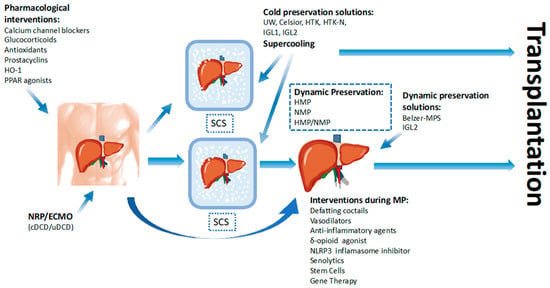
Focusing on Ischemic Reperfusion Injury in the New Era of Dynamic Machine Perfusion in Liver Transplantation
Abstract
1. Introduction
2. Graft Vulnerability before Preservation: Pre Preservation Injury
2.1. Liver Steatosis
2.2. Aged Liver
2.3. Injury Associated with Cardiopulmonary Arrest or Hypotension before Donation
2.4. Injury during Organ Procurement
2.5. Warm Ischemia Injury in Donation after Cardiac Death
3. Graft Injury Prevention during Organ Preservation
3.1. Static Cold Graft Storage Injury (SCSI)
3.2. Organ Preservation Solutions for Preventing SCSI
4. Supercooling Strategies for Organ Preservation
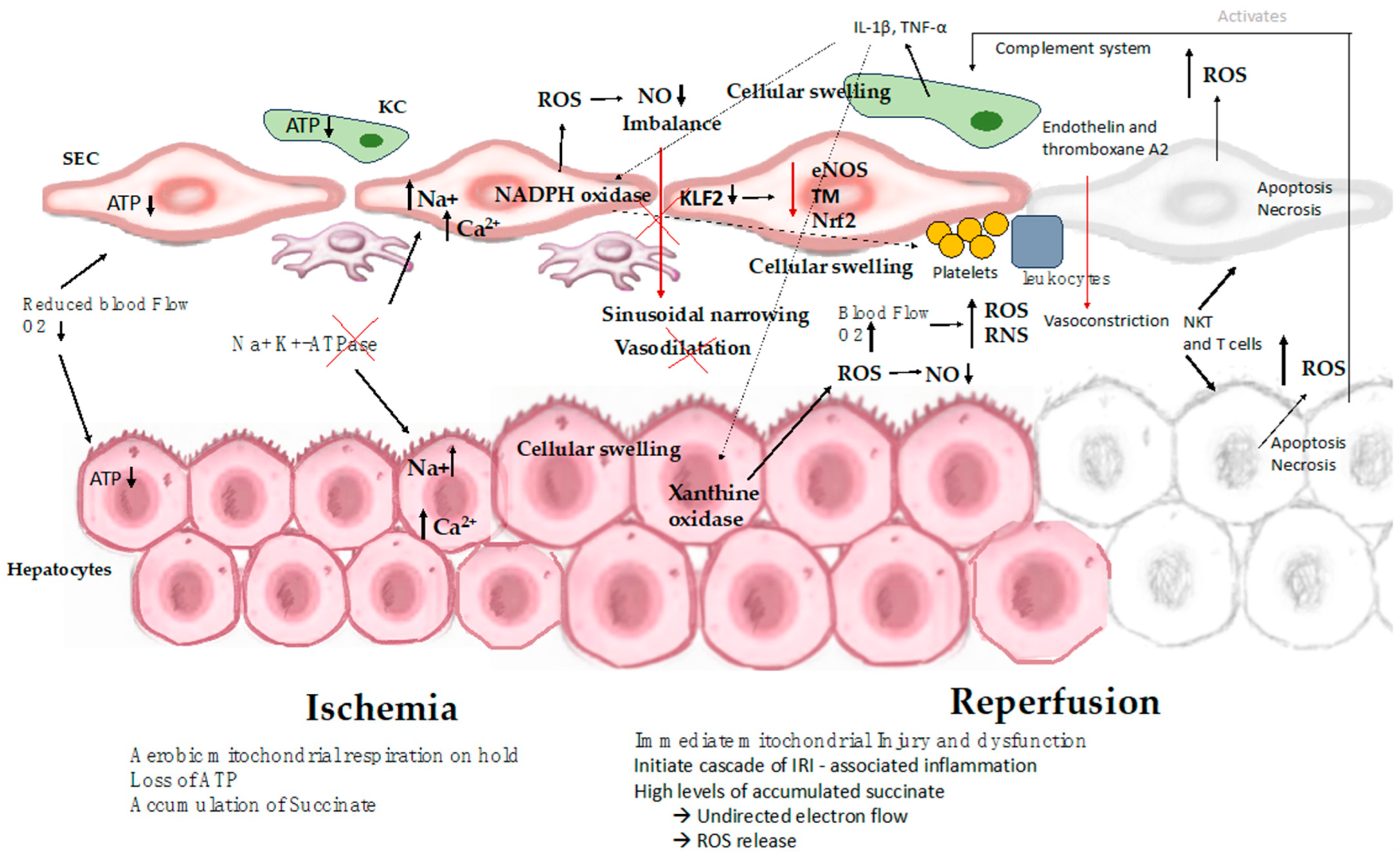
5. Pathophysiological Mechanisms of IRI
5.1. Pathophysiology of the Ischemic Cascade
5.1.1. Hypothermia-Induced Cell Swelling
5.1.2. Sinusoidal Endothelial Cell Injury
5.1.3. The Role of Mitochondria in IRI
5.2. Rewarming Injury
5.3. Reperfusion Injury
5.3.1. Events during Reperfusion
5.3.2. Mitochondrial Injury and Cell Death
5.3.3. Endothelial Cell Injury Associated with Platelet and Leukocyte Adhesion
5.3.4. Injury Caused by Sterile Inflammatory Immune Response
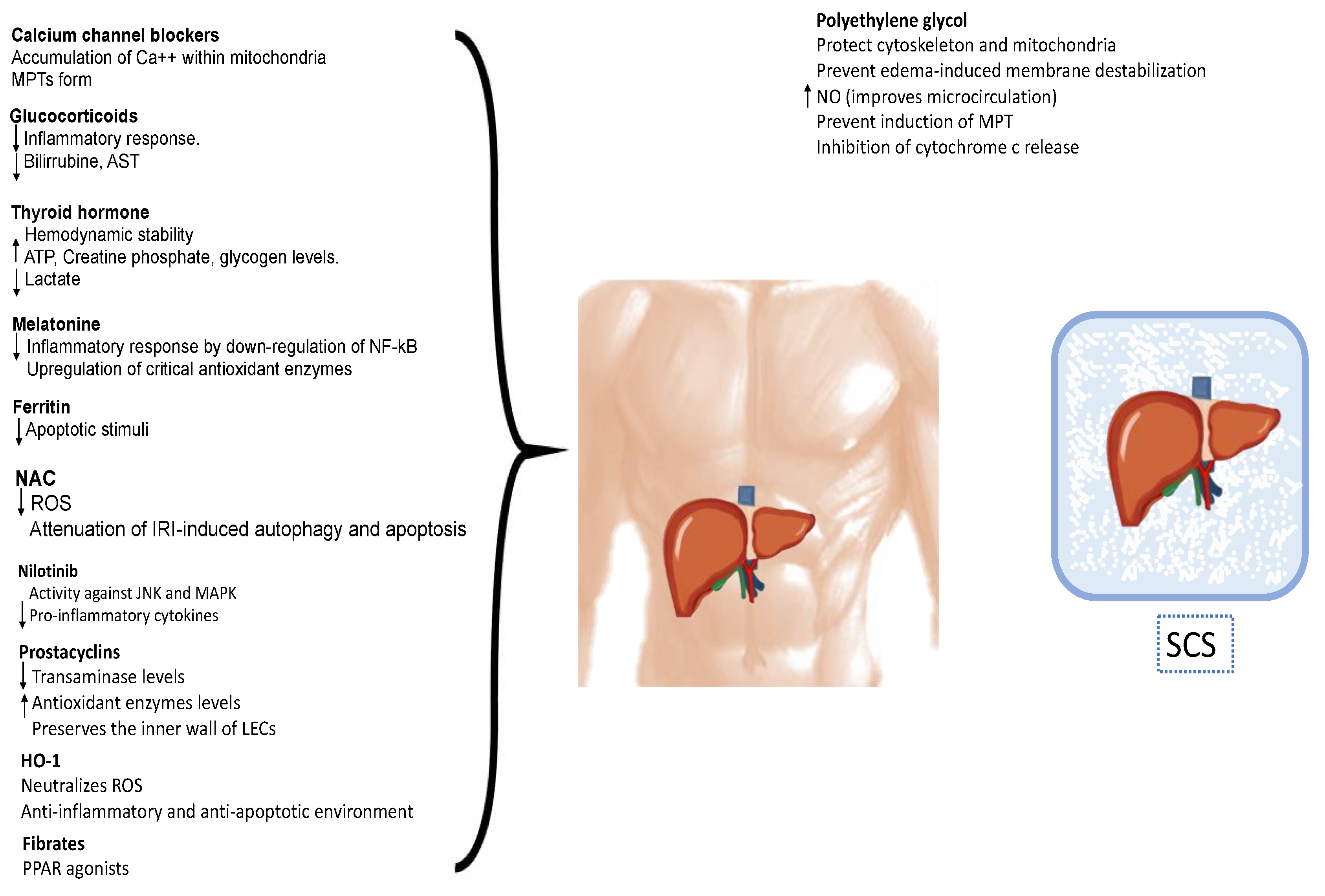
6. Pharmacological Strategies for IRI Modulation
6.1. Calcium Channel Blockers
6.2. Hormones
6.3. Antioxidants
6.4. Polyethylene Glycol
6.5. Other Agents
7. Dynamic Preservation
7.1. In Situ Normothermic Regional Perfusion
7.2. Ex Situ Normothermic Machine Perfusion
7.3. Ex Situ Hypothermic Machine Perfusion
7.4. Ex Situ Combined Hypothermic and Normothermic Dynamic Perfusion
8. Therapeutics Agents during Ex Vivo Machine Perfusion
8.1. Defatting Cocktail
8.2. Vasodilators
8.3. Other Therapeutics Agents
8.4. Senolytics
8.5. Gene Therapy
8.6. Mesenchymal Stem Cells
9. Summary
10. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Quaresima, S.; Melandro, F.; Giovanardi, F.; Shah, K.; De Peppo, V.; Mennini, G.; Ghinolfi, D.; Limkemann, A.; Pawlik, T.M.; Lai, Q. New Insights in the Setting of Transplant Oncology. Medicina 2023, 59, 568. [Google Scholar] [CrossRef] [PubMed]
- Vodkin, I.; Kuo, A. Extended Criteria Donors in Liver Transplantation. Clin. Liver Dis. 2017, 21, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.S.; Cassim, S.; Raymond, V.A.; Gottschalk, S.; Merlen, G.; Zwingmann, C.; Lapierre, P.; Darby, P.; Mazer, C.D.; Bilodeau, M. Upregulation of Krebs cycle and anaerobic glycolysis activity early after onset of liver ischemia. PLoS ONE 2018, 13, e0199177. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Naini, B.V.; Markovic, D.; Aziz, A.; Younan, S.; Lu, M.; Hirao, H.; Kadono, K.; Kojima, H.; DiNorcia, J.; et al. Ischemia-reperfusion injury and its relationship with early allograft dysfunction in liver transplant patients. Am. J. Transplant. 2021, 21, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Busuttil, R.W.; Tanaka, K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003, 9, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.F.; Palmeira, C.M.; Rolo, A.P. Preservation of Mitochondrial Health in Liver Ischemia/Reperfusion Injury. Biomedicines 2023, 11, 948. [Google Scholar] [CrossRef]
- Liu, J.; Man, K. Mechanistic Insight and Clinical Implications of Ischemia/Reperfusion Injury Post Liver Transplantation. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 1463–1474. [Google Scholar] [CrossRef]
- Schlegel, A.; Muller, X.; Mueller, M.; Stepanova, A.; Kron, P.; de Rougemont, O.; Muiesan, P.; Clavien, P.A.; Galkin, A.; Meierhofer, D.; et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. EBioMedicine. 2020, 60, 103014. [Google Scholar] [CrossRef]
- Tara, A.; Dominic, J.L.; Patel, J.N.; Garg, I.; Yeon, J.; Memon, M.S.; Gergal Gopalkrishna Rao, S.R.; Bugazia, S.; Dhandapani, T.P.M.; Kannan, A.; et al. Mitochondrial Targeting Therapy Role in Liver Transplant Preservation Lines: Mechanism and Therapeutic Strategies. Cureus 2021, 13, e16599. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Z.J.; Ma, X.; Li, K.; Li, X.Y.; Tang, Y.; Peng, C. Mitochondrial quality control in hepatic ischemia-reperfusion injury. Heliyon 2023, 9, e17702. [Google Scholar] [CrossRef]
- McCully, J.D.; Del Nido, P.J.; Emani, S.M. Mitochondrial transplantation for organ rescue. Mitochondrion 2022, 64, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Petrowsky, H.; Hong, J.C.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Ischaemia-reperfusion injury in liver transplantation—From bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 79–89. [Google Scholar] [CrossRef]
- Nair, A.; Hashimoto, K. Extended criteria donors in liver transplantation-from marginality to mainstream. Hepatobiliary Surg. Nutr. 2018, 7, 386–388. [Google Scholar] [CrossRef]
- Berthiaume, F.; Barbe, L.; Mokuno, Y.; MacDonald, A.D.; Jindal, R.; Yarmush, M.L. Steatosis reversibly increases hepatocyte sensitivity to hypoxia-reoxygenation injury. J. Surg. Res. 2009, 152, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Fukumori, T.; Ohkohchi, N.; Tsukamoto, S.; Satomi, S. The mechanism of injury in a steatotic liver graft during cold preservation. Transplantation 1999, 67, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Tien, C.; Remulla, D.; Kwon, Y.; Emamaullee, J. Contemporary strategies to assess and manage liver donor steatosis: A review. Curr. Opin. Organ Transplant. 2021, 26, 474–481. [Google Scholar] [CrossRef]
- Durand, F.; Renz, J.F.; Alkofer, B.; Burra, P.; Clavien, P.A.; Porte, R.J.; Freeman, R.B.; Belghiti, J. Report of the Paris consensus meeting on expanded criteria donors in liver transplantation. Liver Transpl. 2008, 14, 1694–1707. [Google Scholar] [CrossRef]
- de Graaf, E.L.; Kench, J.; Dilworth, P.; Shackel, N.A.; Strasser, S.I.; Joseph, D.; Pleass, H.; Crawford, M.; McCaughan, G.W.; Verran, D.J. Grade of deceased donor liver macrovesicular steatosis impacts graft and recipient outcomes more than the Donor Risk Index. J. Gastroenterol. Hepatol. 2012, 27, 540–546. [Google Scholar] [CrossRef]
- Spitzer, A.L.; Lao, O.B.; Dick, A.A.; Bakthavatsalam, R.; Halldorson, J.B.; Yeh, M.M.; Upton, M.P.; Reyes, J.D.; Perkins, J. The biopsied donor liver: Incorporating macrosteatosis into high-risk donor assessment. Liver Transpl. 2010, 16, 874–884. [Google Scholar] [CrossRef]
- Maeso-Díaz, R.; Ortega-Ribera, M.; Fernández-Iglesias, A.; Hide, D.; Muñoz, L.; Hessheimer, A.J.; Vila, S.; Francés, R.; Fonde-vila, C.; Albillos, A.; et al. Effects of aging on liver microcirculatory function and sinusoidal phenotype. Aging Cell. 2018, 17, e12829. [Google Scholar] [CrossRef]
