Untargeted Metabolomic Biomarker Discovery for the Detection of Ectopic Pregnancy
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Population and Sample Collection
4.2. Sample Collection
4.3. Sample Extraction and LC-MS/MS Mass Spectrometry
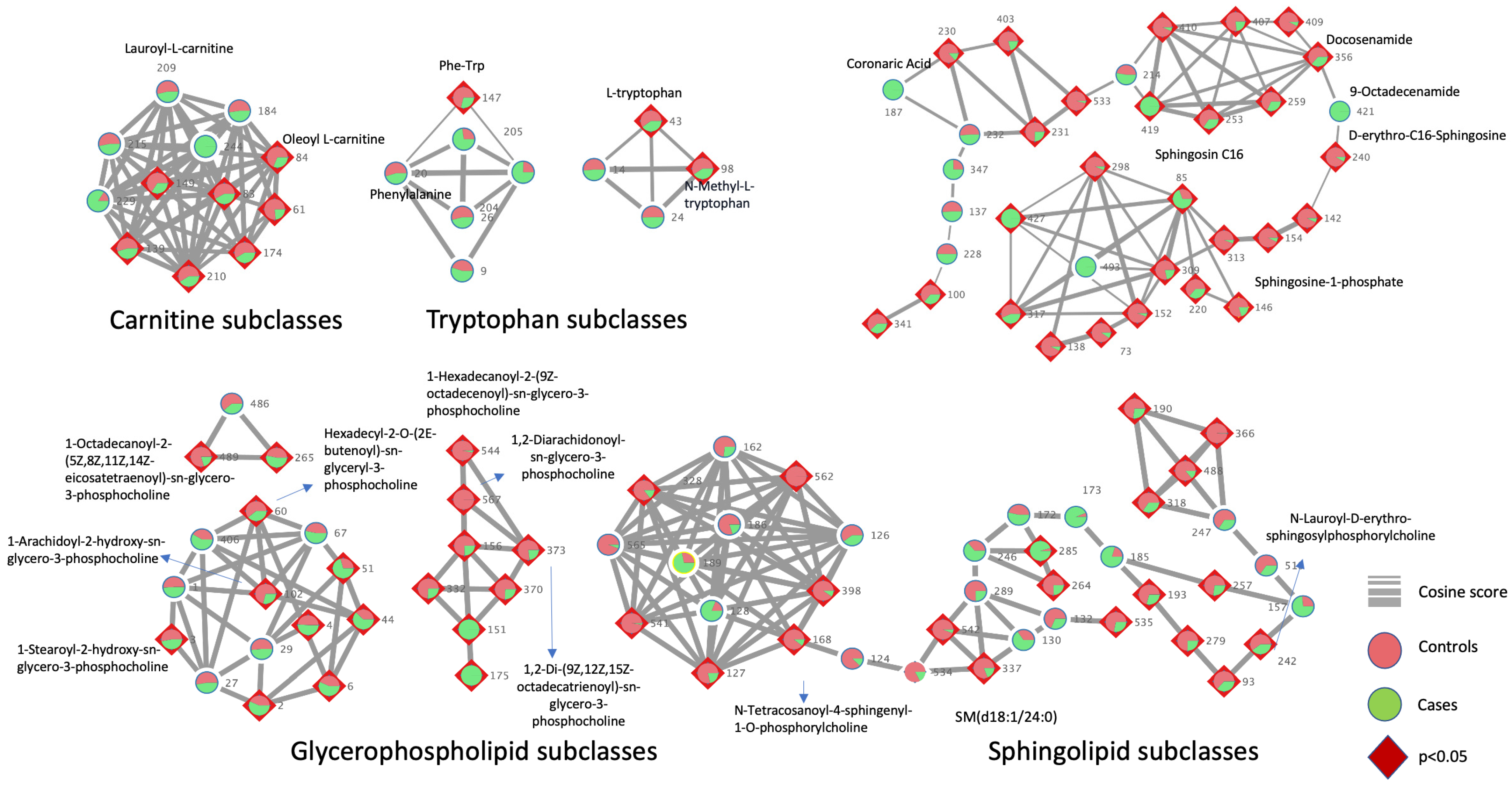
4.4. Feature-Based Molecular Networking Using GNPS and Compound Annotation
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farquhar, C.M. Ectopic pregnancy. Lancet 2005, 366, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.M.; Rouse, D.J.; Varner, E.; Austin, J.M., Jr. Treatment of the small unruptured ectopic pregnancy: A cost analysis of methotrexate versus laparoscopy. Obstet. Gynecol. 1996, 88, 123–127. [Google Scholar] [CrossRef]
- Goyaux, N.; Leke, R.; Keita, N.; Thonneau, P. Ectopic pregnancy in African developing countries. Acta Obstet. Et Gynecol. Scand. 2003, 82, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, J.; Coste, J.; Fernandez, H.; Pouly, J.L.; Job-Spira, N. Sites of ectopic pregnancy: A 10 year population-based study of 1800 cases. Hum. Reprod. 2002, 17, 3224–3230. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Ectopic Pregnancy—United States, 1990–1992. MMWR Morb. Mortal. Wkly. Rep. 1995, 44, 46–48. [Google Scholar]
- Alkatout, I.; Honemeyer, U.; Strauss, A.; Tinelli, A.; Malvasi, A.; Jonat, W.; Mettler, L.; Schollmeyer, T. Clinical diagnosis and treatment of ectopic pregnancy. Obstet. Gynecol. Surv. 2013, 68, 571–581. [Google Scholar] [CrossRef]
- Atri, M.; Leduc, C.; Gillett, P.; Bret, P.M.; Reinhold, C.; Kintzen, G.; Aldis, A.E.; Thibodeau, M. Role of endovaginal sonography in the diagnosis and management of ectopic pregnancy. RadioGraphics 1996, 16, 755–774, discussion 775. [Google Scholar] [CrossRef]
- Dogra, V.; Paspulati, R.M.; Bhatt, S. First trimester bleeding evaluation. Ultrasound Q. 2005, 21, 69–85, quiz 149–150, 153–154. [Google Scholar]
- Barnhart, K.T.; Fay, C.A.; Suescum, M.; Sammel, M.D.; Appleby, D.; Shaunik, A.; Dean, A.J. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet. Gynecol. 2011, 117, 299–306. [Google Scholar] [CrossRef]
