Carvacrol Encapsulation in Chitosan–Carboxymethylcellulose–Alginate Nanocarriers for Postharvest Tomato Protection
Abstract
1. Introduction
2. Results
2.1. Morphological Analysis by Transmission Electron Microscopy
2.2. Bioactive Compound Encapsulation and Release Efficiencies
2.3. Energy-Dispersive X-ray Analysis
2.4. X-ray Powder Diffraction Study
2.5. Infrared Vibrational Study
2.6. Thermal Analysis
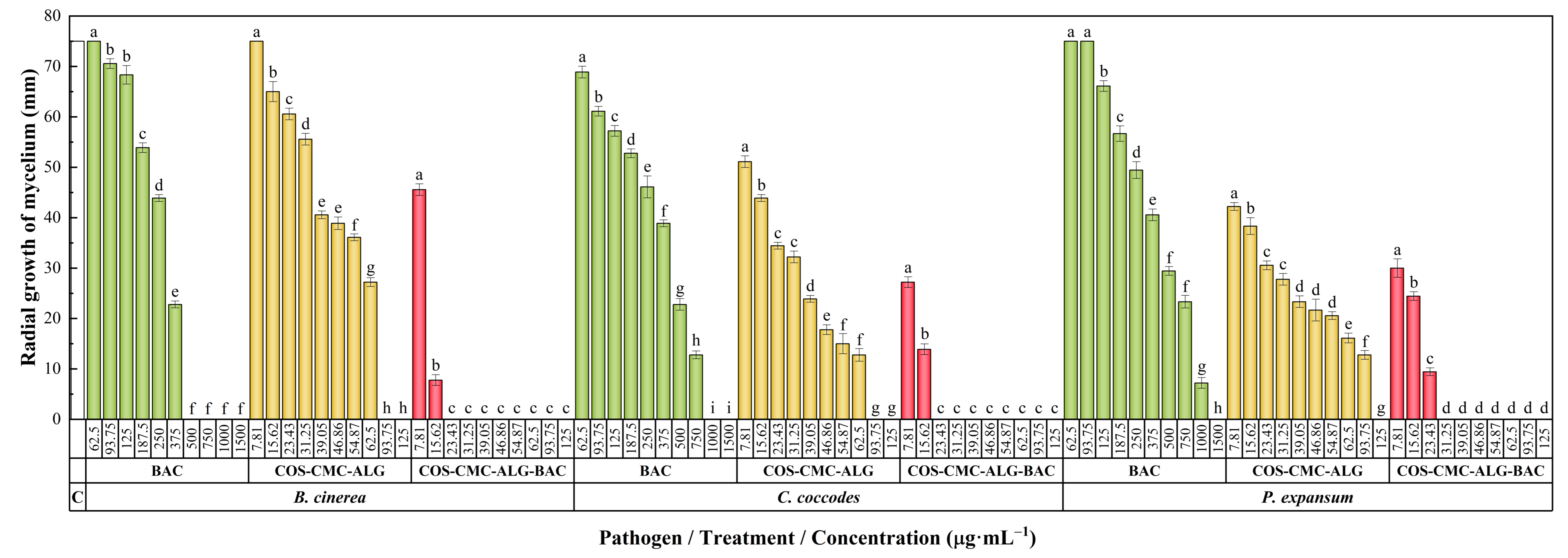
2.7. Antifungal Activity
2.7.1. In Vitro Antifungal Activity
2.7.2. Ex Situ Postharvest Protection Tests
3. Discussion
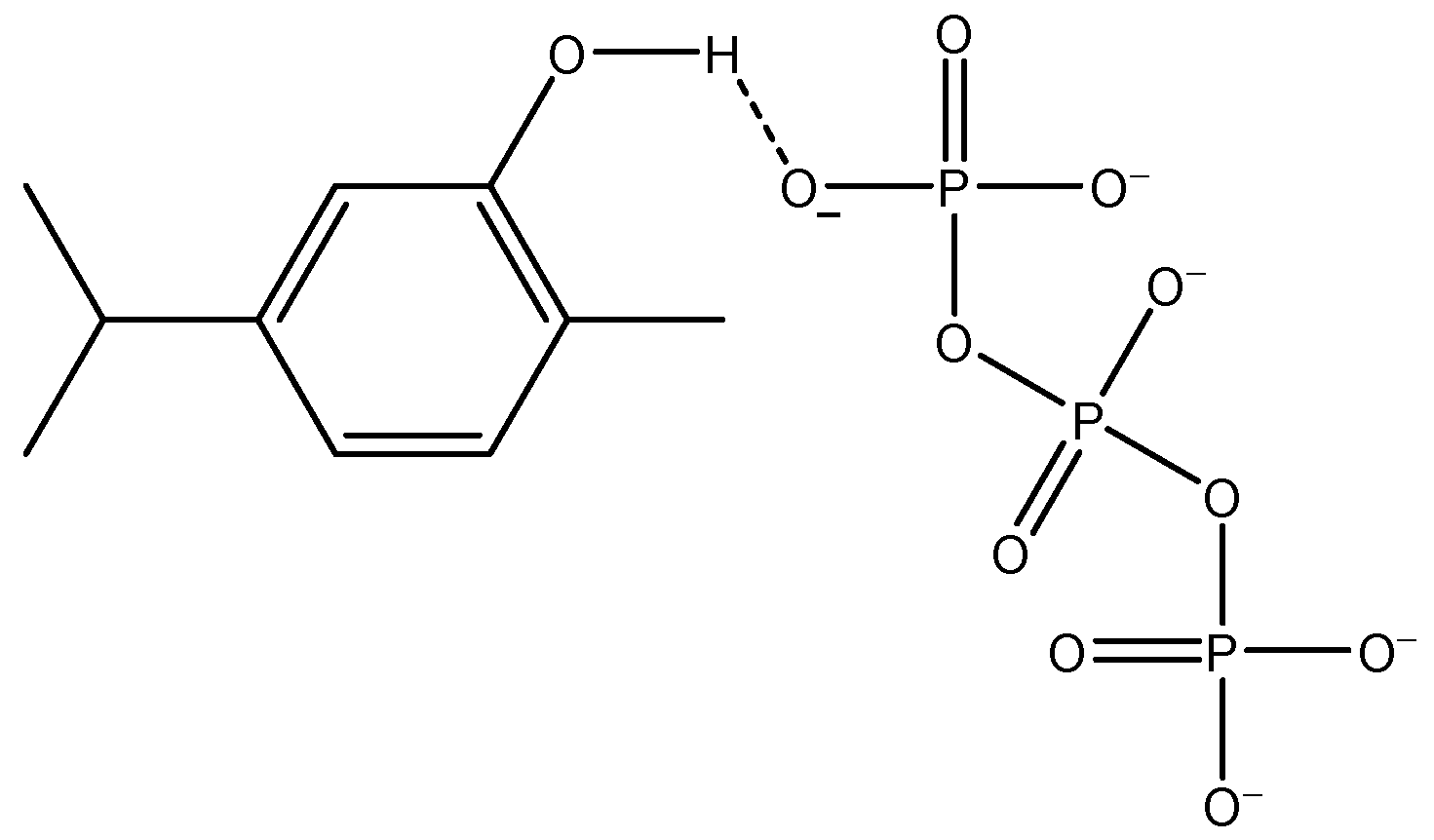
3.1. On the COS–CMC–ALG System Assemblage and the Carvacrol Encapsulation Mechanism
3.2. Comparison of Carvacrol Antimicrobial Activity
3.3. Comparison of Efficacy with Conventional Fungicides
| Commercial Fungicide | Pathogen | Radial Growth of Mycelium (mm) | Inhibition (%) | Ref. ** | ||
|---|---|---|---|---|---|---|
| Rd/10 | Rd * | Rd/10 | Rd * | |||
| Azoxystrobin | B. cinerea | 12 | 51 | 84 | 32 | [65] |
| C. coccodes | 30.6 | 24.4 | 59.2 | 67.5 | [66] | |
| P. expansum | 38.9 | 25.6 | 48.1 | 65.9 | [65] | |
| Mancozeb | B. cinerea | 0 | 0 | 100 | 100 | [65] |
| C. coccodes | 0 | 0 | 100 | 100 | [66] | |
| P. expansum | 0 | 0 | 100 | 100 | [65] | |
| Fosetyl-Al | B. cinerea | 38 | 0 | 49.3 | 100 | [65] |
| C. coccodes | 0 | 0 | 100 | 100 | [66] | |
| P. expansum | 67.2 | 26.1 | 10.4 | 65.2 | [65] | |
4. Materials and Methods
4.1. Reagents and Fungal Isolates
4.2. Synthesis Procedure
4.3. Bioactive Compound Encapsulation and Release
4.4. Nanocarriers Characterization
4.5. Antifungal Activity Assessment
4.5.1. In Vitro Antifungal Activity
4.5.2. Preparation of Fungal Conidial Suspension
4.5.3. Ex Situ Protection of Tomato Fruits
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chavan, P.; Lata, K.; Kaur, T.; Rezek Jambrak, A.; Sharma, S.; Roy, S.; Sinhmar, A.; Thory, R.; Pal Singh, G.; Aayush, K.; et al. Recent advances in the preservation of postharvest fruits using edible films and coatings: A comprehensive review. Food Chem. 2023, 418, 135916. [Google Scholar] [CrossRef] [PubMed]
- Liyanapathiranage, A.; Dassanayake, R.S.; Gamage, A.; Karri, R.R.; Manamperi, A.; Evon, P.; Jayakodi, Y.; Madhujith, T.; Merah, O. Recent developments in edible films and coatings for fruits and vegetables. Coatings 2023, 13, 1177. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation techniques for food bioactive components: A review. Food Bioprocess Technol. 2012, 6, 628–647. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Vatankhah, M.; Hassanisaadi, M.; Kennedy, J.F. Chitosan-based nanocomposites as coatings and packaging materials for the postharvest improvement of agricultural product: A review. Carbohydr. Polym. 2023, 309, 120666. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, R.; Rhim, J.-W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Bacterial cellulose as a biodegradable food packaging material: A review. Food Hydrocoll. 2021, 113, 106530. [Google Scholar] [CrossRef]