- Jiménez-Romero, C.; Caso Maestro, O.; Cambra Molero, F.; Justo Alonso, I.; Alegre Torrado, C.; Manrique Municio, A.; Calvo Pulido, J.; Loinaz Segurola, C.; Moreno González, E. Using old liver grafts for liver transplantation: Where are the limits? World J. Gastroenterol. 2014, 20, 10691–10702. [Google Scholar] [CrossRef] [PubMed]
- von Horn, C.; Zlatev, H.; Pletz, J.; Lüer, B.; Minor, T. Comparison of thermal variations in post-retrieval graft conditioning on rat livers. Artif. Organs. 2022, 46, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Castro, M.B.; Cornide-Petronio, M.E.; Gracia-Sancho, J.; Peralta, C. Inflammasome-Mediated Inflammation in Liver Ischemia-Reperfusion Injury. Cells 2019, 8, 1131. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, S.; Shin, T.; Huber, N.; Eismann, T.; Galloway, E.; Schuster, R.; Blanchard, J.; Edwards, M.J.; Lentsch, A.B. Hepatocyte signaling through CXC chemokine receptor-2 is detrimental to liver recovery after ischemia/reperfusion in mice. Hepatology 2008, 48, 1213–1223. [Google Scholar] [CrossRef]
- Heylen, L.; Pirenne, J.; Naesens, M.; Sprangers, B.; Jochmans, I. “Time is tissue”-A minireview on the importance of donor nephrectomy, donor hepatectomy, and implantation times in kidney and liver transplantation. Am. J. Transplant. 2021, 21, 2653–2661. [Google Scholar] [CrossRef]
- de Vera, M.E.; Lopez-Solis, R.; Dvorchik, I.; Campos, S.; Morris, W.; Demetris, A.J.; Fontes, P.; Marsh, J.W. Liver transplantation using donation after cardiac death donors: Long-term follow-up from a single center. Am. J. Transplant. 2009, 9, 773–781. [Google Scholar] [CrossRef]
- Ho, K.J.; Owens, C.D.; Johnson, S.R.; Khwaja, K.; Curry, M.P.; Pavlakis, M.; Mandelbrot, D.; Pomposelli, J.J.; Shah, S.A.; Saidi, R.F.; et al. Donor postextubation hypotension and age correlate with outcome after donation after cardiac death transplantation. Transplantation 2008, 85, 1588–1594. [Google Scholar] [CrossRef]
- Balibrea, J.M.; Núñez-Peña, J.R.; García-Martín, M.C.; Olmedilla, Y.; Martín-Antona, E.; Berthuin, J.; Rancan, L.; Vara, E.; Balibrea, J.L. The differential tissue expression of inflammatory, oxidative stress, and apoptosis markers in human uncontrolled non-heart-beating donors. Transplantation 2013, 95, 1346–1353. [Google Scholar] [CrossRef]
- Southard, J.H.; Belzer, F.O. Organ preservation. Annu. Rev. Med. 1995, 46, 235–247. [Google Scholar] [CrossRef]
- Belzer, F.O.; Southard, J.H. Principles of solid-organ preservation by cold storage. Transplantation 1988, 45, 673–676. [Google Scholar] [CrossRef]
- Hertl, M.; Chartrand, P.B.; West, D.D.; Harvey, P.R.; Strasberg, S.M. The effects of hepatic preservation at 0 degrees C compared to 5 degrees C: Influence of antiproteases and periodic flushing. Cryobiology 1994, 31, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Villa, R.; Fondevila, C.; Erill, I.; Guimerà, A.; Bombuy, E.; Gómez-Suárez, C.; Sacristán, J.C.; García-Valdecasas, J.C. Real-time direct measurement of human liver allograft temperature from recovery to transplantation. Transplantation 2006, 81, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Hamamoto, I.; Nakamura, K.; Tanaka, K.; Ozawa, K. Isolated perfusion of rat livers: Effect of temperature on O2 consumption, enzyme release, energy store, and morphology. Nihon Geka Hokan 1993, 62, 58–70. [Google Scholar] [PubMed]
- Adam, R.; Delvart, V.; Karam, V.; Ducerf, C.; Navarro, F.; Letoublon, C.; Belghiti, J.; Pezet, D.; Castaing, D.; Le Treut, Y.P.; et al. Compared efficacy of preservation solutions in liver transplantation: A long-term graft outcome study from the European Liver Transplant Registry. Am. J. Transplant. 2015, 15, 395–406. [Google Scholar] [CrossRef]
- Kox, J.; Moers, C.; Monbaliu, D.; Strelniece, A.; Treckmann, J.; Jochmans, I.; Leuvenink, H.; Van Heurn, E.; Pirenne, J.; Paul, A.; et al. The Benefits of Hypothermic Machine Preservation and Short Cold Ischemia Times in Deceased Donor Kidneys. Transplantation 2018, 102, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, R.; Schurink, I.J.; de Vries, Y.; van den Berg, A.P.; Cortes Cerisuelo, M.; Darwish Murad, S.; Erdmann, J.I.; Gilbo, N.; de Haas, R.J.; Heaton, N.; et al. Hypothermic Machine Perfusion in Liver Transplantation—A Randomized Trial. N. Engl. J. Med. 2021, 384, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Czigany, Z.; Pratschke, J.; Froněk, J.; Guba, M.; Schöning, W.; Raptis, D.A.; Andrassy, J.; Kramer, M.; Strnad, P.; Tolba, R.H.; et al. Hypothermic Oxygenated Machine Perfusion Reduces Early Allograft Injury and Improves Post-transplant Outcomes in Extended Criteria Donation Liver Transplantation from Donation After Brain Death: Results from a Multicenter Randomized Controlled Trial (HOPE ECD-DBD). Ann. Surg. 2021, 274, 705–712. [Google Scholar] [CrossRef]
- de Vries, R.J.; Tessier, S.N.; Banik, P.D.; Nagpal, S.; Cronin, S.E.J.; Ozer, S.; Hafiz, E.O.A.; van Gulik, T.M.; Yarmush, M.L.; Markmann, J.F.; et al. Supercooling extends preservation time of human livers. Nat. Biotechnol. 2019, 37, 1131–1136. [Google Scholar] [CrossRef]
- Berendsen, T.A.; Bruinsma, B.G.; Puts, C.F.; Saeidi, N.; Usta, O.B.; Uygun, B.E.; Izamis, M.L.; Toner, M.; Yarmush, M.L.; Uygun, K. Supercooling enables long-term transplantation survival following 4 days of liver preservation. Nat. Med. 2014, 20, 790–793. [Google Scholar] [CrossRef]
- Goutard, M.; de Vries, R.J.; Tawa, P.; Pendexter, C.A.; Rosales, I.A.; Tessier, S.N.; Burlage, L.C.; Lantieri, L.; Randolph, M.A.; Lellouch, A.G.; et al. Exceeding the Limits of Static Cold Storage in Limb Transplantation Using Subnormothermic Machine Perfusion. J. Reconstr. Microsurg. 2023, 39, 350–360. [Google Scholar] [CrossRef]
- de Vries, R.J.; Tessier, S.N.; Banik, P.D.; Nagpal, S.; Cronin, S.E.J.; Ozer, S.; Hafiz, E.O.A.; van Gulik, T.M.; Yarmush, M.L.; Markmann, J.F.; et al. Subzero non-frozen preservation of human livers in the supercooled state. Nat. Protoc. 2020, 15, 2024–2040. [Google Scholar] [CrossRef] [PubMed]
- Puts, C.F.; Berendsen, T.A.; Bruinsma, B.G.; Ozer, S.; Luitje, M.; Usta, O.B.; Yarmush, M.L.; Uygun, K. Polyethylene glycol protects primary hepatocytes during supercooling preservation. Cryobiology 2015, 71, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M. Biology of cell survival in the cold: The basis for biopreservation of tissue and organs. In Advances in Biopreservation; CRC Press: Boca Raton, FL, USA, 2007; pp. 15–62. [Google Scholar]