- Doubilet, P.M.; Benson, C.B.; Bourne, T.; Blaivas, M.; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Intrauterine Pregnancy; Barnhart, K.T.; Benacerraf, B.R.; Brown, D.L.; Filly, R.A.; Fox, J.C.; et al. Diagnostic criteria for nonviable pregnancy early in the first trimester. N. Engl. J. Med. 2013, 369, 1443–1451. [Google Scholar] [CrossRef]
- Connolly, A.; Ryan, D.H.; Stuebe, A.M.; Wolfe, H.M. Reevaluation of discriminatory and threshold levels for serum beta-hCG in early pregnancy. Obstet. Gynecol. 2013, 121, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Rana, P.; Kazmi, I.; Singh, R.; Afzal, M.; Al-Abbasi, F.A.; Aseeri, A.; Singh, R.; Khan, R.; Anwar, F. Ectopic pregnancy: A review. Arch. Gynecol. Obstet. 2013, 288, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Rausch, M.E.; Barnhart, K.T. Serum biomarkers for detecting ectopic pregnancy. Clin. Obstet. Gynecol. 2012, 55, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Birkhahn, R.H.; Gaeta, T.J.; Leo, P.J.; Bove, J.J. The utility of maternal creatine kinase in the evaluation of ectopic pregnancy. Am. J. Emerg. Med. 2000, 18, 695–697. [Google Scholar] [CrossRef]
- Rausch, M.E.; Sammel, M.D.; Takacs, P.; Chung, K.; Shaunik, A.; Barnhart, K.T. Development of a multiple marker test for ectopic pregnancy. Obstet. Gynecol. 2011, 117, 573–582. [Google Scholar] [CrossRef]
- Florio, P.; Severi, F.M.; Bocchi, C.; Luisi, S.; Mazzini, M.; Danero, S.; Torricelli, M.; Petraglia, F. Single serum activin a testing to predict ectopic pregnancy. J. Clin. Endocrinol. Metab. 2007, 92, 1748–1753. [Google Scholar] [CrossRef]
- Daniel, Y.; Geva, E.; Lerner-Geva, L.; Eshed-Englender, T.; Gamzu, R.; Lessing, J.B.; Bar-Am, A.; Amit, A. Levels of vascular endothelial growth factor are elevated in patients with ectopic pregnancy: Is this a novel marker? Fertil. Steril. 1999, 72, 1013–1017. [Google Scholar] [CrossRef]
- Gebeh, A.K.; Willets, J.M.; Marczylo, E.L.; Taylor, A.H.; Konje, J.C. Ectopic pregnancy is associated with high anandamide levels and aberrant expression of FAAH and CB1 in fallopian tubes. J. Clin. Endocrinol. Metab. 2012, 97, 2827–2835. [Google Scholar] [CrossRef]
- Horne, A.W.; Phillips, J.A., 3rd; Kane, N.; Lourenco, P.C.; McDonald, S.E.; Williams, A.R.; Simon, C.; Dey, S.K.; Critchley, H.O. CB1 expression is attenuated in Fallopian tube and decidua of women with ectopic pregnancy. PLoS ONE 2008, 3, e3969. [Google Scholar] [CrossRef]
