Iodine Rearrangements of Tetraallylsilane and Synthesis of Silicon-Stereogenic Organosilanes
Abstract
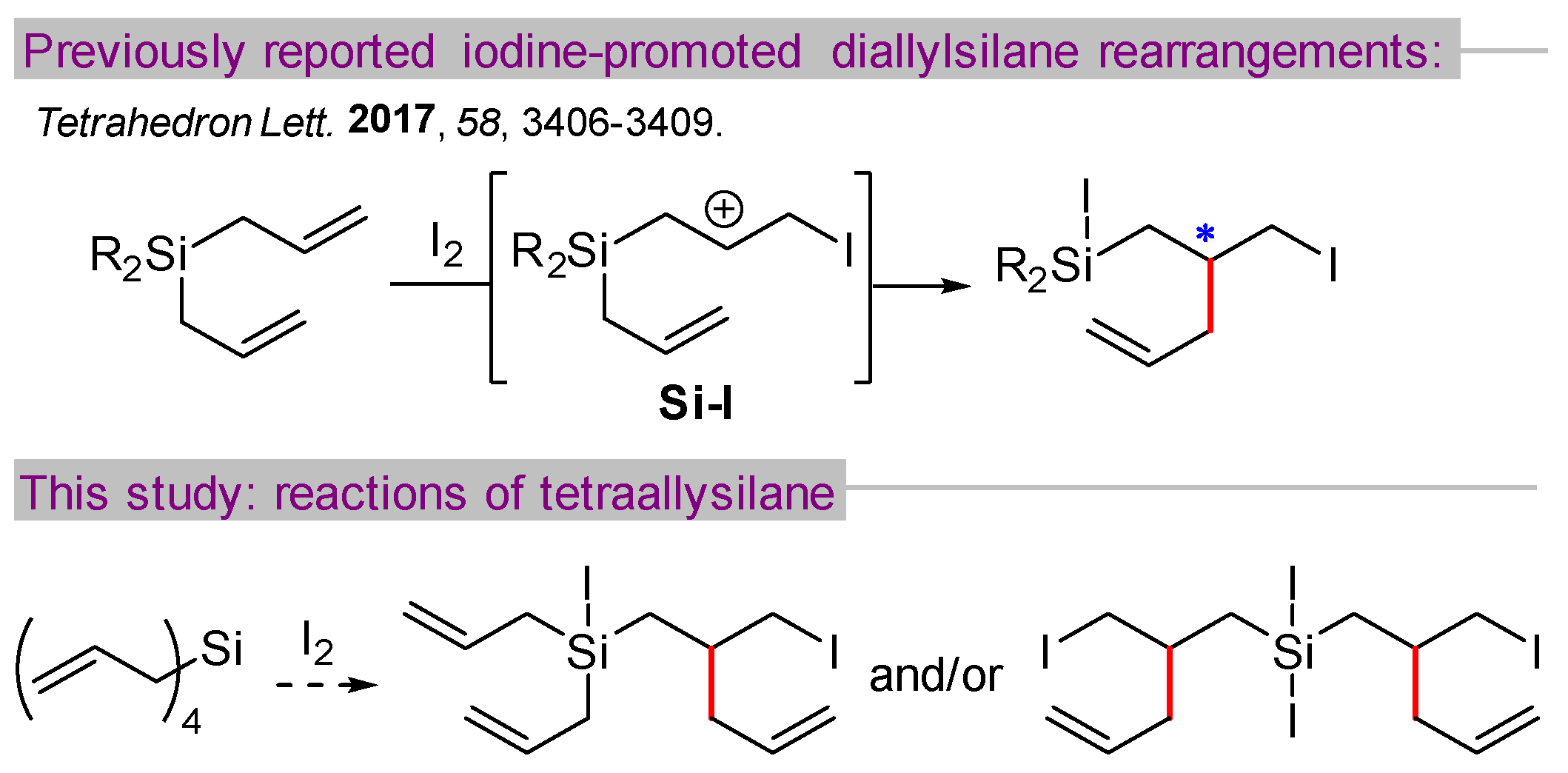
1. Introduction
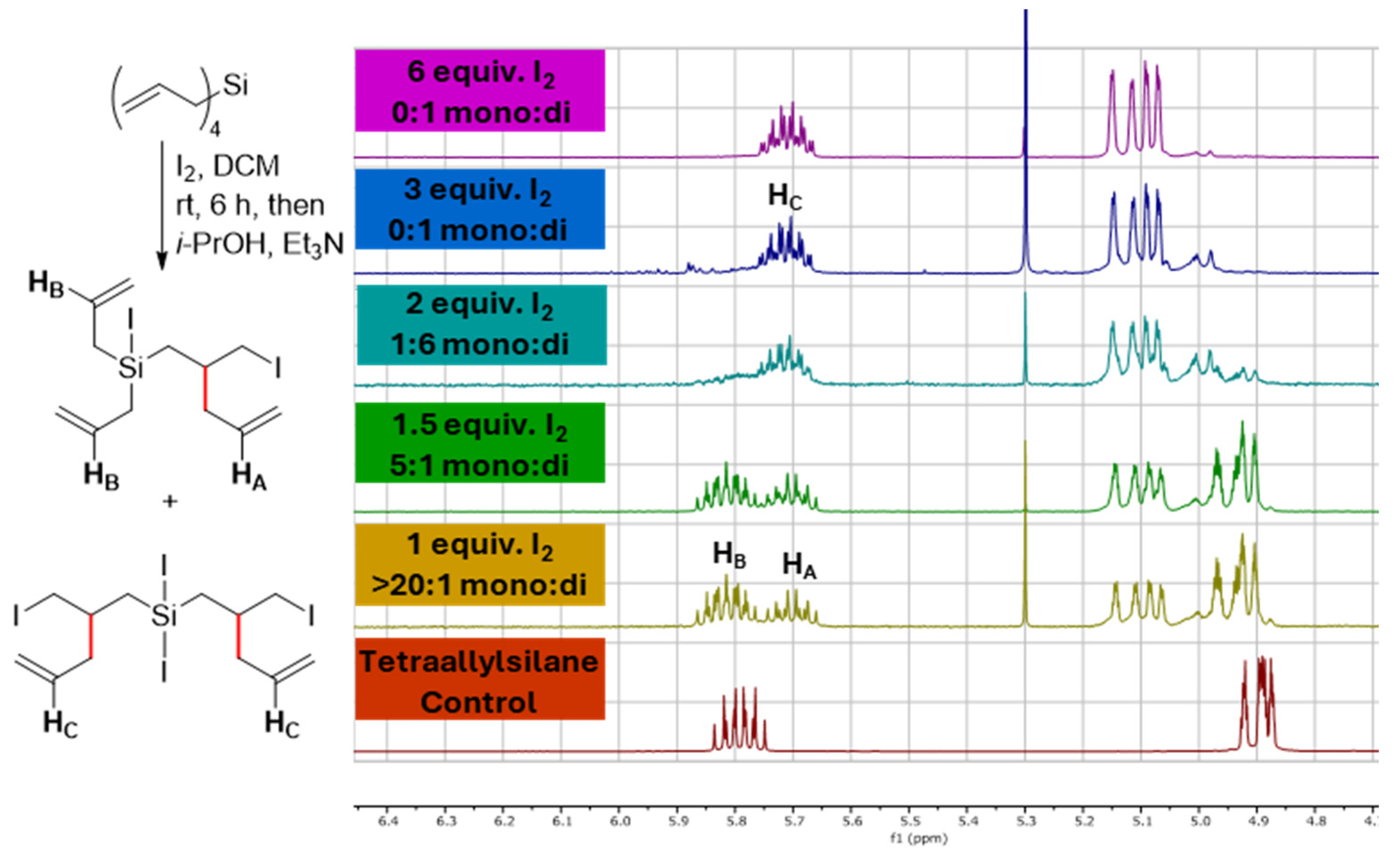
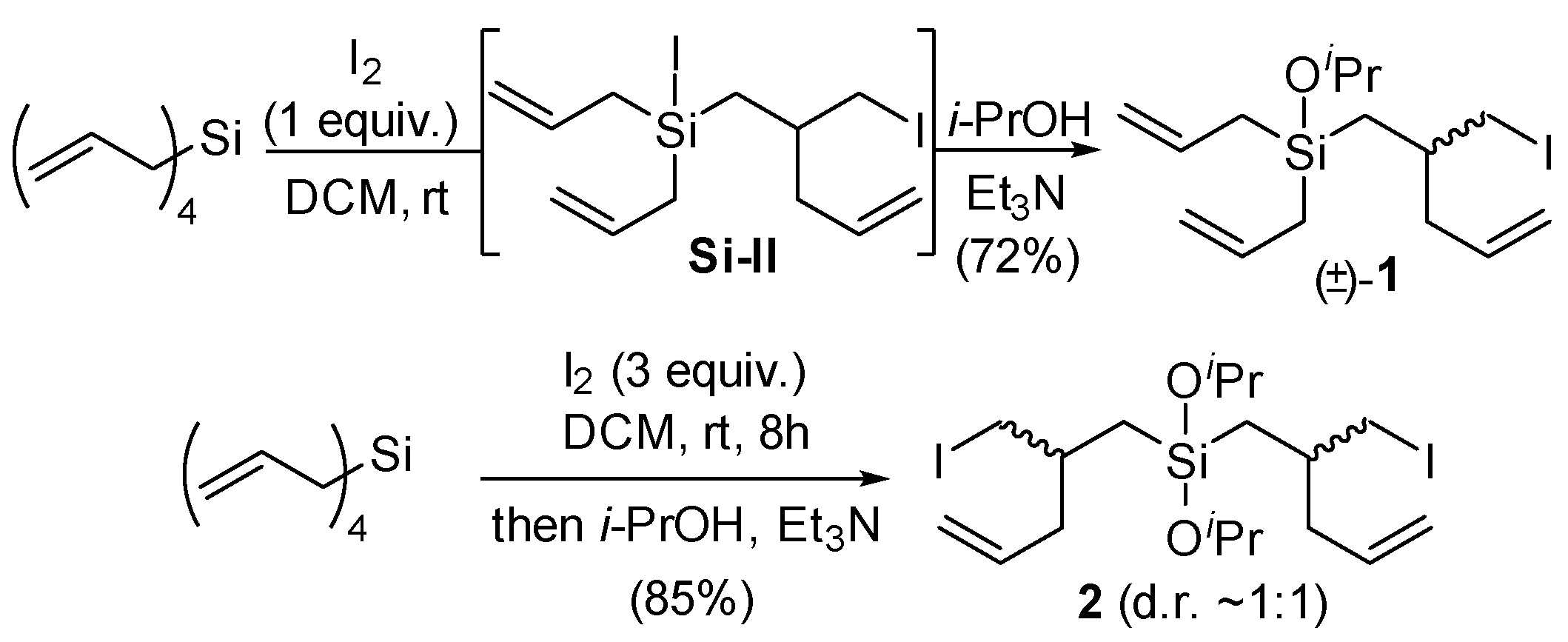
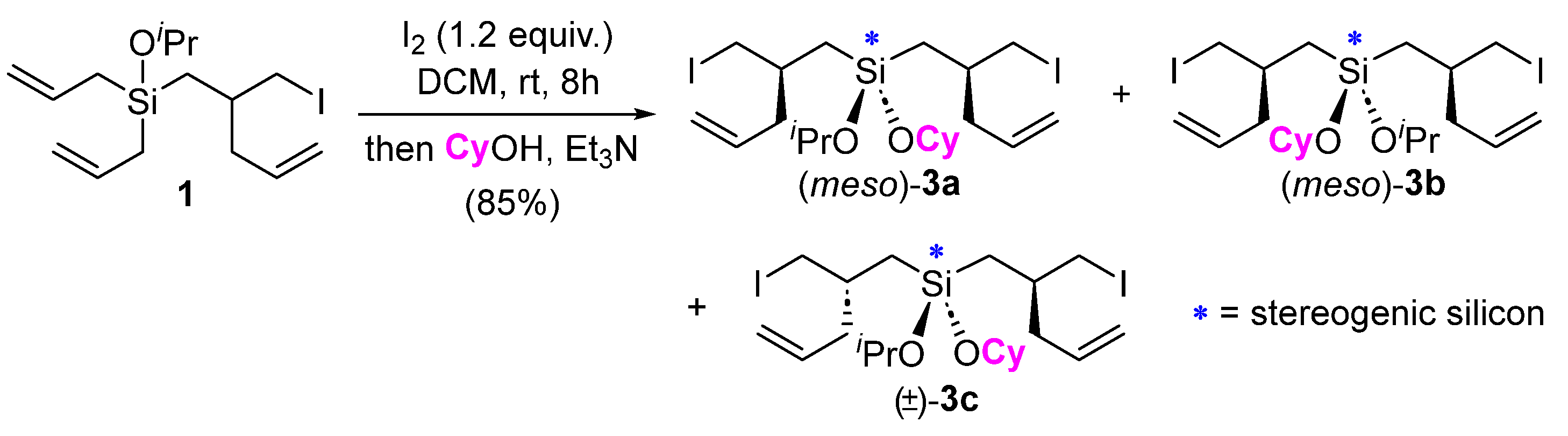
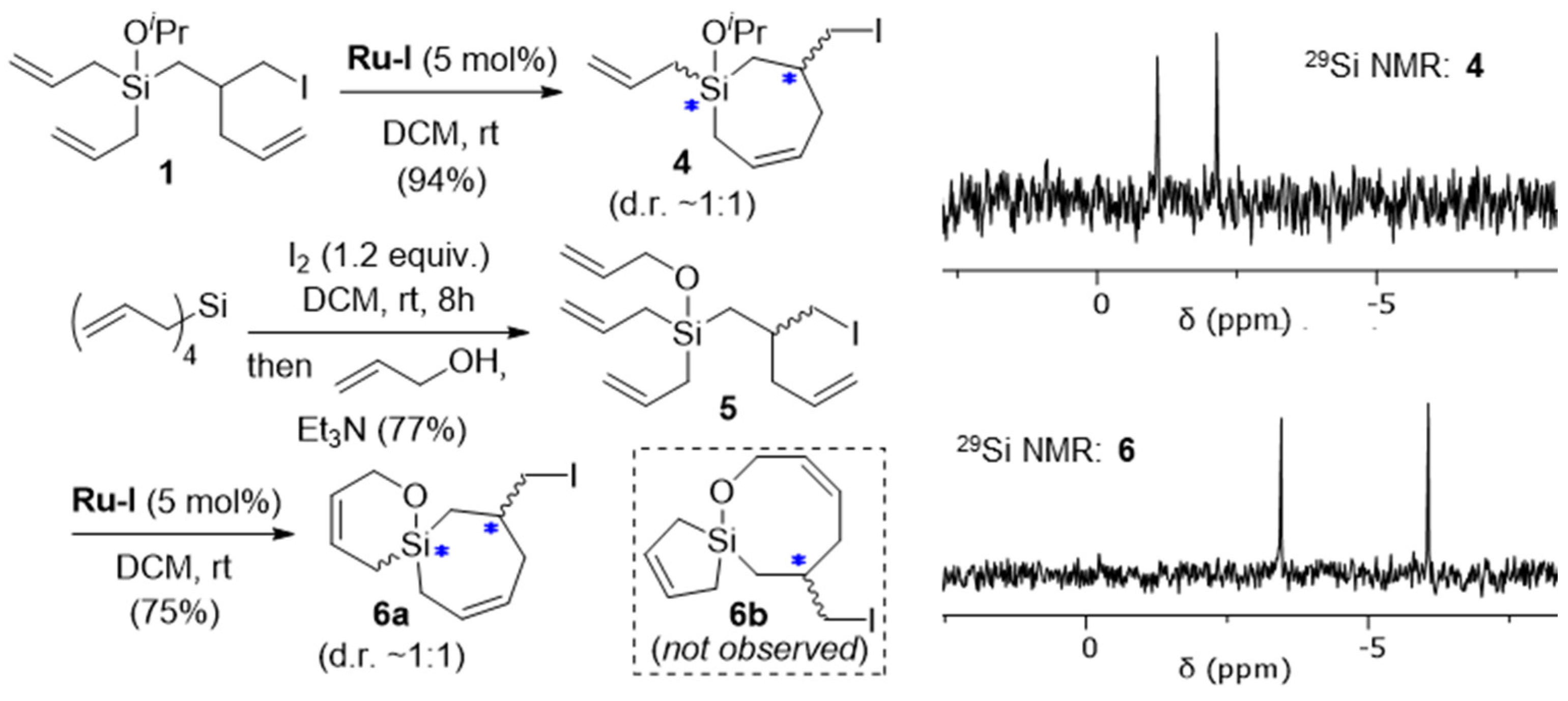
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Compound Syntheses and Spectral Data
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- O’Neil, G.W.; Cummins, E.J. Iodine-mediated rearrangements of diallylsilanes. Tetrahedron Lett. 2017, 58, 3406–3409. [Google Scholar] [PubMed]
- Hosomi, A.; Sakurai, H. Protection of alcohols and acids with allylsilanes catalyzed by iodine or iodotrimethylsilane in chlorinated hydrocarbons. Chem. Lett. 1981, 10, 85–88. [Google Scholar]
- Jones, R.G.; Cragg, R.H.; Swain, A.C. Structure and mechanism in the cyclopolymerization of diallylsilanes. Eur. Polym. J. 1992, 28, 651–655. [Google Scholar]
- Suslova, E.N.; Albanov, B.A.; Shainyan, J. Unusual product of the Si-C bond cleavage in diallyldiphenylsilane. Russ. J. Gen. Chem. 2008, 78, 1016–1017. [Google Scholar]