- Russo, L.; Gracia-Sancho, J.; García-Calderó, H.; Marrone, G.; García-Pagán, J.C.; García-Cardeña, G.; Bosch, J. Addition of simvastatin to cold storage solution prevents endothelial dysfunction in explanted rat livers. Hepatology 2012, 55, 921–930. [Google Scholar] [CrossRef]
- Peralta, C.; Jiménez-Castro, M.B.; Gracia-Sancho, J. Hepatic ischemia and reperfusion injury: Effects on the liver sinusoidal milieu. J. Hepatol. 2013, 59, 1094–1106. [Google Scholar] [CrossRef]
- Pfanner, N.; Warscheid, B.; Wiedemann, N. Mitochondrial proteins: From biogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 2019, 20, 267–284, Correction in Nat. Rev. Mol. Cell Biol. 2021, 22, 367. [Google Scholar] [CrossRef]
- Oanh, N.T.K.; Lee, H.S.; Kim, Y.H.; Min, S.; Park, Y.J.; Heo, J.; Park, Y.Y.; Lim, W.C.; Cho, H. Regulation of nuclear DNA damage response by mitochondrial morphofunctional pathway. Nucleic Acids Res. 2022, 50, 9247–9259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yan, Q.; Wang, X.; Chen, X.; Chen, Y.; Du, J.; Chen, L. The Role of Mitochondria in Liver Ischemia-Reperfusion Injury: From Aspects of Mitochondrial Oxidative Stress, Mitochondrial Fission, Mitochondrial Membrane Permeable Transport Pore Formation, Mitophagy, and Mitochondria-Related Protective Measures. Oxid. Med. Cell Longev. 2021, 2021, 6670579. [Google Scholar] [CrossRef]
- Martin, J.L.; Costa, A.S.H.; Gruszczyk, A.V.; Beach, T.E.; Allen, F.M.; Prag, H.A.; Hinchy, E.C.; Mahbubani, K.; Hamed, M.; Tronci, L.; et al. Succinate accumulation drives ischaemia-reperfusion injury during organ transplantation. Nat. Metab. 2019, 1, 966–974. [Google Scholar] [CrossRef]
- Bouma, H.R.; Ketelaar, M.E.; Yard, B.A.; Ploeg, R.J.; Henning, R.H. AMP-activated protein kinase as a target for preconditioning in transplantation medicine. Transplantation 2010, 90, 353–358. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- van Golen, R.F.; van Gulik, T.M.; Heger, M. Mechanistic overview of reactive species-induced degradation of the endothelial glycocalyx during hepatic ischemia/reperfusion injury. Free Radic. Biol. Med. 2012, 52, 1382–1402. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Ferreira, J.C.; Gross, E.R.; Mochly-Rosen, D. Targeting aldehyde dehydrogenase 2: New therapeutic opportunities. Physiol. Rev. 2014, 94, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Forte, M.; Bianchi, F.; Cotugno, M.; Marchitti, S.; Stanzione, R.; Maglione, V.; Sciarretta, S.; Valenti, V.; Carnevale, R.; Versaci, F.; et al. An interplay between UCP2 and ROS protects cells from high-salt-induced injury through autophagy stimulation. Cell Death Dis. 2021, 12, 919. [Google Scholar] [CrossRef]
- Hirao, H.; Nakamura, K.; Kupiec-Weglinski, J.W. Liver ischaemia-reperfusion injury: A new understanding of the role of innate immunity. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 239–256. [Google Scholar] [CrossRef]
- Corbitt, N.; Kimura, S.; Isse, K.; Specht, S.; Chedwick, L.; Rosborough, B.R.; Lunz, J.G.; Murase, N.; Yokota, S.; Demetris, A.J.; et al. Gut bacteria drive Kupffer cell expansion via MAMP-mediated ICAM-1 induction on sinusoidal endothelium and influence preservation-reperfusion injury after orthotopic liver transplantation. Am. J. Pathol. 2013, 182, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Corbitt, N.; Kimura, S.; Isse, K.; Specht, S.; Chedwick, L.; Rosborough, B.R.; Lunz, J.G.; Murase, N.; Yokota, S.; Demetris, A.J. The membrane attack complex (C5b-9) in liver cold ischemia and reperfusion injury. Liver Transpl. 2008, 14, 1133–1141. [Google Scholar] [CrossRef]
- Trocha, M.; Szelag, A. The role of calcium and calcium channel blocking drugs in damage to the liver preserved for transplantation. Ann. Transplant. 2004, 9, 5–11. [Google Scholar]
- Saidi, R.F.; Chang, J.; Verb, S.; Brooks, S.; Nalbantoglu, I.; Adsay, V.; Jacobs, M.J. The effect of methylprednisolone on warm ischemia-reperfusion injury in the liver. Am. J. Surg. 2007, 193, 345–348. [Google Scholar] [CrossRef]
- Orci, L.A.; Toso, C.; Mentha, G.; Morel, P.; Majno, P.E. Systematic review and meta-analysis of the effect of perioperative steroids on ischaemia-reperfusion injury and surgical stress response in patients undergoing liver resection. Br. J. Surg. 2013, 100, 600–609. [Google Scholar] [CrossRef]
- Saidi, R.F.; Chang, J.; Verb, S.; Brooks, S.; Nalbantoglu, I.; Adsay, V.; Jacobs, M.J. Long-term normothermic perfusion of human livers for longer than 12 days. Artif. Organs 2022, 46, 2504–2510. [Google Scholar] [CrossRef]
- Salim, A.; Vassiliu, P.; Velmahos, G.C.; Sava, J.; Murray, J.A.; Belzberg, H.; Asensio, J.A.; Demetriades, D. The role of thyroid hormone administration in potential organ donors. Arch. Surg. 2001, 136, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo, R.A.; Van Erp, A.C.; Ottens, P.J.; Wiersema-Buist, J.; Leuvenink, H.G.; Romanque, P. Anti-Apoptotic Effects of 3,3’,5-Triiodo-L-Thyronine in the Liver of Brain-Dead Rats. PLoS ONE 2015, 10, e0138749. [Google Scholar] [CrossRef] [PubMed]
- Novitzky, D.; Mi, Z.; Sun, Q.; Collins, J.F.; Cooper, D.K. Thyroid hormone therapy in the management of 63,593 brain-dead organ donors: A retrospective analysis. Transplantation 2014, 98, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Z.T.; Jin, L.; Lin, J.; Fan, Z.L.; Zeng, Z.; Huang, H.F. Melatonin attenuates hepatic ischemia-reperfusion injury in rats by inhibiting NF-κB signaling pathway. Hepatobiliary Pancreat. Dis. Int. 2021, 20, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Li, B.; Duan, W.; Jing, L.; Zhang, B.; Zhang, M.; Yu, L.; Liu, Z.; Yu, B.; Ren, K.; et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J. Pineal Res. 2017, 63, 12419. [Google Scholar] [CrossRef]
- Coskun, A.; Yegen, C.; Arbak, S.; Attaallah, W.; Gunal, O.; Elmas, M.A.; Ucal, Y.; Can, O.; Baş, B.; Yildirim, Z.; et al. Melatonin in preservation solutions prevents ischemic injury in rat kidneys. PLoS ONE 2022, 17, e0273921. [Google Scholar] [CrossRef]
- Katwal, G.; Baral, D.; Fan, X.; Weiyang, H.; Zhang, X.; Ling, L.; Xiong, Y.; Ye, Q.; Wang, Y. SIRT3 a Major Player in Attenuation of Hepatic Ischemia-Reperfusion Injury by Reducing ROS via Its Downstream Mediators: SOD2, CYP-D, and HIF-1α. Oxid. Med. Cell. Longev. 2018, 2018, 2976957. [Google Scholar] [CrossRef]
