Glutathione Reductase Expression and Its Prognostic Significance in Colon Cancer
Abstract
1. Introduction
2. Results
2.1. A Description of the Studied Group
2.2. Clinically Relevant Parameters (as Independent Variables) Correlated with GR Immunohistochemical Expression
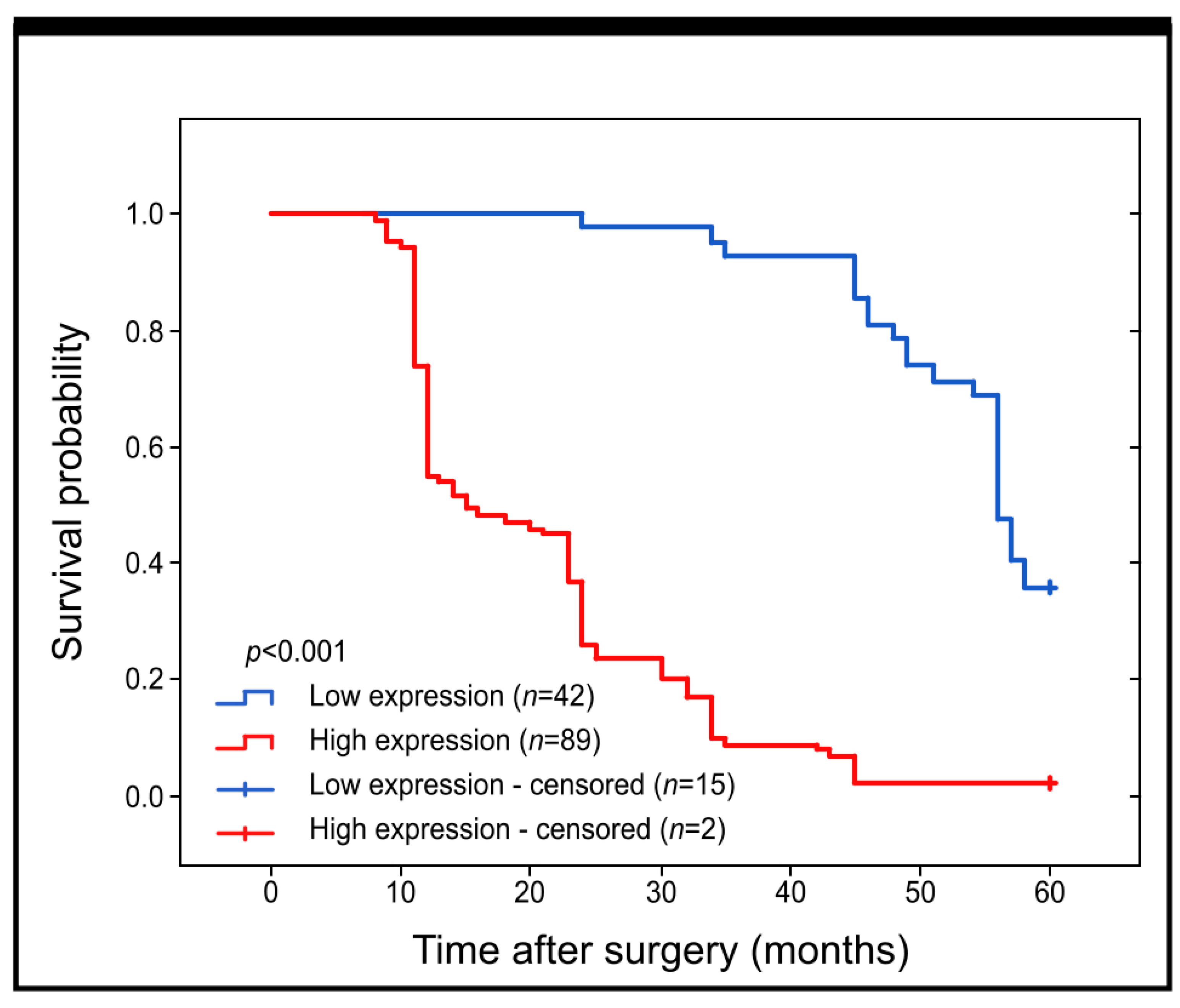
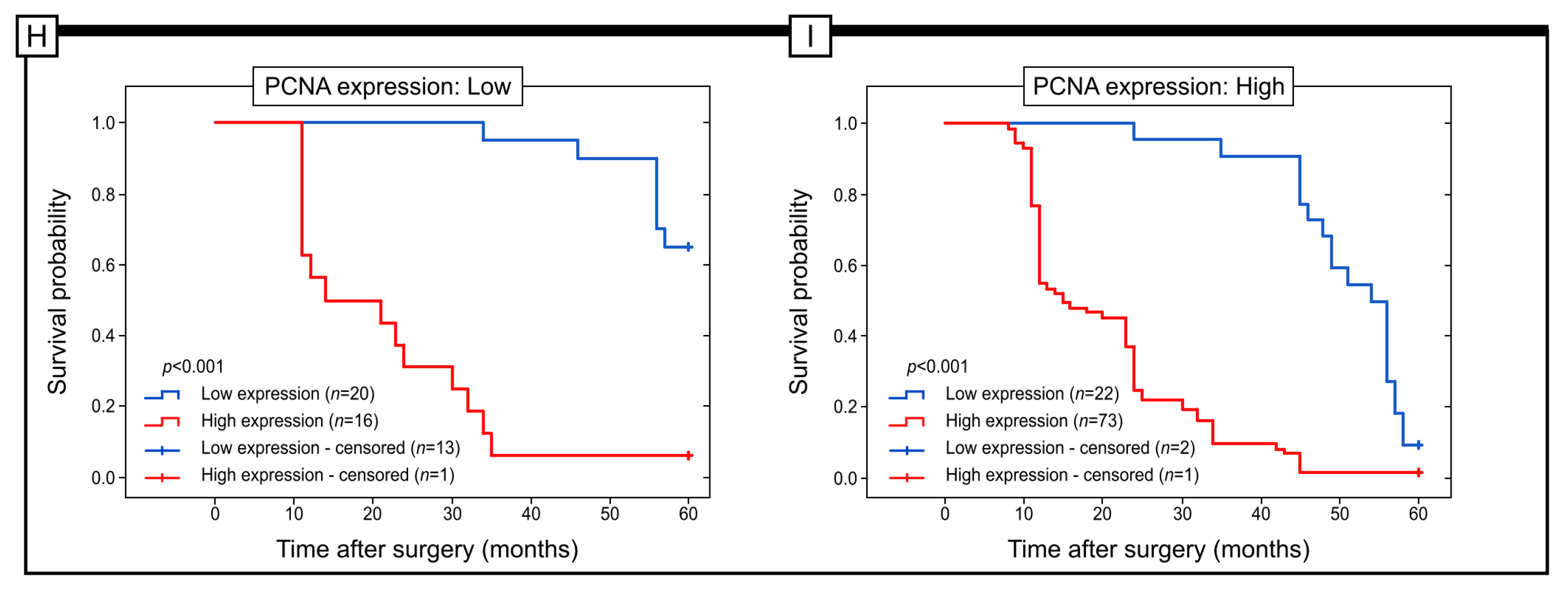
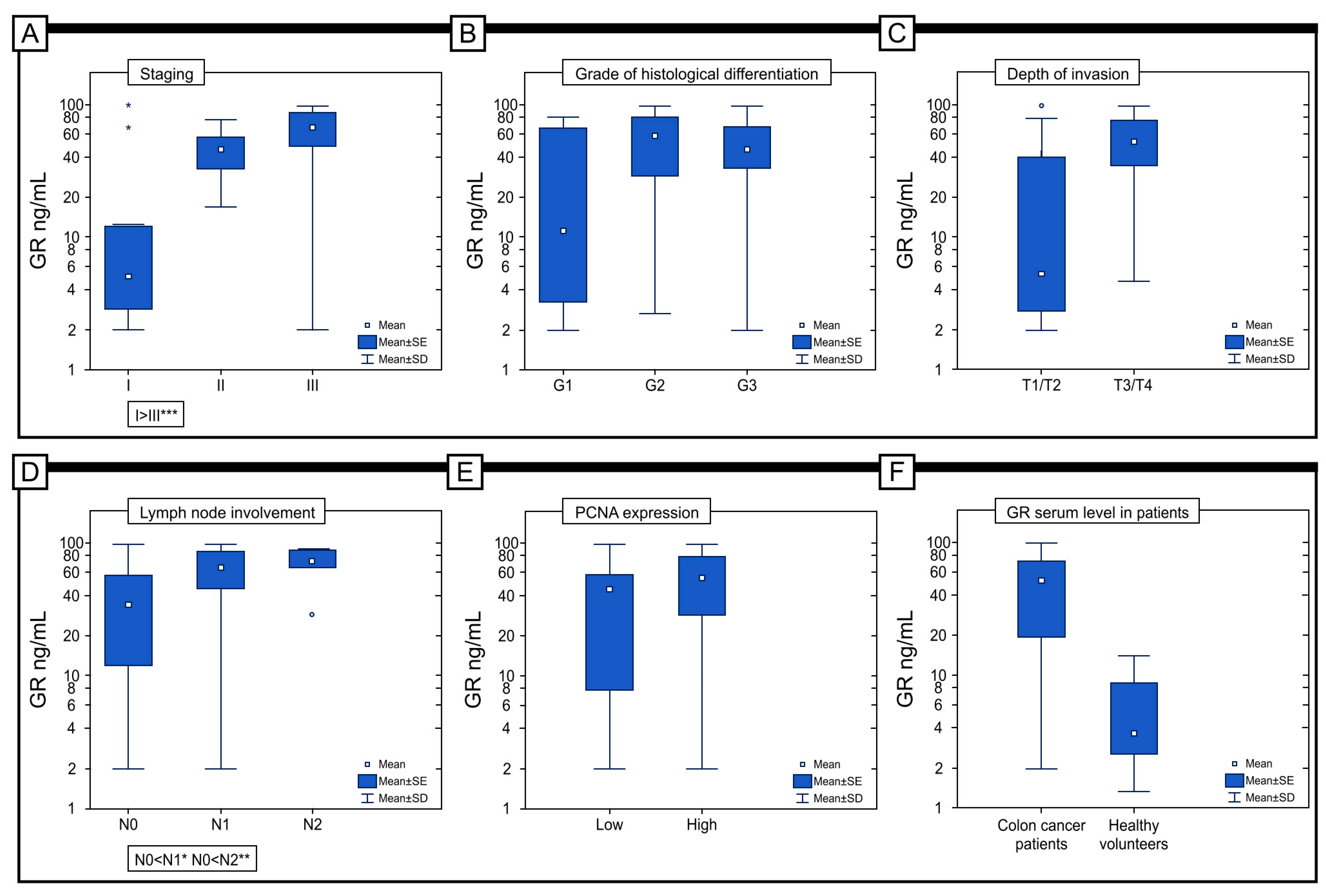
2.3. Prognostic Role of GR Protein Expression in Colon Adenocarcinoma
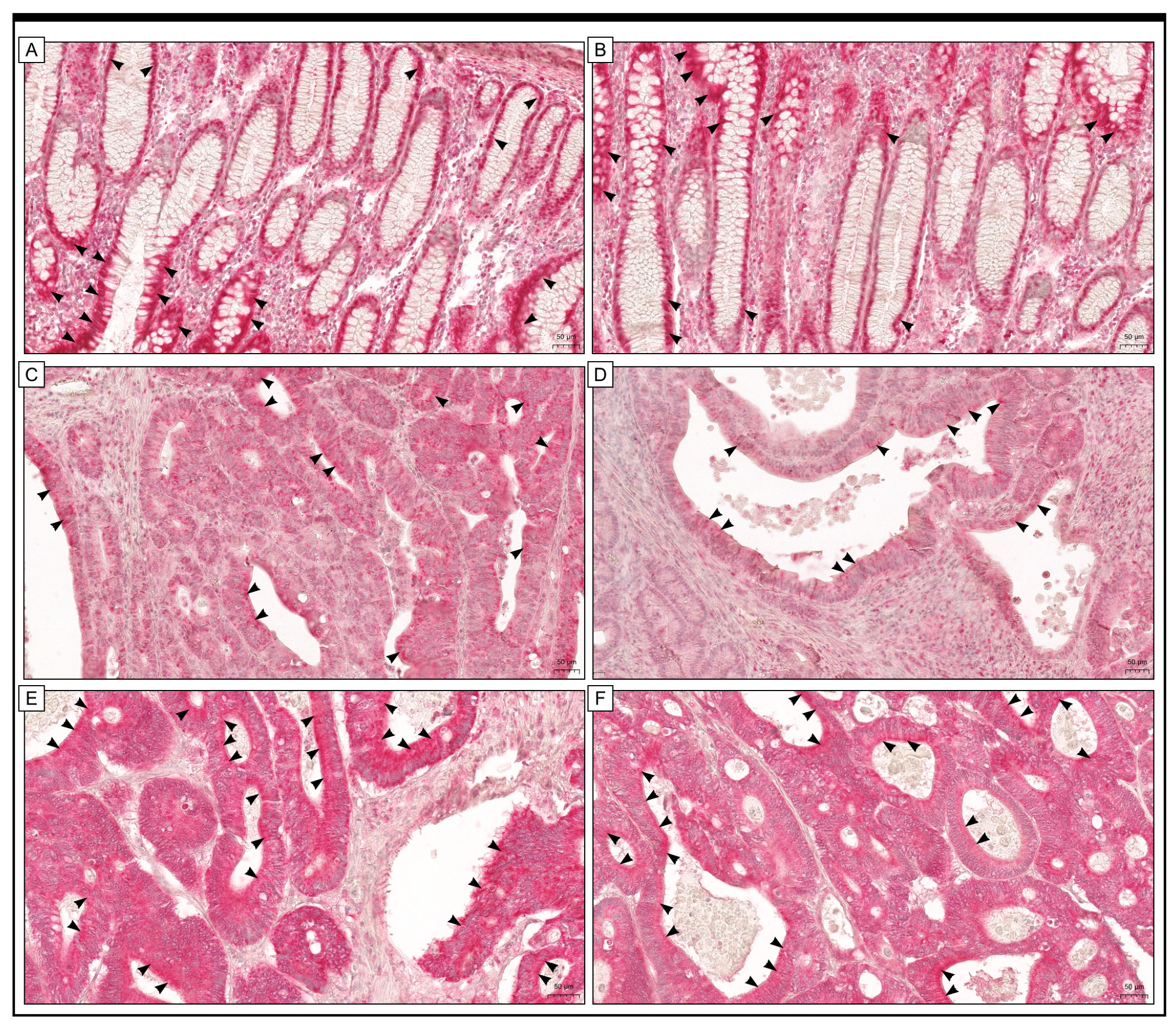
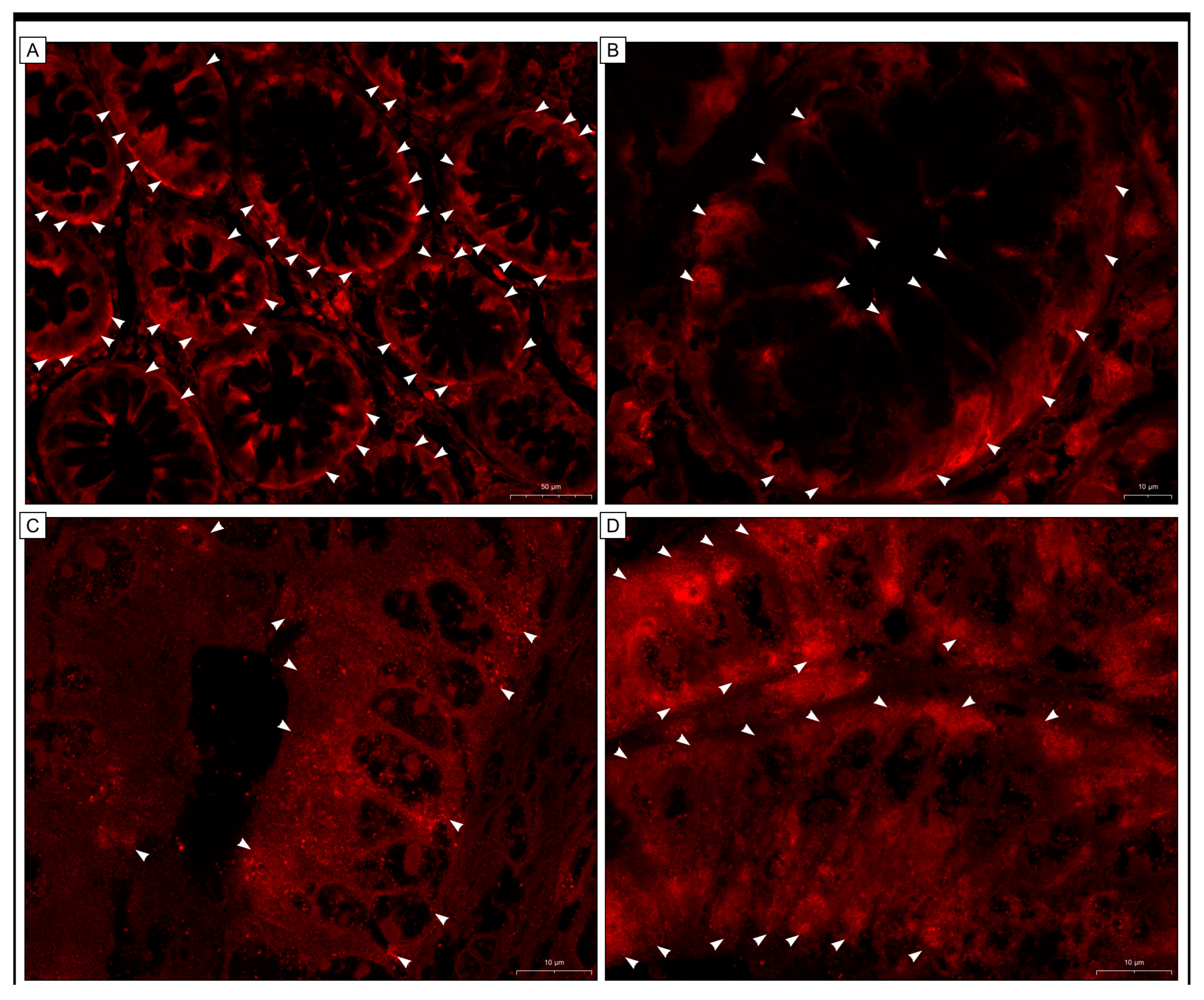
2.4. Immunofluorescence Staining
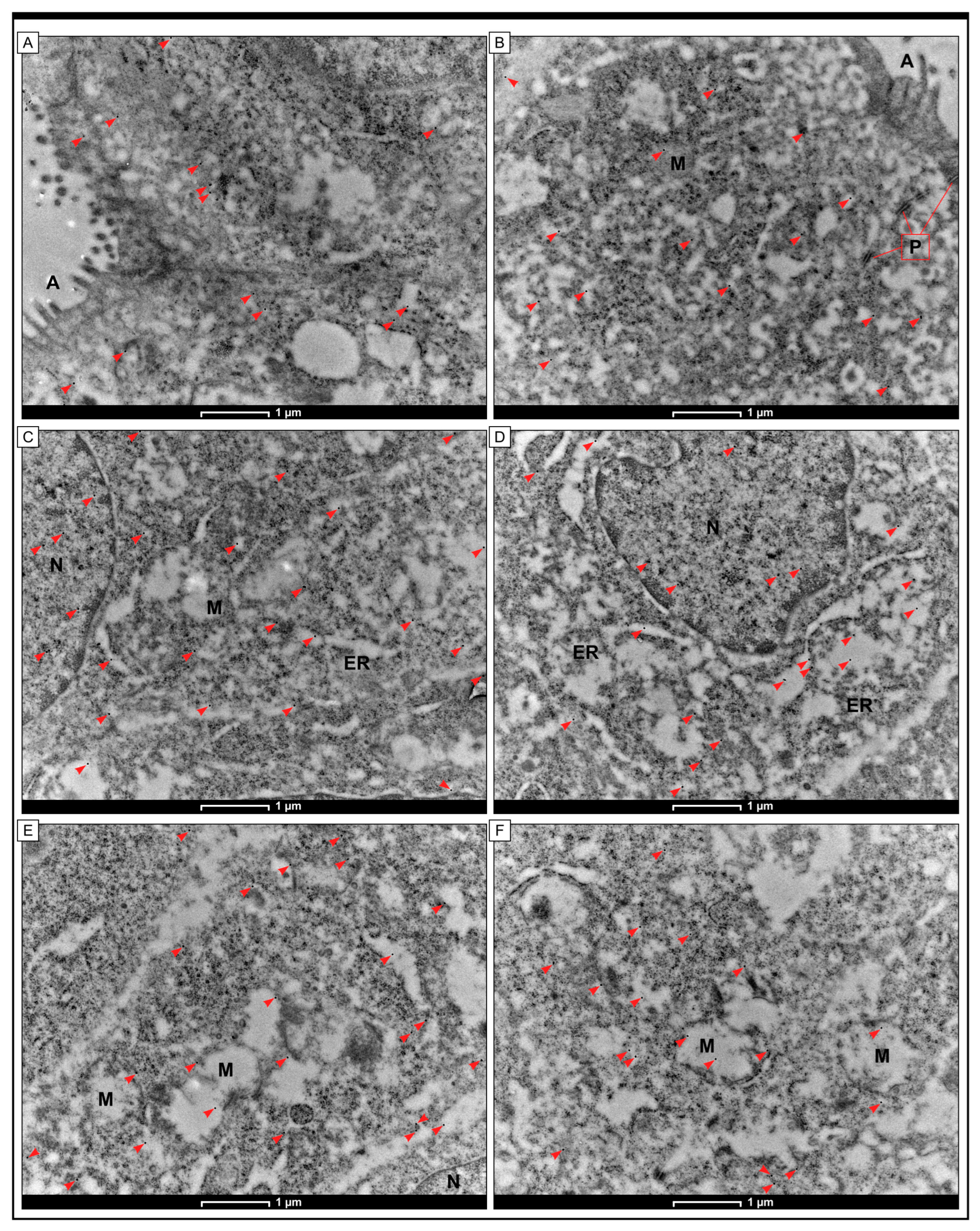
2.5. Intracellular Localization of GR Protein by the Method of Immunogold Labelling with the Use of Transmission Electron Microscopy (TEM)
2.6. GR Protein Expression in Colorectal Cancer Cell Lines Detected by the WB Method
2.7. Serum Level of GR in Patients
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Samples from Tumours and Patients
5.2. Immunohistochemical and Immunofluorescence Staining
5.3. Immunogold Electron Microscopy
5.4. Colorectal Cancer Cell Lines
5.5. GR Expression in Colorectal Cancer Cell Lines
5.6. Serum Level of GR in Colon Adenocarcinoma Patients
5.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewandowska, A.; Rudzki, G.; Lewandowski, T.; Stryjkowska-Góra, A.; Rudzki, S. Risk Factors for the Diagnosis of Colorectal Cancer. Cancer Control 2022, 29, 10732748211056692. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, E.M.; Murphy, C.C. Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology 2020, 158, 341–353. [Google Scholar] [CrossRef]
- Healy, M.A.; Thirumurthi, S.; You, Y.N. Screening high-risk populations for colon and rectal cancers. J. Surg. Oncol. 2019, 120, 858–863. [Google Scholar] [CrossRef]
- Pino, M.S.; Chung, D.C. The chromosomal instability pathway in colon cancer. Gastroenterology 2010, 138, 2059–2072. [Google Scholar] [CrossRef] [PubMed]
- Limoli, C.L.; Giedzinski, E. Induction of chromosomal instability by chronic oxidative stress. Neoplasia 2003, 5, 339–346. [Google Scholar] [CrossRef]
- Mena, S.; Ortega, A.; Estrela, J.M. Oxidative stress in environmental-induced carcinogenesis. Mutat. Res. 2009, 674, 36–44. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Arnér, E.S.; Holmgren, A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000, 267, 6102–6109. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.; Halliwell, B. Free radicals and hearing. Cause, consequence, and criteria. Ann. N. Y. Acad. Sci. 1999, 884, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.K.; Tamura, T.; Rogers, B.J.; Welty, S.E.; Hansen, T.N.; Smith, C.V. Analyses of glutathione reductase hypomorphic mice indicate a genetic knockout. Toxicol. Sci. 2004, 82, 367–373. [Google Scholar] [CrossRef]
