia, X. Significant Succession of Intestinal Bacterial Community and Function during the Initial 72 Hours of Acute Pancreatitis in Rats. Front. Cell. Infect. Microbiol. 2022, 12, 808991. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, J.R.; Spitofsky, N.; Zamani, A.; Hood, R.; Boggs, A.; Li, X.; Li, M.; Reiner, E.; Ayyaz, A.; Etwebi, Z.; et al. TNFAIP8 controls murine intestinal stem cell homeostasis and regeneration by regulating microbiome-induced Akt signaling. Nat. Commun. 2020, 11, 2591. [Google Scholar] [CrossRef]
- Stevens, B.R.; Goel, R.; Seungbum, K.; Richards, E.M.; Holbert, R.C.; Pepine, C.J.; Raizada, M.K. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 2018, 67, 1555–1557. [Google Scholar] [CrossRef]
- Kamioka, M.; Goto, Y.; Nakamura, K.; Yokoi, Y.; Sugimoto, R.; Ohira, S.; Kurashima, Y.; Umemoto, S.; Sato, S.; Kunisawa, J.; et al. Intestinal commensal microbiota and cytokines regulate Fut2(+) Paneth cells for gut defense. Proc. Natl. Acad. Sci. USA 2022, 119, e2115230119. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, F.; Xiong, Y.; Wu, J.; Li, X.; Li, G.; Bai, T.; Hou, X.; Song, J. Reprogrammed fecal and mucosa-associated intestinal microbiota and weakened mucus layer in intestinal goblet cell- specific Piezo1-deficient mice. Front. Cell. Infect. Microbiol. 2022, 12, 1035386. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xu, H.; Chen, Y.; Wu, J.; Jin, F.; Wu, Q.; Yao, X.M. Therapeutic effect of Bifidobacterium combined with early enteral nutrition in the treatment of severe acute pancreatitis: A pilot study. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4018–4024. [Google Scholar] [CrossRef]
- Davies, S.J.; Mythen, M. Hemodynamic and Intestinal Microcirculatory Changes in a Phenylephrine Corrected Porcine Model of Hemorrhage. Anesth. Analg. 2021, 133, 1060–1069. [Google Scholar] [CrossRef]
- Koike, Y.; Li, B.; Lee, C.; Alganabi, M.; Zhu, H.; Chusilp, S.; Lee, D.; Cheng, S.; Li, Q.; Pierro, A. The intestinal injury caused by ischemia-reperfusion is attenuated by amniotic fluid stem cells via the release of tumor necrosis factor-stimulated gene 6 protein. FASEB J. 2020, 34, 6824–6836. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Benakis, C.; Brea, D.; Caballero, S.; Faraco, G.; Moore, J.; Murphy, M.; Sita, G.; Racchumi, G.; Ling, L.; Pamer, E.G.; et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med. 2016, 22, 516–523. [Google Scholar] [CrossRef]
- Smith, A.B.; Jenior, M.L.; Keenan, O.; Hart, J.L.; Specker, J.; Abbas, A.; Rangel, P.C.; Di, C.; Green, J.; Bustin, K.A.; et al. Enterococci enhance Clostridioides difficile pathogenesis. Nature 2022, 611, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Li, K.L.; Wang, B.Z.; Li, Z.P.; Li, Y.L.; Liang, J.J. Alterations of intestinal flora and the effects of probiotics in children with recurrent respiratory tract infection. World J. Pediatr. 2019, 15, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yilin, H.; Di, W.; Jie, W.; Hanyue, W.; Ya, L.; Jie, P. A nomogram for predicting the risk of mortality in patients with acute pancreatitis and Gram-negative bacilli infection. Front. Cell. Infect. Microbiol. 2022, 12, 1032375. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metab. 2016, 24, 151–157. [Google Scholar] [CrossRef]
- Huang, H.; Li, K.; Lee, Y.; Chen, M. Preventive Effects of Lactobacillus Mixture against Chronic Kidney Disease Progression through Enhancement of Beneficial Bacteria and Downregulation of Gut-Derived Uremic Toxins. J. Agric. Food Chem. 2021, 69, 7353–7366. [Google Scholar] [CrossRef]
- Lei, C.; Sun, R.; Xu, G.; Tan, Y.; Feng, W.; McClain, C.J.; Deng, Z. Enteric VIP-producing neurons maintain gut microbiota homeostasis through regulating epithelium fucosylation. Cell Host Microbe 2022, 30, 1417–1434.e8. [Google Scholar] [CrossRef]
