New Psychoactive Substances Toxicity: A Systematic Review of Acute and Chronic Psychiatric Effects
Abstract
1. Introduction
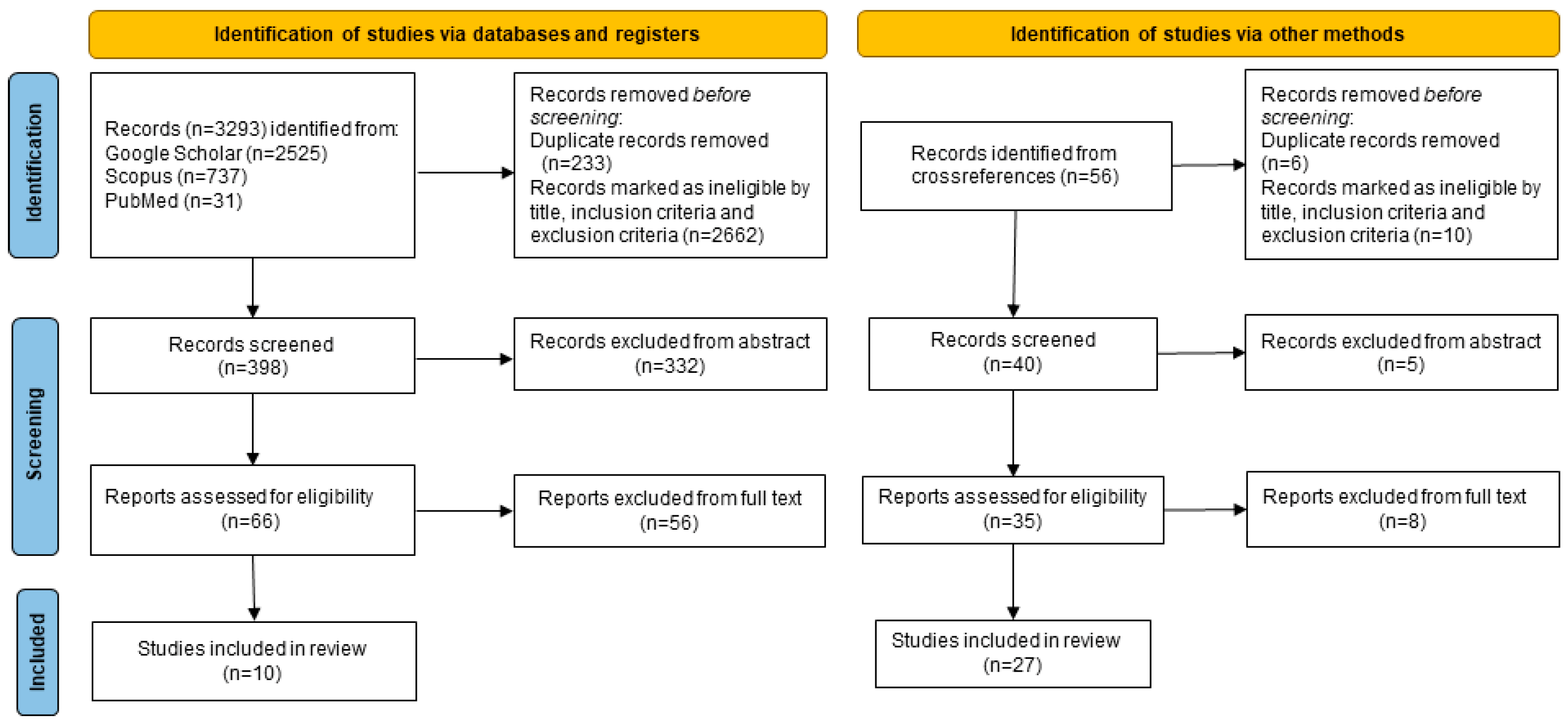
2. Materials and Methods
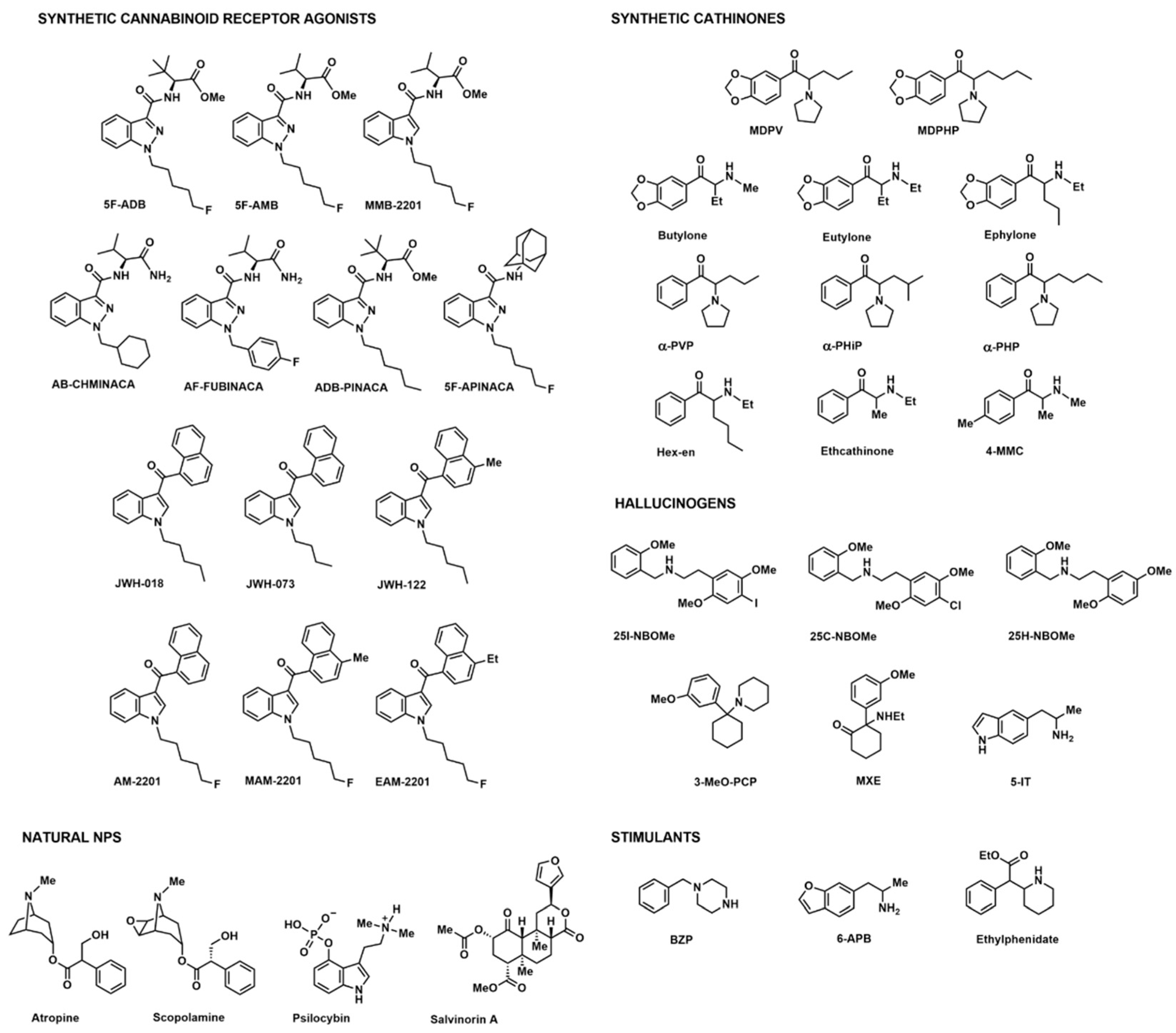
3. Results
| New Psychoactive Substance | Age, Sex Clinical History | Psychiatric Comorbidities | Other Drugs | Psychiatric Symptoms | Year | Ref. |
|---|---|---|---|---|---|---|
| SCRAs = 57 | ||||||
| 5F-ADB | 17 yo, male | na | nr | Psychomotor agitation, confusion, anxiety, psychosis, delirium/hallucinations, | 2017 | [23] |
| 5F-ADB | 17 yo, male | na | nr | Psychomotor agitation, confusion, anxiety psychosis, delirium/hallucination | 2017 | [23] |
| 5F-ADB | 26 yo, male | na | nr | Bizarre behavior, changing moods, incessant oral fluency, nonsensical statements | 2019 | [25] |
| 5F-ADB | 49 yo, male | na | Opioids, fentanyl | Substance-induced psychosis, suicidal behavior | 2019 | [25] |
| 5F-ADB | 31 yo, male | na | nr | Panic attacks | 2023 | [24] |
| 5F-ADB MMB-2201 “Cherry bomb formula 6A” | 14 yo, female | na | THC | Altered consciousness, c, apparent seizures | 2017 | [23] |
| 5F-ADBSpice | 21 yo, male | na | THC | Psychomotor agitation, suicidality, altered language, bradypsychia | 2017 | [23] |
| JWH-122 | 21 yo, male | na | THC, 6-APB | Agitation, paranoid behavior | 2018 | [32] |
| AB-CHMINACA AB-FUBINACA AM-2201 5F-AMB 5F-APINACA EAM-2201 JWH-018 JWH-122 MAM-2201 | 25 yo, male | na | na | Psychosis, anxiety, panic attacks, agitation | 2023 | [24] |
| ADB-PINACA | 25 yo, male | na | nr | Restless and aggressive behavior | 2015 | [26] |
| ADB-PINACA | 24 yo, male | na | nr | Confusion and agitation | 2015 | [26] |
| ADB-PINACA | 30 yo, male regular cocaine user | na | nr | Severely combative and aggressive behavior | 2015 | [26] |
| ADB-PINACA | 16 yo, female | na | nr | Mild agitation and anxiety | 2015 | [26] |
| AM-2201 | 26 yo, male regular THC user | na | nr | Severe panic attacks, recurring visual disturbances, impairment in social and occupational functioning | 2015 | [27] |
| AM-2201 | 35 yo, male | paranoid schizophrenia diagnosis, antisocial personality disorder | nr | Manic, prominent behavioral changes | 2014 | [28] |
| AM-2201 | 21 yo, male | paranoid schizophrenia | nr | Severe agitation, anxiety and presence of paranoid delusions | 2014 | [28] |
| AM-2201 | 27 yo, male | undifferentiated schizophrenia | nr | Hypomanic severe anxiety and agitation, behavioral changes, possible haptic hallucinations | 2014 | [28] |
| AM-2201 | 29 yo, male | undifferentiated schizophrenia | nr | Agitation, severe formal thought symptoms, moderate anxiety | 2014 | [28] |
| AM-2201 | 18 yo, male | na | na | Aggressive behavior | 2023 | [24] |
| AM-2201 JWH-073 JWH-018 | 23 yo, male | na | nr | Psychotic episodes | 2013 | [29] |
| JWH-018 | 17 yo, female | na | Cannabis | Violent behavior, hallucination | 2018 | [32] |
| JWH-018 JWH-122 | 17 yo, male | na | nr | Altered state of mind | 2015 | [30] |
| JWH-122 | 18 yo, male | na | THC | Acute: hallucinations Chronic: occasional visual disorders hallucinations, derealization and body lightness | 2017 | [31] |
| JWH-122 | 18 yo, male | na | na | Hallucinations, perception disorder | 2023 | [24] |
| K2 | 19 yo, male | na | THC | Combativeness | 2013 | [33] |
| K2 | 24 yo, male | na | nr | Anxiety | 2013 | [33] |
| K2 | 22 yo, male | na | nr | Hallucinations, agitation, dream state | 2013 | [33] |
| K2 | 17 yo, male | na | nr | Hallucinations, catatonia, disorganized thoughts | 2018 | [32] |
| K2 | 36 yo, male | na | Ephedrine, pseudoephedrine, promethazine, DXM | Florid persecutory delusions, hallucinations | 2018 | [32] |
| K2 | 36 yo, male THC addiction | schizophrenia | THC | Worsening paranoia, illogical speech, hallucinations | 2018 | [32] |
