The Involvement of Cysteine-X-Cysteine Motif Chemokine Receptors in Skin Homeostasis and the Pathogenesis of Allergic Contact Dermatitis and Psoriasis
Abstract
1. Introduction
2. CXCR-Mediated Cellular Communication
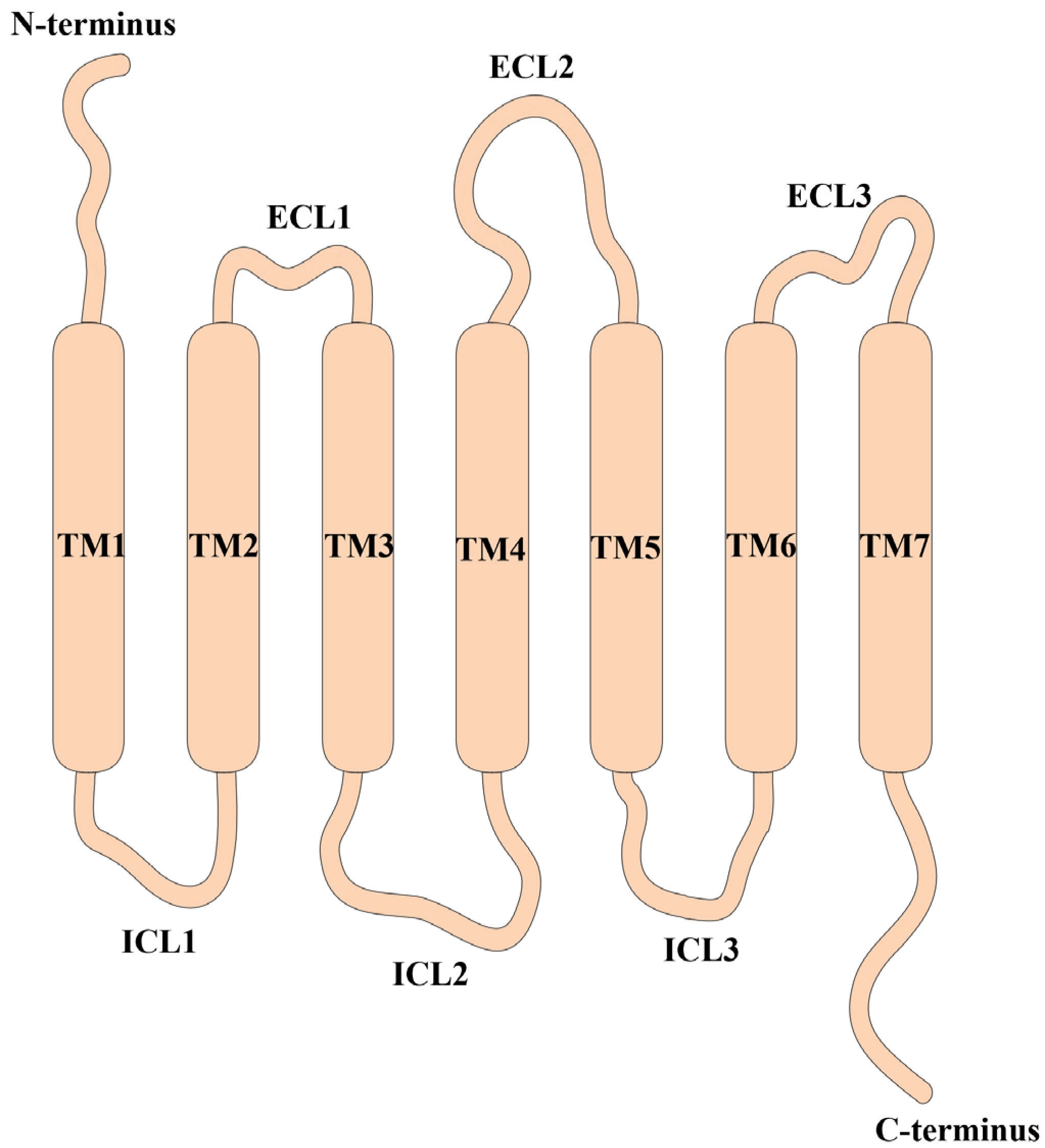
2.1. The Architecture of CXCRs and Their Corresponding Ligands
2.2. Transmissive Pathways of CXCR Signaling
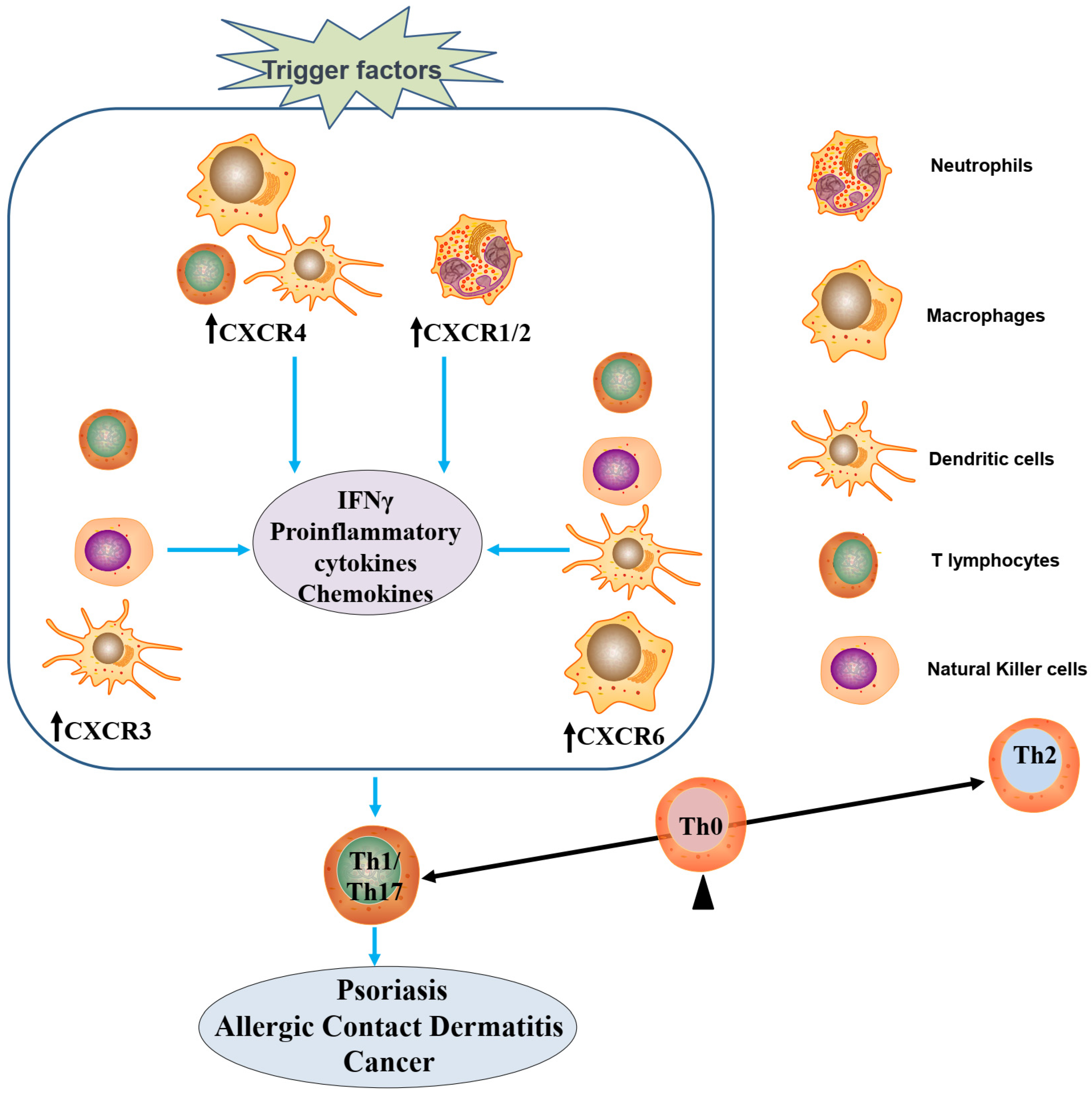
3. CXCRs in Dermatological Inflammatory Conditions
3.1. Expression of CXCRs and Functional Implications in Skin Cells
3.2. Activation of CXCRs and the Onset of ACD
3.3. Activation of CXCRs and Initiation of Psoriatic Inflammation
4. CXCR-Specific Therapies
4.1. Application of CXCR as a Therapeutic Target in Allergic Contact Dermatitis
4.2. Prospect of CXCR Inhibition as a Therapeutic Approach for Psoriasis
4.3. Therapeutic Use of CXCR Ligands for Treating Skin Disorders and Cancer
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, K.Q.; Bao, Z.Y.; Tang, P.; Gong, W.H.; Yoshimura, T.; Wang, J.M. Chemokines in homeostasis and diseases. Cell. Mol. Immunol. 2018, 15, 324–334. [Google Scholar] [CrossRef]
- Weis, W.I.; Kobilka, B.K. The Molecular Basis of G Protein-Coupled Receptor Activation. Annu. Rev. Biochem. 2018, 87, 897–919. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Qiao, Y.H.; Li, Z.J. New Insights into Modes of GPCR Activation. Trends Pharmacol. Sci. 2018, 39, 367–386. [Google Scholar] [CrossRef] [PubMed]
- Billottet, C.; Quemener, C.; Bikfalvi, A. CXCR3, a double-edged sword in tumor progression and angiogenesis. BBA-Rev. Cancer 2013, 1836, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Alsayed, R.K.M.E.; Khan, A.Q.; Ahmad, F.; Ansari, A.W.; Alam, M.A.; Buddenkotte, J.; Steinhoff, M.; Uddin, S.; Ahmad, A. Epigenetic regulation of CXCR4 signaling in cancer pathogenesis and progression. Semin. Cancer Biol. 2022, 86, 697–708. [Google Scholar] [CrossRef]
- Bao, N.D.; Fu, B.; Zhong, X.L.; Jia, S.S.; Ren, Z.Z.; Wang, H.R.; Wang, W.H.; Shi, H.; Li, J.; Ge, F.L.; et al. Role of the CXCR6/CXCL16 axis in autoimmune diseases. Int. Immunopharmacol. 2023, 121, 110530. [Google Scholar] [CrossRef]
- Hogaboam, C.M.; Carpenter, K.J.; Schuh, J.M.; Proudfoot, A.A.E.I.; Bridger, G.; Buckland, K.F. The therapeutic potential in targeting CCR5 and CXCR4 receptors in infectious and allergic pulmonary disease. Pharmacol. Therapeut. 2005, 107, 314–328. [Google Scholar] [CrossRef]
- Lounsbury, N. Advances in CXCR7 Modulators. Pharmaceuticals 2020, 13, 33. [Google Scholar] [CrossRef]
- Tiberio, L.; Del Prete, A.; Schioppa, T.; Sozio, F.; Bosisio, D.; Sozzani, S. Chemokine and chemotactic signals in dendritic cell migration. Cell. Mol. Immunol. 2018, 15, 346–352. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.M.; Cheng, Y.J.; Cao, X.T. Dendritic cell migration in inflammation and immunity. Cell. Mol. Immunol. 2021, 18, 2461–2471. [Google Scholar] [CrossRef]
- Takekoshi, T.; Wu, X.S.; Mitsui, H.; Tada, Y.; Kao, M.C.; Sato, S.; Dwinell, M.B.; Hwang, S.T. CXCR4 Negatively Regulates Keratinocyte Proliferation in IL-23-Mediated Psoriasiform Dermatitis. J. Investig. Dermatol. 2013, 133, 2530–2537. [Google Scholar] [CrossRef]
