Specific Substrate Activity of Lotus Root Polyphenol Oxidase: Insights from Gaussian-Accelerated Molecular Dynamics and Markov State Models
Abstract
1. Introduction
2. Results
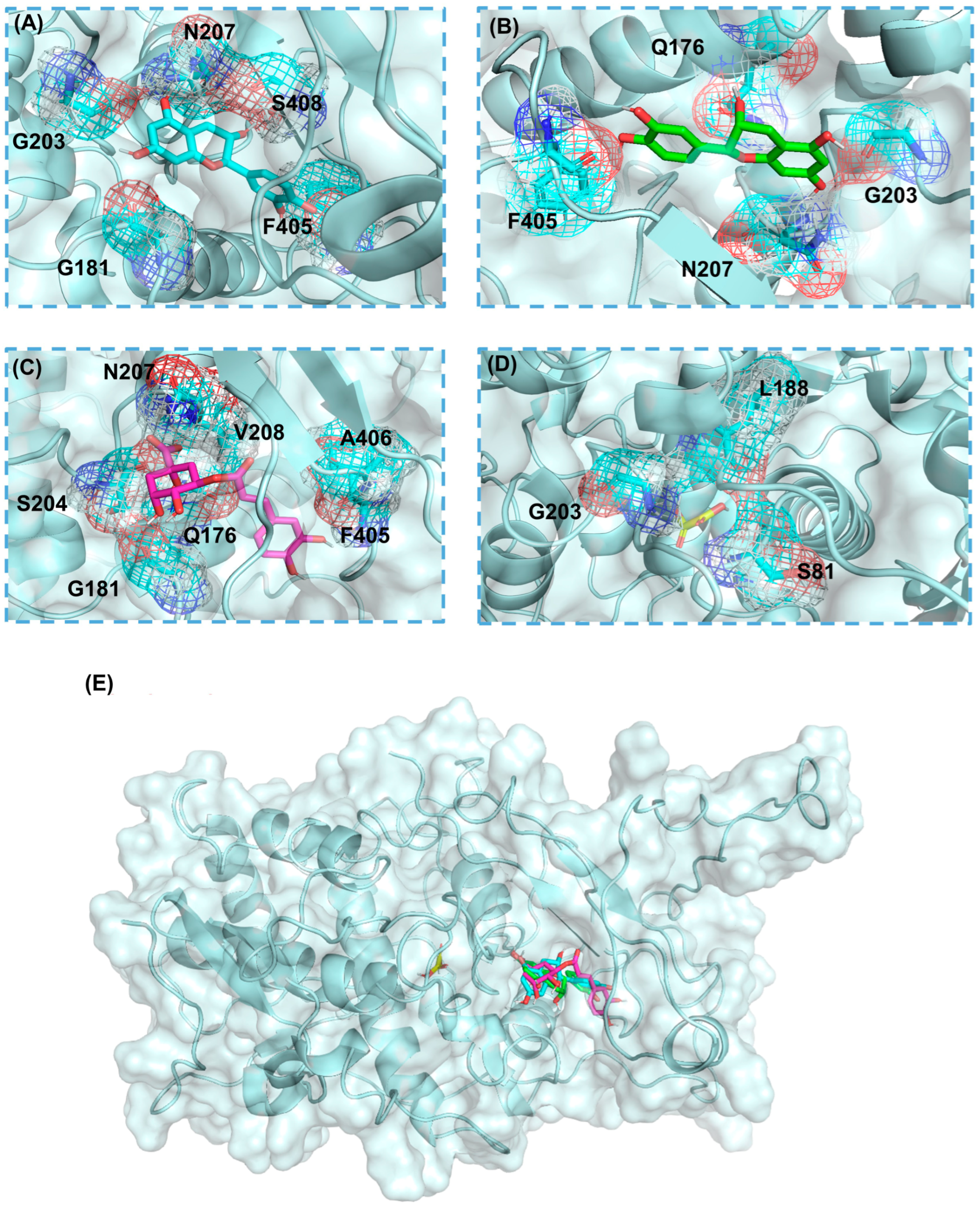
2.1. Molecular Docking Results
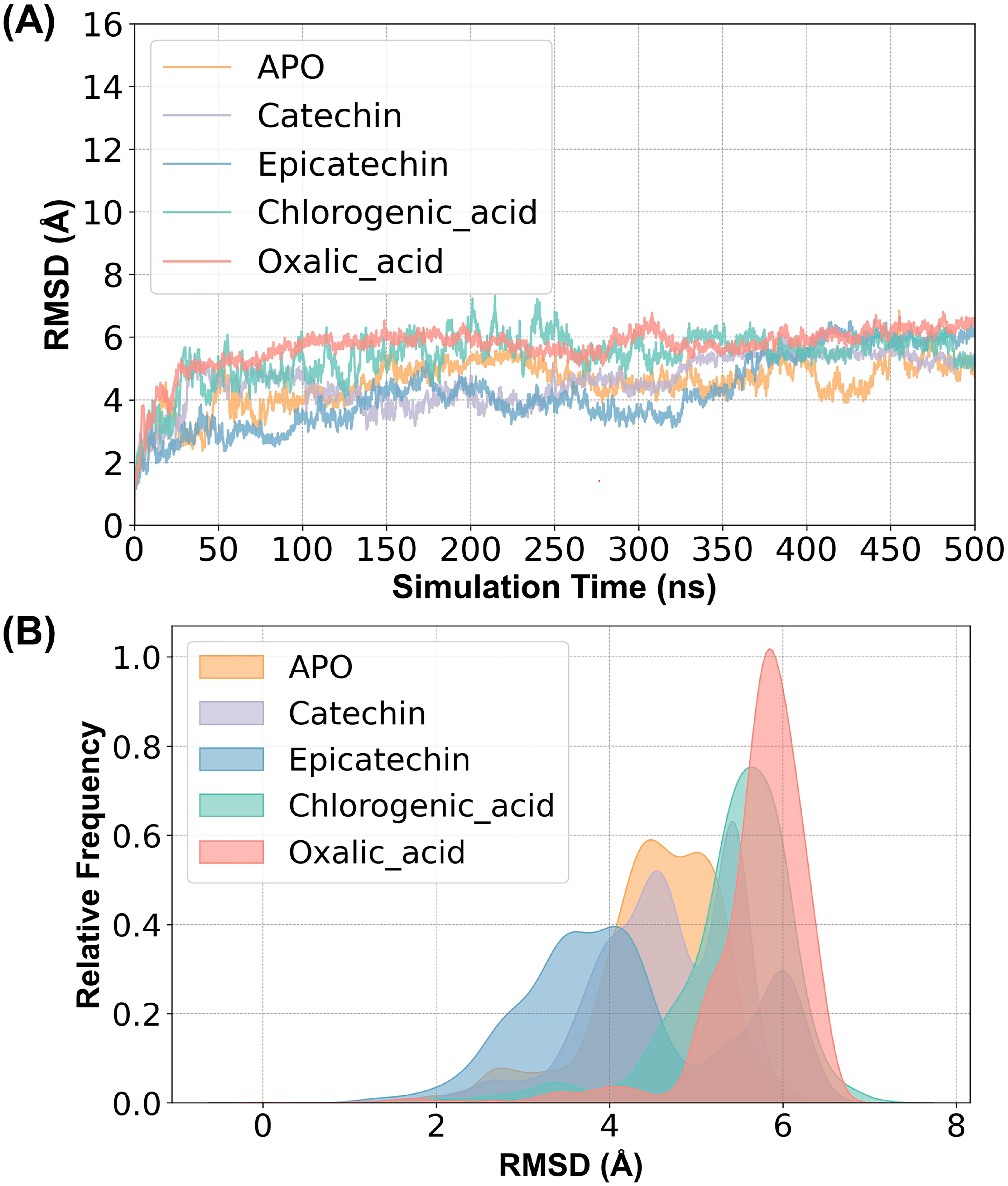
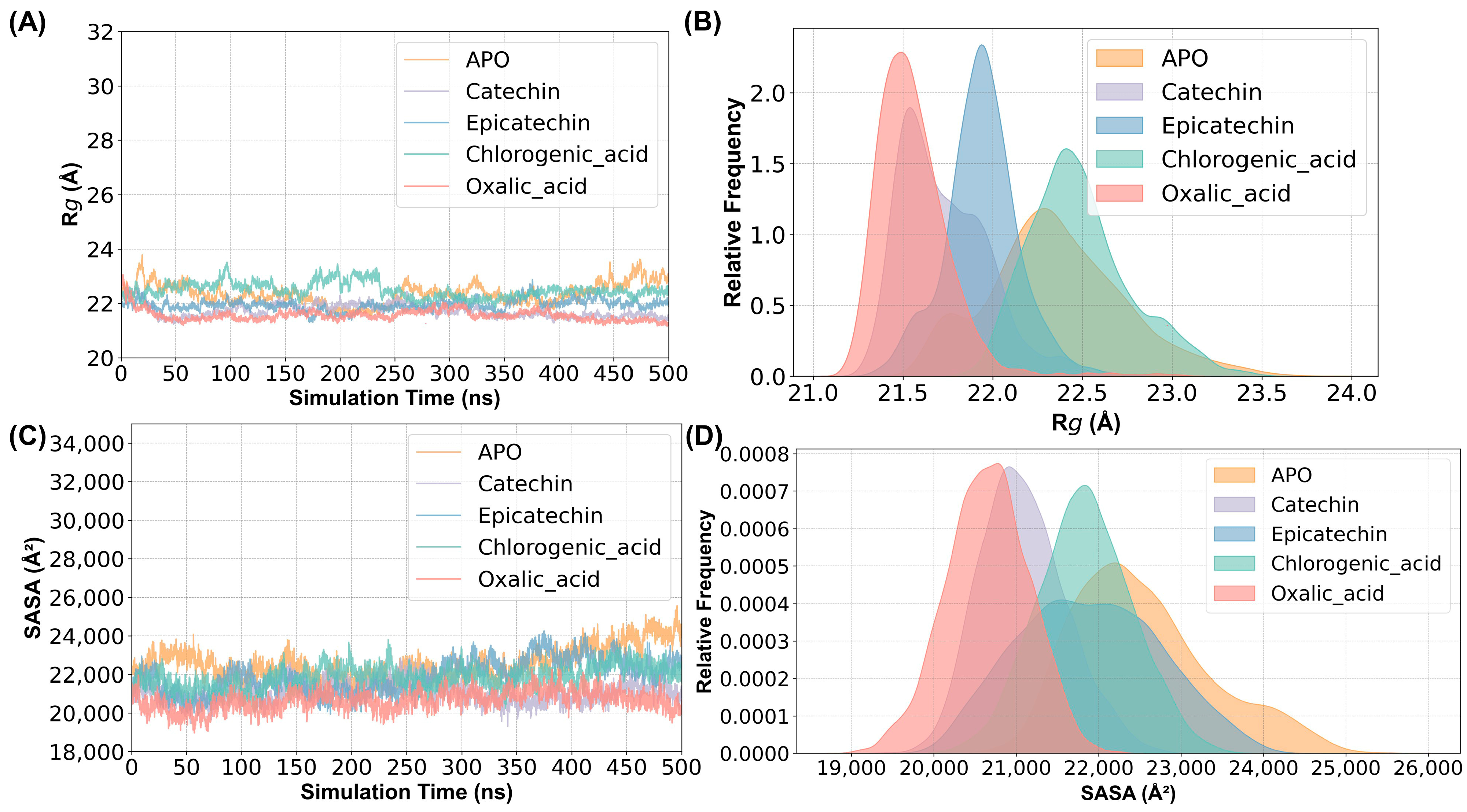
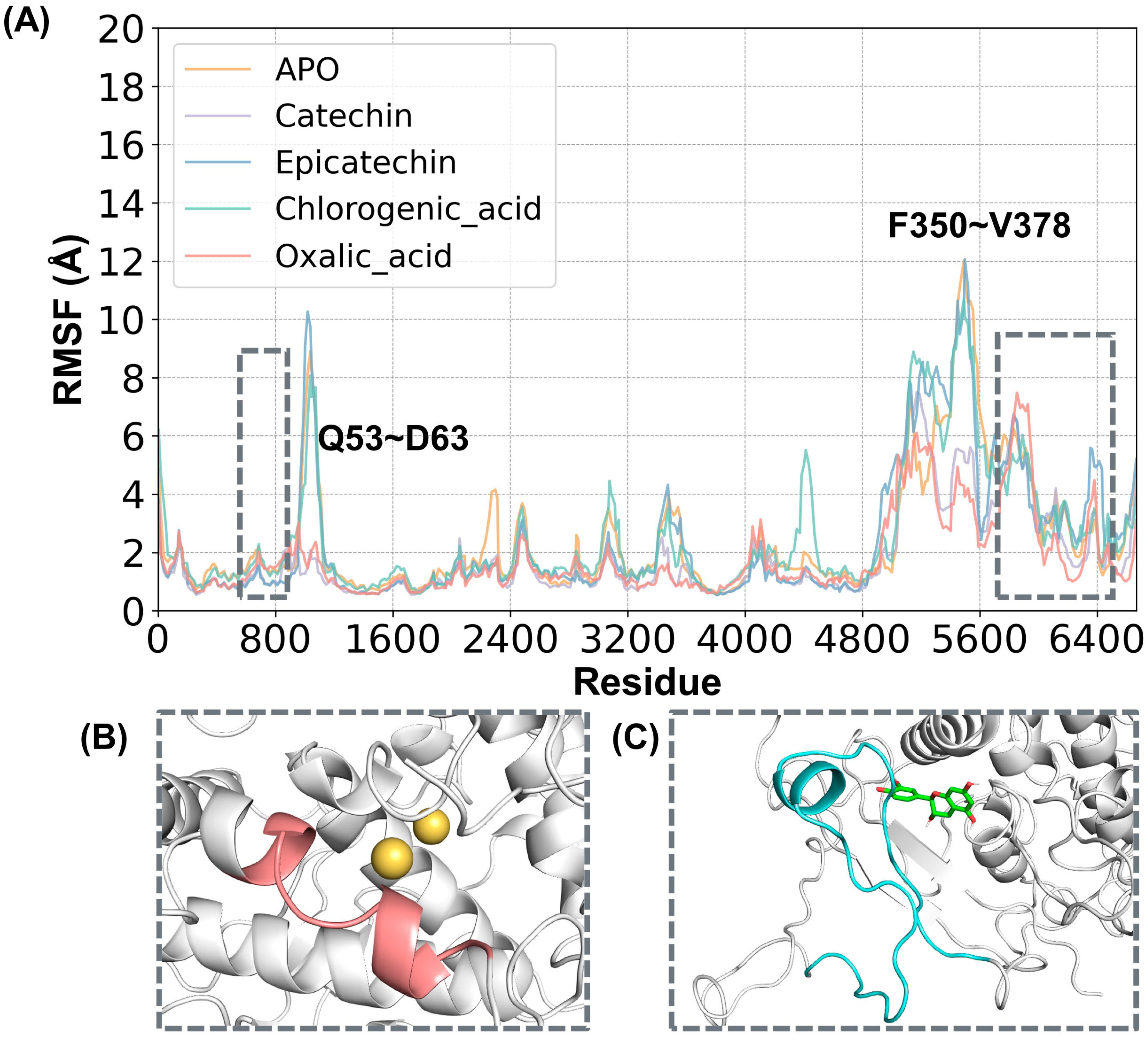
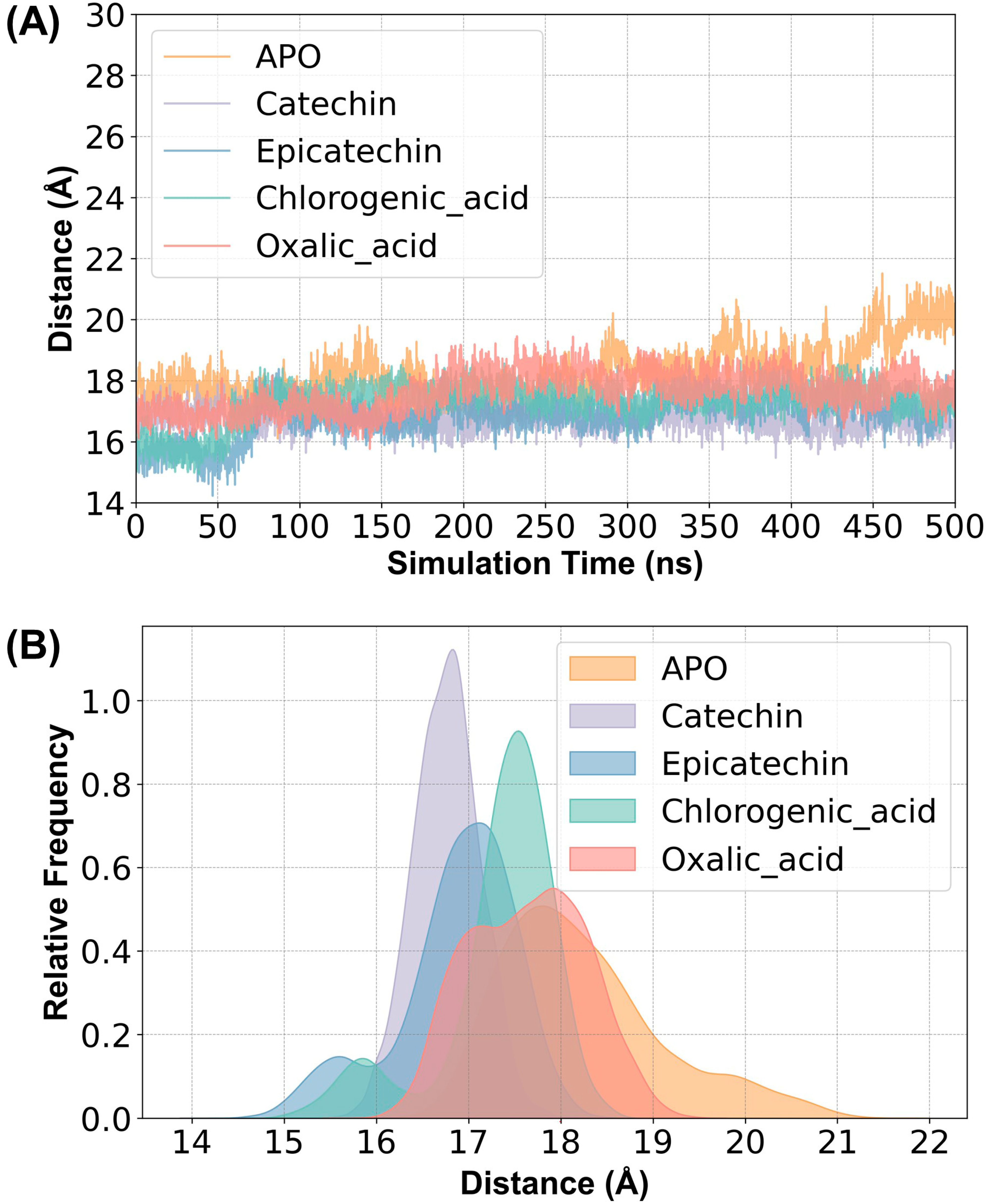
2.2. Structural Stability and Flexibility between Substrates and PPO
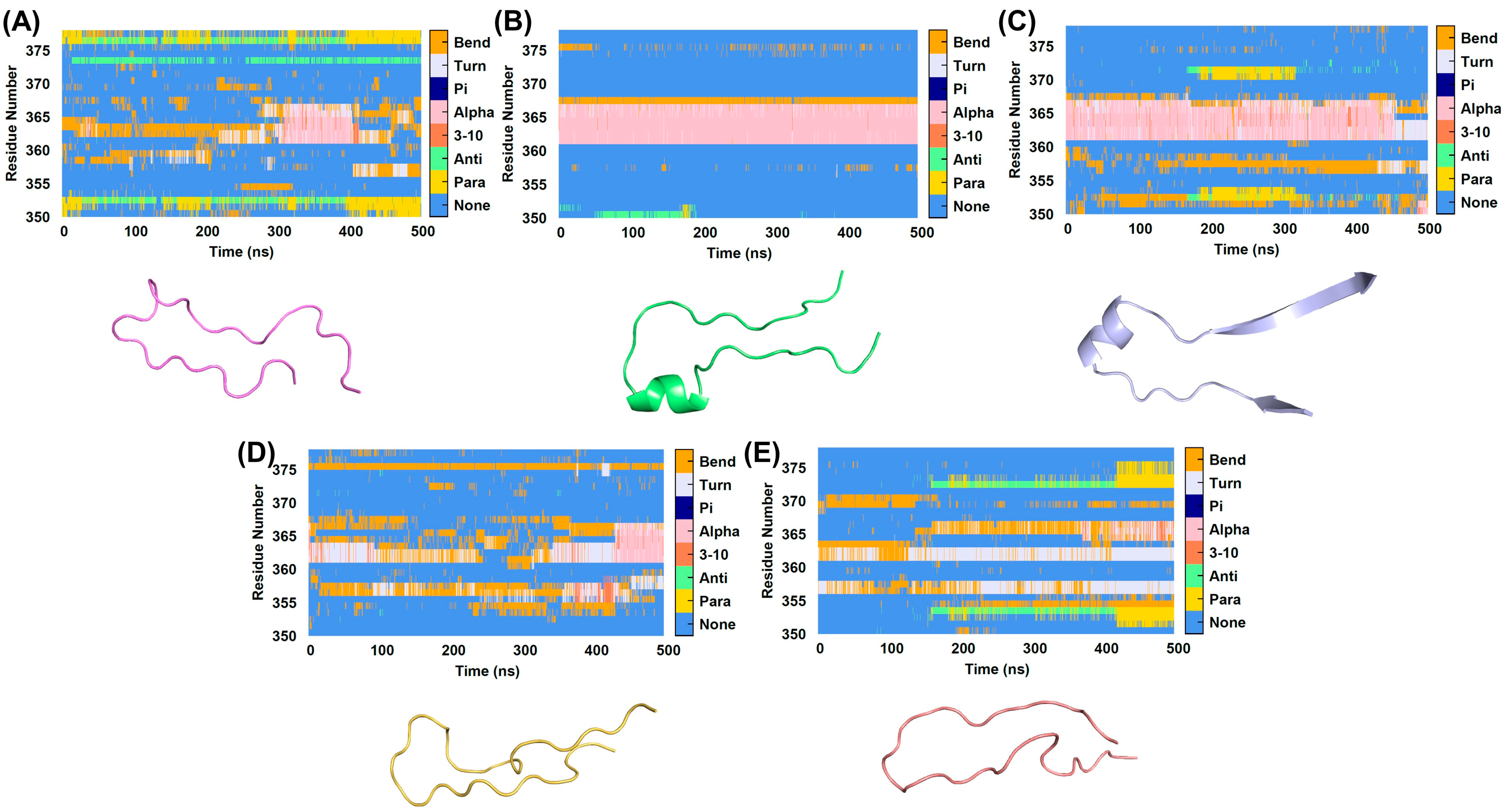
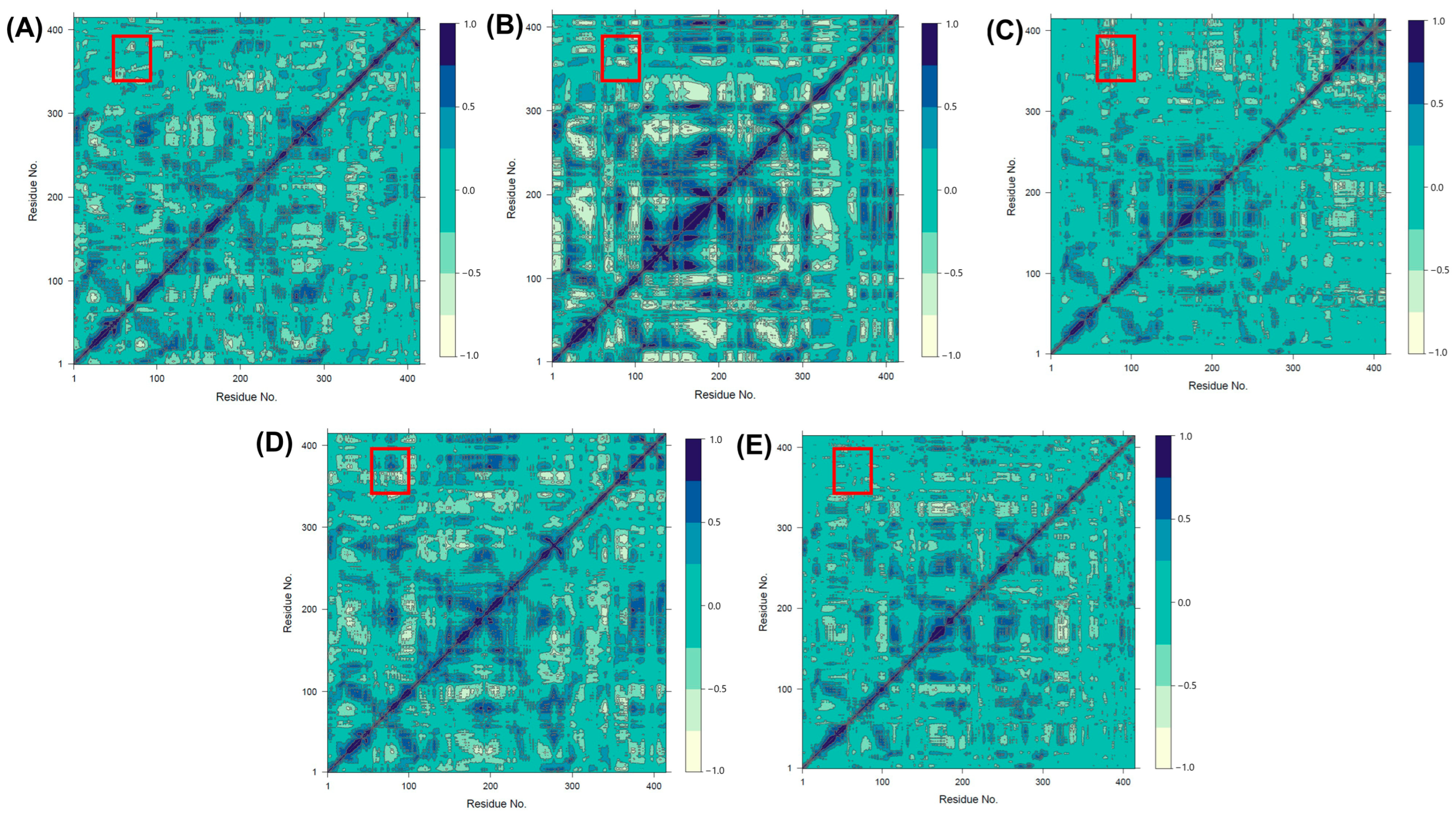
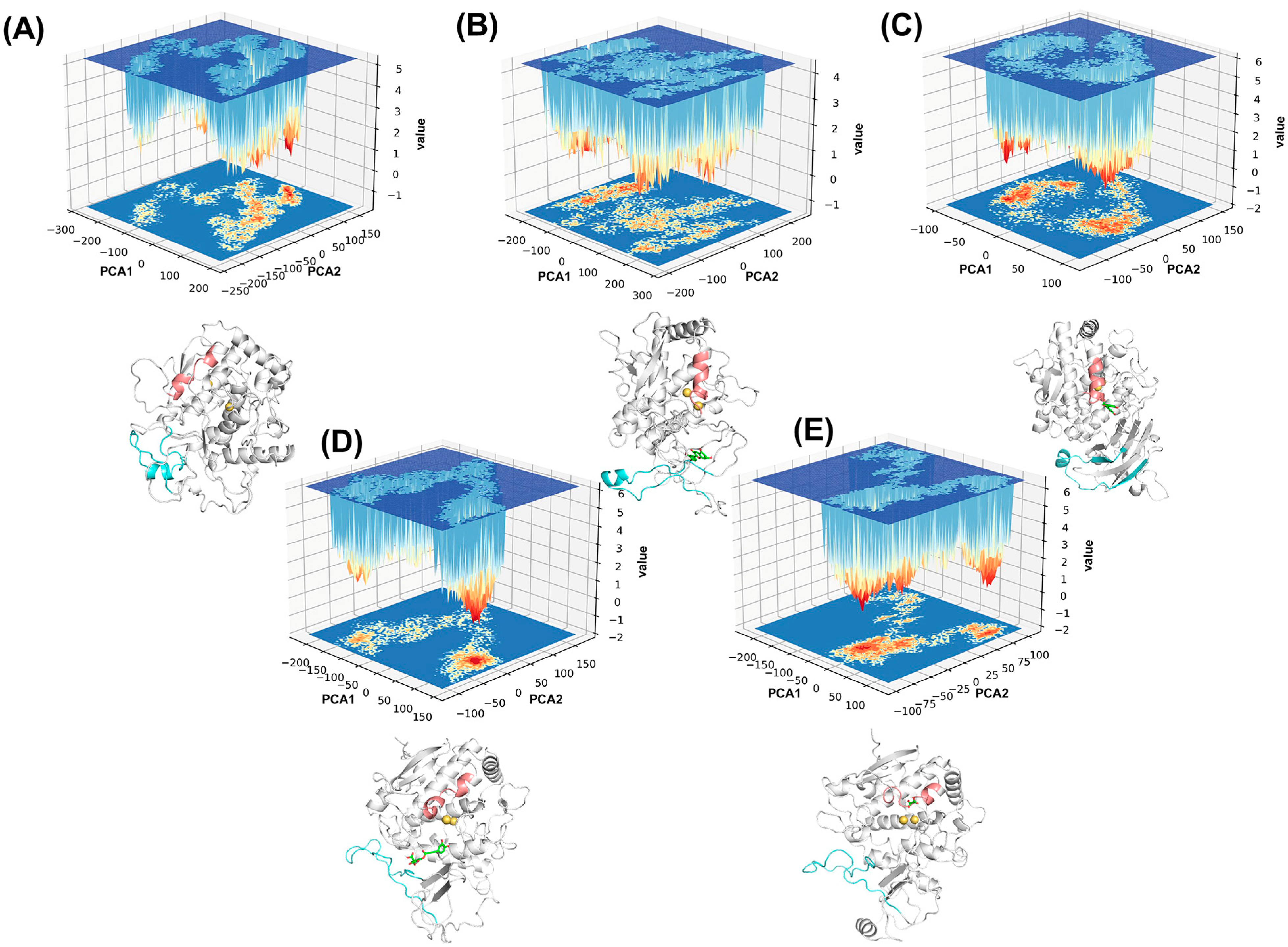
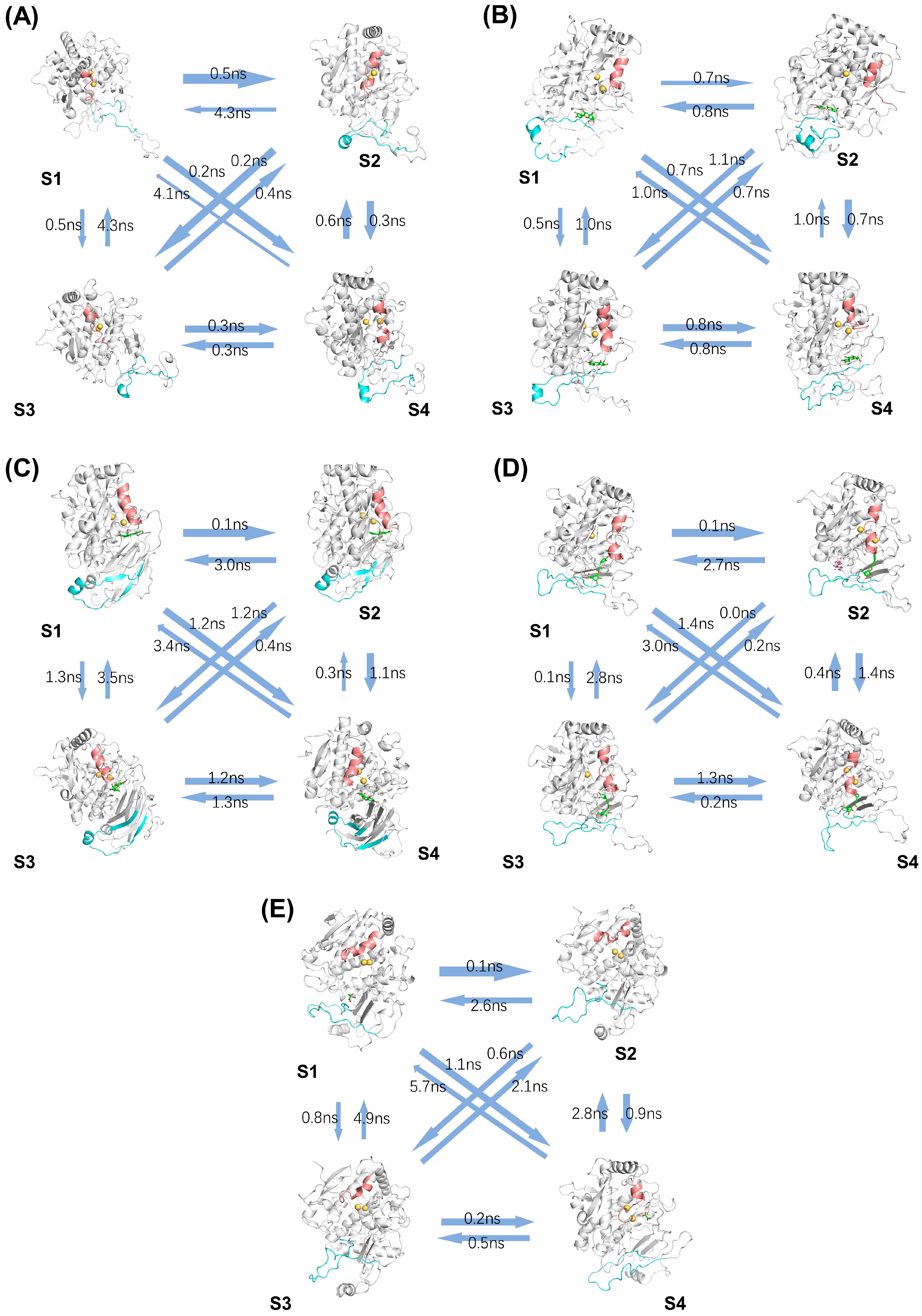
2.3. Analysis of Conformational Changes
2.4. MM-PBSA Analysis
3. Discussion
4. Materials and Methods
4.1. Preparation of Simulated Molecular Systems
4.2. Conventional Molecular Dynamics Simulations
4.3. Gaussian-Accelerated Molecular Dynamics Simulations
4.4. Trajectory Analysis
4.5. MM-PBSA Calculations
4.6. Markov Model Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMBER | Assisted Model Building with Energy Refinement |
| cMD | Conventional Molecular Dynamics |
| DCCM | Dynamic Cross-Correlation Matrix |
| DFT | Density Functional Theory |
| FEL | Free Energy Landscape |
| GaMD | Gaussian-Accelerated Molecular Dynamics |
| HOMO | Highest Occupied Molecular Orbital |
| ITS | Implied Timescales |
| LUMO | Lowest Unoccupied Molecular Orbital |
| MD | Molecular Dynamics |
| MFPT | Mean First Passage Time |
| MM/PBSA | Molecular Mechanics/Poisson–Boltzmann Surface Area |
| MSM | Markov State Model |
| NPT | Isothermal-Isobaric Ensemble (from French: Normale-Isobare) |
| NVT | Constant Number, Volume, and Temperature (from French: Normale-Volume-Température) |
| PCA | Principal Component Analysis |
| PME | Particle Mesh Ewald |
| PPO | Polyphenol Oxidase |
| PBC | Periodic Boundary Conditions |
| RMSD | Root Mean Square Deviation |
| RMSF | Root Mean Square Fluctuation |
| Rg | Radius of Gyration |