| K2 Spice | 18 yo, male | na | THC | Delusions, disorganized behavior | 2018 | [32] |
| K2 (Bayou Blaster) | 19 yo, female | na | nr | Agitation, altered mental status, drowsiness, depression, suicidal ideations | 2013 | [33] |
| SCRAs (Mr. Nice Guy) | 23 yo, male | na | Cannabinoids | Psychotic symptoms, persecutory delusions, agitation, aggression, paranoia, altered mental status and severe agitation | 2015 | [34] |
| SCRAs (Humboldt Gold) | 17 yo, male | na | Cannabinoids | Agitation, hallucinations | 2013 | [33] |
| SCRAs (Space) | 17 yo, male | na | nr | Self-perception disorder, anxiety | 2013 | [33] |
| SCRAs | 18 yo, male | na | BDZs | Anxiety, insomnia, ideas of reference hallucinations | 2013 | [35] |
| SCRAs | 20 yo, male regular THC user | na | nr | Acute: disturbance in consciousness, change in cognition, delirium, psychomotor agitation, thought-blocking, disorganized behaviors and thoughts Chronic: diagnosed with schizophreniform disorder | 2013 | [36] |
| SCRAs | 18 yo, male | na | Illicit stimulants, cannabinoids | Acute: mystical and grandiose delusions, substance-induced bipolar disorder Chronic: mystical and grandiose delusions | 2015 | [37] |
| SCRAs | 28 yo, male | paranoid schizophrenia | nr | Delusional mood, persecutory delusions, hallucinations, disorganized thoughts | 2018 | [38] |
| SCRAs | 32 yo, female regular SCRA, THC, crack cocaine and heroin user | schizoaffective disorder | Aripiprazole, carbolithium | Aggressive behavior, sexual disinhibition, delusional mood, grandiose and persecutory delusions | 2018 | [38] |
| SCRAs | 20 yo, male | na | Polysubstance misuse | Bizarre behavior, substance-induced psychotic episodes, sexual disinhibition, arousal and aggressive behavior | 2018 | [38] |
| SCRAs | 39 yo, male regular polysubstance user | bipolar disorder | nr | Agitation, aggressive behavior, disordered with grandiose delusions | 2018 | [38] |
| SCRAs | 31 yo, male occasional LSD user; ecstasy, cannabinoids and psychostimulants | na | nr | Acute: HPPD Type II Chronic: perception disorders | 2018 | [21] |
| SCRAs | 18 yo, male | na | nr | Self-talking and laughing, delusions, manic symptoms | 2018 | [32] |
| SCRAs | 18 yo, female occasional THC user | na | nr | Hallucinations, soliloquy | 2018 | [32] |
| SCRAs | 17 yo, male regular THC, LSD and ecstasy user | na | nr | Paranoia and disorganized thought, bizarre behavior | 2018 | [32] |
| SCRAs (Black Diamond) | 26 yo, male | na | nr | Paranoid delusions | 2018 | [32] |
| SCRAs (bonsai) | 17 yo, male | na | nr | Capgras syndrome, persecutory delusions, hallucination | 2018 | [32] |
| SCRAs (bonsai) | 31 yo, male | na | nr | Anger, insomnia, delusions | 2018 | [32] |
| SCRAs (kush) | 21 yo, male | na | THC | Catatonia, self-talk, inappropriate laughter | 2018 | [32] |
| SCRAs (Mr. Nice Guy) | 23 yo, male | na | nr | Visibly psychotic, persecutory delusions | 2018 | [32] |
| SCRAs (spice) | 17 yo, male regular THC user | na | nr | Catatonia, delusions | 2018 | [32] |
| SCRAs (spice) | 17 yo, male | na | LSD, psilocybin, SCs, oxycodone | Delusions, hallucinations | 2018 | [32] |
| Spice | 59 yo, male previous regular heroin, cocaine and THC user | PTSD | nr | Hallucinations, disorganized, bizarre behavior | 2018 | [32] |
| Spice | 20 yo, male | na | nr | Paranoia, hallucinations | 2018 | [32] |
| Spice | 23 yo, male | na | Cannabis | Nonsensical speech, paranoia | 2018 | [32] |
| Spice | 25 yo, male | na | nr | Severe psychosis, paranoia | 2018 | [32] |
| SCs (n = 29) | ||||||
| Bath salts | 26 yo, female regular SC user | na | na | After 5 months: visual hallucinations After 8 months: occasional hallucinations, reduced psychotic symptoms | 2013 | [39] |