- Ben Mosbah, I.; Mouchel, Y.; Pajaud, J.; Ribault, C.; Lucas, C.; Laurent, A.; Boudjema, K.; Morel, F.; Corlu, A.; Compagnon, P. Pretreatment with mangafodipir improves liver graft tolerance to ischemia/reperfusion injury in rat. PLoS ONE 2012, 7, e50235. [Google Scholar] [CrossRef]
- Dare, A.J.; Logan, A.; Prime, T.A.; Rogatti, S.; Goddard, M.; Bolton, E.M.; Bradley, J.A.; Pettigrew, G.J.; Murphy, M.P.; Saeb-Parsy, K. The mitochondria-targeted anti-oxidant MitoQ decreases ischemia-reperfusion injury in a murine syngeneic heart transplant model. J. Heart Lung Transplant. 2015, 34, 1471–1480. [Google Scholar] [CrossRef]
- James, A.M.; Sharpley, M.S.; Manas, A.R.; Frerman, F.E.; Hirst, J.; Smith, R.A.; Murphy, M.P. Interaction of the mitochondria-targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J. Biol. Chem. 2007, 282, 14708–14718. [Google Scholar] [CrossRef]
- Firuzi, O.; Miri, R.; Tavakkoli, M.; Saso, L. Antioxidant therapy: Current status and future prospects. Curr. Med. Chem. 2011, 18, 3871–3888. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, K.; Xia, Y.; Dai, W.; Wang, F.; Shen, M.; Cheng, P.; Wang, J.; Lu, J.; Zhang, Y.; et al. N-acetylcysteine attenuates ischemia-reperfusion-induced apoptosis and autophagy in mouse liver via regulation of the ROS/JNK/Bcl-2 pathway. PLoS ONE 2014, 9, e108855. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Panisello-Rosello, A.; Castro-Benitez, C.; Adam, R. Glycocalyx Preservation and NO Production in Fatty Livers-The Protective Role of High. Molecular Polyethylene Glycol in Cold Ischemia Injury. Int. J. Mol. Sci. 2018, 19, 2375. [Google Scholar] [CrossRef] [PubMed]
- Bardallo, R.G.; da Silva, R.T.; Carbonell, T.; Folch-Puy, E.; Palmeira, C.; Roselló-Catafau, J.; Pirenne, J.; Adam, R.; Panisello-Roselló, A. Role of PEG35, Mitochondrial ALDH2, and Glutathione in Cold Fatty Liver Graft Preservation: An IGL-2 Approach. Int. J. Mol. Sci. 2021, 22, 5332. [Google Scholar] [CrossRef]
- Asong-Fontem, N.; Panisello-Rosello, A.; Sebagh, M.; Gonin, M.; Rosello-Catafau, J.; Adam, R. The Role of IGL-2 Preservation Solution on Rat Livers during SCS and HOPE. Int. J. Mol. Sci. 2022, 23, 12615. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.T.; Bardallo, R.G.; Folch-Puy, E.; Carbonell, T.; Palmeira, C.M.; Fondevila, C.; Adam, R.; Roselló-Catafau, J.; Panisello-Roselló, A. IGL-2 as a Unique Solution for Cold Static Preservation and Machine Perfusion in Liver and Mitochondrial Protection. Transplant. Proc. 2022, 54, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Panisello Rosello, A.; Teixeira da Silva, R.; Castro, C.; G Bardallo, R.; Calvo, M.; Folch-Puy, E.; Carbonell, T.; Palmeira, C.; Roselló Catafau, J.; Adam, R. Polyethylene Glycol 35 as a Perfusate Additive for Mitochondrial and Glycocalyx Protection in HOPE Liver Preservation. Int. J. Mol. Sci. 2020, 21, 5703. [Google Scholar] [CrossRef]
- Ocuin, L.M.; Zeng, S.; Cavnar, M.J.; Sorenson, E.C.; Bamboat, Z.M.; Greer, J.B.; Kim, T.S.; Popow, R.; DeMatteo, R.P. Nilotinib protects the murine liver from ischemia/reperfusion injury. J. Hepatol. 2012, 57, 766–773. [Google Scholar] [CrossRef]
- Gedik, E.; Girgin, S.; Obay, B.D.; Ozturk, H.; Ozturk, H.; Buyukbayram, H. Iloprost, a prostacyclin (PGI2) analogue, reduces liver injury in hepatic ischemia-reperfusion in rats. Acta Cir. Bras. 2009, 24, 226–232. [Google Scholar] [CrossRef]
- Ghonem, N.; Yoshida, J.; Stolz, D.B.; Humar, A.; Starzl, T.E.; Murase, N.; Venkataramanan, R. Treprostinil, a prostacyclin analog, ameliorates ischemia-reperfusion injury in rat orthotopic liver transplantation. Am. J. Transplant. 2011, 11, 2508–2516. [Google Scholar] [CrossRef]
- Zhang, M.; Nakamura, K.; Kageyama, S.; Lawal, A.O.; Gong, K.W.; Bhetraratana, M.; Fujii, T.; Sulaiman, D.; Hirao, H.; Bolisetty, S.; et al. Myeloid HO-1 modulates macrophage polarization and protects against ischemia-reperfusion injury. JCI Insight. 2018, 3, e120596. [Google Scholar] [CrossRef]
- Hirao, H.; Dery, K.J.; Kageyama, S.; Nakamura, K.; Kupiec-Weglinski, J.W. Heme Oxygenase-1 in liver transplant ischemia-reperfusion injury: From bench-to-bedside. Free Radic. Biol. Med. 2020, 157, 75–82. [Google Scholar] [CrossRef]
- Kaur, J.; Kaur, T.; Sharma, A.K.; Kaur, J.; Yadav, H.N.; Pathak, D.; Singh, A.P. Fenofibrate attenuates ischemia reperfusion-induced acute kidney injury and associated liver dysfunction in rats. Drug Dev. Res. 2021, 82, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, N.V.; Johnston, P.S.; O’Grady, J.G.; Smith, M.F.; Friend, P.J.; Rolles, K.; Calne, R.Y. Clinical use of UW solution or a simplified liver preservation solution prior to transplantation in 179 human livers, December 1987–July 1989. Transplant Proc. 1990, 22, 2189–2190. [Google Scholar] [PubMed]
- Nasralla, D.; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; García-Valdecasas, J.C.; Heaton, N.; et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Markmann, J.F.; Abouljoud, M.S.; Ghobrial, R.M.; Bhati, C.S.; Pelletier, S.J.; Lu, A.D.; Ottmann, S.; Klair, T.; Eymard, C.; Roll, G.R.; et al. Impact of Portable Normothermic Blood-Based Machine Perfusion on Outcomes of Liver Transplant: The OCS Liver PROTECT Randomized Clinical Trial. JAMA Surg. 2022, 157, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.E.; Kosmoliaptsis, V.; Pley, C.; Randle, L.; Fear, C.; Crick, K.; Gimson, A.E.; Allison, M.; Upponi, S.; Brais, R.; et al. Observations on the ex situ perfusion of livers for transplantation. Am. J. Transplant. 2018, 18, 2005–2020. [Google Scholar] [CrossRef]
- Ravaioli, M.; Germinario, G.; Dajti, G.; Sessa, M.; Vasuri, F.; Siniscalchi, A.; Morelli, M.C.; Serenari, M.; Del Gaudio, M.; Zanfi, C.; et al. Hypothermic oxygenated perfusion in extended criteria donor liver transplantation-A randomized clinical trial. Am. J. Transplant. 2022, 22, 2401–2408. [Google Scholar] [CrossRef]
- Guarrera, J.V.; Henry, S.D.; Samstein, B.; Odeh-Ramadan, R.; Kinkhabwala, M.; Goldstein, M.J.; Ratner, L.E.; Renz, J.F.; Lee, H.T.; Brown, R.S., Jr.; et al. Hypothermic machine preservation in human liver transplantation: The first clinical series. Am. J. Transplant. 2010, 10, 372–381. [Google Scholar] [CrossRef]
- Hessheimer, A.J.; Coll, E.; Torres, F.; Ruíz, P.; Gastaca, M.; Rivas, J.I.; Gómez, M.; Sánchez, B.; Santoyo, J.; Ramírez, P.; et al. Normothermic regional perfusion vs. super-rapid recovery in controlled donation after circulatory death liver transplantation. J. Hepatol. 2019, 70, 658–665. [Google Scholar] [CrossRef]