| SASA | Solvent Accessible Surface Area |
| TPT | Transition Path Theory |
| ΔEele | Electrostatic Energies |
| ΔEMM | Gas Phase Energy |
| ΔEvdW | Van der Waals Energies |
| ΔGsol | Solvation-Free Energy |
| Amino Acid Single-Letter Abbreviation | |
| F | Phenylalanine |
| G | Glycine |
| L | Leucine |
| V | Valine |
| N | Asparagine |
| Q | Glutamine |
| S | Serine |
References
- Zhang, S. Recent Advances of Polyphenol Oxidases in Plants. Molecules 2023, 28, 2158. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.M.; Kwon, E.B.; Lee, B.; Kim, C.Y. Recent Trends in Controlling the Enzymatic Browning of Fruit and Vegetable Products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef] [PubMed]
- Macheix, J.J.; Sapis, J.C.; Fleuriet, A. Phenolic compounds and polyphenoloxidase in relation to browning in grapes and wines. Crit. Rev. Food Sci. Nutr. 1991, 30, 441–486. [Google Scholar] [CrossRef]
- Mai, F.; Glomb, M.A. Isolation of phenolic compounds from iceberg lettuce and impact on enzymatic browning. J. Agric. Food Chem. 2013, 61, 2868–2874. [Google Scholar] [CrossRef]
- Friedman, M. Prevention of adverse effects of food browning. Adv. Exp. Med. Biol. 1991, 289, 171–215. [Google Scholar] [CrossRef]
- Ma, Y.; Hong, T.T.; Xu, D.; Wu, F.F.; Xu, X.M. Inhibition of PPO-related browning in fresh noodles: A combination of chemical and heat treatment. Food Chem. 2023, 404, 134549. [Google Scholar] [CrossRef]
- Tilley, A.; McHenry, M.P.; McHenry, J.A.; Solah, V.; Bayliss, K. Enzymatic browning: The role of substrates in polyphenol oxidase mediated browning. Curr. Res. Food Sci. 2023, 7, 100623. [Google Scholar] [CrossRef]
- Sui, X.; Meng, Z.; Dong, T.; Fan, X.; Wang, Q. Enzymatic browning and polyphenol oxidase control strategies. Curr. Opin. Biotechnol. 2023, 81, 102921. [Google Scholar] [CrossRef]