| Bath salts | 40 yo, male regular THC user | na | nr | Paranoid behavior, hallucinations, psychological delusions, psychosis, aggressive behavior, self-mutilation | 2017 | [48] |
| Bath salts | 29 yo, female | bipolar disorder | Polysubstance abuse | Altered mental state | 2016 | [19] |
| Bath salts | 23 yo, male | na | nr | Bizarre behavior, suicidality, hallucinations | 2016 | [19] |
| MDPV 4-MMC | 18 yo, male | na | Cannabinoids | Acute: hallucinations, agitation, confusion, memory loss After 1 week: memory and speech disorder | 2014 | [40] |
| MDPV Butylone | 28 yo, male | na | na | Acute psychosis | 2016 | [19] |
| Eutylone | 32 yo, male | bipolar affective disorder schizophrenia | Ethanol | Abnormal behavior, unconsciousness | 2023 | [16] |
| MDPHP | nr, male | na | Ethanol, BDZs | Aggressive behavior | 2023 | [16] |
| MDPV | 23 yo, male | psychiatric history | THC | Bizarre behavior, suicidality, hallucinations, agitation | 2023 | [16] |
| MDPV | 40 yo, male | bipolar disorder history | Lidocaine | Aggressive behavior, delusional | 2023 | [16] |
| MDPV 4-MMC | 33 yo, male opiates and MAMP addiction | familial bipolar disorder | nr | Hallucinations (auditory and visual), anxiety, paranoia, withdrawal syndrome | 2013 | [41] |
| MDPV 4-MMC butylone α-PVP | 46 yo, male | na | Zolpidem | Suicidal behavior hallucinations, paranoia, anxiety persecutory delusions | 2014 | [49] |
| 4-MMC | 40 yo, male regular cocaine user | na | nr | Acute: persecution and reference delusions Chronic: delusions | 2016 | [42] |
| 4-MMC | 26 yo, male heavy ethanol userand regular cocaine user | na | nr | Hallucinations | 2016 | [42] |
| 4-MMC | 25 yo, male regular cocaine, KETA, GHB, MDMA, MAMP and poppers user | antisocial behavior | nr | Paranoid behavior, intense emotional and behavioral impact, hallucinations | 2016 | [43] |
| 4-MMC | 36 yo, male | na | Cocaine MDMA BDZs | Aggressive and bizarre behavior | 2023 | [16] |
| 4-MMC α-PVP | 26 yo, male occasional ethanol, cannabinoid, AMP and 4-MMC user | na | nr | Acute: psychotic disorder Chronic: craving, withdrawal syndrome, addiction syndrome, drug tolerance, substance-induced schizophrenia-like psychosis diagnosis | 2023 | [22] |
| Ethcathinone | nr, male | na | AMP | Depressed mood | 2023 | [16] |
| Hex-en | 21 yo, male | na | BDZs, AMP, cannabinoids | Disorientation, aggressive behavior | 2023 | [16] |
| Ephylone | 32 yo, male | na | nr | Psychomotor agitation, aggressive behavior | 2019 | [44] |
| Ephylone | 26 yo, female | na | MDMA | Disconnected speech, episodes of visual hallucinations, | 2019 | [44] |
| Ephylone | 26 yo, male | history of mental disorders | nr | Psychosis, paranoia, inconsistent speech | 2019 | [44] |
| Ephylone | 18 yo, male | na | nr | Psychomotor agitation | 2019 | [44] |
| Ephylone | 29 yo, male regular MDMA and SCs user | history of bipolar disorder | AMP, BDZs, cannabinoids, opiates | Agitation, aggressive behavior | 2017 | [45] |
| SCs | 45 yo, male | paranoid schizophrenia mood disorder | nr | Chronic: increasing agitation | 2016 | [19] |
| α-PHiP | 37 yo, male | na | na | Agitation and bizarre behavior | 2023 | [50] |
| α-PHP | 39 yo, male | na | nr | Hallucinations, delusions, aggressive behavior, anxiety, psychotic symptoms | 2018 | [46] |
| α-PVP | 17 yo, female | na | nr | Altered mental status, agitation, psychotic behaviors, auditory hallucinations | 2016 | [47] |
| α-PVP | 40 yo, male | na | na | Psychotic behavior | 2023 | [50] |
| Hallucinogens (n = 17) | ||||||
| 25I-NBOMe | 29 yo, male | na | AMP MDMA, 2C-I | Agitation, aggressiveness, self-injury | 2013 | [51] |
| 25I-NBOMe | 20 yo, male | na | 2C-I | Agitation, visual hallucinations | 2013 | [51] |
| 25I-NBOMe | 19 yo, male | na | 2C-I | Agitation, auditory and visual hallucinations | 2013 | [51] |