- Suslova, E.N.; Albanov, B.A.; Shainyan, J. Transformations of diallylsilanes under the action of electrophilic reagents. J. Organomet. Chem. 2009, 694, 420–426. [Google Scholar]
- Bols, M.; Skrydstrup, T. Silicon-Tethered Reactions. Chem. Rev. 1995, 95, 1253–1277. [Google Scholar]
- Bracegirdle, S.; Anderson, E.A. Recent advances in the use of temporary silicon tethers in metal-mediated reactions. Chem. Soc. Rev. 2010, 39, 4114–4129. [Google Scholar]
- Moss, G.P. ‘stereogenic unit’. In IUPAC Compendium of Chemical Terminology, 3rd ed.; International Union of Pure and Applied Chemistry: Research Triangle Park, NC, USA, 2006; Online version 3.0.1, 2019. [Google Scholar] [CrossRef]
- Xu, L.-W.; Li, L.; Lai, G.-Q.; Jiang, J.-X. The recent synthesis and application of silicon-stereogenic silanes: A renewed and significant challenge in asymmetric synthesis. Chem. Soc. Rev. 2011, 40, 1777–1790. [Google Scholar]
- Wu, Y.; Zheng, L.; Wang, Y.; Wang, P. Catalytic asymmetric synthesis of silicon-stereogenic organosilanes. Chem 2023, 9, 3461–3514. [Google Scholar]