- Tang, M.G.; Zhang, S.; Xiong, L.G.; Zhou, J.H.; Huang, J.A.; Zhao, A.Q.; Liu, Z.H.; Liu, A.L. A comprehensive review of polyphenol oxidase in tea (Camellia sinensis): Physiological characteristics, oxidation manufacturing, and biosynthesis of functional constituents. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2267–2291. [Google Scholar] [CrossRef]
- Cheng, K.; Dong, W.; Long, Y.; Zhao, J.; Hu, R.; Zhang, Y.; Zhu, K. Evaluation of the impact of different drying methods on the phenolic compounds, antioxidant activity, and in vitro digestion of green coffee beans. Food Sci. Nutr. 2019, 7, 1084–1095. [Google Scholar] [CrossRef]
- Toro-Uribe, S.; Godoy-Chivatá, J.; Villamizar-Jaimes, A.R.; Perea-Flores, M.J.; López-Giraldo, L.J. Insight of Polyphenol Oxidase Enzyme Inhibition and Total Polyphenol Recovery from Cocoa Beans. Antioxidants 2020, 9, 458. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Bi, J.F.; Hu, J.X.; Li, X. Metabolomics comparison of four varieties apple with different browning characters in response to pretreatment during pulp processing. Food Res. Int. 2024, 190, 114600. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Roh, S.H.; Min, S.C. Inactivation of Potato Polyphenol Oxidase Using Microwave Cold Plasma Treatment. J. Food Sci. 2019, 84, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Toledo, L.; Aguirre, C. Enzymatic browning in avocado (Persea americana) revisited: History, advances, and future perspectives. Crit. Rev. Food Sci. Nutr. 2017, 57, 3860–3872. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, F.; Niu, H.; Yuan, L.; Tian, J.; Cai, S.; Bi, X.; Zhou, L. Structural studies and molecular dynamic simulations of polyphenol oxidase treated by high pressure processing. Food Chem. 2022, 372, 131243. [Google Scholar] [CrossRef]
- Zhou, H.; Bie, S.; Li, Z.; Zhou, L. Comparing the Effect of HPP on the Structure and Stability of Soluble and Membrane-Bound Polyphenol Oxidase from ‘Lijiang Snow’ Peach: Multispectroscopic and Molecular Dynamics Simulation. Foods 2023, 12, 1820. [Google Scholar] [CrossRef]