| 25I-NBOMe | 22 yo, male | na | 2C-I | Agitation, visual hallucinations, aggressiveness | 2013 | [51] |
| 25I-NBOMe | 21 yo, male history of 2C-B use | na | 2C-I | Agitation, visual hallucinations, aggressiveness | 2013 | [51] |
| 25I-NBOMe | 20 yo, male regular AMP and MDMA user | na | Ethanol, 2C-I | Visual hallucinations | 2013 | [51] |
| 25I-NBOMe | 20 yo male regular MDMA, cocaine, cannabis and LSD user | na | 2C-I | Visual hallucinations | 2013 | [51] |
| 25I-NBOMe | 18 yo, male | na | Cannabinoids (screening) | Acute: severe agitation, hallucinations Chronic: episodes of aggressiveness | 2013 | [52] |
| 25I-NBOMe 25C-NBOMe 25H-NBOMe | 29 yo, nr, regular THC and LSD user | schizophrenia | nr | After 1 month: persistent memory impairment, significant abnormalities in executive functions | 2016 | [19] |
| 3-MeO-PCP | 29 yo, male | anxious–depressive symptoms in adolescence under pharmacological treatment | nr | After 3 days: mania-like episode with psychotic features, visual and tactile hallucinations, paranoid delusions, severe dissociation, sense of impending doom, psychomotor agitation, aggressive behaviors, persistent psychotic symptoms and behavioral alterations, substance-induced psychosis | 2024 | [54] |
| 5-IT | 24 yo, male | na | nr | Hallucinations, restlessness | 2014 | [53] |
| 5-IT | 53 yo, male | na | MXE, ethanol | Restlessness | 2014 | [53] |
| 5-IT | 21 yo, male | na | Methylphenidate, ritalinic acid, KETA | Agitation, restlessness | 2014 | [53] |
| 5-IT | 27 yo, female | na | MDPV, ethylphenidate, 4-MEC, 5-APB, buprenorphine, ethanol | Hallucinations, restlessness | 2014 | [53] |
| 5-IT | 23 yo, male | na | na | Hallucinations, agitation, restlessness | 2014 | [53] |
| 5-IT | 23 yo, male | na | na | Agitation | 2014 | [53] |
| MXE | 23 yo, male occasional THC, KETA and LSD user | na | nr | Acute: usual visual and auditory hallucinosis, severe dissociative symptoms, detachment from reality and absorption in imaginative thoughts, marked affective withdrawal, motivational anhedonia After 3 months: diagnosis of substance-induced psychotic disorder | 2019 | [55] |
| Natural NPS (n = 3) | ||||||
| Datura stramonium (tropane alkaloids) | 32 yo, male regular hallucinogen user | paranoid schizophrenia with mental and behavioral disorders | na | Paranoid schizophrenia, mental and behavioral disorders | 2016 | [19] |
| Psilocybe spp. (psilocybin) | 23 yo, male regular THC user | na | Ethanol, THC | Acute: hallucinations Chronic: HPPD Type I | 2018 | [21] |
| Salvia divinorum (salvinorin A) | 24 yo, female chronic salvinorin A user | bipolar I disorder, occasional psychotic symptoms | nr | Psychotic symptoms, auditory hallucinations, persecutory and religious delusions | 2016 | [19] |
| Stimulants (n = 3) | ||||||
| BZP | 48 yo, male | schizophrenia | nr | Aggressive behavior, incoherent speech | 2016 | [19] |
| 6-APB | 21 yo, male | na | THC | Acute psychosis, agitation, paranoid behavior | 2023 | [24] |
| Ethylphenidate | 30 yo, male | paranoid schizophrenia | Benzocaine | Severe thought disorder, chaotic and bizarre behavior pattern | 2016 | [19] |
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- UNODC World Drug Report 2024—Drug Market Patterns and Trends. Available online: https://www.unodc.org/unodc/en/data-and-analysis/wdr2024-drug-market-trends.html (accessed on 10 July 2024).
- EUDA Drug Supply, Production and Precursors—The Current Situation in Europe (European Drug Report 2024). 2024. Available online: https://www.euda.europa.eu/publications/european-drug-report/2024/drug-supply-production-and-precursors_en (accessed on 10 July 2024).
- Zapata, F.; Matey, J.M.; Montalvo, G.; García-Ruiz, C. Chemical Classification of New Psychoactive Substances (NPS). Microchem. J. 2021, 163, 105877. [Google Scholar] [CrossRef]