- Ye, Z.-T.; Wu, Z.-W.; Zhang, X.-X.; Zhou, J.; Yu, J.-S. Organocatalytic enantioselective construction of Si-stereocenters: Recent advances and perspectives. Chem. Soc. Rev. 2024, 53, 8546–8562. [Google Scholar]
- Tomooka, K.; Nakazaki, A.; Nakai, T. A Novel Aryl Migration from Silicon to Carbon: An Efficient Approach to Asymmetric Synthesis of r-Aryl â-Hydroxy Cyclic Amines and Silanols. J. Am. Chem. Soc. 2000, 122, 408–409. [Google Scholar]
- Igawa, K.; Takada, J.; Shimono, T.; Tomooka, K. Enantioselective Synthesis of Silanol. J. Am. Chem. Soc. 2008, 130, 16132–16133. [Google Scholar] [PubMed]
- Bauer, J.O.; Strohmann, C. Stereocontrol in Nucleophilic Substitution Reactions at Silicon: The Role of Permutation in Generating Silicon-Centered Chirality. J. Am. Chem. Soc. 2015, 137, 4304–4307. [Google Scholar]
- Bai, X.-F.; Zou, J.-F.; Chen, M.-Y.; Xu, Z.; Li, L.; Cui, Y.-M.; Zheng, Z.-J.; Xu, L.-W. Lewis-Base-Mediated Diastereoselective Silylations of Alcohols: Synthesis of Silicon-Stereogenic Dialkoxysilanes Controlled by Chiral Aryl BINMOLs. Chem. Asian J. 2017, 12, 1730–1735. [Google Scholar]