- Hassan, M.; Abbas, Q.; Ashraf, Z.; Moustafa, A.A.; Seo, S.Y. Pharmacoinformatics exploration of polyphenol oxidases leading to novel inhibitors by virtual screening and molecular dynamic simulation study. Comput. Biol. Chem. 2017, 68, 131–142. [Google Scholar] [CrossRef]
- Gacche, R.N.; Gond, D.S.; Dhole, N.A.; Dawane, B.S. Coumarin Schiff-bases: As antioxidant and possibly anti-inflammatory agents. J. Enzym. Inhib. Med. Chem. 2006, 21, 157–161. [Google Scholar] [CrossRef]
- Mayer, A.M. Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef]
- Yang, H.; He, S.; Feng, Q.; Liu, Z.; Xia, S.; Zhou, Q.; Wu, Z.; Zhang, Y. Lotus (Nelumbo nucifera): A multidisciplinary review of its cultural, ecological, and nutraceutical significance. Bioresour. Bioprocess. 2024, 11, 18. [Google Scholar] [CrossRef]
- Deng, X.; Huang, J.; Zhang, M.; Wei, X.; Song, H.; Wang, Y.; Xin, J.; Sun, H.; Liu, J.; Yang, D.; et al. Metabolite profiling and screening of callus browning-related genes in lotus (Nelumbo nucifera). Physiol. Plant. 2023, 175, e14027. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Deng, Z.Y.; Dong, H.; Tsao, R.; Liu, X. Substrate specificity of polyphenol oxidase and its selectivity towards polyphenols: Unlocking the browning mechanism of fresh lotus root (Nelumbo nucifera Gaertn.). Food Chem. 2023, 424, 136392. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Arantes, P.R.; Bhattarai, A.; Hsu, R.V.; Pawnikar, S.; Huang, Y.M.; Palermo, G.; Miao, Y. Gaussian accelerated molecular dynamics (GaMD): Principles and applications. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2021, 11, e1521. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, A.; Miao, Y. Gaussian accelerated molecular dynamics for elucidation of drug pathways. Expert. Opin. Drug Discov. 2018, 13, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Collier, T.A.; Piggot, T.J.; Allison, J.R. Molecular Dynamics Simulation of Proteins. Methods Mol. Biol. 2020, 2073, 311–327. [Google Scholar] [CrossRef]