- Günther, C.; Carballido-Perrig, N.; Kaesler, S.; Carballido, J.M.; Biedermann, T. CXCL16 and CXCR6 Are Upregulated in Psoriasis and Mediate Cutaneous Recruitment of Human CD8 T Cells. J. Investig. Dermatol. 2012, 132, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.M.; Hill, R.Z.; Schwendinger-Schreck, J.; Deguine, J.; Brock, E.C.; Kucirek, N.; Rifi, Z.; Wei, J.; Gronert, K.; Brem, R.B.; et al. Neutrophils promote CXCR3-dependent itch in the development of atopic dermatitis. eLife 2019, 8, e48448. [Google Scholar] [CrossRef]
- Smith, J.S.; Rajagopal, S.; Atwater, A.R. Chemokine Signaling in Allergic Contact Dermatitis: Toward Targeted Therapies. Dermatitis 2018, 29, 179–186. [Google Scholar] [CrossRef]
- Sebastiani, S.; Albanesi, C.; Nasorri, F.; Girolomoni, G.; Cavani, A. Nickel-specific CD4 and CD8 T cells display distinct migratory responses to chemokines produced during allergic contact dermatitis. J. Investig. Dermatol. 2002, 118, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Rashighi, M.; Agarwal, P.; Richmond, J.M.; Harris, T.H.; Dresser, K.; Su, M.W.; Zhou, Y.W.; Deng, A.; Hunter, C.A.; Luster, A.D.; et al. CXCL10 Is Critical for the Progression and Maintenance of Depigmentation in a Mouse Model of Vitiligo. Sci. Transl. Med. 2014, 6, 223ra23. [Google Scholar] [CrossRef]
- He, S.; Xu, J.H.; Wu, J.F. The Promising Role of Chemokines in Vitiligo: From Oxidative Stress to the Autoimmune Response. Oxid. Med. Cell. Longev. 2022, 2022, 8796735. [Google Scholar] [CrossRef] [PubMed]
- Doron, H.; Amer, M.; Ershaid, N.; Blazquez, R.; Shani, O.; Lahav, T.G.; Cohen, N.; Adler, O.; Hakim, Z.; Pozzi, S.; et al. Inflammatory Activation of Astrocytes Facilitates Melanoma Brain Tropism via the CXCL10-CXCR3 Signaling Axis. Cell. Rep. 2019, 28, 1785–1798. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Hirschfeld, J.; Kantarci, A.; Wilensky, A.; Shapira, L. The role of the host-Neutrophil biology. Periodontol 2000 2023, 1–47. [Google Scholar] [CrossRef]
- Xie, Y.S.; Kuang, W.B.; Wang, D.W.; Yuan, K.; Yang, P. Expanding role of CXCR2 and therapeutic potential of CXCR2 antagonists in inflammatory diseases and cancers. Eur. J. Med. Chem. 2023, 250, 115175. [Google Scholar] [CrossRef]
- Karin, N. CXCR3 Ligands in Cancer and Autoimmunity, Chemoattraction of Effector T Cells, and Beyond. Front. Immunol. 2020, 11, 976. [Google Scholar] [CrossRef]
- Groom, J.R. Regulators of T-cell fate: Integration of cell migration, differentiation and function. Immunol. Rev. 2019, 289, 101–114. [Google Scholar] [CrossRef]
- Janssens, R.; Struyf, S.; Proost, P. The unique structural and functional features of CXCL12. Cell. Mol. Immunol. 2018, 15, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Janssens, R.; Struyf, S.; Proost, P. Pathological roles of the homeostatic chemokine CXCL12. Cytokine Growth Factor Rev. 2018, 44, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.H.; Wang, M.N.; Ao, D.Y.; Wei, X.W. CXCL13-CXCR5 axis: Regulation in inflammatory diseases and cancer. BBA-Rev. Cancer 2022, 1877, 188799. [Google Scholar] [CrossRef]