- Glaubitz, J.; Wilden, A.; Frost, F.; Ameling, S.; Homuth, G.; Mazloum, H.; Rühlemann, M.C.; Bang, C.; Aghdassi, A.A.; Budde, C.; et al. Activated regulatory T-cells promote duodenal bacterial translocation into necrotic areas in severe acute pancreatitis. Gut 2023, 72, 1355–1369. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Lou, L.; Fan, J.; Huang, C.; Mei, Q.; Wu, J.; Guo, Y.; Lu, Y.; Wang, X.; Zeng, Y. Commensal Escherichia coli Aggravates Acute Necrotizing Pancreatitis through Targeting of Intestinal Epithelial Cells. Appl. Environ. Microbiol. 2019, 85, e00059-19. [Google Scholar] [CrossRef]
- Wen, Y.; Han, C.; Liu, T.; Wang, R.; Cai, W.; Yang, J.; Liang, G.; Yao, L.; Shi, N.; Fu, X.; et al. Chaiqin chengqi decoction alleviates severity of acute pancreatitis via inhibition of TLR4 and NLRP3 inflammasome: Identification of bioactive ingredients via pharmacological sub-network analysis and experimental validation. Phytomedicine 2020, 79, 153328. [Google Scholar] [CrossRef]
- Jiao, J.; Liu, J.; Li, Q.; Zhang, G.; Pan, C.; Luo, F.; Zhang, Q.; Qi, B.; Zhao, L.; Yin, P.; et al. Gut Microbiota-Derived Diaminopimelic Acid Promotes the NOD1/RIP2 Signaling Pathway and Plays a Key Role in the Progression of Severe Acute Pancreatitis. Front. Cell. Infect. Microbiol. 2022, 12, 838340. [Google Scholar] [CrossRef]
- Liu, L.W.; Xie, Y.; Li, G.Q.; Zhang, T.; Sui, Y.H.; Zhao, Z.J.; Zhang, Y.Y.; Yang, W.B.; Geng, X.L.; Xue, D.B.; et al. Gut microbiota-derived nicotinamide mononucleotide alleviates acute pancreatitis by activating pancreatic SIRT3 signalling. Br. J. Pharmacol. 2023, 180, 647–666. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xie, J.; Guo, X.; Yang, G.; Cai, B.; Liu, J.; Yue, M.; Tang, Y.; Wang, G.; Chen, S.; et al. Bifidobacterium spp. and their metabolite lactate protect against acute pancreatitis via inhibition of pancreatic and systemic inflammatory responses. Gut Microbes 2022, 14, 2127456. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Han, Z.; Zhou, R.; Su, W.; Gong, L.; Yang, Z.; Song, X.; Zhang, S.; Shu, H.; Wu, D. Altered gut microbiota in the early stage of acute pancreatitis were related to the occurrence of acute respiratory distress syndrome. Front. Cell. Infect. Microbiol. 2023, 13, 1127369. [Google Scholar] [CrossRef] [PubMed]
- Qi-Xiang, M.; Yang, F.; Ze-Hua, H.; Nuo-Ming, Y.; Rui-Long, W.; Bin-Qiang, X.; Jun-Jie, F.; Chun-Lan, H.; Yue, Z. Intestinal TLR4 deletion exacerbates acute pancreatitis through gut microbiota dysbiosis and Paneth cells deficiency. Gut Microbes 2022, 14, 2112882. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, C.; Li, N.; Ding, L.; Chen, H.; Wan, J.; Yang, X.; Xia, L.; He, W.; Xiong, H.; et al. The interplay between the gut microbiota and NLRP3 activation affects the severity of acute pancreatitis in mice. Gut Microbes 2020, 11, 1774–1789. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, D.; Zhu, H.; Zhu, J.; Weng, S.; Dong, L.; Liu, T.; Hu, Y.; Shen, X. Berberine treatment increases Akkermansia in the gut and improves high-fat diet-induced atherosclerosis in Apoe(−/−) mice. Atherosclerosis 2018, 268, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Ling, Z.; Huang, Y.; Cao, Y.; Liu, Q.; Cai, T.; Yuan, H.; Liu, C.; Li, Y.; Xu, K. Dysbiosis of Intestinal Microbiota Associated With Inflammation Involved in the Progression of Acute Pancreatitis. Pancreas 2015, 44, 868–875. [Google Scholar] [CrossRef]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietilä, T.E.; Kainulainen, V.; Klievink, J.; Huuskonen, L.; Aalvink, S.; Skurnik, M.; Boeren, S.; et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE 2017, 12, e0173004. [Google Scholar] [CrossRef]