- Priya Aarthy, A.; Sen, S.; Srinivasan, M.; Muthukumar, S.; Madhanraj, P.; Akbarsha, M.A.; Archunan, G. Ectopic pregnancy: Search for biomarker in salivary proteome. Sci. Rep. 2023, 13, 16828. [Google Scholar] [CrossRef]
- Brown, J.K.; Lauer, K.B.; Ironmonger, E.L.; Inglis, N.F.; Bourne, T.H.; Critchley, H.O.; Horne, A.W. Shotgun proteomics identifies serum fibronectin as a candidate diagnostic biomarker for inclusion in future multiplex tests for ectopic pregnancy. PLoS ONE 2013, 8, e66974. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Horne, A.W.; Brown, J.K.; Tong, S.; Kaitu’u-Lino, T. Evaluation of ADAM-12 as a diagnostic biomarker of ectopic pregnancy in women with a pregnancy of unknown location. PLoS ONE 2012, 7, e41442. [Google Scholar] [CrossRef] [PubMed]
- Boschetti, E.; Righetti, P.G. The ProteoMiner in the proteomic arena: A non-depleting tool for discovering low-abundance species. J. Proteom. 2008, 71, 255–264. [Google Scholar] [CrossRef]
- Barnhart, K.T.; Bollig, K.J.; Senapati, S.; Takacs, P.; Robins, J.C.; Haisenleder, D.J.; Beer, L.A.; Savaris, R.F.; Koelper, N.C.; Speicher, D.W.; et al. Multiplexed serum biomarkers to discriminate nonviable and ectopic pregnancy. Fertil. Steril. 2024, 122, 482–493. [Google Scholar] [CrossRef] [PubMed]
- German, J.B.; Hammock, B.D.; Watkins, S.M. Metabolomics: Building on a century of biochemistry to guide human health. Metabolomics 2005, 1, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, H.; Sounderajah, V.; Glen, R.; Ebbels, T.; Blaise, B.J.; Kalra, D.; Kultima, K.; Spjuth, O.; Tenori, L.; Salek, R.M.; et al. Metabolomics: The Stethoscope for the Twenty-First Century. Med. Princ. Pract. 2021, 30, 301–310. [Google Scholar] [CrossRef]
- Bahado-Singh, R.O.; Akolekar, R.; Chelliah, A.; Mandal, R.; Dong, E.; Kruger, M.; Wishart, D.S.; Nicolaides, K. Metabolomic analysis for first-trimester trisomy 18 detection. Am. J. Obstet. Gynecol. 2013, 209, 65 e61–69. [Google Scholar] [CrossRef]
- Bahado-Singh, R.O.; Syngelaki, A.; Akolekar, R.; Mandal, R.; Bjondahl, T.C.; Han, B.; Dong, E.; Bauer, S.; Alpay-Savasan, Z.; Graham, S.; et al. Validation of metabolomic models for prediction of early-onset preeclampsia. Am. J. Obstet. Gynecol. 2015, 213, 530.e1. [Google Scholar] [CrossRef]