- Pan, Z.J.; Zhu, T.; Liu, Y.J.; Zhang, N.N. Role of the CXCL13/CXCR5 axis in autoimmune diseases. Front. Immunol. 2022, 13, 1061939. [Google Scholar] [CrossRef] [PubMed]
- La Porta, C.A.M. CXCR6: The Role of Environment in Tumor Progression. Challenges for Therapy. Stem. Cell Rev. Rep. 2012, 8, 1282–1285. [Google Scholar] [CrossRef]
- Huynh, C.; Dingemanse, J.; Schwabedissen, H.E.M.Z.; Sidharta, P.N. Relevance of the CXCR4/CXCR7-CXCL12 axis and its effect in pathophysiological conditions. Pharmacol. Res. 2020, 161, 105092. [Google Scholar] [CrossRef]
- Villalvilla, A.; Gomez, R.; Roman-Blas, J.A.; Largo, R.; Herrero-Beaumont, G. SDF-1 signaling: A promising target in rheumatic diseases. Expert Opin. Ther. Targets 2014, 18, 1077–1087. [Google Scholar] [CrossRef]
- Eck, S.M.; Blackburn, J.S.; Schmucker, A.C.; Burrage, P.S.; Brinckerhoff, C.E. Matrix metalloproteinase and G protein coupled receptors: Co-conspirators in the pathogenesis of autoimmune disease and cancer. J. Autoimmun. 2009, 33, 214–221. [Google Scholar] [CrossRef][Green Version]
- Erazo-Martínez, V.; Tobón, G.J.; Cañas, C.A. Circulating and skin biopsy-present cytokines related to the pathogenesis of cutaneous lupus erythematosus. Autoimmun. Rev. 2023, 22, 103262. [Google Scholar] [CrossRef]
- Wenzel, J. Cutaneous lupus erythematosus: New insights into pathogenesis and therapeutic strategies. Nat. Rev. Rheumatol. 2019, 15, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.F.; Wang, M.; Cao, Z.W. Reduced CD4 T Cell CXCR3 Expression in Patients With Allergic Rhinitis. Front. Immunol. 2020, 11, 581180. [Google Scholar] [CrossRef]
- Purohit, A.; Saxena, S.; Varney, M.; Prajapati, D.R.; Kozel, J.A.; Lazenby, A.; Singh, R.K. Host-Dependent Regulation of Pancreatic Cancer Growth, Angiogenesis, and Metastasis. Am. J. Pathol. 2021, 191, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Bekker, L.G.; Beyrer, C.; Mgodi, N.; Lewin, S.R.; Delany-Moretlwe, S.; Taiwo, B.; Masters, M.C.; Lazarus, J.V. HIV infection. Nat. Rev. Dis. Primers 2023, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Israr, M.; DeVoti, J.A.; Papayannakos, C.J.; Bonagura, V.R. Role of chemokines in HPV-induced cancers. Semin. Cancer Biol. 2022, 87, 170–183. [Google Scholar] [CrossRef]
- Wu, X.; Qian, L.; Zhao, H.D.; Lei, W.R.; Liu, Y.Q.; Xu, X.L.; Li, J.W.; Yang, Z.; Wang, D.; Zhang, Y.C.; et al. CXCL12/CXCR4: An amazing challenge and opportunity in the fight against fibrosis. Ageing Res. Rev. 2023, 83, 101809. [Google Scholar] [CrossRef]
- Xu, H.L.; Lin, S.Y.; Zhou, Z.Y.; Li, D.D.; Zhang, X.T.; Yu, M.H.; Zhao, R.Y.; Wang, Y.H.; Qian, J.R.; Li, X.Y.; et al. New genetic and epigenetic insights into the chemokine system: The latest discoveries aiding progression toward precision medicine. Cell. Mol. Immunol. 2023, 20, 739–776. [Google Scholar] [CrossRef]
- Bünemann, E.; Hoff, N.P.; Buhren, B.A.; Wiesner, U.; Meller, S.; Bölke, E.; Muller-Homey, A.; Kubitza, R.; Ruzicka, T.; Zlotnik, A.; et al. Chemokine ligand-receptor interactions critically regulate cutaneous wound healing. Eur. J. Med. Res. 2018, 23, 4. [Google Scholar] [CrossRef]