- Fondevila, C.; Hessheimer, A.J.; Ruiz, A.; Calatayud, D.; Ferrer, J.; Charco, R.; Fuster, J.; Navasa, M.; Rimola, A.; Taurá, P.; et al. Liver transplant using donors after unexpected cardiac death: Novel preservation protocol and acceptance criteria. Am. J. Transplant. 2007, 7, 1849–1855. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Hunt, F.; Messer, S.; Currie, I.; Large, S.; Sutherland, A.; Crick, K.; Wigmore, S.J.; Fear, C.; Cornateanu, S.; et al. In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. Am. J. Transplant. 2019, 19, 1745–1758. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, R.; Butler, A.J.; Kosmoliaptsis, V.; Mumford, L.; Fear, C.; Swift, L.; Fedotovs, A.; Upponi, S.; Khwaja, S.; Richards, J.; et al. Liver Transplantation Outcomes from Controlled Circulatory Death Donors: SCS vs in situ NRP vs ex situ NMP. Ann. Surg. 2022, 275, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Oniscu, G.C.; Mehew, J.; Butler, A.J.; Sutherland, A.; Gaurav, R.; Hogg, R.; Currie, I.; Jones, M.; Watson, C.J.E.l. Improved Organ Utilization and Better Transplant Outcomes with In Situ Normothermic Regional Perfusion in Controlled Donation After Circulatory Death. Transplantation. 2023, 107, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Mergental, H.; Laing, R.W.; Kirkham, A.J.; Perera, M.T.P.R.; Boteon, Y.L.; Attard, J.; Barton, D.; Curbishley, S.; Wilkhu, M.; Neil, D.A.H.; et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat. Commun. 2020, 11, 2939. [Google Scholar] [CrossRef] [PubMed]
- Bral, M.; Dajani, K.; Leon Izquierdo, D.; Bigam, D.; Kneteman, N.; Ceresa, C.D.L.; Friend, P.J.; Shapiro, A.M.J. A Back-to-Base Experience of Human. Normothermic Ex. Situ Liver Perfusion: Does the Chill Kill? Liver Transplant. 2019, 25, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Ghinolfi, D.; Rreka, E.; De Tata, V.; Franzini, M.; Pezzati, D.; Fierabracci, V.; Masini, M.; Cacciatoinsilla, A.; Bindi, M.L.; Marselli, L.; et al. Pilot, Open, Randomized, Prospective Trial for Normothermic Machine Perfusion Evaluation in Liver Transplantation From Older Donors. Liver Transplant. 2019, 25, 436–449. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Gaurav, R.; Fear, C.; Swift, L.; Selves, L.; Ceresa, C.D.L.; Upponi, S.S.; Brais, R.; Allison, M.; Macdonald-Wallis, C.; et al. Predicting Early Allograft Function After Normothermic Machine Perfusion. Transplantation 2022, 106, 2391–2398. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, Q.; Huang, S.; Huang, C.; Wang, D.; Yang, L.; Zhang, J.; Chen, M.; Wu, L.; Zhang, Z.; et al. Ischaemia-free liver transplantation in humans: A first-in-human trial. Lancet Reg. Health West. Pac. 2021, 16, 100260. [Google Scholar] [CrossRef]
- Clavien, P.A.; Dutkowski, P.; Mueller, M.; Eshmuminov, D.; Bautista Borrego, L.; Weber, A.; Muellhaupt, B.; Sousa Da Silva, R.X.; Burg, B.R.; Rudolf von Rohr, P.; et al. Transplantation of a human liver following 3 days of ex situ normothermic preservation. Nat. Biotechnol. 2022, 40, 1610–1616. [Google Scholar] [CrossRef]
- Eshmuminov, D.; Becker, D.; Bautista Borrego, L.; Hefti, M.; Schuler, M.J.; Hagedorn, C.; Muller, X.; Mueller, M.; Onder, C.; Graf, R.; et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat. Biotechnol. 2020, 38, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Parente, A.; Flores Carvalho, M.; Schlegel, A. Endothelial Cells and Mitochondria: Two Key Players in Liver Transplantation. Int. J. Mol. Sci. 2023, 24, 10091. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; de Rougemont, O.; Graf, R.; Clavien, P.A.; Dutkowski, P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J. Hepatol. 2013, 58, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Patrono, D.; Cussa, D.; Sciannameo, V.; Montanari, E.; Panconesi, R.; Berchialla, P.; Lepore, M.; Gambella, A.; Rizza, G.; Catalano, G.; et al. Outcome of liver transplantation with grafts from brain-dead donors treated with dual hypothermic oxygenated machine perfusion, with particular reference to elderly donors. Am. J. Transplant. 2022, 22, 1382–1395. [Google Scholar] [CrossRef]
- Jochmans, I.; Brat, A.; Davies, L.; Hofker, H.S.; van de Leemkolk, F.E.M.; Leuvenink, H.G.D.; Knight, S.R.; Pirenne, J.; Ploeg, R.J. Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): A randomised, double-blind, paired, phase 3 trial. Lancet 2020, 396, 1653–1662, Correction in Lancet 2021, 396, 1978. [Google Scholar] [CrossRef]
- Wyss, R.K.; Méndez Carmona, N.; Arnold, M.; Segiser, A.; Mueller, M.; Dutkowski, P.; Carrel, T.P.; Longnus, S.L. Hypothermic, oxygenated perfusion (HOPE) provides cardioprotection via succinate oxidation prior to normothermic perfusion in a rat model of donation after circulatory death (DCD). Am. J. Transplant. 2021, 21, 1003–1011. [Google Scholar] [CrossRef]
- Muller, X.; Schlegel, A.; Kron, P.; Eshmuminov, D.; Würdinger, M.; Meierhofer, D.; Clavien, P.A.; Dutkowski, P. Novel Real-time Prediction of Liver Graft Function During Hypothermic Oxygenated Machine Perfusion Before Liver Transplantation. Ann. Surg. 2019, 270, 783–790. [Google Scholar] [CrossRef]
- Scholz, R.; Thurman, R.G.; Williamson, J.R.; Chance, B.; Bücher, T. Flavin and pyridine nucleotide oxidation-reduction changes in perfused rat liver. I. Anoxia and subcellular localization of fluorescent flavoproteins. J. Biol. Chem. 1969, 244, 2317–2324. [Google Scholar] [CrossRef]
- Minor, T.; Efferz, P.; Fox, M.; Wohlschlaeger, J.; Lüer, B. Controlled oxygenated rewarming of cold stored liver grafts by thermally graduated machine perfusion prior to reperfusion. Am. J. Transplant. 2013, 13, 1450–1460. [Google Scholar] [CrossRef]
- an Leeuwen, O.B.; Bodewes, S.B.; Lantinga, V.A.; Haring, M.P.D.; Thorne, A.M.; Brüggenwirth, I.M.A.; van den Berg, A.P.; de Boer, M.T.; de Jong, I.E.M.; de Kleine, R.H.J.; et al. Sequential hypothermic and normothermic machine perfusion enables safe transplantation of high-risk donor livers. Am. J. Transplant. 2022, 22, 1658–1670. [Google Scholar] [CrossRef]