- Xie, F. Biopolymer-based multilayer films and coatings for food preservation: An update of the recent development. Curr. Food Sci. Technol. Rep. 2023, 1, 1–12. [Google Scholar] [CrossRef]
- Ashfaq, A.; Khursheed, N.; Fatima, S.; Anjum, Z.; Younis, K. Application of nanotechnology in food packaging: Pros and cons. J. Agric. Food Res. 2022, 7, 100270. [Google Scholar] [CrossRef]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2017, 16, 101–112. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Gholizadeh Vazvani, M.; Hassanisaadi, M.; Skorik, Y.A. Micro-/nano-carboxymethyl cellulose as a promising biopolymer with prospects in the agriculture sector: A review. Polymers 2023, 15, 440. [Google Scholar] [CrossRef]
- Severino, P.; da Silva, C.F.; Andrade, L.N.; de Lima Oliveira, D.; Campos, J.; Souto, E.B. Alginate nanoparticles for drug delivery and targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef]
- Cui, Z.; Mumper, R.J. Chitosan-based nanoparticles for topical genetic immunization. J. Control. Release 2001, 75, 409–419. [Google Scholar] [CrossRef]
- Sohail, R.; Abbas, S.R. Evaluation of amygdalin-loaded alginate-chitosan nanoparticles as biocompatible drug delivery carriers for anticancerous efficacy. Int. J. Biol. Macromol. 2020, 153, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Agnihotri, S.; Goyal, D. Fabrication of chitosan–alginate nanospheres for controlled release of cartap hydrochloride. Nanotechnology 2021, 33, 025701. [Google Scholar] [CrossRef] [PubMed]
- Salama, H.E.; Abdel Aziz, M.S.; Alsehli, M. Carboxymethyl cellulose/sodium alginate/chitosan biguanidine hydrochloride ternary system for edible coatings. Int. J. Biol. Macromol. 2019, 139, 614–620. [Google Scholar] [CrossRef]
- Wang, M.; Zang, Y.; Hong, K.; Zhao, X.; Yu, C.; Liu, D.; An, Z.; Wang, L.; Yue, W.; Nie, G. Preparation of pH-sensitive carboxymethyl cellulose/chitosan/alginate hydrogel beads with reticulated shell structure to deliver Bacillus subtilis natto. Int. J. Biol. Macromol. 2021, 192, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Han, B.; Wang, Z.; Gao, C.; Peng, C.; Shen, J. Hollow chitosan-alginate multilayer microcapsules as drug delivery vehicle: Doxorubicin loading and in vitro and in vivo studies. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Morozkina, S.; Strekalovskaya, U.; Vanina, A.; Snetkov, P.; Krasichkov, A.; Polyakova, V.; Uspenskaya, M. The fabrication of alginate–carboxymethyl cellulose-based composites and drug release profiles. Polymers 2022, 14, 3604. [Google Scholar] [CrossRef]
- Karzar Jeddi, M.; Mahkam, M. Magnetic nano carboxymethyl cellulose-alginate/chitosan hydrogel beads as biodegradable devices for controlled drug delivery. Int. J. Biol. Macromol. 2019, 135, 829–838. [Google Scholar] [CrossRef]