- Kamerbeek, N.M.; van Zwieten, R.; de Boer, M.; Morren, G.; Vuil, H.; Bannink, N.; Lincke, C.; Dolman, K.M.; Becker, K.; Schirmer, R.H.; et al. Molecular basis of glutathione reductase deficiency in human blood cells. Blood 2007, 109, 3560–3566. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-León, D.; Monzó-Beltrán, L.; Gómez-Abril, S.Á.; Estañ-Capell, N.; Camarasa-Lillo, N.; Pérez-Ebri, M.L.; Escandón-Álvarez, J.; Alonso-Iglesias, E.; Santaolaria-Ayora, M.L.; Carbonell-Moncho, A.; et al. The Effectiveness of Glutathione Redox Status as a Possible Tumor Marker in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 6183. [Google Scholar] [CrossRef] [PubMed]
- Moghadamyeghaneh, Z.; Hanna, M.H.; Carmichael, J.C.; Mills, S.D.; Pigazzi, A.; Stamos, M.J. Preoperative leukocytosis in colorectal cancer patients. J. Am. Coll. Surg. 2015, 221, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Tang, Z.H.; Liu, S.; Guo, S.S. Clinicopathological significance of overexpression of interleukin-6 in colorectal cancer. World J. Gastroenterol. 2017, 23, 1780–1786. [Google Scholar] [CrossRef]
- Jagust, P.; Alcalá, S.; Sainz Jr, B.; Heeschen, C.; Sancho, P. Glutathione metabolism is essential for self-renewal and chemoresistance of pancreatic cancer stem cells. World J. Stem Cells 2020, 12, 1410–1428. [Google Scholar] [CrossRef]
- Abdel Hadi, N.; Reyes-Castellanos, G.; Carrier, A. Targeting Redox Metabolism in Pancreatic Cancer. Int. J. Mol. Sci. 2021, 22, 1534. [Google Scholar] [CrossRef]
- Nishizawa, S.; Araki, H.; Ishikawa, Y.; Kitazawa, S.; Hata, A.; Soga, T.; Hara, T. Low tumor glutathione level as a sensitivity marker for glutamate-cysteine ligase inhibitors. Oncol. Lett. 2018, 15, 8735–8743. [Google Scholar] [CrossRef]
- Kim, A.D.; Zhang, R.; Han, X.; Kang, K.A.; Piao, M.J.; Maeng, Y.H.; Chang, W.Y.; Hyun, J.W. Involvement of glutathione and glutathione metabolizing enzymes in human colorectal cancer cell lines and tissues. Mol. Med. Rep. 2015, 12, 4314–4319. [Google Scholar] [CrossRef] [PubMed]