- Kroeze, K.L.; Boink, M.A.; Sampat-Sardjoepersad, S.C.; Waaijman, T.; Scheper, R.J.; Gibbs, S. Autocrine Regulation of Re-Epithelialization After Wounding by Chemokine Receptors CCR1, CCR10, CXCR1, CXCR2, and CXCR3. J. Investig. Dermatol. 2012, 132, 216–225. [Google Scholar] [CrossRef]
- Caproni, M.; Torchia, D.; Antiga, E.; Terranova, M.; Volpi, W.; Del Bianco, E.; D’Agata, A.; Fabbri, P. The comparative effects of tacrolimus and hydrocortisone in adult atopic dermatitis: An immunohistochemical study. Br. J. Dermatol. 2007, 156, 312–319. [Google Scholar] [CrossRef]
- Peddibhotla, S.; Caples, K.; Mehta, A.; Chen, Q.Y.; Hu, J.Y.; Idlett-Ali, S.; Zhang, L.P.; Zgheib, C.; Xu, J.W.; Liechty, K.W.; et al. Triazolothiadiazine derivative positively modulates CXCR4 signaling and improves diabetic wound healing. Biochem. Pharmacol. 2023, 216, 115764. [Google Scholar] [CrossRef]
- Peiffer, B.J.; Qi, L.; Ahmadi, A.R.; Wang, Y.F.; Guo, Z.F.; Peng, H.J.; Sun, Z.L.; Liu, J.O. Activation of BMP Signaling by FKBP12 Ligands Synergizes with Inhibition of CXCR4 to Accelerate Wound Healing. Cell. Chem. Biol. 2019, 26, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Gallais Serezal, I.; Tajpara, P.; Schonfeldt, T.; Ignatov, B.; Sortebech, D.; Hoffer, E.; Zhang, T.; Rooijackers, E.; Ehrstrom, M.; Nylen, S.; et al. T cells in resolved allergic contact dermatitis steer tissue inflammation and MMP-12-driven tissue modulation. Allergy 2022, 77, 3680. [Google Scholar] [CrossRef]
- Ma, F.Y.; Plazyo, O.; Billi, A.C.; Tsoi, L.C.; Xing, X.Y.; Wasikowski, R.; Gharaee-Kermani, M.; Hile, G.; Jiang, Y.Y.; Harms, P.W.; et al. Single cell and spatial sequencing define processes by which keratinocytes and fibroblasts amplify inflammatory responses in psoriasis. Nat. Commun. 2023, 14, 3455. [Google Scholar] [CrossRef] [PubMed]
- Dhayni, K.; Zibara, K.; Issa, H.; Kamel, S.; Bennis, Y. Targeting CXCR1 and CXCR2 receptors in cardiovascular diseases. Pharmacol. Therapeut. 2022, 237, 108257. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.Y.; Hsiao, Y.W.; Liu, H.L.; Fan, X.J.; Wan, X.B.; Liu, T.L.; Hung, S.J.; Chen, Y.T.; Liang, H.Y.; Wang, J.M. Fibroblast CEBPD/SDF4 axis in response to chemotherapy-induced angiogenesis through CXCR4. Cell Death Discov. 2021, 7, 94. [Google Scholar] [CrossRef]
- Smadja, D.M.; Dorfmüller, P.; Guerin, C.L.; Bieche, I.; Badoual, C.; Boscolo, E.; Kambouchner, M.; Cazes, A.; Mercier, O.; Humbert, M.; et al. Cooperation between human fibrocytes and endothelial colony-forming cells increases angiogenesis via the CXCR4 pathway. Thromb. Haemost. 2014, 112, 1002–1013. [Google Scholar] [CrossRef]
- de Alba, C.G.; Buendia-Roldán, I.; Salgado, A.; Becerril, C.; Ramírez, R.; González, Y.; Checa, M.; Navarro, C.; Ruiz, V.; Pardo, A.; et al. Fibrocytes Contribute to Inflammation and Fibrosis in Chronic Hypersensitivity Pneumonitis through Paracrine Effects. Am. J. Respir. Crit. Care Med. 2015, 191, 427–436. [Google Scholar] [CrossRef]
- McCully, M.L.; Kouzeli, A.; Moser, B. Peripheral Tissue Chemokines: Homeostatic Control of Immune Surveillance T Cells. Trends Immunol. 2018, 39, 734–747. [Google Scholar] [CrossRef]