- Zhang, X.-X.; Gao, Y.; Zhang, Y.-X.; Zhou, J.; Yu, J.-S. Highly Enantioselective Construction of Multifunctional Silicon- Stereogenic Silacycles by Asymmetric Enamine Catalysis. Angew. Chem. Int. Ed. 2023, e202217724. [Google Scholar]
- Bains, W.; Tacke, R. Silicon chemistry as a novel source of chemical diversity in drug design. Curr. Opin. Drug Discovery Dev. 2003, 6, 526–543. [Google Scholar]
- Fotie, J.; Matherne, C.M.; Wroblewski, J.E. Silicon switch: Carbon-silicon Bioisosteric replacement as a strategy to modulate the selectivity, physicochemical, and drug-like properties in anticancer pharmacophores. Chem. Biol. Drug. Des. 2023, 102, 235–254. [Google Scholar]
- Kawakami, Y.; Kakihana, Y.; Ooi, O.; Oishi, M.; Suzuki, K.; Shinke, S.; Uenishi, K. Control of stereochemical structures of silicon-containing polymeric systems. Polym. Int. 2009, 58, 279–284. [Google Scholar]
- Fomina, I.A.; Myers, C.R.; Soumis, C.L.M.; Scheuermann, M.L.; McCarty, J.; Clark, T.B.; O’Neil, G.W. Regiodivergent Medium-Ring Oxasilacycle Synthesis from Diallylsilanes. Heterocycles 2022, 104, 1966–1993. [Google Scholar] [CrossRef]