- Maffei, F.; Angeloni, C.; Malaguti, M.; Moraga, J.M.; Pasqui, F.; Poli, C.; Colecchia, A.; Festi, D.; Hrelia, P.; Hrelia, S. Plasma antioxidant enzymes and clastogenic factors as possible biomarkers of colorectal cancer risk. Mutat. Res. 2011, 714, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Feng, J.; Yang, Y.; Dai, C.; Lu, A.; Li, J.; Liao, Y.; Xiang, M.; Huang, Q.; Wang, D.; et al. Significance of Serum Total Oxidant/Antioxidant Status in Patients with Colorectal Cancer. PLoS ONE. 2017, 12, e0170003. [Google Scholar] [CrossRef] [PubMed]
- Cecerska-Heryć, E.; Surowska, O.; Heryć, R.; Serwin, N.; Napiontek-Balińska, S.; Dołęgowska, B. Are antioxidant enzymes essential markers in the diagnosis and monitoring of cancer patients—A review. Clin. Biochem. 2021, 93, 1–8. [Google Scholar] [CrossRef]
- Gopčević, K.R.; Rovčanin, B.R.; Tatić, S.B.; Krivokapić, Z.V.; Gajić, M.M.; Dragutinović, V.V. Activity of superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase in different stages of colorectal carcinoma. Dig. Dis. Sci. 2013, 58, 2646–2652. [Google Scholar] [CrossRef] [PubMed]
- Skrzydlewska, E.; Sulkowski, S.; Koda, M.; Zalewski, B.; Kanczuga-Koda, L.; Sulkowska, M. Lipid peroxidation and antioxidant status in colorectal cancer. World J. Gastroenterol. 2005, 11, 403–406. [Google Scholar] [CrossRef]
- Skrzydlewska, E.; Stankiewicz, A.; Sulkowska, M.; Sulkowski, S.; Kasacka, I. Antioxidant status and lipid peroxidation in colorectal cancer. J. Toxicol. Environ. Health A 2001, 64, 213–222. [Google Scholar] [CrossRef]
- Zińczuk, J.; Maciejczyk, M.; Zaręba, K.; Romaniuk, W.; Markowski, A.; Kędra, B.; Zalewska, A.; Pryczynicz, A.; Matowicka-Karna, J.; Guzińska-Ustymowicz, K. Antioxidant Barrier, Redox Status, and Oxidative Damage to Biomolecules in Patients with Colorectal Cancer. Can Malondialdehyde and Catalase Be Markers of Colorectal Cancer Advancement? Biomolecules 2019, 9, 637. [Google Scholar] [CrossRef]
- Gaya-Bover, A.; Hernández-López, R.; Alorda-Clara, M.; Ibarra de la Rosa, J.M.; Falcó, E.; Fernández, T.; Company, M.M.; Torrens-Mas, M.; Roca, P.; Oliver, J.; et al. Antioxidant enzymes change in different non-metastatic stages in tumoral and peritumoral tissues of colorectal cancer. Int. J. Biochem. Cell Biol. 2020, 120, 105698. [Google Scholar] [CrossRef]
- Strzelczyk, J.K.; Wielkoszyński, T.; Krakowczyk, Ł.; Adamek, B.; Zalewska-Ziob, M.; Gawron, K.; Kasperczyk, J.; Wiczkowski, A. The activity of antioxidant enzymes in colorectal adenocarcinoma and corresponding normal mucosa. Acta Biochim. Pol. 2012, 59, 549–556. [Google Scholar] [CrossRef]
- Brzozowa-Zasada, M.; Piecuch, A.; Michalski, M.; Matysiak, N.; Kucharzewski, M.; Łos, M.J. The Clinical Application of Immunohistochemical Expression of Notch4 Protein in Patients with Colon Adenocarcinoma. Int. J. Mol. Sci. 2023, 24, 7502. [Google Scholar] [CrossRef]