- Krohn, I.K.; Aerts, J.L.; Breckpot, K.; Goyvaerts, C.; Knol, E.; Van Wijk, F.; Gutermuth, J. T-cell subsets in the skin and their role in inflammatory skin disorders. Allergy 2022, 77, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G.E.; Harris, J.E.; Richmond, J.M. Resident Memory T Cells in Autoimmune Skin Diseases. Front. Immunol. 2021, 12, 652191. [Google Scholar] [CrossRef] [PubMed]
- Cambier, S.; Gouwy, M.; Proost, P. The chemokines CXCL8 and CXCL12: Molecular and functional properties, role in disease and efforts towards pharmacological intervention. Cell Mol. Immunol. 2023, 20, 217–251. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.D.; Bonefeld, C.M.; Schwensen, J.F.B.; Thyssen, J.P.; Uter, W. Novel insights into contact dermatitis. J. Allergy Clin. Immunol. 2022, 149, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Alinaghi, F.; Bennike, N.H.; Egeberg, A.; Thyssen, J.P.; Johansen, J.D. Prevalence of contact allergy in the general population: A systematic review and meta-analysis. Contact Dermat. 2019, 80, 77–85. [Google Scholar] [CrossRef]
- Neale, H.; Garza-Mayers, A.C.; Tam, I.; Yu, J. Pediatric allergic contact dermatitis. Part I: Clinical features and common contact allergens in children. J. Am. Acad. Dermatol. 2021, 84, 235–244. [Google Scholar] [CrossRef]
- Brites, G.S.; Ferreira, I.; Sebastiao, A.I.; Silva, A.; Carrascal, M.; Neves, B.M.; Cruz, M.T. Allergic contact dermatitis: From pathophysiology to development of new preventive strategies. Pharmacol. Res. 2020, 162, 105282. [Google Scholar] [CrossRef]
- De Graaf, N.P.J.; Sanne, R.; Sue, G.; Kleverlaan, C.J.; Marta, L.G.; Thomas, R.; Feilzer, A.J.; Bontkes, H.J. Nickel allergy is associated with a broad spectrum cytokine response. Contact Dermat. 2022, 88, 10–17. [Google Scholar] [CrossRef]
- Lefevre, M.A.; Nosbaum, A.; Rozieres, A.; Lenief, V.; Mosnier, A.; Cortial, A.; Prieux, M.; De Bernard, S.; Nourikyan, J.; Jouve, P.E.; et al. Unique molecular signatures typify skin inflammation induced by chemical allergens and irritants. Allergy 2021, 76, 3697–3712. [Google Scholar] [CrossRef]
- Dilley, M.; Geng, B. Immediate and Delayed Hypersensitivity Reactions to Antibiotics: Aminoglycosides, Clindamycin, Linezolid, and Metronidazole. Clin. Rev. Allerg Immunol. 2022, 62, 463–475. [Google Scholar] [CrossRef]
- Zirwas, M.J. Contact Dermatitis to Cosmetics. Clin. Rev. Allerg Immunol. 2019, 56, 119–128. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Yiannias, J.A. Contact Dermatitis to Medications and Skin Products. Clin. Rev. Allerg Immunol. 2019, 56, 41–59. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, S.M.; White, I.R.; Kimber, I.; McFadden, J.P.; Tziotzios, C. Contact allergy across the human lifespan. J. Allergy Clin. Immunol. 2020, 145, 1352–1354. [Google Scholar] [CrossRef]
- Brys, A.K.; Rodriguez-Homs, L.G.; Suwanpradid, J.; Atwater, A.R.; MacLeod, A.S. Shifting Paradigms in Allergic Contact Dermatitis: The Role of Innate Immunity. J. Investig. Dermatol. 2020, 140, 21–28. [Google Scholar] [CrossRef]