- Lindorff-Larsen, K.; Piana, S.; Dror, R.O.; Shaw, D.E. How fast-folding proteins fold. Science 2011, 334, 517–520. [Google Scholar] [CrossRef]
- da Fonseca, A.M.; Caluaco, B.J.; Madureira, J.M.C.; Cabongo, S.Q.; Gaieta, E.M.; Djata, F.; Colares, R.P.; Neto, M.M.; Fernandes, C.F.C.; Marinho, G.S.; et al. Screening of Potential Inhibitors Targeting the Main Protease Structure of SARS-CoV-2 via Molecular Docking, and Approach with Molecular Dynamics, RMSD, RMSF, H-Bond, SASA and MMGBSA. Mol. Biotechnol. 2024, 66, 1919–1933. [Google Scholar] [CrossRef]
- Tian, M.; Xu, Y.-Y.; Li, Y.-N.; Yu, S.; Wang, Y.-L.; Ma, X.-L.; Zhang, Y.-W. Engineering of Substrate-Binding Domain to Improve Catalytic Activity of Chondroitin B Lyase with Semi-Rational Design. Curr. Issues Mol. Biol. 2024, 46, 9916–9927. [Google Scholar] [CrossRef]
- Kumar, S.U.; Sankar, S.; Kumar, D.T.; Younes, S.; Younes, N.; Siva, R.; Doss, C.G.P.; Zayed, H. Molecular dynamics, residue network analysis, and cross-correlation matrix to characterize the deleterious missense mutations in GALE causing galactosemia III. Cell Biochem. Biophys. 2021, 79, 201–219. [Google Scholar] [CrossRef]
- Blaschek, L.; Pesquet, E. Phenoloxidases in Plants—How Structural Diversity Enables Functional Specificity. Front. Plant Sci. 2021, 12, 754601. [Google Scholar] [CrossRef]
- Khan, M.T.; Ali, S.; Zeb, M.T.; Kaushik, A.C.; Malik, S.I.; Wei, D.Q. Gibbs Free Energy Calculation of Mutation in PncA and RpsA Associated With Pyrazinamide Resistance. Front. Mol. Biosci. 2020, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Golla, V.K.; Prajapati, J.D.; Joshi, M.; Kleinekathöfer, U. Exploration of Free Energy Surfaces Across a Membrane Channel Using Metadynamics and Umbrella Sampling. J. Chem. Theory Comput. 2020, 16, 2751–2765. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res 2023, 51, D523–D531. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Senoo, N.; Chinthapalli, D.K.; Baile, M.G.; Golla, V.K.; Saha, B.; Oluwole, A.O.; Ogunbona, O.B.; Saba, J.A.; Munteanu, T.; Valdez, Y.; et al. Functional diversity among cardiolipin binding sites on the mitochondrial ADP/ATP carrier. EMBO J. 2024, 43, 2979–3008. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Jabłońska, J.; Pravda, L.; Vařeková, R.S.; Thornton, J.M. PDBsum: Structural summaries of PDB entries. Protein Sci. 2018, 27, 129–134. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Suzek, T.O.; Zhang, J.; Wang, J.; Zhou, Z.; Han, L.; Karapetyan, K.; Dracheva, S.; Shoemaker, B.A.; et al. PubChem’s BioAssay Database. Nucleic Acids Res. 2012, 40, D400–D412. [Google Scholar] [CrossRef]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Case, D.A.; Belfon, K.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E., III; Cruzeiro, V.W.D.; Darden, T.A.; Duke, R.E.; Ghoreishi, D.; et al. AMBER 2022; University of California: San Francisco, CA, USA, 2022. [Google Scholar]
- Harvey, M.J.; Giupponi, G.; Fabritiis, G.D. ACEMD: Accelerating Biomolecular Dynamics in the Microsecond Time Scale. J. Chem. Theory Comput. 2009, 5, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Kasavajhala, K.; Belfon, K.A.A.; Raguette, L.; Huang, H.; Migues, A.N.; Bickel, J.; Wang, Y.; Pincay, J.; Wu, Q.; et al. ff19SB: Amino-Acid-Specific Protein Backbone Parameters Trained against Quantum Mechanics Energy Surfaces in Solution. J. Chem. Theory Comput. 2020, 16, 528–552. [Google Scholar] [CrossRef] [PubMed]
- Izadi, S.; Anandakrishnan, R.; Onufriev, A.V. Building Water Models: A Different Approach. J. Phys. Chem. Lett. 2014, 5, 3863–3871. [Google Scholar] [CrossRef] [PubMed]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N.log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E., 3rd. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Roe, D.R.; Cheatham, T.E., 3rd. Parallelization of CPPTRAJ enables large scale analysis of molecular dynamics trajectory data. J. Comput. Chem. 2018, 39, 2110–2117. [Google Scholar] [CrossRef]