- Brzozowa-Zasada, M.; Ianaro, A.; Piecuch, A.; Michalski, M.; Matysiak, N.; Stęplewska, K. Immunohistochemical Expression of Glutathione Peroxidase-2 (Gpx-2) and Its Clinical Relevance in Colon Adenocarcinoma Patients. Int. J. Mol. Sci. 2023, 24, 14650. [Google Scholar] [CrossRef] [PubMed]
- Frithiof, H.; Welinder, C.; Larsson, A.M.; Rydén, L.; Aaltonen, K. A novel method for downstream characterization of breast cancer circulating tumor cells following CellSearch isolation. J. Transl. Med. 2015, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- Assi, M. The differential role of reactive oxygen species in early and late stages of cancer. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R646–R653. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P. Radical-free biology of oxidative stress. Am. J. Physiol.-Cell Physiol. 2008, 295, C849–C868. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Perse, M. Oxidative stress in the pathogenesis of colorectal cancer: Cause or consequence? Biomed. Res. Int. 2013, 2013, 725710. [Google Scholar] [CrossRef]
- Aboushousha, R.; van der Velden, J.; Hamilton, N.; Peng, Z.; MacPherson, M.; Erickson, C.; White, S.; Wouters, E.F.M.; Reynaert, N.L.; Seward, D.J.; et al. Glutaredoxin attenuates glutathione levels via deglutathionylation of Otub1 and subsequent destabilization of system xC. Sci Adv. 2023, 9, eadi5192. [Google Scholar] [CrossRef]
- Kennedy, L.; Sandhu, J.K.; Harper, M.E.; Cuperlovic-Culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef]
- Zhao, Y.; Seefeldt, T.; Chen, W.; Carlson, L.; Stoebner, A.; Hanson, S.; Foll, R.; Matthees, D.P.; Palakurthi, S.; Guan, X. Increase in thiol oxidative stress via glutathione reductase inhibition as a novel approach to enhance cancer sensitivity to X-ray irradiation. Free Radic. Biol. Med. 2009, 47, 176–183. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L.F.; Schmitz, A.E.; da Silva, L.C.S.; de Oliveira, K.A.; Nedel, C.B.; Tasca, C.I.; de Bem, A.F.; Farina, M.; Dafre, A.L. Inhibition of reductase systems by 2-AAPA modulates peroxiredoxin oxidation and mitochondrial function in A172 glioblastoma cells. Toxicol. In Vitro 2017, 42, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, Z.; Feng, J.; Zhang, X.; Wu, J.; Chen, W. 2-Acetylamino-3-[4-(2-acetylamino-2-carboxyethylsulfanylcarbonylamino) phenyl carbamoylsulfanyl] propionic acid, a glutathione reductase inhibitor, induces G2/M cell cycle arrest through generation of thiol oxidative stress in human esophageal cancer cells. Oncotarget 2017, 8, 61846–61860. [Google Scholar] [CrossRef]