- Han, B.; Lv, X.; Liu, G.; Li, S.; Fan, J.; Chen, L.; Huang, Z.; Lin, G.; Xu, X.; Huang, Z.; et al. Gut microbiota-related bile acid metabolism-FXR/TGR5 axis impacts the response to anti-α4β7-integrin therapy in humanized mice with colitis. Gut Microbes 2023, 15, 2232143. [Google Scholar] [CrossRef]
- Mouries, J.; Brescia, P.; Silvestri, A.; Spadoni, I.; Sorribas, M.; Wiest, R.; Mileti, E.; Galbiati, M.; Invernizzi, P.; Adorini, L.; et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 2019, 71, 1216–1228. [Google Scholar] [CrossRef]
- Tran, Q.T.; Sendler, M.; Wiese, M.L.; Doller, J.; Zierke, L.; Gischke, M.; Glaubitz, J.; Tran, V.H.; Lalk, M.; Bornscheuer, U.T.; et al. Systemic Bile Acids Affect the Severity of Acute Pancreatitis in Mice Depending on Their Hydrophobicity and the Disease Pathogenesis. Int. J. Mol. Sci. 2022, 23, 13592. [Google Scholar] [CrossRef]
- Pallagi, P.; Görög, M.; Papp, N.; Madácsy, T.; Varga, Á.; Crul, T.; Szabó, V.; Molnár, M.; Dudás, K.; Grassalkovich, A.; et al. Bile acid- and ethanol-mediated activation of Orai1 damages pancreatic ductal secretion in acute pancreatitis. J. Physiol. 2022, 600, 1631–1650. [Google Scholar] [CrossRef] [PubMed]
- Vonlaufen, A.; Xu, Z.; Daniel, B.; Kumar, R.K.; Pirola, R.; Wilson, J.; Apte, M.V. Bacterial endotoxin: A trigger factor for alcoholic pancreatitis? Evidence from a novel, physiologically relevant animal model. Gastroenterology 2007, 133, 1293–1303. [Google Scholar] [CrossRef]
- Dohnalová, L.; Lundgren, P.; Carty, J.R.E.; Goldstein, N.; Wenski, S.L.; Nanudorn, P.; Thiengmag, S.; Huang, K.P.; Litichevskiy, L.; Descamps, H.C.; et al. A microbiome-dependent gut-brain pathway regulates motivation for exercise. Nature 2022, 612, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Inserra, A.; Giorgini, G.; Lacroix, S.; Bertazzo, A.; Choo, J.; Markopolous, A.; Grant, E.; Abolghasemi, A.; De Gregorio, D.; Flamand, N.; et al. Effects of repeated lysergic acid diethylamide (LSD) on the mouse brain endocannabinoidome and gut microbiome. Br. J. Pharmacol. 2023, 180, 721–739. [Google Scholar] [CrossRef]
- Shen, S.Y.; Yu, R.; Li, W.; Liang, L.F.; Han, Q.Q.; Huang, H.J.; Li, B.; Xu, S.F.; Wu, G.C.; Zhang, Y.Q.; et al. The neuroprotective effects of GPR55 against hippocampal neuroinflammation and impaired adult neurogenesis in CSDS mice. Neurobiol. Dis. 2022, 169, 105743. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Deng, J.; Zhou, X.; Cai, B.; Zhang, B.; Chen, X.; Chen, Z.; Wang, W. Sitagliptin activates the p62-Keap1-Nrf2 signalling pathway to alleviate oxidative stress and excessive autophagy in severe acute pancreatitis-related acute lung injury. Cell Death Dis. 2021, 12, 928. [Google Scholar] [CrossRef]
- Pallagi, P.; Madácsy, T.; Varga, Á.; Maléth, J. Intracellular Ca(2+) Signalling in the Pathogenesis of Acute Pancreatitis: Recent Advances and Translational Perspectives. Int. J. Mol. Sci. 2020, 21, 4005. [Google Scholar] [CrossRef]
- Ait Djebbara, S.; McHeik, S.; Percier, P.; Segueni, N.; Poncelet, A.; Truyens, C. The macrophage infectivity potentiator of Trypanosoma cruzi induces innate IFN-γ and TNF-α production by human neonatal and adult blood cells through TLR2/1 and TLR4. Front. Immunol. 2023, 14, 1180900. [Google Scholar] [CrossRef]
- Zhu, Z.; Yi, B.; Tang, Z.; Chen, X.; Li, M.; Xu, T.; Zhao, Z.; Tang, C. Lactobacillus casei combined with Lactobacillus reuteri alleviate pancreatic cancer by inhibiting TLR4 to promote macrophage M1 polarization and regulate gut microbial homeostasis. BMC Cancer 2023, 23, 1044. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Dawra, R.; Wasiluk, K.; Phillips, P.; Dudeja, V.; Kurt-Jones, E.; Finberg, R.; Saluja, A. Impact of toll-like receptor 4 on the severity of acute pancreatitis and pancreatitis-associated lung injury in mice. Gut 2009, 58, 813–819. [Google Scholar] [CrossRef]