- Ichiye, T.; Karplus, M. Collective motions in proteins: A covariance analysis of atomic fluctuations in molecular dynamics and normal mode simulations. Proteins 1991, 11, 205–217. [Google Scholar] [CrossRef]
- Srivastava, G.; Tripathi, S.; Kumar, A.; Sharma, A. Molecular investigation of active binding site of isoniazid (INH) and insight into resistance mechanism of S315T-MtKatG in Mycobacterium tuberculosis. Tuberculosis 2017, 105, 18–27. [Google Scholar] [CrossRef]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef]
- Homeyer, N.; Gohlke, H. Free Energy Calculations by the Molecular Mechanics Poisson-Boltzmann Surface Area Method. Mol. Inf. 2012, 31, 114–122. [Google Scholar] [CrossRef]
- Wang, C.; Greene, D.; Xiao, L.; Qi, R.; Luo, R. Recent Developments and Applications of the MMPBSA Method. Front. Mol. Biosci. 2017, 4, 87. [Google Scholar] [CrossRef] [PubMed]
- Kollman, P.A.; Massova, I.; Reyes, C.; Kuhn, B.; Huo, S.; Chong, L.; Lee, M.; Lee, T.; Duan, Y.; Wang, W.; et al. Calculating structures and free energies of complex molecules: Combining molecular mechanics and continuum models. Acc. Chem. Res. 2000, 33, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, K.; Ma, Y.; Han, W. Probing the Mechanisms of Inhibitors Binding to Presenilin Homologue Using Molecular Dynamics Simulations. Molecules 2023, 28, 2076. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.K.; Trendelkamp-Schroer, B.; Paul, F.; Pérez-Hernández, G.; Hoffmann, M.; Plattner, N.; Wehmeyer, C.; Prinz, J.H.; Noé, F. PyEMMA 2: A Software Package for Estimation, Validation, and Analysis of Markov Models. J. Chem. Theory Comput. 2015, 11, 5525–5542. [Google Scholar] [CrossRef]

| S1–S2 | S1–S3 | S1–S4 | S2–S3 | S2–S4 | S3–S4 | |
|---|---|---|---|---|---|---|
| Ligand-free protein | 3.95 | 3.69 | 3.18 | 2.83 | 3.23 | 3.09 |