- Ahmad, I.; Falck-Pederson, M.L.; Undheim, K. Synthesis of silacycloalkenes and silaspirenes by Ru(II)-catalyzed ring-closing metathesis reactions. J. Organomet. Chem. 2001, 625, 160–172. [Google Scholar]
- Mahieux, C.; Laguerre, M.; Landais, Y.; Pianet, I. Multinuclear magnetic resonance and molecular modeling investigations as unambiguous methods for the determination of silacycle 3D structures. Magn. Reson. Chem. 2004, 42, 467–473. [Google Scholar] [PubMed]
- Anhaus, J.T.; Gibson, V.C.; Clegg, W.; Collingwood, S.P. Silicon-Containing Macrocycles and Polymers via Metathesis: X-ray Crystal Structures of cis,cis- and trans,trans-1,1,6,6,Tetraphenyl-1,6-disilacyclodeca-3,8-diene. Organometallics 1993, 12, 1780–1798. [Google Scholar]
- Kulkarni, A.A.; Diver, S.T. Cycloheptadiene Ring Synthesis by Tandem Intermolecular Enyne Metathesis. Org. Lett. 2003, 5, 3463–3466. [Google Scholar]
- Shieh, P.; Nguyen, H.V.-T.; Johnson, J.A. Tailored silyl ether monomers enable backbond-degradable polynorbornene-based linear, bottlebrush and star copolymers through ROMP. Nature Chem. 2019, 11, 1124–1132. [Google Scholar]