- Lan, W.; He, L.; Liu, Y. Preparation and properties of sodium carboxymethyl cellulose/sodium alginate/chitosan composite film. Coatings 2018, 8, 291. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Langa-Lomba, N.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Santiago-Aliste, A.; Torres-Sánchez, S.; Martín-Ramos, P. Lignin–chitosan nanocarriers for the delivery of bioactive natural products against wood-decay phytopathogens. Agronomy 2022, 12, 461. [Google Scholar] [CrossRef]
- Santiago-Aliste, A.; Sánchez-Hernández, E.; Langa-Lomba, N.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Martín-Ramos, P. Multifunctional nanocarriers based on chitosan oligomers and graphitic carbon nitride assembly. Materials 2022, 15, 8981. [Google Scholar] [CrossRef] [PubMed]
- Laurienzo, P.; Malinconico, M.; Mattia, G.; Russo, R.; Rotonda, M.I.L.; Quaglia, F.; Capitani, D.; Mannina, L. Novel alginate–acrylic polymers as a platform for drug delivery. J. Biomed. Mater. Res. Part A 2006, 78A, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Soullard, L.; Bayle, P.-A.; Lancelon-Pin, C.; Rolere, S.; Texier, I.; Jean, B.; Nonglaton, G. Optimization of the methacrylation of carboxymethylcellulose and use for the design of hydrogels and cryogels with controlled structure and properties. Cellulose 2023, 30, 6203–6217. [Google Scholar] [CrossRef]
- Goycoolea, F.M.; Lollo, G.; Remuñán-López, C.; Quaglia, F.; Alonso, M.J. Chitosan-alginate blended nanoparticles as carriers for the transmucosal delivery of macromolecules. Biomacromolecules 2009, 10, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Burt, S.A.; Vlielander, R.; Haagsman, H.P.; Veldhuizen, E.J.A. Increase in activity of essential oil components carvacrol and thymol against Escherichia coli O157:H7 by addition of food stabilizers. J. Food Prot. 2005, 68, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Gandova, V.; Lazarov, A.; Fidan, H.; Dimov, M.; Stankov, S.; Denev, P.; Ercisli, S.; Stoyanova, A.; Gulen, H.; Assouguem, A.; et al. Physicochemical and biological properties of carvacrol. Open Chem. 2023, 21, 20220319. [Google Scholar] [CrossRef]
- Mączka, W.; Twardawska, M.; Grabarczyk, M.; Wińska, K. Carvacrol—A natural phenolic compound with antimicrobial properties. Antibiotics 2023, 12, 824. [Google Scholar] [CrossRef]
- Keawchaoon, L.; Yoksan, R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B Biointerfaces 2011, 84, 163–171. [Google Scholar] [CrossRef]
- Sadeghi, R.; Etemad, S.G.; Keshavarzi, E.; Haghshenasfard, M. Investigation of alumina nanofluid stability by UV–vis spectrum. Microfluid. Nanofluidics 2015, 18, 1023–1030. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential–a critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Obaidat, R.M.; Tashtoush, B.M.; Bayan, M.F.; Al Bustami, R.T.; Alnaief, M. Drying using supercritical fluid technology as a potential method for preparation of chitosan aerogel microparticles. AAPS PharmSciTech 2015, 16, 1235–1244. [Google Scholar] [CrossRef]