- Turkoglu, O.; Zeb, A.; Graham, S.; Szyperski, T.; Szender, J.B.; Odunsi, K.; Bahado-Singh, R. Metabolomics of biomarker discovery in ovarian cancer: A systematic review of the current literature. Metabolomics 2016, 12, 60. [Google Scholar] [CrossRef]
- Turkoglu, O.; Citil, A.; Katar, C.; Mert, I.; Kumar, P.; Yilmaz, A.; Uygur, D.S.; Erkaya, S.; Graham, S.F.; Bahado-Singh, R.O. Metabolomic identification of novel diagnostic biomarkers in ectopic pregnancy. Metabolomics 2019, 15, 143. [Google Scholar] [CrossRef]
- Nothias, L.F.; Petras, D.; Schmid, R.; Duhrkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-based molecular networking in the GNPS analysis environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Fakhr, Y.; Brindley, D.N.; Hemmings, D.G. Physiological and pathological functions of sphingolipids in pregnancy. Cell. Signal. 2021, 85, 110041. [Google Scholar] [CrossRef] [PubMed]
- Santana, P.; Llanes, L.; Hernandez, I.; Gonzalez-Robayna, I.; Tabraue, C.; Gonzalez-Reyes, J.; Quintana, J.; Estevez, F.; Ruiz de Galarreta, C.M.; Fanjul, L.F. Interleukin-1 beta stimulates sphingomyelin hydrolysis in cultured granulosa cells: Evidence for a regulatory role of ceramide on progesterone and prostaglandin biosynthesis. Endocrinology 1996, 137, 2480–2489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Norman, R.J.; Buck, R.H.; Kemp, M.A.; Joubert, S.M. Impaired corpus luteum function in ectopic pregnancy cannot be explained by altered human chorionic gonadotropin. J. Clin. Endocrinol. Metab. 1988, 66, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ning, N.; Kong, B.; Xu, Y.; Xu, H.; Zhou, C.; Li, S.; Shao, Y.; Qiu, J.; Li, J. Aberrant sphingolipid metabolism in the human fallopian tube with ectopic pregnancy. Lipids 2013, 48, 989–995. [Google Scholar] [CrossRef]
- Winter, S.C.; Linn, L.S.; Helton, E. Plasma carnitine concentrations in pregnancy, cord blood, and neonates and children. Clin. Chim. Acta 1995, 243, 87–93. [Google Scholar] [CrossRef]
- Lindsay, K.L.; Hellmuth, C.; Uhl, O.; Buss, C.; Wadhwa, P.D.; Koletzko, B.; Entringer, S. Longitudinal Metabolomic Profiling of Amino Acids and Lipids across Healthy Pregnancy. PLoS ONE 2015, 10, e0145794. [Google Scholar] [CrossRef]