| Catechin-bound protein | 3.78 | 3.70 | 3.82 | 3.86 | 3.77 | 3.96 |
| Epicatechin-bound protein | 2.31 | 4.28 | 4.26 | 4.13 | 4.17 | 4.25 |
| Chlorogenic acid-bound protein | 2.96 | 2.62 | 4.36 | 1.78 | 4.33 | 4.25 |
| Oxalic acid-bound protein | 2.83 | 3.99 | 4.18 | 3.77 | 4.04 | 2.87 |
| Catechin | Epicatechin | Chlorogenic Acid | Oxalic Acid | |
|---|---|---|---|---|
| ∆EvdW | −19.32 ± 1.17 | −22.50 ± 2.10 | −51.40 ± 1.17 | −0.76 ± 0.44 |
| ∆Eele | −51.65 ± 2.97 | −46.71 ± 5.13 | −22.35 ± 3.00 | −3.57 ± 2.53 |
| ∆Ggas | −70.96 ± 2.83 | −69.21 ± 3.91 | −73.76 ± 3.82 | −4.33 ± 2.94 |
| ∆Gsolv | 59.43 ± 2.07 | 58.46 ± 2.80 | 45.73 ± 3.27 | 4.02 ± 2.62 |
| ∆Gtotal | −11.53 ± 1.00 | −10.75 ± 1.29 | −28.03 ± 1.04 | −0.31 ± 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zheng, S.; Tang, Y.; Han, W.; Li, W.; Li, T. Specific Substrate Activity of Lotus Root Polyphenol Oxidase: Insights from Gaussian-Accelerated Molecular Dynamics and Markov State Models. Int. J. Mol. Sci. 2024, 25, 10074. https://doi.org/10.3390/ijms251810074
Liu M, Zheng S, Tang Y, Han W, Li W, Li T. Specific Substrate Activity of Lotus Root Polyphenol Oxidase: Insights from Gaussian-Accelerated Molecular Dynamics and Markov State Models. International Journal of Molecular Sciences. 2024; 25(18):10074. https://doi.org/10.3390/ijms251810074
Chicago/Turabian StyleLiu, Minghao, Siyun Zheng, Yijia Tang, Weiwei Han, Wannan Li, and Tao Li. 2024. "Specific Substrate Activity of Lotus Root Polyphenol Oxidase: Insights from Gaussian-Accelerated Molecular Dynamics and Markov State Models" International Journal of Molecular Sciences 25, no. 18: 10074. https://doi.org/10.3390/ijms251810074
APA StyleLiu, M., Zheng, S., Tang, Y., Han, W., Li, W., & Li, T. (2024). Specific Substrate Activity of Lotus Root Polyphenol Oxidase: Insights from Gaussian-Accelerated Molecular Dynamics and Markov State Models. International Journal of Molecular Sciences, 25(18), 10074. https://doi.org/10.3390/ijms251810074