- Shameem, A.; Devendran, P.; Siva, V.; Venkatesh, K.S.; Manikandan, A.; Bahadur, S.A.; Nallamuthu, N. Dielectric investigation of NaLiS nanoparticles loaded on alginate polymer matrix synthesized by single pot microwave irradiation. J. Inorg. Organomet. Polym. Mater. 2017, 28, 671–678. [Google Scholar] [CrossRef]
- Manjubala, I.; Basu, P.; Narendrakumar, U. In situ synthesis of hydroxyapatite/carboxymethyl cellulose composites for bone regeneration applications. Colloid. Polym. Sci. 2018, 296, 1729–1737. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Y.; Yang, H.; Wang, Q.; Zhang, Z. Controlled synthesis of mesocrystal magnesium oxide parallelogram and its catalytic performance. CrystEngComm 2015, 17, 2642–2650. [Google Scholar] [CrossRef]
- Iswandana, R.; Putri, K.S.S.; Dwiputra, R.; Yanuari, T.; Sari, S.P.; Djajadisastra, J. Formulation of chitosan tripolyphosphate-tetrandrine beads using ionic gelation method: In vitro and in vivo evaluation. Int. J. Appl. Pharm. 2017, 9, 109. [Google Scholar] [CrossRef][Green Version]
- Chen, C.-C.; Wang, J.-M.; Huang, Y.-R.; Yu, Y.-H.; Wu, T.-M.; Ding, S.-J. Synergistic effect of thermoresponsive and photocuring methacrylated chitosan-based hybrid hydrogels for medical applications. Pharmaceutics 2023, 15, 1090. [Google Scholar] [CrossRef]
- Baysal, K.; Aroguz, A.Z.; Adiguzel, Z.; Baysal, B.M. Chitosan/alginate crosslinked hydrogels: Preparation, characterization and application for cell growth purposes. Int. J. Biol. Macromol. 2013, 59, 342–348. [Google Scholar] [CrossRef]
- Silvestro, I.; Francolini, I.; Di Lisio, V.; Martinelli, A.; Pietrelli, L.; Scotto d’Abusco, A.; Scoppio, A.; Piozzi, A. Preparation and characterization of TPP-chitosan crosslinked scaffolds for tissue engineering. Materials 2020, 13, 3577. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Amodio, M.L.; Colelli, G. Carvacrol-loaded chitosan nanoparticles maintain quality of fresh-cut carrots. Innov. Food Sci. Emerg. Technol. 2017, 41, 56–63. [Google Scholar] [CrossRef]
- Sim, J.X.F.; Khazandi, M.; Chan, W.Y.; Trott, D.J.; Deo, P. Antimicrobial activity of thyme oil, oregano oil, thymol and carvacrol against sensitive and resistant microbial isolates from dogs with otitis externa. Vet. Dermatol. 2019, 30, 524-e159. [Google Scholar] [CrossRef] [PubMed]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2019, 60, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R.; Zhou, T. Antifungal activity of monoterpenoids against postharvest pathogens Botrytis cinerea and Monilinia fructicola. J. Essent. Oil Res. 2000, 12, 113–121. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Sharifzadeh, A.; Shokri, H.; Khosravi, A.R.; Abbaszadeh, A. Antifungal efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to control the growth of food-relevant fungi. J. De Mycol. Médicale 2014, 24, e51–e56. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zhang, X.; Zhao, T.; Zhou, L. Effects of Origanum vulgare essential oil and its two main components, carvacrol and thymol, on the plant pathogen Botrytis cinerea. PeerJ 2020, 8, e9626. [Google Scholar] [CrossRef]