- Ku, C.W.; Tan, Z.W.; Lim, M.K.; Tam, Z.Y.; Lin, C.H.; Ng, S.P.; Allen, J.C.; Lek, S.M.; Tan, T.C.; Tan, N.S. Spontaneous miscarriage in first trimester pregnancy is associated with altered urinary metabolite profile. BBA Clin. 2017, 8, 48–55. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Z.; Heng, J.; Tian, M.; Chen, J.; Chen, F.; Guan, W. L-carnitine increases cell proliferation and amino acid transporter expression via the activation of insulin-like growth factor I signaling pathway in rat trophoblast cells. Food Sci. Nutr. 2020, 8, 3298–3307. [Google Scholar] [CrossRef]
- Kokawa, K.; Shikone, T.; Nakano, R. Apoptosis in human chorionic villi and decidua in normal and ectopic pregnancy. Mol. Hum. Reprod. 1998, 4, 87–91. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kucera, E.; Konig, F.; Tangl, S.; Grosschmidt, K.; Kainz, C.; Sliutz, G. Bcl-2 expression as a novel immunohistochemical marker for ruptured tubal ectopic pregnancy. Hum. Reprod. 2001, 16, 1286–1290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seol, T.K.; Lee, W.; Park, S.; Kim, K.N.; Kim, T.Y.; Oh, Y.N.; Jun, J.H. Effect of palmitoylethanolamide on inflammatory and neuropathic pain in rats. Korean J. Anesthesiol. 2017, 70, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Soriano, D.; Hugol, D.; Quang, N.T.; Darai, E. Serum concentrations of interleukin-2R (IL-2R), IL-6, IL-8, and tumor necrosis factor alpha in patients with ectopic pregnancy. Fertil. Steril. 2003, 79, 975–980. [Google Scholar] [CrossRef]
- Rees, A.; Edwards, I.C.Z.; Richards, O.; Raikes, M.E.; Angelini, R.; Thornton, C.A. The dynamic inflammatory profile of pregnancy can be monitored using a novel lipid-based mass spectrometry technique. Mol. Omics 2023, 19, 340–350. [Google Scholar] [CrossRef]
- Friesen, R.W.; Novak, E.M.; Hasman, D.; Innis, S.M. Relationship of dimethylglycine, choline, and betaine with oxoproline in plasma of pregnant women and their newborn infants. J. Nutr. 2007, 137, 2641–2646. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Otten, W.; Merlot, E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids 2011, 41, 1195–1205. [Google Scholar] [CrossRef]