- EUDA New Psychoactive Substances—The Current Situation in Europe (European Drug Report 2024). 2024. Available online: https://www.euda.europa.eu/publications/european-drug-report/2024/drug-situation-in-europe-up-to-2024_en (accessed on 10 July 2024).
- Graziano, S.; Zaami, S.; Tittarelli, R.; Pichini, S.; Sciotti, M.; Berretta, P.; Busardò, F.P.; Marinelli, E.; Tini, A.; Scaravaggi, G.; et al. The Complex and Constantly Evolving Public Health Threat of New Psychoactive Substances in Italy: Addressing the Main Functions of a National Observatory of Drugs. Ann. Ist. Super. Sanita 2021, 57, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Pantano, F.; Tittarelli, R.; Mannocchi, G.; Zaami, S.; Ricci, S.; Giorgetti, R.; Terranova, D.; Busardò, F.P.; Marinelli, E. Hepatotoxicity Induced by “the 3Ks”: Kava, Kratom and Khat. Int. J. Mol. Sci. 2016, 17, 580. [Google Scholar] [CrossRef]
- Schifano, N.; Chiappini, S.; Mosca, A.; Miuli, A.; Santovito, M.C.; Pettorruso, M.; Capogrosso, P.; Dehò, F.; Martinotti, G.; Schifano, F. Recreational Drug Misuse and Its Potential Contribution to Male Fertility Levels’ Decline: A Narrative Review. Brain Sci. 2022, 12, 1582. [Google Scholar] [CrossRef]
- Orsolini, L.; Chiappini, S.; Papanti, D.; De Berardis, D.; Corkery, J.M.; Schifano, F. The Bridge Between Classical and “Synthetic”/Chemical Psychoses: Towards a Clinical, Psychopathological, and Therapeutic Perspective. Front. Psychiatry 2019, 10, 851. [Google Scholar] [CrossRef]
- Bersani, G.; Prevete, E. Novel Psychoactive Substances (NPS) Use in Severe Mental Illness (SMI) Patients: Potential Changes in the Phenomenology of Psychiatric Diseases. Hum. Psychopharmacol. 2017, 32, e2591. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.L.; Mogford, D.V.; Lawrence, R.J.; Lawrie, S.M. Use of Novel Psychoactive Substances by Inpatients on General Adult Psychiatric Wards. BMJ Open 2016, 6, e009430. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. New Psychoactive Substances: A Review and Updates. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320967197. [Google Scholar] [CrossRef] [PubMed]
- Schifano, F.; Orsolini, L.; Duccio Papanti, G.; Corkery, J.M. Novel Psychoactive Substances of Interest for Psychiatry. World Psychiatry 2015, 14, 15–26. [Google Scholar] [CrossRef]
- Peglow, S.; Buchner, J.; Briscoe, G. Synthetic Cannabinoid Induced Psychosis in a Previously Nonpsychotic Patient. Am. J. Addict. 2012, 21, 287–288. [Google Scholar] [CrossRef]
- Daswani, R.R.; Choles, C.M.; Kim, D.D.; Barr, A.M. A Systematic Review and Meta-Analysis of Synthetic Cathinone Use and Psychosis. Psychopharmacology 2024, 241, 875–896. [Google Scholar] [CrossRef]
- Stiles, B.M.; Fish, A.F.; Cook, C.A.; Silva, V. Bath Salt-Induced Psychosis: Nursing Assessment, Diagnosis, Treatment, and Outcomes. Perspect. Psychiatr. Care 2016, 52, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Daziani, G.; Lo Faro, A.F.; Montana, V.; Goteri, G.; Pesaresi, M.; Bambagiotti, G.; Montanari, E.; Giorgetti, R.; Montana, A. Synthetic Cathinones and Neurotoxicity Risks: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 6230. [Google Scholar] [CrossRef]
- Chiappini, S.; Mosca, A.; Miuli, A.; Santovito, M.C.; Orsolini, L.; Corkery, J.M.; Guirguis, A.; Pettorruso, M.; Martinotti, G.; Di Giannantonio, M.; et al. New Psychoactive Substances and Suicidality: A Systematic Review of the Current Literature. Medicina 2021, 57, 580. [Google Scholar] [CrossRef]