- Goldaracena, N.; Echeverri, J.; Spetzler, V.N.; Kaths, J.M.; Barbas, A.S.; Louis, K.S.; Adeyi, O.A.; Grant, D.R.; Selzner, N.; Selzner, M. Anti-inflammatory signaling during ex vivo liver perfusion improves the preservation of pig liver grafts before transplantation. Liver Transplant. 2016, 22, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Beal, E.W.; Kim, J.L.; Reader, B.F.; Akateh, C.; Maynard, K.; Washburn, W.K.; Zweier, J.L.; Whitson, B.A.; Black, S.M. [D-Ala2, D-Leu5] Enkephalin Improves Liver Preservation During Normothermic Ex Vivo Perfusion. J. Surg. Res. 2019, 241, 323–335. [Google Scholar] [CrossRef]
- Yu, Y.; Cheng, Y.; Pan, Q.; Zhang, Y.J.; Jia, D.G.; Liu, Y.F. Effect of the Selective NLRP3 Inflammasome Inhibitor mcc950 on Transplantation Outcome in a Pig Liver Transplantation Model With Organs From Donors After Circulatory Death Preserved by Hypothermic Machine Perfusion. Transplantation 2019, 103, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Akamatsu, Y.; Maida, K.; Kashiwadate, T.; Kobayashi, Y.; Ohuchi, N.; Satomi, S. A new liver graft preparation method for uncontrolled non-heart-beating donors, combining short oxygenated warm perfusion and prostaglandin E1. J. Surg. Res. 2013, 184, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Maida, K.; Akamatsu, Y.; Hara, Y.; Tokodai, K.; Miyagi, S.; Kashiwadate, T.; Miyazawa, K.; Kawagishi, N.; Ohuchi, N. Short Oxygenated Warm Perfusion With Prostaglandin E1 Administration Before Cold Preservation as a Novel Resuscitation Method for Liver Grafts From Donors After Cardiac Death in a Rat In Vivo Model. Transplantation 2016, 100, 1052–1058. [Google Scholar] [CrossRef]
- Nassar, A.; Liu, Q.; Farias, K.; D’Amico, G.; Buccini, L.; Urcuyo, D.; Kelly, D.; Hashimoto, K.; Eghtesad, B.; Uso, T.D.; et al. Role of vasodilation during normothermic machine perfusion of DCD porcine livers. Int. J. Artif. Organs 2014, 37, 165–172. [Google Scholar] [CrossRef]
- Echeverri, J.; Goldaracena, N.; Kaths, J.M.; Linares, I.; Roizales, R.; Kollmann, D.; Hamar, M.; Urbanellis, P.; Ganesh, S.; Adeyi, O.A.; et al. Comparison of BQ123, Epoprostenol, and Verapamil as Vasodilators During Normothermic Ex Vivo Liver Machine Perfusion. Transplantation 2018, 102, 601–608. [Google Scholar] [CrossRef]
- Nagrath, D.; Xu, H.; Tanimura, Y.; Zuo, R.; Berthiaume, F.; Avila, M.; Yarmush, R.; Yarmush, M.L. Metabolic preconditioning of donor organs: Defatting fatty livers by normothermic perfusion ex vivo. Metab. Eng. 2009, 11, 274–283. [Google Scholar] [CrossRef]
- Liu, Q.; Berendsen, T.; Izamis, M.L.; Uygun, B.; Yarmush, M.L.; Uygun, K. Perfusion defatting at subnormothermic temperatures in steatotic rat livers. Transplant. Proc. 2013, 45, 3209–3213. [Google Scholar] [CrossRef]
- Boteon, Y.L.; Attard, J.; Boteon, A.P.C.S.; Wallace, L.; Reynolds, G.; Hubscher, S.; Mirza, D.F.; Mergental, H.; Bhogal, R.H.; Afford, S. Manipulation of Lipid Metabolism During Normothermic Machine Perfusion: Effect of Defatting Therapies on Donor Liver Functional Recovery. Liver Transplant. 2019, 25, 1007–1022. [Google Scholar] [CrossRef]
- Lin, F.; Zhen, F.; Yan, X.; Shaojun, Y.; Guizhu, P.; Yanfeng, W.; Qifa, Y. Hypothermic oxygenated perfusion with defatting cocktail further improves steatotic liver grafts in a transplantation rat model. Artif. Organs 2021, 45, E304–E316. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, H.; Kuroda, S.; Mikuriya, Y.; Ohdan, H. Ischemia–reperfusion injury in patients with fatty liver and the clinical impact of steatotic liver on hepatic surgery. Surg. Today 2014, 44, 1611–1625. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, R.W.; Zilvetti, M.; Roy, D.; Hughes, D.; Morovat, A.; Coussios, C.C.; Friend, P.J. Hepatic steatosis and normothermic perfusion-preliminary experiments in a porcine model. Transplantation 2011, 92, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Seal, J.B.; Gewertz, B.L. Vascular dysfunction in ischemia-reperfusion injury. Ann. Vasc. Surg. 2005, 19, 572–584. [Google Scholar] [CrossRef]
- Reddy, S.P.; Bhattacharjya, S.; Maniakin, N.; Greenwood, J.; Guerreiro, D.; Hughes, D.; Imber, C.J.; Pigott, D.W.; Fuggle, S.; Taylor, R.; et al. Preservation of porcine non-heart-beating donor livers by sequential cold storage and warm perfusion. Transplantation 2004, 77, 1328–1332. [Google Scholar] [CrossRef] [PubMed]
- Korolchuk, V.I.; Miwa, S.; Carroll, B.; von Zglinicki, T. Mitochondria in Cell Senescence: Is Mitophagy the Weakest Link? EBioMedicine 2017, 21, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Bernadotte, A.; Mikhelson, V.M.; Spivak, I.M. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging 2016, 8, 3–11. [Google Scholar] [CrossRef]
- Gasek, N.S.; Kuchel, G.A.; Kirkland, J.L.; Xu, M. Strategies for Targeting Senescent Cells in Human Disease. Nat. Aging 2021, 1, 870–879. [Google Scholar] [CrossRef]
- Matsunaga, T.; Iske, J.; Schroeter, A.; Azuma, H.; Zhou, H.; Tullius, S.G. The potential of Senolytics in transplantation. Mech. Ageing Dev. 2021, 200, 111582. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, S.; Shi, Y.; Tian, N.; Zhou, Y.; Zhang, X. Senolytics: Eliminating Senescent Cells and Alleviating Intervertebral Disc Degeneration. Front. Bioeng. Biotechnol. 2022, 10, 823945. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef] [PubMed]
- Iske, J.; Seyda, M.; Heinbokel, T.; Maenosono, R.; Minami, K.; Nian, Y.; Quante, M.; Falk, C.S.; Azuma, H.; Martin, F.I.; et al. Senolytics prevent mt-DNA-induced inflammation and promote the survival of aged organs following transplantation. Nat. Commun. 2020, 11, 4289. [Google Scholar] [CrossRef]
- Thompson, W.S.; Mondal, G.; Vanlith, C.J.; Kaiser, R.A.; Lillegard, J.B. The future of gene-targeted therapy for hereditary tyrosinemia type 1 as a lead indication among the inborn errors of metabolism. Expert. Opin. Orphan Drugs. 2020, 8, 245–256. [Google Scholar] [CrossRef]
- Goldaracena, N.; Spetzler, V.N.; Echeverri, J.; Kaths, J.M.; Cherepanov, V.; Persson, R.; Hodges, M.R.; Janssen, H.L.; Selzner, N.; Grant, D.R.; et al. Inducing Hepatitis C Virus Resistance After Pig Liver Transplantation-A Proof of Concept of Liver Graft Modification Using Warm Ex Vivo Perfusion. Am. J. Transplant. 2017, 17, 970–978. [Google Scholar] [CrossRef]
- Gillooly, A.R.; Perry, J.; Martins, P.N. First Report of siRNA Uptake (for RNA Interference) During Ex Vivo Hypothermic and Normothermic Liver Machine Perfusion. Transplantation 2019, 103, e56–e57. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, M.F.; Brüggenwirth, I.M.A.; Gillooly, A.; Khvorova, A.; Kowalik, T.F.; Martins, P.N. Gene Silencing With siRNA (RNA Interference): A New Therapeutic Option During Ex Vivo Machine Liver Perfusion Preservation. Liver Transplant. 2019, 25, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi-Riani, E.; Gillooly, A.R.; Iesari, S.; Brüggenwirth, I.M.A.; Ferguson, C.M.; Komuta, M.; Xhema, D.; Daumerie, A.; Maistriaux, L.; Leuvenink, H.; et al. Delivering siRNA Compounds During HOPE to Modulate Organ Function: A Proof-of-concept Study in a Rat Liver Transplant Model. Transplantation 2022, 106, 1565–1576. [Google Scholar] [CrossRef]