- Morontsev, A.; Gringolts, M.; Lakhtin, V.; Finkelshtein, E. Synthesis of high-molecular weight poly(1,1-dimethyl-1-silapentene) by olefin metathesis polymerization in the presence of Grubbs catalysts. J. Organomet. Chem. 2020, 911, 121156. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, E.D.; Wier, K.E.; O’Neil, G.W. Iodine Rearrangements of Tetraallylsilane and Synthesis of Silicon-Stereogenic Organosilanes. Int. J. Mol. Sci. 2024, 25, 9996. https://doi.org/10.3390/ijms25189996
Tan ED, Wier KE, O’Neil GW. Iodine Rearrangements of Tetraallylsilane and Synthesis of Silicon-Stereogenic Organosilanes. International Journal of Molecular Sciences. 2024; 25(18):9996. https://doi.org/10.3390/ijms25189996
Chicago/Turabian StyleTan, Elliott D., Kerry E. Wier, and Gregory W. O’Neil. 2024. "Iodine Rearrangements of Tetraallylsilane and Synthesis of Silicon-Stereogenic Organosilanes" International Journal of Molecular Sciences 25, no. 18: 9996. https://doi.org/10.3390/ijms25189996
APA StyleTan, E. D., Wier, K. E., & O’Neil, G. W. (2024). Iodine Rearrangements of Tetraallylsilane and Synthesis of Silicon-Stereogenic Organosilanes. International Journal of Molecular Sciences, 25(18), 9996. https://doi.org/10.3390/ijms25189996