- Adebayo, O.; Dang, T.; Bélanger, A.; Khanizadeh, S. Antifungal studies of selected essential oils and a commercial formulation against Botrytis cinerea. J. Food Res. 2013, 2, 217. [Google Scholar] [CrossRef]
- Álvarez-García, S.; Moumni, M.; Romanazzi, G. Antifungal activity of volatile organic compounds from essential oils against the postharvest pathogens Botrytis cinerea, Monilinia fructicola, Monilinia fructigena, and Monilinia laxa. Front. Plant Sci. 2023, 14, 1274770. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Pérez-Pérez, J.C.; Varillas-Torres, J.M.; Navarro-Cruz, A.R.; Hernández-Carranza, P.; Munguía-Pérez, R.; Cid-Pérez, T.S.; Avila-Sosa, R. Starch edible films/coatings added with carvacrol and thymol: In vitro and in vivo evaluation against Colletotrichum gloeosporioides. Foods 2021, 10, 175. [Google Scholar] [CrossRef]
- Lee, D.-H.; Lee, M.-W.; Cho, S.B.; Hwang, K.; Park, I.-K. Antifungal mode of action of bay, allspice, and ajowan essential oils and their constituents against Colletotrichum gloeosporioides via overproduction of reactive oxygen species and downregulation of ergosterol biosynthetic genes. Ind. Crops Prod. 2023, 197, 116684. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, A.; Ren, M.; Wei, G.; Xu, H. First report on Colletotrichum fructicola causing anthracnose in Chinese sorghum and its management using phytochemicals. J. Fungi 2023, 9, 279. [Google Scholar] [CrossRef]
- Pei, S.; Liu, R.; Gao, H.; Chen, H.; Wu, W.; Fang, X.; Han, Y. Inhibitory effect and possible mechanism of carvacrol against Colletotrichum fructicola. Postharvest Biol. Technol. 2020, 163, 111126. [Google Scholar] [CrossRef]
- Duduk, N.; Markovic, T.; Vasic, M.; Duduk, B.; Vico, I.; Obradovic, A. Antifungal activity of three essential oils against Colletotrichum acutatum, the causal agent of strawberry anthracnose. J. Essent. Oil Bear. Plants 2015, 18, 529–537. [Google Scholar] [CrossRef]
- Araújo, E.R.; Costa-Carvalho, R.R.; Fontes, M.G.; Laranjeira, D.; Blank, A.F.; Alves, P.B. Antifungal activity of essential oils of Lippia species of Colletotrichum sp. in vitro. Acta Hortic. 2018, 1198, 9–16. [Google Scholar] [CrossRef]
- Kadoglidou, K.; Lagopodi, A.; Karamanoli, K.; Vokou, D.; Bardas, G.A.; Menexes, G.; Constantinidou, H.-I.A. Inhibitory and stimulatory effects of essential oils and individual monoterpenoids on growth and sporulation of four soil-borne fungal isolates of Aspergillus terreus, Fusarium oxysporum, Penicillium expansum, and Verticillium dahliae. Eur. J. Plant Pathol. 2011, 130, 297–309. [Google Scholar] [CrossRef]
- Felšöciová, S.; Vukovic, N.; Jeżowski, P.; Kačániová, M. Antifungal activity of selected volatile essential oils against Penicillium sp. Open Life Sci. 2020, 15, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Moussa, H.; El Omari, B.; Chefchaou, H.; Tanghort, M.; Mzabi, A.; Chami, N.; Remmal, A. Action of thymol, carvacrol and eugenol on Penicillium and Geotrichum isolates resistant to commercial fungicides and causing postharvest citrus decay. Can. J. Plant Pathol. 2020, 43, 26–34. [Google Scholar] [CrossRef]