- Lei, Y.; Tang, L.; Liu, S.; Hu, S.; Wu, L.; Liu, Y.; Yang, M.; Huang, S.; Tang, X.; Tang, T.; et al. Parabacteroides produces acetate to alleviate heparanase-exacerbated acute pancreatitis through reducing neutrophil infiltration. Microbiome 2021, 9, 115. [Google Scholar] [CrossRef]
- van den Berg, F.F.; van Dalen, D.; Hyoju, S.K.; van Santvoort, H.C.; Besselink, M.G.; Wiersinga, W.J.; Zaborina, O.; Boermeester, M.A.; Alverdy, J. Western-type diet influences mortality from necrotising pancreatitis and demonstrates a central role for butyrate. Gut 2021, 70, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Eisses, J.F.; Criscimanna, A.; Dionise, Z.R.; Orabi, A.I.; Javed, T.A.; Sarwar, S.; Jin, S.; Zhou, L.; Singh, S.; Poddar, M.; et al. Valproic Acid Limits Pancreatic Recovery after Pancreatitis by Inhibiting Histone Deacetylases and Preventing Acinar Redifferentiation Programs. Am. J. Pathol. 2015, 185, 3304–3315. [Google Scholar] [CrossRef]
- Wang, Y.; Kuo, S.; Shu, M.; Yu, J.; Huang, S.; Dai, A.; Two, A.; Gallo, R.L.; Huang, C.M. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: Implications of probiotics in acne vulgaris. Appl. Microbiol. Biotechnol. 2014, 98, 411–424. [Google Scholar] [CrossRef]
- Gu, Y.; Qin, X.; Zhou, G.; Wang, C.; Mu, C.; Liu, X.; Zhong, W.; Xu, X.; Wang, B.; Jiang, K.; et al. Lactobacillus rhamnosus GG supernatant promotes intestinal mucin production through regulating 5-HT4R and gut microbiota. Food Funct. 2022, 13, 12144–12155. [Google Scholar] [CrossRef] [PubMed]
- Lutgendorff, F.; Trulsson, L.M.; van Minnen, L.P.; Rijkers, G.T.; Timmerman, H.M.; Franzén, L.E.; Gooszen, H.G.; Akkermans, L.M.; Söderholm, J.D.; Sandström, P.A. Probiotics enhance pancreatic glutathione biosynthesis and reduce oxidative stress in experimental acute pancreatitis. Am. J. Physiol. Liver Physiol. 2008, 295, G1111–G1121. [Google Scholar] [CrossRef] [PubMed]
- Magryś, A.; Pawlik, M. Postbiotic Fractions of Probiotics Lactobacillus plantarum 299v and Lactobacillus rhamnosus GG Show Immune-Modulating Effects. Cells 2023, 12, 2538. [Google Scholar] [CrossRef]
- Tzikos, G.; Tsalkatidou, D.; Stavrou, G.; Thoma, G.; Chorti, A.; Tsilika, M.; Michalopoulos, A.; Papavramidis, T.; Giamarellos-Bourboulis, E.J.; Kotzampassi, K. A Four-Probiotic Regime to Reduce Surgical Site Infections in Multi-Trauma Patients. Nutrients 2022, 14, 2620. [Google Scholar] [CrossRef]
- Choi, S.M.; Lin, H.; Xie, W.; Chu, I.K. Study of Potential Synergistic Effect of Probiotic Formulas on Acrylamide Reduction. Int. J. Mol. Sci. 2023, 24, 4693. [Google Scholar] [CrossRef]
- Shin, M.; Truong, V.-L.; Lee, M.; Kim, D.; Kim, M.S.; Cho, H.; Jung, Y.H.; Yang, J.; Jeong, W.S.; Kim, Y. Investigation of phenyllactic acid as a potent tyrosinase inhibitor produced by probiotics. Curr. Res. Food Sci. 2023, 6, 100413. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.D.; Zhu, R.X.; Bian, Z.Z.; Sun, T.W. Effect of probiotics on length of hospitalization in mild acute pancreatitis: A randomized, double-blind, placebo-controlled trial. World J. Gastroenterol. 2021, 27, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; de Vries, R.B.; Rovers, M.M.; Gooszen, H.G.; Ritskes-Hoitinga, M. The effects of probiotic supplementation on experimental acute pancreatitis: A systematic review and meta-analysis. PLoS ONE 2012, 7, e48811. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Harris, L.A.; Lacy, B.E.; Quigley, E.M.M.; Moayyedi, P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 48, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Bafeta, A.; Koh, M.; Riveros, C.; Ravaud, P. Harms Reporting in Randomized Controlled Trials of Interventions Aimed at Modifying Microbiota: A Systematic Review. Ann. Intern. Med. 2018, 169, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Yang, Z.; Fan, Y.; Gong, L.; Han, Z.; Ji, L.; Hu, X.; Wu, D. Gut microbiota on admission as predictive biomarker for acute necrotizing pancreatitis. Front. Immunol. 2022, 13, 988326. [Google Scholar] [CrossRef] [PubMed]
- Sisodiya, S.M. Precision medicine and therapies of the future. Epilepsia 2021, 62 (Suppl. S2), S90–S105. [Google Scholar] [CrossRef]
- Tsin Wen, Y.; Cheng, A.C.; Podin, Y. Precision Medicine for Sepsis Management in Low- and Middle-Income Countries—Melioidosis as a Model? Am. J. Respir. Crit. Care Med. 2023. [Google Scholar] [CrossRef]
- Ben-Ammar, I.; Rousseau, A.; Nicolle, R.; Tarabay, A.; Boige, V.; Valery, M.; Pudlarz, T.; Malka, D.; Gelli, M.; Fernandez-De-Sevilla, E.; et al. Precision medicine for KRAS wild-type pancreatic adenocarcinomas. Eur. J. Cancer 2023, 197, 113497. [Google Scholar] [CrossRef]
- Kouidrat, Y.; Collen, L.L.; Vaxillaire, M.; Dechaume, A.; Toussaint, B.; Vaillant, E.; Amanzougarene, S.; Derhourhi, M.; Delemer, B.; Azahaf, M.; et al. Dominant PDX1 deficiency causes highly penetrant diabetes at different ages, associated with obesity and exocrine pancreatic deficiency: Lessons for precision medicine. Diabetes Metab. 2023, 101507. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, T.; Sui, Y.; Li, G.; Liu, L.; Lu, T.; Tan, H.; Sun, B.; Li, X.; Li, L. Gut microbiota affects pancreatic fibrotic progression through immune modulation in chronic pancreatitis. Microb. Pathog. 2023, 177, 106035. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Qin, X.; Ran, T.; Pan, Y.; Hong, Y.; Wang, J.; Zhang, X.; Shen, X.; Liu, C.; Lu, X.; et al. Causal link between gut microbiota and four types of pancreatitis: A genetic association and bidirectional Mendelian randomization study. Front. Microbiol. 2023, 14, 1290202. [Google Scholar] [CrossRef] [PubMed]
- Jaber, S.; Garnier, M.; Asehnoune, K.; Bounes, F.; Buscail, L.; Chevaux, J.B.; Dahyot-Fizelier, C.; Darrivere, L.; Jabaudon, M.; Joannes-Boyau, O.; et al. Guidelines for the management of patients with severe acute pancreatitis, 2021. Anaesth. Crit. Care Pain Med. 2022, 41, 101060. [Google Scholar] [CrossRef]
- Awla, D.; Abdulla, A.; Regnér, S.; Thorlacius, H. TLR4 but not TLR2 regulates inflammation and tissue damage in acute pancreatitis induced by retrograde infusion of taurocholate. Inflamm. Res. 2011, 60, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Shao, F.; Yu, D.; Zhang, J.; Liu, Z.; Ma, J.; Xia, P.; Wang, S. Maturation and specialization of group 2 innate lymphoid cells through the lung-gut axis. Nat. Commun. 2022, 13, 7600. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Yang, J.; Cramer, J.V.; Malik, R.; Liesz, A. Detection of cytokine-induced sickness behavior after ischemic stroke by an optimized behavioral assessment battery. Brain Behav. Immun. 2021, 91, 668–672. [Google Scholar] [CrossRef]
- Guan, H.; Zhang, X.; Kuang, M.; Yu, J. The gut-liver axis in immune remodeling of hepatic cirrhosis. Front. Immunol. 2022, 13, 946628. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, R.; Li, H.; Zhao, X.; Sun, Y.; Fan, Y.; Zhang, S. Alterations of Gut Microbiome and Serum Metabolome in Coronary Artery Disease Patients Complicated with Non-alcoholic Fatty Liver Disease Are Associated With Adverse Cardiovascular Outcomes. Front. Cardiovasc. Med. 2021, 8, 805812. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).