- Chiappini, S.; Vaccaro, G.; Mosca, A.; Miuli, A.; Stigliano, G.; Stefanelli, G.; Giovannetti, G.; Carullo, R.; d’Andrea, G.; Di Carlo, F.; et al. New Trends of Drug Abuse in Custodial Settings: A Systematic Review on the Misuse of over-the-Counter Drugs, Prescription-Only-Medications, and New Psychoactive Substances. Neurosci. Biobehav. Rev. 2024, 162, 105691. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.; Bressington, D.; Hughes, E.; Ivanecka, A. A Systematic Review of the Effects of Novel Psychoactive Substances “legal Highs” on People with Severe Mental Illness. J. Psychiatr. Ment. Health Nurs. 2016, 23, 267–281. [Google Scholar] [CrossRef]
- Page, M.J.; Mckenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Skryabin, V.Y.; Vinnikova, M.; Nenastieva, A.; Alekseyuk, V. Hallucinogen Persisting Perception Disorder: A Literature Review and Three Case Reports. J. Addict. Dis. 2018, 37, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Severtsev, V.; Budanova, A. Recurrent Psychotic Episodes Induced by Synthetic Cathinones in a Monozygotic Twin with Drug Addiction: A Case Report. Consort. Psychiatr. 2023, 4, 58–65. [Google Scholar] [CrossRef]
- Barceló, B.; Pichini, S.; López-Corominas, V.; Gomila, I.; Yates, C.; Busardò, F.P.; Pellegrini, M. Acute Intoxication Caused by Synthetic Cannabinoids 5F-ADB and MMB-2201: A Case Series. Forensic Sci. Int. 2017, 273, e10–e14. [Google Scholar] [CrossRef]
- de Oliveira, M.C.; Vides, M.C.; Lassi, D.L.S.; Torales, J.; Ventriglio, A.; Bombana, H.S.; Leyton, V.; Périco, C.D.A.M.; Negrão, A.B.; Malbergier, A.; et al. Toxicity of Synthetic Cannabinoids in K2/Spice: A Systematic Review. Brain Sci. 2023, 13, 990. [Google Scholar] [CrossRef]
- Kraemer, M.; Fels, H.; Dame, T.; Musshoff, F.; Halter, S.; Mogler, L.; Hess, C.; Madea, B.; Maas, A. Mono-/Polyintoxication with 5F-ADB: A Case Series. Forensic Sci. Int. 2019, 301, e29–e37. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.D.; Trecki, J.; Edison, L.A.; Steck, A.R.; Arnold, J.K.; Gerona, R.R. A Common Source Outbreak of Severe Delirium Associated with Exposure to the Novel Synthetic Cannabinoid ADB-PINACA. J. Emerg. Med. 2015, 48, 573–580. [Google Scholar] [CrossRef]
- Besli, G.E.; Ikiz, M.A.; Yildirim, S.; Saltik, S. Synthetic Cannabinoid Abuse in Adolescents: A Case Series. J. Emerg. Med. 2015, 49, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Celofiga, A.; Koprivsek, J.; Klavz, J. Use of Synthetic Cannabinoids in Patients with Psychotic Disorders: Case Series. J. Dual Diagn. 2014, 10, 168–173. [Google Scholar] [CrossRef]
- Patton, A.L.; Chimalakonda, K.C.; Moran, C.L.; Mccain, K.R.; Radominska-Pandya, A.; James, L.P.; Kokes, C.; Moran, J.H. K2 Toxicity: Fatal Case of Psychiatric Complications Following AM2201 Exposure. J. Forensic Sci. 2013, 58, 1676–1680. [Google Scholar] [CrossRef]
- Naviglio, S.; Papanti, D.; Moressa, V.; Ventura, A. An Adolescent with an Altered State of Mind. BMJ 2015, 350, h299. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coppola, M.; Mondola, R. JWH-122 Consumption Adverse Effects: A Case of Hallucinogen Persisting Perception Disorder Five-Year Follow-Up. J. Psychoact. Drugs 2017, 49, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Verrico, C.D.; Kosten, T.R.; Nielsen, D.A. Psychosis and Synthetic Cannabinoids. Psychiatry Res. 2018, 268, 400–412. [Google Scholar] [CrossRef]
- Harris, C.R.; Brown, A. Synthetic Cannabinoid Intoxication: A Case Series and Review. J. Emerg. Med. 2013, 44, 360–366. [Google Scholar] [CrossRef]
- Durand, D.; Delgado, L.; de la Parra-Pellot, D.M.; Nichols-Vinueza, D. Psychosis and Severe Rhabdomyolysis with Synthetic Cannabinoid Use: A Case Report. Clin. Schizophr. Relat. Psychoses 2015, 8, 205–208. [Google Scholar] [CrossRef]
- Papanti, D.; Schifano, F.; Botteon, G.; Bertossi, F.; Mannix, J.; Vidoni, D.; Impagnatiello, M.; Pascolo-Fabrici, E.; Bonavigo, T. “spiceophrenia”: A Systematic Overview of “Spice”-Related Psychopathological Issues and a Case Report. Hum. Psychopharmacol. 2013, 28, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Berry-Cabán, C.S.; Ee, J.; Ingram, V.; Berry, C.E.; Kim, E.H. Synthetic Cannabinoid Overdose in a 20-Year-Old Male US Soldier. Subst. Abus. 2013, 34, 70–72. [Google Scholar] [CrossRef]