- Střelková, T.; Nemes, B.; Kovács, A.; Novotný, D.; Božik, M.; Klouček, P. Inhibition of fungal strains isolated from cereal grains via vapor phase of essential oils. Molecules 2021, 26, 1313. [Google Scholar] [CrossRef]
- Schlösser, I.; Prange, A. Antifungal activity of selected natural preservatives against the foodborne molds Penicillium verrucosum and Aspergillus westerdijkiae. FEMS Microbiol. Lett. 2018, 365, 125. [Google Scholar] [CrossRef]
- Markovic, T.; Chatzopoulou, P.; Siljegovic, J.; Nikolic, M.; Glamoclija, J.; Ciric, A.; Sokovic, M. Chemical analysis and antimicrobial activities of the essential oils of Satureja thymbra L. and Thymbra spicata L. and their main components. Arch. Biol. Sci. 2011, 63, 457–464. [Google Scholar] [CrossRef]
- Lu, C.; Hou, K.; Zhou, T.; Wang, X.; Zhang, J.; Cheng, C.; Du, Z.; Li, B.; Wang, J.; Wang, J.; et al. Characterization of the responses of soil micro-organisms to azoxystrobin and the residue dynamics of azoxystrobin in wheat–corn rotation fields over two years. Chemosphere 2023, 318, 137918. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Torres, P. Molecular mechanisms underlying fungicide resistance in citrus postharvest green mold. J. Fungi 2021, 7, 783. [Google Scholar] [CrossRef] [PubMed]
- Cocco, P. Time for re-evaluating the human carcinogenicity of ethylenedithiocarbamate fungicides? A systematic review. Int. J. Environ. Res. Public Health 2022, 19, 2632. [Google Scholar] [CrossRef]
- Emara, A.R.; Ibrahim, H.M.; Masoud, S.A. The role of storage on Mancozeb fungicide formulations and their antifungal activity against Fusarium oxysporium and Rhizoctonia solani. Arab. J. Chem. 2021, 14, 103322. [Google Scholar] [CrossRef]
- Santiago-Aliste, A.; Sánchez-Hernández, E.; Buzón-Durán, L.; Marcos-Robles, J.L.; Martín-Gil, J.; Martín-Ramos, P. Uncaria tomentosa-loaded chitosan oligomers–hydroxyapatite–carbon nitride nanocarriers for postharvest fruit protection. Agronomy 2023, 13, 2189. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Álvarez-Martínez, J.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Martín-Ramos, P. Helichrysum stoechas (L.) Moench inflorescence extract for tomato disease management. Molecules 2023, 28, 5861. [Google Scholar] [CrossRef]
- Machado, T.O.; Beckers, S.J.; Fischer, J.; Sayer, C.; de Araújo, P.H.H.; Landfester, K.; Wurm, F.R. Cellulose nanocarriers via miniemulsion allow pathogen-specific agrochemical delivery. J. Colloid Interface Sci. 2021, 601, 678–688. [Google Scholar] [CrossRef]