- Meller, S.; Lauerma, A.I.; Kopp, F.M.; Winterberg, F.; Anthoni, M.; Müller, A.; Gombert, M.; Haahtela, A.; Alenius, H.; Rieker, J.; et al. Chemokine responses distinguish chemical-induced allergic from irritant skin inflammation:: Memory T cells make the difference. J. Allergy Clin. Immunol. 2007, 119, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Kim, N.Y.; Kim, J.H. Current Understanding of the Roles of CD1a-Restricted T Cells in the Immune System. Mol. Cells 2021, 44, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Sasson, S.C.; Gordon, C.L.; Christo, S.N.; Klenerman, P.; Mackay, L.K. Local heroes or villains: Tissue-resident memory T cells in human health and disease. Cell Mol. Immunol. 2020, 17, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Funch, A.B.; Mraz, V.; Gadsboll, A.O.; Jee, M.H.; Weber, J.F.; Odum, N.; Woetmann, A.; Johansen, J.D.; Geisler, C.; Bonefeld, C.M. CD8(+) tissue-resident memory T cells recruit neutrophils that are essential for flare-ups in contact dermatitis. Allergy 2022, 77, 513–524. [Google Scholar] [CrossRef]
- Margraf, A.; Lowell, C.A.; Zarbock, A. Neutrophils in acute inflammation: Current concepts and translational implications. Blood 2022, 139, 2130–2144. [Google Scholar] [CrossRef]
- Smith, J.S.; Nicholson, L.T.; Suwanpradid, J.; Glenn, R.A.; Knape, N.M.; Alagesan, P.; Gundry, J.N.; Wehrman, T.S.; Atwater, A.R.; Gunn, M.D.; et al. Biased agonists of the chemokine receptor CXCR3 differentially control chemotaxis and inflammation. Sci. Signal 2018, 11, eaaq1075. [Google Scholar] [CrossRef]
- Bieber, T. Disease modification in inflammatory skin disorders: Opportunities and challenges. Nat. Rev. Drug Discov. 2023, 22, 935. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis A Review. JAMA-J. Am. Med. Assoc. 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, H.Y.; Lin, W.R.; Lu, L.X.; Su, J.; Chen, X. Signaling pathways and targeted therapies for psoriasis. Signal Transduct. Target. Ther. 2023, 8, 437. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Das, D.; Akhtar, S.; Kurra, S.; Gupta, S.; Sharma, A. Emerging role of immune cell network in autoimmune skin disorders: An update on pemphigus, vitiligo and psoriasis. Cytokine Growth Factor Rev. 2019, 45, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Su, Y.W.; Li, S.Y.; Chen, H.; Wu, R.F.; Wu, H.J. The roles of T cells in psoriasis. Front. Immunol. 2023, 14, 1081256. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Wang, M.Y.; Gao, H.; Zheng, A.; Li, J.H.; Mu, D.Z.; Tong, J.Y. The Role of Helper T Cells in Psoriasis. Front. Immunol. 2021, 12, 788940. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Balato, A.; Enerbäck, C.; Sabat, R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet 2021, 397, 754–766. [Google Scholar] [CrossRef]
- Majumder, S.; McGeachy, M.J. IL-17 in the Pathogenesis of Disease: Good Intentions Gone Awry. Annu. Rev. Immunol. 2021, 39, 537–556. [Google Scholar] [CrossRef]
- Bravo, A.; Kavanaugh, A. Bedside to bench: Defining the immunopathogenesis of psoriatic arthritis. Nat. Rev. Rheumatol. 2019, 15, 645–656. [Google Scholar] [CrossRef]
- Uttarkar, S.; Brembilla, N.C.; Boehncke, W.H. Regulatory cells in the skin: Pathophysiologic role and potential targets for anti-inflammatory therapies. J. Allergy Clin. Immunol. 2019, 143, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.D.; Zhao, Q.X.; Wang, X.Y.; Zhou, H.; Hu, J.; Gu, L.N.; Hu, Y.W.; Zeng, F.L.; Zhao, F.L.; Yue, C.C.; et al. Pathogenesis, multi-omics research, and clinical treatment of psoriasis. J. Autoimmun. 2022, 133, 102916. [Google Scholar] [CrossRef]