- Badawy, A.A. The tryptophan utilization concept in pregnancy. Obstet. Gynecol. Sci. 2014, 57, 249–259. [Google Scholar] [CrossRef]
- Wedderburn, C.J.; Warner, P.; Graham, B.; Duncan, W.C.; Critchley, H.O.; Horne, A.W. Economic evaluation of diagnosing and excluding ectopic pregnancy. Hum. Reprod. 2010, 25, 328–333. [Google Scholar] [CrossRef]
- Barnhart, K.; van Mello, N.M.; Bourne, T.; Kirk, E.; Van Calster, B.; Bottomley, C.; Chung, K.; Condous, G.; Goldstein, S.; Hajenius, P.J.; et al. Pregnancy of unknown location: A consensus statement of nomenclature, definitions, and outcome. Fertil. Steril. 2011, 95, 857–866. [Google Scholar] [CrossRef]
- Raghuvanshi, R.; Grayson, A.G.; Schena, I.; Amanze, O.; Suwintono, K.; Quinn, R.A. Microbial Transformations of Organically Fermented Foods. Metabolites 2019, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Xia, J.; Mandal, R.; Sinelnikov, I.V.; Broadhurst, D.; Wishart, D.S. MetaboAnalyst 2.0—A comprehensive server for metabolomic data analysis. Nucleic Acids Res. 2012, 40, W127–W133. [Google Scholar] [CrossRef]
- Deng, L.; Chang, D.; Foshaug, R.R.; Eisner, R.; Tso, V.K.; Wishart, D.S.; Fedorak, R.N. Development and Validation of a High-Throughput Mass Spectrometry Based Urine Metabolomic Test for the Detection of Colonic Adenomatous Polyps. Metabolites 2017, 7, 32. [Google Scholar] [CrossRef]
- Spicer, R.A.; Salek, R.; Steinbeck, C. Compliance with minimum information guidelines in public metabolomics repositories. Sci. Data 2017, 4, 170137. [Google Scholar] [CrossRef]
- Landsberg, A.; Sharma, A.; Gibson, I.W.; Rush, D.; Wishart, D.S.; Blydt-Hansen, T.D. Non-invasive staging of chronic kidney allograft damage using urine metabolomic profiling. Pediatr. Transplant. 2018, 22, e13226. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression Shrinkage and Selection via the Lasso. J. R. Stat. Society. Ser. B Stat. Methodol. 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Xia, J.; Broadhurst, D.I.; Wilson, M.; Wishart, D.S. Translational biomarker discovery in clinical metabolomics: An introductory tutorial. Metabolomics 2013, 9, 280–299. [Google Scholar] [CrossRef]

| Discovery Group | Validation Group | |||||
|---|---|---|---|---|---|---|