- Lin, M.; Fang, S.; Zhao, X.; Liang, X.; Wu, D. Natamycin-loaded zein nanoparticles stabilized by carboxymethyl chitosan: Evaluation of colloidal/chemical performance and application in postharvest treatments. Food Hydrocoll. 2020, 106, 105871. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, P.; Xu, Y.; Wang, B.; Liu, P.; Hao, J.; Liu, X. Encapsulation of fluazinam to extend efficacy duration in controlling Botrytis cinerea on cucumber. Pest Manag. Sci. 2021, 77, 2836–2842. [Google Scholar] [CrossRef]
- Liang, W.; Zhang, J.; Wurm, F.R.; Wang, R.; Cheng, J.; Xie, Z.; Li, X.; Zhao, J. Lignin-based non-crosslinked nanocarriers: A promising delivery system of pesticide for development of sustainable agriculture. Int. J. Biol. Macromol. 2022, 220, 472–481. [Google Scholar] [CrossRef]
- Santos-Moriano, P.; Fernandez-Arrojo, L.; Mengibar, M.; Belmonte-Reche, E.; Peñalver, P.; Acosta, F.N.; Ballesteros, A.O.; Morales, J.C.; Kidibule, P.; Fernandez-Lobato, M.; et al. Enzymatic production of fully deacetylated chitooligosaccharides and their neuroprotective and anti-inflammatory properties. Biocatal. Biotransform. 2017, 36, 57–67. [Google Scholar] [CrossRef]
- Tian, M.; Tan, H.; Li, H.; You, C. Molecular weight dependence of structure and properties of chitosan oligomers. RSC Adv. 2015, 5, 69445–69452. [Google Scholar] [CrossRef]
- Gupta, B.; Gupta, A.K. Photocatalytic performance of 3D engineered chitosan hydrogels embedded with sulfur-doped C3N4/ZnO nanoparticles for Ciprofloxacin removal: Degradation and mechanistic pathways. Int. J. Biol. Macromol. 2022, 198, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Beckers, S.J.; Yiamsawas, D.; Thines, E.; Landfester, K.; Wurm, F.R. Targeted drug delivery in plants: Enzyme-responsive lignin nanocarriers for the curative treatment of the worldwide grapevine trunk disease Esca. Adv. Sci. 2019, 6, 1802315. [Google Scholar] [CrossRef]
- Shekarchi, M.; Khanavi, M.; Adib, N.; Amri, M.; Hajimehdipoor, H. A validated high performance liquid chromatography method for the analysis of thymol and carvacrol in Thymus vulgaris L. volatile oil. Pharmacogn. Mag. 2010, 6, 154. [Google Scholar] [CrossRef] [PubMed]
- Tiunov, I.A.; Gorbachevskyy, M.V.; Kopitsyn, D.S.; Kotelev, M.S.; Ivanov, E.V.; Vinokurov, V.A.; Novikov, A.A. Synthesis of large uniform gold and core–shell gold–silver nanoparticles: Effect of temperature control. Russ. J. Phys. Chem. A 2016, 90, 152–157. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef]
- Levy, Y.; Benderly, M.; Cohen, Y.; Gisi, U.; Bassand, D. The joint action of fungicides in mixtures: Comparison of two methods for synergy calculation. EPPO Bull. 1986, 16, 651–657. [Google Scholar] [CrossRef]


| Wavenumber (cm−1) | Assignment | Component | |
|---|---|---|---|
| Empty NCs | Loaded NCs | ||
| 3350 | H–bonded OH stretching | carvacrol | |
| 3265 | 3266 | asymmetrical stretching of the –NH group | COS |
| 2960 | CH stretching branched alkane | carvacrol | |
| 1704 | C=O asymmetric stretching of phenolic acids | carvacrol | |
| 1632 | 1628 | overlapping stretching of alkenes (C=C) and carbonyl (C=O) | COS, CMC |