- Kamata, M.; Tada, Y. Crosstalk: Keratinocytes and immune cells in psoriasis. Front. Immunol. 2023, 14, 1286344. [Google Scholar] [CrossRef] [PubMed]
- Cibrian, D.; de la Fuente, H.; Sánchez-Madrid, F. Metabolic Pathways That Control Skin Homeostasis and Inflammation. Trends Mol. Med. 2020, 26, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Yadav, T.C.; Khera, H.K.; Mishra, P.; Raghuwanshi, N.; Pruthi, V.; Prasad, R. Insights into interplay of immunopathophysiological events and molecular mechanistic cascades in psoriasis and its associated comorbidities. J. Autoimmun. 2021, 118, 102614. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.T.; Fu, K.; Yang, J.; Shimada, S.G.; LaMotte, R.H. CXCR3 chemokine receptor signaling mediates itch in experimental allergic contact dermatitis. Pain 2015, 156, 1737–1746. [Google Scholar] [CrossRef]
- Jing, P.B.; Cao, D.L.; Li, S.S.; Zhu, M.X.; Bai, X.Q.; Wu, X.B.; Gao, Y.J. Chemokine Receptor CXCR3 in the Spinal Cord Contributes to Chronic Itch in Mice. Neurosci. Bull. 2018, 34, 54–63. [Google Scholar] [CrossRef]
- Dwyer, M.P.; Yu, Y.N.; Chao, J.P.; Aki, C.; Chao, J.H.; Biju, P.; Girijavallabhan, V.; Rindgen, D.; Bond, R.; Mayer-Ezel, R.; et al. Discovery of 2-hydroxy-,-dimethyl-3-{2-[-1-(5-methylfuran-2-yl)propyl]amino]-3,4-dioxocyclobut-1-enylamino}benzamide (SCH 527123): A potent, orally bioavailable CXCR2/CXCR1 receptor antagonist. J. Med. Chem. 2006, 49, 7603–7606. [Google Scholar] [CrossRef]
- Lazaar, A.L.; Sweeney, L.E.; MacDonald, A.J.; Alexis, N.E.; Chen, C.; Tal-Singer, R. SB-656933, a novel CXCR2 selective antagonist, inhibits neutrophil activation and ozone-induced airway inflammation in humans. Br. J. Clin. Pharmacol. 2011, 72, 282–293. [Google Scholar] [CrossRef]
- Mehrpouri, M. The contributory roles of the CXCL12/CXCR4/CXCR7 axis in normal and malignant hematopoiesis: A possible therapeutic target in hematologic malignancies. Eur. J. Pharmacol. 2022, 920, 174831. [Google Scholar] [CrossRef]
- Martin, M.; Mayer, I.A.; Walenkamp, A.M.E.; Lapa, C.; Andreeff, M.; Bobirca, A. At the bedside: Profiling and treating patients with CXCR4-expressing cancers. J. Leukoc. Biol. 2021, 109, 953–967. [Google Scholar] [CrossRef] [PubMed]
- Ngamsri, K.C.; Jans, C.; Putri, R.A.; Schindler, K.; Gamper-Tsigaras, J.; Eggstein, C.; Köhler, D.; Konrad, F.M. Inhibition of CXCR4 and CXCR7 Is Protective in Acute Peritoneal Inflammation. Front. Immunol. 2020, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Bockorny, B.; Semenisty, V.; Macarulla, T.; Borazanci, E.; Wolpin, B.M.; Stemmer, S.M.; Golan, T.; Geva, R.; Borad, M.J.; Pedersen, K.S.; et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: The COMBAT trial. Nat. Med. 2020, 26, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, J.H.; Jin, W.J.; Kim, H.H.; Ha, H.; Lee, Z.H. JN-2, a C-X-C motif chemokine receptor 3 antagonist, ameliorates arthritis progression in an animal model. Eur. J. Pharmacol. 2018, 823, 1–10. [Google Scholar] [CrossRef]