| Parameter | Cases | Controls | p-Value | Cases | Controls | p-Value |
| Number of patients | 30 | 30 | 20 | 20 | ||
| Age, mean (SD) | 25.7 (5.9) | 26.1 (6.7) | 0.147 ^ | 26.7 (5.2) | 25.1 (5.7) | 0.647 ^ |
| Nullipara, n | 13 | 16 | 0.343 * | 18 | 22 | 0.248 * |
| BMI, mean (SD) | 27.2 (5.3) | 26.1 (5.9) | 0.865 ^ | 25.2 (5.5) | 25.1 (4.9) | 0.955 ^ |
| Risk Factors, n | ||||||
| Previous EP | 6 | 3 | 0.044 * | 4 | 1 | 0.025 * |
| Previous Adnexal Surgery | 7 | 3 | 0.046 * | 5 | 2 | 0.038 * |
| Previous pelvic /abdominal Surgery | 8 | 10 | 0.423 * | 6 | 7 | 0.263 * |
| History of Infertility | 3 | 4 | 0.520 * | 1 | 4 | 0.608 * |
| History of PID | 6 | 5 | 0.378 * | 1 | 2 | 0.198 * |
| Smoking | 7 | 5 | 0.102 * | 8 | 9 | 0.092 * |
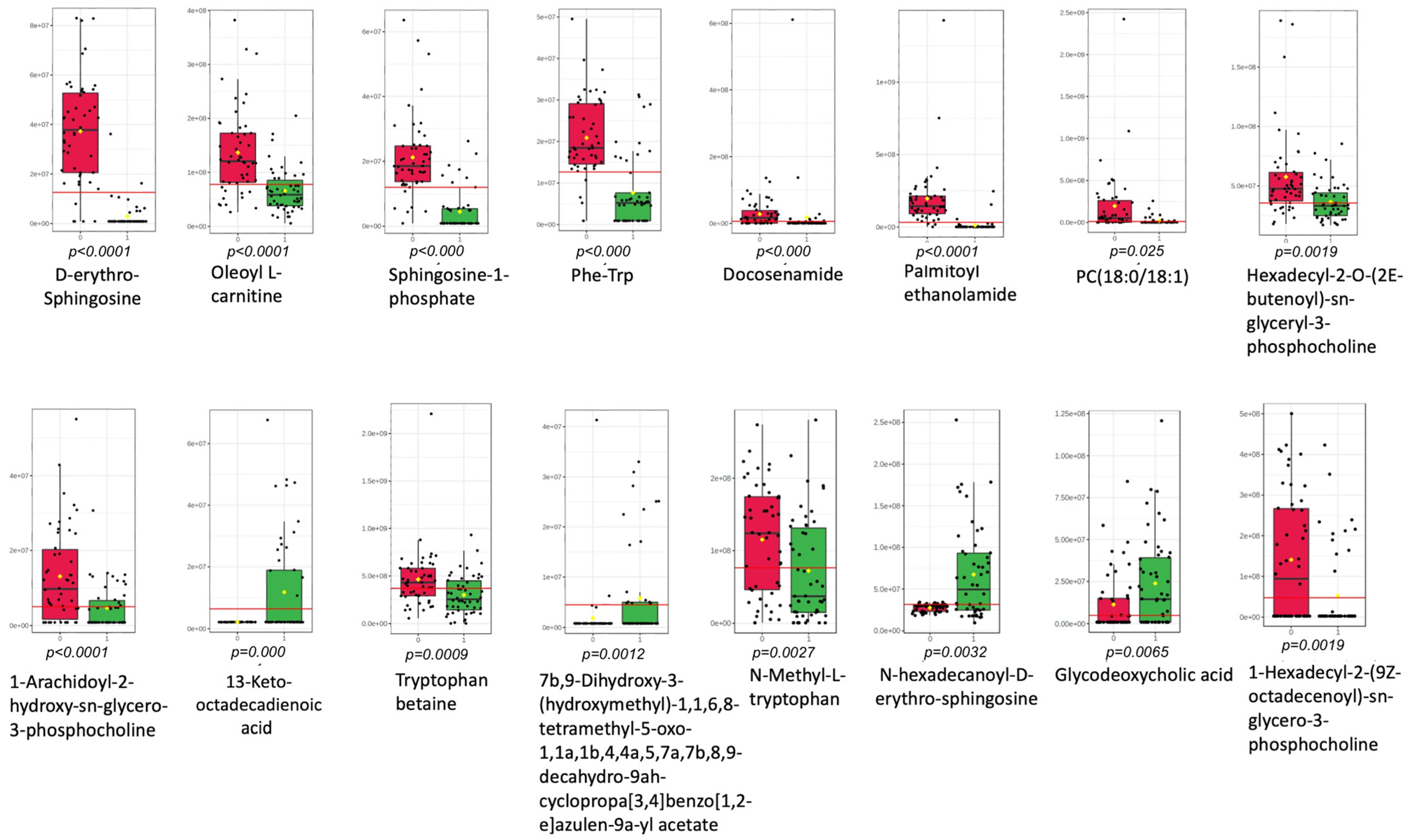
| Compound Name | Annotation Level | Raw Formula | m/z [M + H]+ | Feature ID | EP vs. IUP | p-Value |
|---|---|---|---|---|---|---|
| D-erythro-Sphingosine | 1 | C18H37NO2 | 300.289 | 240 | Down | <0.0001 |
| Sphingosine 1-phosphate | 1 | C18H38NO5P | 380.255 | 146 | Down | <0.0001 |
| Oleoyl L-carnitine | 1 | C25H47NO4 | 426.357 | 84 | Down | <0.0001 |
| Phe-Trp | 2 | C20H21N3O3 | 352.165 | 147 | Down | <0.0001 |
| Docosenamide | 2 | C22H43NO | 338.341 | 356 | Down | <0.0001 |
| Hexadecyl-2-O-(2E-butenoyl)-sn-glyceryl-3-phosphocholine | 3 | C28H56NO7P | 550.386 | 60 | Down | <0.0001 |
| 1-Arachidoyl-2-hydroxy-sn-glycero-3-phosphocholine | 3 | C28H58NO7P | 552.402 | 102 | Down | <0.0001 |
| Palmitoyl ethanolamide | 3 | C18H37NO2 | 300.289 | 73 | Down | <0.0001 |
| 1,2-Di-(9Z,12Z,15Z-octadecatrienoyl)-sn-glycero-3-phosphocholine | 2 | PC(18:3(9Z,12Z,15Z)/ 18:3(9Z,12Z,15Z)) | 778.538 | 373 | Down | 0.0001 |
| 13-ketooctadecadienoic acid | 3 | C18H30O3 | 295.226 | 89 | Up | 0.0001 |
| N-Tetracosanoyl-4-sphingenyl-1-O-phosphorylcholine | 3 | C41H83N2O6P | 815.697 | 328 | Down | 0.0003 |
| Tryptophan betaine | 3 | C14H18N2O2 | 247.143 | 43 | Down | 0.0009 |
| 7b,9-Dihydroxy-3-(hydroxymethyl)-1,1,6,8-tetramethyl-5-oxo-1,1a,1b,4,4a,5,7a,7b,8,9-decahydro-9ah-cyclopropa[3,4]benzo[1,2-e]azulen-9a-yl acetate | 1 | C22H30O6 | 432.238 | 432 | Up | 0.0012 |
| 1-Hexadecyl-2-(9Z-octadecenoyl)-sn-glycero-3-phosphocholine | 3 | C42H84NO7P | 746.606 | 544 | Down | 0.0019 |