- Zhao, G.; Fu, C.; Wang, L.; Zhu, L.; Yan, Y.; Xiang, Y.; Zheng, F.; Gong, F.; Chen, S.; Chen, G. Down-regulation of nuclear HMGB1 reduces ischemia-induced HMGB1 translocation and release and protects against liver ischemia-reperfusion injury. Sci. Rep. 2017, 7, 46272. [Google Scholar] [CrossRef]
- Contreras, J.L.; Vilatoba, M.; Eckstein, C.; Bilbao, G.; Anthony Thompson, J.; Eckhoff, D.E. Caspase-8 and caspase-3 small interfering RNA decreases ischemia/reperfusion injury to the liver in mice. Surgery 2004, 136, 390–400. [Google Scholar] [CrossRef]
- Wang, G.; Wan, L.; Zhang, L.; Yan, C.; Zhang, Y. MicroRNA-133a Regulates the Viability and Differentiation Fate of Bone Marrow Mesenchymal Stem Cells via MAPK/ERK Signaling Pathway by Targeting FGFR1. DNA Cell Biol. 2021, 40, 1112–1123. [Google Scholar] [CrossRef]
- Andersson, P.; Gidlöf, O.; Braun, O.O.; Götberg, M.; van der Pals, J.; Olde, B.; Erlinge, D. Plasma levels of liver-specific miR-122 is massively increased in a porcine cardiogenic shock model and attenuated by hypothermia. Shock 2012, 37, 234–238. [Google Scholar] [CrossRef]
- Van Caster, P.; Brandenburger, T.; Strahl, T.; Metzger, S.; Bauer, I.; Pannen, B.; Braun, S. Circulating microRNA-122, -21 and -223 as potential markers of liver injury following warm ischaemia and reperfusion in rats. Mol. Med. Rep. 2015, 12, 3146–3150. [Google Scholar] [CrossRef] [PubMed]
- Vandermeulen, M.; Erpicum, P.; Weekers, L.; Briquet, A.; Lechanteur, C.; Detry, O.; Beguin, Y.; Jouret, F. Mesenchymal Stromal Cells in Solid Organ Transplantation. Transplantation 2020, 104, 923–936. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef]
- Gao, L.; Mei, S.; Zhang, S.; Qin, Q.; Li, H.; Liao, Y.; Fan, H.; Liu, Z.; Zhu, H. Cardio-renal Exosomes in Myocardial Infarction Serum Regulate Proangiogenic Paracrine Signaling in Adipose Mesenchymal Stem Cells. Theranostics 2020, 10, 1060–1073. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, F.; Todeschini, M.; Azzollini, N.; Cravedi, P.; Cassis, P.; Solini, S.; Fiori, S.; Rota, C.; Karachi, A.; Carrara, C.; et al. Effect of Timing and Complement Receptor Antagonism on Intragraft Recruitment and Protolerogenic Effects of Mesenchymal Stromal Cells in Murine Kidney Transplantation. Transplantation 2019, 103, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Sasajima, H.; Miyagi, S.; Kakizaki, Y.; Kamei, T.; Unno, M.; Satomi, S.; Goto, M. Cytoprotective Effects of Mesenchymal Stem Cells During Liver Transplantation from Donors After Cardiac Death in Rats. Transplant. Proc. 2018, 50, 2815–2820. [Google Scholar] [CrossRef]
- Rigo, F.; De Stefano, N.; Navarro-Tableros, V.; David, E.; Rizza, G.; Catalano, G.; Gilbo, N.; Maione, F.; Gonella, F.; Roggio, D.; et al. Extracellular Vesicles from Human Liver Stem Cells Reduce Injury in an Ex Vivo Normothermic Hypoxic Rat Liver Perfusion Model. Transplantation 2018, 102, e205–e210. [Google Scholar] [CrossRef]
- Yang, L.; Cao, H.; Sun, D.; Hou, B.; Lin, L.; Shen, Z.Y.; Song, H.L. Bone marrow mesenchymal stem cells combine with normothermic machine perfusion to improve rat donor liver quality-the important role of hepatic microcirculation in donation after circulatory death. Cell Tissue Res. 2020, 381, 239–254. [Google Scholar] [CrossRef]
- Verstegen, M.M.A.; Mezzanotte, L.; Ridwan, R.Y.; Wang, K.; de Haan, J.; Schurink, I.J.; Sierra Parraga, J.M.; Hoogduijn, M.; Kessler, B.M.; Huang, H.; et al. First Report on Ex Vivo Delivery of Paracrine Active Human Mesenchymal Stromal Cells to Liver Grafts During Machine Perfusion. Transplantation 2020, 104, e5–e7. [Google Scholar] [CrossRef] [PubMed]
- Laing, R.W.; Stubblefield, S.; Wallace, L.; Roobrouck, V.D.; Bhogal, R.H.; Schlegel, A.; Boteon, Y.L.; Reynolds, G.M.; Ting, A.E.; Mirza, D.F.; et al. The Delivery of Multipotent Adult Progenitor Cells to Extended Criteria Human Donor Livers Using Normothermic Machine Perfusion. Front. Immunol. 2020, 11, 1226. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Yang, L.; Zheng, W.; Cao, H.; Wu, L.; Song, H. Protective Effects of Bone Marrow Mesenchymal Stem Cells (BMMSCS) Combined with Normothermic Machine Perfusion on Liver Grafts Donated After Circulatory Death via Reducing the Ferroptosis of Hepatocytes. Med. Sci. Monit. 2021, 27, e930258. [Google Scholar] [CrossRef]
- De Stefano, N.; Navarro-Tableros, V.; Roggio, D.; Calleri, A.; Rigo, F.; David, E.; Gambella, A.; Bassino, D.; Amoroso, A.; Patrono, D.; et al. Human liver stem cell-derived extracellular vesicles reduce injury in a model of normothermic machine perfusion of rat livers previously exposed to a prolonged warm ischemia. Transpl. Int. 2021, 34, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Karangwa, S.A.; Dutkowski, P.; Fontes, P.; Friend, P.J.; Guarrera, J.V.; Markmann, J.F.; Mergental, H.; Minor, T.; Quintini, C.; Selzner, M.; et al. Machine Perfusion of Donor Livers for Transplantation: A Proposal for Standardized Nomenclature and Reporting Guidelines. Am. J. Transplant. 2016, 16, 2932–2942. [Google Scholar] [CrossRef]

| BEL-GEN (UW) | PERF-GEN (Belzer MPS) * | CE | HTK | HTK-N | IGL-1 | IGL-2 | |
|---|---|---|---|---|---|---|---|
| Electrolytes (mmol/L) Potassium | 125 | 25 | 100 | 10 | 10 | 30 | 25 |
| Sodium | 30 | 120 | 15 | 15 | 16 | 120 | 125 |
| Magnesium | 5 | 5 | 13 | 4 | 8 | 5 | 5 |
| Chloride | 1 | 50 | 30 | 20 | |||
| Calcium | 0.25 | 0.015 | 0.02 | ||||
| Zinc | 0.091 | ||||||
| Impermeant substances (mmol/L) | |||||||
| Lactobionic acid | 100 | 80 | 100 | 100 | |||
| Mannitol | 30 | 60 | 60 | ||||
| Gluconate | 85 | 30 | |||||
| Ribose | 5 | ||||||
| Raffinose | 30 | 30 | |||||
| Glucose | 10 | 33 | |||||
| Oncotic agents (g/L) | |||||||
| HES | 50 | 50 | |||||
| PEG-35 | 1 | 5 | |||||
| Buffers | Phosphate Sulfate | Phosphate HEPES | Histidine Bicarbonate | Histidine | Histidine N-acetylhistidine | Phosphate | Phosphate |
| HEPES | |||||||
| Histidine | |||||||
| Sulfate | |||||||