- Li, X.; Wu, J.; Zhang, X.; Chen, W. Glutathione reductase-mediated thiol oxidative stress suppresses metastasis of murine melanoma cells. Free Radic. Biol. Med. 2018, 129, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Piecuch, A.; Kurek, J.; Kucharzewski, M.; Wyrobiec, G.; Jasiński, D.; Brzozowa-Zasada, M. Catalase immunoexpression in colorectal lesions. Prz. Gastroenterol. 2020, 15, 330–337. [Google Scholar] [CrossRef]
- Piecuch, A.; Brzozowa-Zasada, M.; Dziewit, B.; Segiet, O.; Kurek, J.; Kowalczyk-Ziomek, G.; Wojnicz, R.; Helewski, K. Immunohistochemical assessment of mitochondrial superoxide dismutase (MnSOD) in colorectal premalignant and malignant lesions. Prz. Gastroenterol. 2016, 11, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Nozoe, T.; Honda, M.; Inutsuka, S.; Yasuda, M.; Korenaga, D. Significance of immunohistochemical expression of manganese superoxide dismutase as a marker of malignant potential in colorectal carcinoma. Oncol. Rep. 2003, 10, 39–43. [Google Scholar] [CrossRef]
- Brzozowa-Zasada, M.; Piecuch, A.; Bajdak-Rusinek, K.; Janelt, K.; Michalski, M.; Klymenko, O.; Matysiak, N. Immunohistochemical Expression of Glutathione Peroxidase 1 (Gpx-1) as an Independent Prognostic Factor in Colon Adenocarcinoma Patients. Pharmaceuticals 2023, 16, 740. [Google Scholar] [CrossRef]
- Robbins, D.; Zhao, Y. Manganese superoxide dismutase in cancer prevention. Antioxid Redox Signal. 2014, 20, 1628–1645. [Google Scholar] [CrossRef]
- Chandel, N.S. Mitochondrial regulation of oxygen sensing. Adv. Exp. Med. Biol. 2010, 661, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Preci, D.P.; Almeida, A.; Weiler, A.L.; Mukai Franciosi, M.L.; Cardoso, A.M. Oxidative damage and antioxidants in cervical cancer. Int. J. Gynecol. Cancer 2021, 31, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Karginova, O.; Parker, J.S.; Fan, C.; He, X.; Bixby, L.; Harrell, J.C.; Roman, E.; Adamo, B.; Troester, M.; et al. Characterization of cell lines derived from breast cancers and normal mammary tissues for the study of the intrinsic molecular subtypes. Breast Cancer Res. Treat. 2013, 142, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Ertel, A.; Verghese, A.; Byers, S.W.; Ochs, M.; Tozeren, A. Pathway-specific differences between tumor cell lines and normal and tumor tissue cells. Mol. Cancer. 