| 1536 | 1538 | N–H bending of N–acetylated residues (amide II) (after binding to alginate); asymmetrical stretching of COO– groups | COS, CMC |
| 1412 | 1418 | N–H stretching (amide and ether bonds); symmetrical stretching of COO– groups | COS, ALG, CMC |
| 1381 | 1381 | N–H stretching (amide III band) | COS |
| 1248 | 1250 | C–O–C stretching | carvacrol |
| 1149 | 1149 | symmetric and antisymmetric stretching in the PO4 group | TPP |
| 1065 | 1065 | –C–O–C bonds | COS |
| 1015 | 1028 | C–O–C stretching; C–O stretching attributed to the saccharide structure | ALG, COS |
| 947 | 942 | C–O stretching vibration of uronic acid residues | ALG |
| 871 | aromatic rings | carvacrol | |
| 810 | typical of p-substituted aromatic rings | carvacrol | |
| Treatment | EC | B. cinerea | C. coccodes | P. expansum |
|---|---|---|---|---|
| BAC | EC50 | 282.4 | 369.8 | 406.6 |
| EC90 | 456.2 | 823.7 | 1122.7 | |
| COS–CMC–ALG | EC50 | 50.8 | 22.7 | 17.0 |
| EC90 | 84.6 | 73.1 | 104.7 | |
| COS–CMC–ALG–BAC | EC50 | 8.9 | 5.3 | 6.4 |
| EC90 | 18.0 | 18.3 | 25.6 |
| Pathogen | Treatment | LD (mm) | LSR (%) |
|---|---|---|---|
| − | Negative control | 0 | 100 |
| B. cinerea | Positive control | 25.4 ± 2.8 | 0 |
| BAC-loaded NCs at 50 µg·mL−1 | 17.3 ± 2.3 | 31.9 | |
| BAC-loaded Ns at 100 µg·mL−1 | 0 | 100 | |
| C. coccodes | Positive control | 24.2 ± 3.7 | 0 |
| BAC-loaded NCs at 50 µg·mL−1 | 21.5 ± 1.6 | 78.8 | |
| BAC-loaded NCs at 100 µg·mL−1 | 1.8 ± 1.1 | 92.6 | |
| P. expansum | Positive control | 16.7 ± 1.2 | 0 |
| BAC-loaded NCs at 50 µg·mL−1 | 0 | 100 | |
| BAC-loaded NCs at 100 µg·mL−1 | 0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Hernández, E.; Santiago-Aliste, A.; Correa-Guimarães, A.; Martín-Gil, J.; Gavara-Clemente, R.J.; Martín-Ramos, P. Carvacrol Encapsulation in Chitosan–Carboxymethylcellulose–Alginate Nanocarriers for Postharvest Tomato Protection. Int. J. Mol. Sci. 2024, 25, 1104. https://doi.org/10.3390/ijms25021104
Sánchez-Hernández E, Santiago-Aliste A, Correa-Guimarães A, Martín-Gil J, Gavara-Clemente RJ, Martín-Ramos P. Carvacrol Encapsulation in Chitosan–Carboxymethylcellulose–Alginate Nanocarriers for Postharvest Tomato Protection. International Journal of Molecular Sciences. 2024; 25(2):1104. https://doi.org/10.3390/ijms25021104
Chicago/Turabian StyleSánchez-Hernández, Eva, Alberto Santiago-Aliste, Adriana Correa-Guimarães, Jesús Martín-Gil, Rafael José Gavara-Clemente, and Pablo Martín-Ramos. 2024. "Carvacrol Encapsulation in Chitosan–Carboxymethylcellulose–Alginate Nanocarriers for Postharvest Tomato Protection" International Journal of Molecular Sciences 25, no. 2: 1104. https://doi.org/10.3390/ijms25021104
APA StyleSánchez-Hernández, E., Santiago-Aliste, A., Correa-Guimarães, A., Martín-Gil, J., Gavara-Clemente, R. J., & Martín-Ramos, P. (2024). Carvacrol Encapsulation in Chitosan–Carboxymethylcellulose–Alginate Nanocarriers for Postharvest Tomato Protection. International Journal of Molecular Sciences, 25(2), 1104. https://doi.org/10.3390/ijms25021104