- Ustundag, M.F.; Ozhan Ibis, E.; Yucel, A.; Ozcan, H. Synthetic Cannabis-Induced Mania. Case Rep. Psychiatry 2015, 2015, 310930. [Google Scholar] [CrossRef]
- Bonaccorso, S.; Metastasio, A.; Ricciardi, A.; Stewart, N.; Jamal, L.; Rujully, N.U.D.; Theleritis, C.; Ferracuti, S.; Ducci, G.; Schifano, F. Synthetic Cannabinoid Use in a Case Series of Patients with Psychosis Presenting to Acute Psychiatric Settings: Clinical Presentation and Management Issues. Brain Sci. 2018, 8, 133. [Google Scholar] [CrossRef]
- Penders, T.M.; Lang, M.C.; Pagano, J.J.; Gooding, Z.S. Electroconvulsive Therapy Improves Persistent Psychosis after Repeated Use of Methylenedioxypyrovalerone (“bath Salts”). J. ECT 2013, 29, e59–e60. [Google Scholar] [CrossRef] [PubMed]
- Zamengo, L.; Frison, G.; Bettin, C.; Sciarrone, R. Understanding the Risks Associated with the Use of New Psychoactive Substances (NPS): High Variability of Active Ingredients Concentration, Mislabelled Preparations, Multiple Psychoactive Substances in Single Products. Toxicol. Lett. 2014, 229, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Winder, G.S.; Stern, N.; Hosanagar, A. Are “Bath Salts” the next Generation of Stimulant Abuse? J. Subst. Abus. Treat. 2013, 44, 42–45. [Google Scholar] [CrossRef]
- Barrios, L.; Grison-Hernando, H.; Boels, D.; Bouquie, R.; Monteil-Ganiere, C.; Clement, R. Death Following Ingestion of Methylone. Int. J. Leg. Med. 2016, 130, 381–385. [Google Scholar] [CrossRef]
- Dolengevich-Segal, H.; Rodríguez-Salgado, B.; Gómez-Arnau, J.; Sánchez-Mateos, D. Severe Psychosis, Drug Dependence, and Hepatitis C Related to Slamming Mephedrone. Case Rep. Psychiatry 2016, 2016, 8379562. [Google Scholar] [CrossRef]
- Costa, J.L.; Cunha, K.F.; Lanaro, R.; Cunha, R.L.; Walther, D.; Baumann, M.H. Analytical Quantification, Intoxication Case Series, and Pharmacological Mechanism of Action for N-Ethylnorpentylone (N-Ethylpentylone or Ephylone). Drug Test Anal. 2019, 11, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Thirakul, P.; Hair, L.S.; Bergen, K.L.; Pearson, J.M. Clinical Presentation, Autopsy Results and Toxicology Findings in an Acute N-Ethylpentylone Fatality. J. Anal. Toxicol. 2017, 41, 342–346. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fujita, Y.; Mita, T.; Usui, K.; Kamijo, Y.; Kikuchi, S.; Onodera, M.; Fujino, Y.; Inoue, Y. Toxicokinetics of the Synthetic Cathinone α-Pyrrolidinohexanophenone. J. Anal. Toxicol. 2018, 42, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Crespi, C. Flakka-Induced Prolonged Psychosis. Case Rep. Psychiatry 2016, 2016, 3460849. [Google Scholar] [CrossRef]
- John, M.E.; Thomas-Rozea, C.; Hahn, D. Bath Salts Abuse Leading to New-Onset Psychosis and Potential for Violence. Clin. Schizophr. Relat. Psychoses 2017, 11, 120–124. [Google Scholar] [CrossRef]
- Dragogna, F.; Oldani, L.; Buoli, M.; Altamura, A.C. A Case of Severe Psychosis Induced by Novel Recreational Drugs. F1000Research 2014, 3, 21. [Google Scholar] [CrossRef]
- Albishri, S.; Alotaibi, A.; Alzoubaidi, F.; El-Serafy, O. Flakka: “The Zombie Drug” a Medicolegal Concern: An Updated Review of α-Pyrrolidinopentiophenone. Saudi J. Forensic Med. Sci. 2023, 3, 1–8. [Google Scholar] [CrossRef]
- Hill, S.L.; Doris, T.; Gurung, S.; Katebe, S.; Lomas, A.; Dunn, M.; Blain, P.; Thomas, S.H.L. Severe Clinical Toxicity Associated with Analytically Confirmed Recreational Use of 25I-NBOMe: Case Series. Clin. Toxicol. 2013, 51, 487–492. [Google Scholar] [CrossRef]
- Rose, S.R.; Poklis, J.L.; Poklis, A. A Case of 251-NBOMe (25-1) Intoxication: A New Potent 5-HT2A Agonist Designer Drug. Clin. Toxicol. 2013, 51, 174–177. [Google Scholar] [CrossRef]