2006, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Gostimskaya, I.; Grant, C.M. Yeast mitochondrial glutathione is an essential antioxidant with mitochondrial thioredoxin providing a back-up system. Free Radic. Biol. Med. 2016, 94, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.M.; Jones, D.P. Redox control systems in the nucleus: Mechanisms and functions. Antioxid. Redox Signal. 2010, 13, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Santiago-Arteche, R.; Muñiz, P.; Cavia-Saiz, M.; Garcia-Giron, C.; García-Gonzalez, M.; Llorente-Ayala, B.; Corral, M.J. Cancer chemotherapy reduces plasma total polyphenols and total antioxidants capacity in colorectal cancer patients. Mol. Biol. Rep. 2012, 39, 9355–9360. [Google Scholar] [CrossRef]
- Simone, C.B., 2nd; Simone, N.L.; Simone, V.; Simone, C.B. Antioxidants and other nutrients do not interfere with chemotherapy or radiation therapy and can increase kill and increase survival, part 1. Altern. Ther. Health Med. 2007, 13, 22–28. [Google Scholar]
- Jiang, H.; Zuo, J.; Li, B.; Chen, R.; Luo, K.; Xiang, X.; Lu, S.; Huang, C.; Liu, L.; Tang, J.; et al. Drug-induced oxidative stress in cancer treatments: Angel or devil? Redox Biol. 2023, 63, 102754. [Google Scholar] [CrossRef]
- Xiong, Y.; Xiao, C.; Li, Z.; Yang, X. Engineering nanomedicine for glutathione depletion-augmented cancer therapy. Chem. Soc. Rev. 2021, 50, 6013–6041. [Google Scholar] [CrossRef] [PubMed]
- Tormos, C.; Javier Chaves, F.; Garcia, M.J.; Garrido, F.; Jover, R.; O’Connor, J.E.; Iradi, A.; Oltra, A.; Oliva, M.R.; Sáez, G.T. Role of glutathione in the induction of apoptosis and c-fos and c-jun mRNAs by oxidative stress in tumor cells. Cancer Lett. 2004, 208, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Chiang, F.F.; Huang, S.C.; Yu, P.T.; Chao, T.H.; Huang, Y.C. Oxidative Stress Induced by Chemotherapy: Evaluation of Glutathione and Its Related Antioxidant Enzyme Dynamics in Patients with Colorectal Cancer. Nutrients 2023, 15, 5104. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Zhang, H.; Dinavahi, R.; Li, F.; Xiang, Y.; Raman, V.; Bhujwalla, Z.M.; Felsher, D.W.; Cheng, L.; Pevsner, J.; et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell 2007, 12, 230–238. [Google Scholar] [CrossRef]
- Piskounova, E.; Agathocleous, M.; Murphy, M.M.; Hu, Z.; Huddlestun, S.E.; Zhao, Z.; Leitch, A.M.; Johnson, T.M.; DeBerardinis, R.J.; Morrison, S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 2015, 527, 186–191. [Google Scholar] [CrossRef]