| N-Methyl-L-tryptophan | 3 | C12H14N2O2 | 188.070 | 98 | Down | 0.0027 |
| N-hexadecanoyl-D-erythro-sphingosine | 1 | C34H67NO3 | 274.274 | 85 | Down | 0.0032 |
| Glycodeoxycholic acid | 1 | C26H43NO5 | 450.321 | 41 | Up | 0.0065 |
| 1-Stearoyl-2-hydroxy-sn-glycero-3-phosphocholine | 2 | C26H54NO7P | 524.371 | 3 | Down | 0.0158 |
| Sphingomyelin (d18:1/24:0) | 2 | SM (d18:1/24:0) | 814.688 | 542 | Down | 0.0199 |
| 1-Octadecanoyl-2-(5Z,8Z,11Z,14Z-eicosatetraenoyl)-sn-glycero-3-phosphocholine | 3 | PC (18:0/18:1) | 810.597 | 488 | Down | 0.0251 |
| N-Lauroyl-D-erythro-sphingosylphosphorylcholine | 2 | 12:0 SM (d18:1/12:0) | 647.512 | 242 | Down | 0.0259 |
| Metabolite Algorithms | AUC (95% CI) | Sensitivity | Specificity |
|---|---|---|---|
| D-erythro-C18-Sphingosine + Oleoyl L-carnitine | 0.962 (0.910–1.000) | 100% | 95.9% |
| Palmitoyl ethanolamide + D-erythro-C18-Sphingosine | 0.963 (0.914–1.000) | 98.0% | 94.0% |
| N-Palmitoylethanolamine + D-erythro-C18-Sphingosine + Sphingosine 1-phosphate + Phenylalanyl tryptophan | 0.955 (0.902–1.000) | 92.0% | 98.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turkoglu, O.; Citil, A.; Katar, C.; Mert, I.; Quinn, R.A.; Bahado-Singh, R.O.; Graham, S.F. Untargeted Metabolomic Biomarker Discovery for the Detection of Ectopic Pregnancy. Int. J. Mol. Sci. 2024, 25, 10333. https://doi.org/10.3390/ijms251910333
Turkoglu O, Citil A, Katar C, Mert I, Quinn RA, Bahado-Singh RO, Graham SF. Untargeted Metabolomic Biomarker Discovery for the Detection of Ectopic Pregnancy. International Journal of Molecular Sciences. 2024; 25(19):10333. https://doi.org/10.3390/ijms251910333
Chicago/Turabian StyleTurkoglu, Onur, Ayse Citil, Ceren Katar, Ismail Mert, Robert A. Quinn, Ray O. Bahado-Singh, and Stewart F. Graham. 2024. "Untargeted Metabolomic Biomarker Discovery for the Detection of Ectopic Pregnancy" International Journal of Molecular Sciences 25, no. 19: 10333. https://doi.org/10.3390/ijms251910333
APA StyleTurkoglu, O., Citil, A., Katar, C., Mert, I., Quinn, R. A., Bahado-Singh, R. O., & Graham, S. F. (2024). Untargeted Metabolomic Biomarker Discovery for the Detection of Ectopic Pregnancy. International Journal of Molecular Sciences, 25(19), 10333. https://doi.org/10.3390/ijms251910333