| Antioxidants | Glutathione Allopurinol | Glutathione | Glutathione | Tryptophan | Tryptophan | Glutathione Allopurinol | Glutathione |
| Metabolic precursors | Adenosine | Adenine | α-ketoglutarate | α-ketoglutarate | Adenosine | Adenosine Sodium nitrite | |
| Alanine | |||||||
| Arginine | |||||||
| Aspartate | |||||||
| Glycine | |||||||
| Osmolarity (mOsm/L) | 320 | 300 | 320–360 | 310 | 305 | 320 | 360 |
| pH | 7.4 | 7.4 | 7.3 | 7.2 | 7.0 | 7.4 | 7.4 |
| Viscosity (cP) | 5.7 | 2.40 | 1.15 | 1.8 | 1.8 | 1.28 | 1.7 |
| Author/Year | Function | Therapeutic Agents | Perfusion | Model | Time in MP | Outcomes |
|---|---|---|---|---|---|---|
| Goldaracena et al., 2016 [112] | Anti-inflammatory agents |
| SNMP | Pig | 4 h |
|
| Beal et al., 2019 [113] | δ-opioid agonist | Enkephalin | NMP | Murine | 4 h |
|
| Yu et al., 2019 [114] | NLRP3 inflammasome inhibitor | mcc950 | HMP | Pig | 2 h |
|
| Hara et al., 2013 [115] | Vasodilators | Prostaglandin E1 | NMP | Murine | 1 h |
|
| Maida et al., 2016 [116] | Vasodilators | Prostaglandin E1 | NMP | Murine | 30 min |
|
| Nassar et al., 2014 [117] | Vasodilators | Prostacyclin (epoprostenol) | NMP | Pig | 10 h |
|
| Echeverri et al., 2018 [118] | Vasodilators | BQ123, epoprostenol, and verapamil | NMP | Pig | 3 h |
|
| Nagrath et al., 2009 [119] | Defatting cocktail | Forskolin, GW7647, scoparone, hypericin, visfatin, and GW501516. | NMP CFH | Murine | 3 h |
|
| Liu et al., 2013 [120] | Defatting cocktail | Forskolin, GW7647, scoparone, hypericin, visfatin, and GW501516. | SNMP | Murine | 6 h |
|
| Boteon et al., 2019 [121] | Defatting cocktail | Forskolin, GW7647, scoparone, hypericin, visfatin, and GW501516. | NMP | Discarded human livers | 12 h |
|
| Lin et al., 2021 [122] | Defatting cocktail | Forskolin, GW7647, scoparone, hypericin, visfatin, and GW501516. | HMP | Murine | 3 h |
|
| Author/Year | Function | Therapeutic Agents | Infusion | Perfusion | Model | MP Time | Outcomes |
|---|---|---|---|---|---|---|---|
| Goldaracena et al., 2017 [135] | Sequesters miRNA-122 and inhibits HCV replication. | ASOs (miravirsen) | Delivered in perfusion solution. | NMP | Pig | 12 h | NMP improved miravirsen uptake versus SCS. Significant miR-122 sequestration and miR-122 target gene depression. Suppression of HCV replication after established infection and prevention of HCV infection. |
| Gillooy et al., 2019 [136] | The Fas receptor expressed in liver signals hepatocytes to apoptosis. | siRNA (against Fas receptor) | Delivered in perfusion solution via portal vein cannulation. | NMP and HMP | Murine | 4 h | siRNA added directly to perfusion solution is absorbed into rat livers during NMP and HMP. |
| Thijssen et al., 2017 [137] | p53 tumor suppressor, a transcription factor which can induce cell apoptosis. | siRNA (against the p53 gene) | - | NMP | Murine | 6 h | siRNA is capable of reaching and penetrating liver cells. HE stains showed less vacuolization and less cell infiltration. Less positive cells in immunofluorescence for p53. Lower levels of inflammatory cytokines (IL-1, IL-6, and TNFα), neutrophil infiltration, and lipoperoxidation. |
| Bonaccorsi-Riani et al., 2022 [138] | siRNA (against Fas receptor) | Added to the perfusate. | HMP | Murine | 1 h | Increased anti-inflammatory cytokines. |
| Author/Year | MSC Sources | Model | Type of Machine | Time | Infusion | Outcomes |
|---|---|---|---|---|---|---|
| Sasajima et al., 2018 [149] | Swine adipose MSC | 30 min warm ischemia murine, DCD | NMP | 2 h | Injected into the portal vein | Increase bile production; improve narrowing of the sinusoidal space |
| Rigo et al., 2018 [150] | Human HLSC-EVs | Murine | NMP | 4 h | Added to the circuit 15 min after starting perfusion | Lower AST and LDH; reduced histological damage; reduced hepatocyte apoptosis |
| Yang et al., 2020 [151] | Rat BMMSCs | 30 min warm ischemia murine, DCD | NMP | 8 h | Injected via portal vein | Improved liver function markers and liver histological damage, reduced hepatocyte apoptosis, and repaired hepatocyte mitochondrial damage. |
| Verstegen et al., 2020 [152] | Human BMMSCs | 15–45 min warm ischemia pig, DCD | HMP NMP | 0.5 h 4 h | Infused during HMP | Increased IL-6 and IL-8 Immunomodulatory effects |
| Laing et al., 2020 [153] | Human MAPCs | Discarded human livers | NMP | 6 h | Infused directly into the right lobe via the hepatic artery or portal vein | Immunomodulatory effects |
| Sun et al., 2021 [154] | Rat BMMSCs | 30 min warm ischemia murine, DCD | NMP | 6 h | Added to the perfusate | Lower Suzuki’s score Reduced the level of ROS and free Fe2+ Increased bile production |
| De Stefano et al., 2021 [155] | Human HLSC-EVs | 60 min warm ischemia murine, DCD | NMP | 6 h | Added to the circuit 15 min after starting perfusion | Reduced transaminase release Enhanced liver metabolism Higher bile production (higher dose) Lower intrahepatic resistance (higher dose) Reduced necrosis and enhanced proliferation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chullo, G.; Panisello-Rosello, A.; Marquez, N.; Colmenero, J.; Brunet, M.; Pera, M.; Rosello-Catafau, J.; Bataller, R.; García-Valdecasas, J.C.; Fundora, Y. Focusing on Ischemic Reperfusion Injury in the New Era of Dynamic Machine Perfusion in Liver Transplantation. Int. J. Mol. Sci. 2024, 25, 1117. https://doi.org/10.3390/ijms25021117
Chullo G, Panisello-Rosello A, Marquez N, Colmenero J, Brunet M, Pera M, Rosello-Catafau J, Bataller R, García-Valdecasas JC, Fundora Y. Focusing on Ischemic Reperfusion Injury in the New Era of Dynamic Machine Perfusion in Liver Transplantation. International Journal of Molecular Sciences. 2024; 25(2):1117. https://doi.org/10.3390/ijms25021117
Chicago/Turabian StyleChullo, Gabriela, Arnau Panisello-Rosello, Noel Marquez, Jordi Colmenero, Merce Brunet, Miguel Pera, Joan Rosello-Catafau, Ramon Bataller, Juan Carlos García-Valdecasas, and Yiliam Fundora. 2024. "Focusing on Ischemic Reperfusion Injury in the New Era of Dynamic Machine Perfusion in Liver Transplantation" International Journal of Molecular Sciences 25, no. 2: 1117. https://doi.org/10.3390/ijms25021117
APA StyleChullo, G., Panisello-Rosello, A., Marquez, N., Colmenero, J., Brunet, M., Pera, M., Rosello-Catafau, J., Bataller, R., García-Valdecasas, J. C., & Fundora, Y. (2024). Focusing on Ischemic Reperfusion Injury in the New Era of Dynamic Machine Perfusion in Liver Transplantation. International Journal of Molecular Sciences, 25(2), 1117. https://doi.org/10.3390/ijms25021117