- Glasauer, A.; Chandel, N.S. Targeting antioxidants for cancer therapy. Biochem. Pharmacol. 2014, 92, 90–101. [Google Scholar] [CrossRef]




| N (Number of Cases) | % | ||
|---|---|---|---|
| Gender | Females | 67 | 51.15 |
| Males | 64 | 48.85 | |
| Age (years) | ≤60 years | 51 | 38.93 |
| 61–75 years | 42 | 32.06 | |
| >75 years | 38 | 29.01 | |
| M ± SD | 64.56 ± 13.27 | ||
| Me [Q1–Q3] | 65 [age range: 55–77] | ||
| Min–Max | 33–89 | ||
| Grade of histological differentiation | G1 | 21 | 16.03 |
| G2 | 69 | 52.67 | |
| G3 | 41 | 31.30 | |
| Depth of invasion | T1 | 13 | 9.92 |
| T2 | 19 | 14.51 | |
| T3 | 78 | 59.54 | |
| T4 | 21 | 16.03 | |
| Regional lymph node involvement | N0 | 54 | 41.22 |
| N1 | 45 | 34.35 | |
| N2 | 32 | 24.43 | |
| Location of tumour | Proximal | 70 | 53.44 |
| Distal | 61 | 46.56 | |
| Angioinvasion | No | 27 | 20.61 |
| Yes | 104 | 79.39 | |
| PCNA immunohistochemical expression | Low | 36 | 27.48 |
| High | 95 | 72.52 | |
| Staging | I | 22 | 16.79 |
| II | 35 | 26.72 | |
| III | 74 | 56.49 | |
| The Immunoexpression Level of GR | p-Value | |||||
|---|---|---|---|---|---|---|
| Low | High | |||||
| Age (years) | ≤60 years | 14 | (27.45%) | 37 | (72.55%) | p = 0.548 |
| 61–75 years | 16 | (38.1%) | 26 | (61.9%) | ||
| >75 years | 12 | (31.58%) | 26 | (68.42%) | ||
| Gender | Females | 20 | (29.85%) | 47 | (70.15%) | p = 0.579 |
| Males | 22 | (34.38%) | 42 | (65.63%) | ||
| Grade of histological differentiation | G1 | 12 | (57.14%) | 9 | (42.86%) | p = 0.007 |
| G2 | 15 | (21.74%) | 54 | (78.26%) | ||
| G3 | 15 | (36.59%) | 26 | (63.41%) | ||
| Depth of invasion | T1/T2 | 21 | (65.63%) | 11 | (34.38%) | p < 0.001 |
| T3 | 15 | (19.23%) | 63 | (80.77%) | ||
| T4 | 6 | (28.57%) | 15 | (71.43%) | ||
| Regional lymph node involvement | N0 | 32 | (59.26%) | 22 | (40.74%) | p < 0.001 |
| N1/N2 | 10 | (12.99%) | 67 | (87.01%) | ||
| Location of tumour | Proximal | 23 | (32.86%) | 47 | (67.14%) | p = 0.834 |
| Distal | 19 | (31.15%) | 42 | (68.85%) | ||
| Angioinvasion | No | 12 | (44.44%) | 15 | (55.56%) | p = 0.188 |
| Yes | 30 | (28.85%) | 74 | (71.15%) | ||
| PCNA immunohistochemical expression | Low | 20 | (55.56%) | 16 | (44.44%) | p < 0.001 |
| High | 22 | (23.16%) | 73 | (76.84%) | ||
| Staging | I | 21 | (95.45%) | 1 | (4.55%) | p < 0.001 |
| II | 13 | (37.14%) | 22 | (62.86%) | ||
| III | 8 | (10.81%) | 66 | (89.19%) | ||
| The Immunoexpression Level of GR | p-Value | |||||
|---|---|---|---|---|---|---|
| Low | High | |||||
| PCNA Immunoexpression | Low | 20 | (15.27%) | 16 | (12.21%) | p = 0.860 |