- Bäckberg, M.; Beck, O.; Hultén, P.; Rosengren-Holmberg, J.; Helander, A. Intoxications of the New Psychoactive Substance 5-(2-Aminopropyl)Indole (5-IT): A Case Series from the Swedish STRIDA Project. Clin. Toxicol. 2014, 52, 618–624. [Google Scholar] [CrossRef]
- Pepe, M.; Di Nicola, M.; Cocciolillo, F.; Chiappini, S.; Martinotti, G.; Calcagni, M.L.; Sani, G. 3-Methoxy-Phencyclidine Induced Psychotic Disorder: A Literature Review and an 18F-FDG PET/CT Case Report. Pharmaceuticals 2024, 17, 452. [Google Scholar] [CrossRef]
- Moccia, L.; Tofani, A.; Mazza, M.; Covino, M.; Martinotti, G.; Schifano, F.; Janiri, L.; Di Nicola, M. Dorsolateral Prefrontal Cortex Impairment in Methoxetamine-Induced Psychosis: An 18F-FDG PET/CT Case Study. J. Psychoact. Drugs 2019, 51, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Castaneto, M.S.; Gorelick, D.A.; Desrosiers, N.A.; Hartman, R.L.; Pirard, S.; Huestis, M.A. Synthetic Cannabinoids: Epidemiology, Pharmacodynamics, and Clinical Implications. Drug Alcohol Depend. 2014, 144, 12–41. [Google Scholar] [CrossRef]
- Wells, D.L.; Ott, C.A. The “New” Marijuana. Ann. Pharmacother. 2011, 45, 414–417. [Google Scholar] [CrossRef]
- Migliaro, M.; Ruiz-Contreras, A.E.; Herrera-Solís, A.; Méndez-Díaz, M.; Prospéro-García, O.E. Endocannabinoid System and Aggression across Animal Species. Neurosci. Biobehav. Rev. 2023, 153, 105375. [Google Scholar] [CrossRef]
- La Maida, N.; Di Trana, A.; Giorgetti, R.; Tagliabracci, A.; Busardò, F.P.; Huestis, M.A. A Review of Synthetic Cathinone-Related Fatalities From 2017 to 2020. Ther. Drug Monit. 2021, 43, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Fantegrossi, W.E.; Murnane, K.S.; Reissig, C.J. The Behavioral Pharmacology of Hallucinogens. Biochem. Pharmacol. 2008, 75, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, A.L. Pharmacology and Toxicology of N-Benzylphenethylamine (“NBOMe”) Hallucinogens. In Neuropharmacology of New Psychoactive Substances (NPS); Springer: Cham, Switzerland, 2017; pp. 283–311. [Google Scholar] [CrossRef]
- Di Trana, A.; Berardinelli, D.; Montanari, E.; Berretta, P.; Basile, G.; Huestis, M.A.; Busardò, F.P. Molecular Insights and Clinical Outcomes of Drugs of Abuse Adulteration: New Trends and New Psychoactive Substances. Int. J. Mol. Sci. 2022, 23, 4619. [Google Scholar] [CrossRef] [PubMed]
- Jonikas, J.A.; Cook, J.A.; Rosen, C.; Laris, A.; Kim, J.-B. Brief Reports: A Program to Reduce Use of Physical Restraint in Psychiatric Inpatient Facilities. Psychiatr. Serv. 2004, 55, 818–820. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taflaj, B.; La Maida, N.; Tittarelli, R.; Di Trana, A.; D’Acquarica, I. New Psychoactive Substances Toxicity: A Systematic Review of Acute and Chronic Psychiatric Effects. Int. J. Mol. Sci. 2024, 25, 9484. https://doi.org/10.3390/ijms25179484
Taflaj B, La Maida N, Tittarelli R, Di Trana A, D’Acquarica I. New Psychoactive Substances Toxicity: A Systematic Review of Acute and Chronic Psychiatric Effects. International Journal of Molecular Sciences. 2024; 25(17):9484. https://doi.org/10.3390/ijms25179484
Chicago/Turabian StyleTaflaj, Beldisa, Nunzia La Maida, Roberta Tittarelli, Annagiulia Di Trana, and Ilaria D’Acquarica. 2024. "New Psychoactive Substances Toxicity: A Systematic Review of Acute and Chronic Psychiatric Effects" International Journal of Molecular Sciences 25, no. 17: 9484. https://doi.org/10.3390/ijms25179484
APA StyleTaflaj, B., La Maida, N., Tittarelli, R., Di Trana, A., & D’Acquarica, I. (2024). New Psychoactive Substances Toxicity: A Systematic Review of Acute and Chronic Psychiatric Effects. International Journal of Molecular Sciences, 25(17), 9484. https://doi.org/10.3390/ijms25179484