- Cao, J.M.; Zhu, W.; Yu, D.J.; Pan, L.; Zhong, L.; Xiao, Y.J.; Gao, Y.Y.; Jiao, Y.; Zhang, Q.; Ji, J.; et al. The Involvement of SDF-1α/CXCR4 Axis in Radiation-Induced Acute Injury and Fibrosis of Skin. Radiat. Res. 2019, 192, 410–421. [Google Scholar] [CrossRef]
- Liao, Z.K.; Hu, S.H.; Han, B.Y.; Qiu, X.; Jiang, S.; Lei, T.C. Pro-pigmentary action of 5-fluorouracil through the stimulated secretion of CXCL12 by dermal fibroblasts. Chin. Med. J.-Peking 2021, 134, 2475–2482. [Google Scholar] [CrossRef]
- Byrne, S.N.; Sarchio, S.N.E. AMD3100 protects from UV-induced skin cancer. Oncoimmunology 2014, 3, e27562. [Google Scholar] [CrossRef][Green Version]

| Biological Drugs | Target sites | Categories | Therapeutic Applications | References |
|---|---|---|---|---|
| AMG487 | CXCR3 | Antagonist | Block CXCR3 signaling and inhibit the migration of inflammatory cells | [86] |
| NBI74330 | CXCR3 | Antagonist | Block CXCR3 signaling and inhibit the migration of inflammatory cells | [87] |
| SCH527123 | CXCR2 | Antagonist | Reduce inflammation and symptoms | [88] |
| SB656933 | CXCR2 | Antagonist | Reduce inflammation and symptoms | [89] |
| Ulocuplumab | CXCR4 | Monoclonal antibody | Block CXCR4 signaling and inhibit immune cell migration | [90,91] |
| Plerixafor | CXCR4 | Monoclonal antibody | Block CXCR4 signaling and inhibit immune cell migration | [90,91] |
| LY2510924 | Dual CXCR3 /CXCR4 | Antagonists | Clinical studies of inflammatory diseases | [90] |
| CCX771 | Dual CXCR3 /CXCR4 | Antagonists | Clinical studies of inflammatory diseases | [92] |
| LY2510924 | CXCR4 | Monoclonal antibody | A potential treatment for inflammatory diseases | [91] |
| BL8040 | CXCR4 | Antagonist | A potential therapy for cancer | [93] |
| MDX1100 | CXCL10 | Monoclonal antibody | A potential therapy for arthritis | [94] |
| AMD3100 | CXCR4 | Inhibitor | Radiation skin damage and fibrosis reduction, hematopoietic stem cell mobilization for cancer patients’ transplantation | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W. The Involvement of Cysteine-X-Cysteine Motif Chemokine Receptors in Skin Homeostasis and the Pathogenesis of Allergic Contact Dermatitis and Psoriasis. Int. J. Mol. Sci. 2024, 25, 1005. https://doi.org/10.3390/ijms25021005
Liu W. The Involvement of Cysteine-X-Cysteine Motif Chemokine Receptors in Skin Homeostasis and the Pathogenesis of Allergic Contact Dermatitis and Psoriasis. International Journal of Molecular Sciences. 2024; 25(2):1005. https://doi.org/10.3390/ijms25021005
Chicago/Turabian StyleLiu, Wenjie. 2024. "The Involvement of Cysteine-X-Cysteine Motif Chemokine Receptors in Skin Homeostasis and the Pathogenesis of Allergic Contact Dermatitis and Psoriasis" International Journal of Molecular Sciences 25, no. 2: 1005. https://doi.org/10.3390/ijms25021005
APA StyleLiu, W. (2024). The Involvement of Cysteine-X-Cysteine Motif Chemokine Receptors in Skin Homeostasis and the Pathogenesis of Allergic Contact Dermatitis and Psoriasis. International Journal of Molecular Sciences, 25(2), 1005. https://doi.org/10.3390/ijms25021005