| High | 22 | (16.79%) | 73 | (55.73%) | p < 0.001 | |
| Prognostic Parameter | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Gender | 1.033 | 0.715–1.491 | 0.864 | – | – | – |
| Age | 0.984 | 0.782–1.237 | 0.885 | – | – | – |
| Staging (S) | 2.093 | 1.598–2.741 | <0.001 | 1.217 | 0.705–2.100 | 0.480 |
| Grade of histological differentiation (G) | 1.392 | 1.074–1.805 | 0.012 | 1.584 | 1.068–2.350 | 0.022 |
| Depth of invasion (T) | 1.397 | 1.122–1.739 | 0.003 | 0.752 | 0.553–1.023 | 0.070 |
| Nodal status (N) | 1.633 | 1.289–2.069 | <0.001 | 0.799 | 0.545–1.173 | 0.252 |
| Localisation | 0.984 | 0.681–1.423 | 0.931 | – | – | – |
| GR expression | 8.061 | 4.890–13.290 | <0.001 | 9.704 | 4.911–19.173 | <0.001 |
| Angioinvasion | 2.076 | 1.235–3.491 | 0.006 | 1.223 | 0.586–2.550 | 0.591 |
| PCNA expression | 2.806 | 1.740–4.527 | <0.001 | 1.400 | 0.776–2.526 | 0.264 |
| GR Serum Level in Colon Adenocarcinoma Patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | M | Me | Min | Max | Q1 | Q3 | SD | |
| x ≤ Q1 | 16 | 25.00 | 6.83 | 4.82 | 1.98 | 19.03 | 2.78 | 11.00 | 5.52 |
| Q1 < x < Q3 | 32 | 50.00 | 48.73 | 51.49 | 19.78 | 67.85 | 34.84 | 58.33 | 14.70 |
| x ≥ Q3 | 16 | 25.00 | 85.85 | 86.75 | 76.41 | 98.16 | 78.94 | 90.57 | 7.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brzozowa-Zasada, M.; Piecuch, A.; Bajdak-Rusinek, K.; Michalski, M.; Klymenko, O.; Matysiak, N.; Janelt, K.; Czuba, Z. Glutathione Reductase Expression and Its Prognostic Significance in Colon Cancer. Int. J. Mol. Sci. 2024, 25, 1097. https://doi.org/10.3390/ijms25021097
Brzozowa-Zasada M, Piecuch A, Bajdak-Rusinek K, Michalski M, Klymenko O, Matysiak N, Janelt K, Czuba Z. Glutathione Reductase Expression and Its Prognostic Significance in Colon Cancer. International Journal of Molecular Sciences. 2024; 25(2):1097. https://doi.org/10.3390/ijms25021097
Chicago/Turabian StyleBrzozowa-Zasada, Marlena, Adam Piecuch, Karolina Bajdak-Rusinek, Marek Michalski, Olesya Klymenko, Natalia Matysiak, Kamil Janelt, and Zenon Czuba. 2024. "Glutathione Reductase Expression and Its Prognostic Significance in Colon Cancer" International Journal of Molecular Sciences 25, no. 2: 1097. https://doi.org/10.3390/ijms25021097
APA StyleBrzozowa-Zasada, M., Piecuch, A., Bajdak-Rusinek, K., Michalski, M., Klymenko, O., Matysiak, N., Janelt, K., & Czuba, Z. (2024). Glutathione Reductase Expression and Its Prognostic Significance in Colon Cancer. International Journal of Molecular Sciences, 25(2), 1097. https://doi.org/10.3390/ijms25021097







