Fenestrated Endothelial Cells across Organs: Insights into Kidney Function and Disease
Abstract
1. Introduction
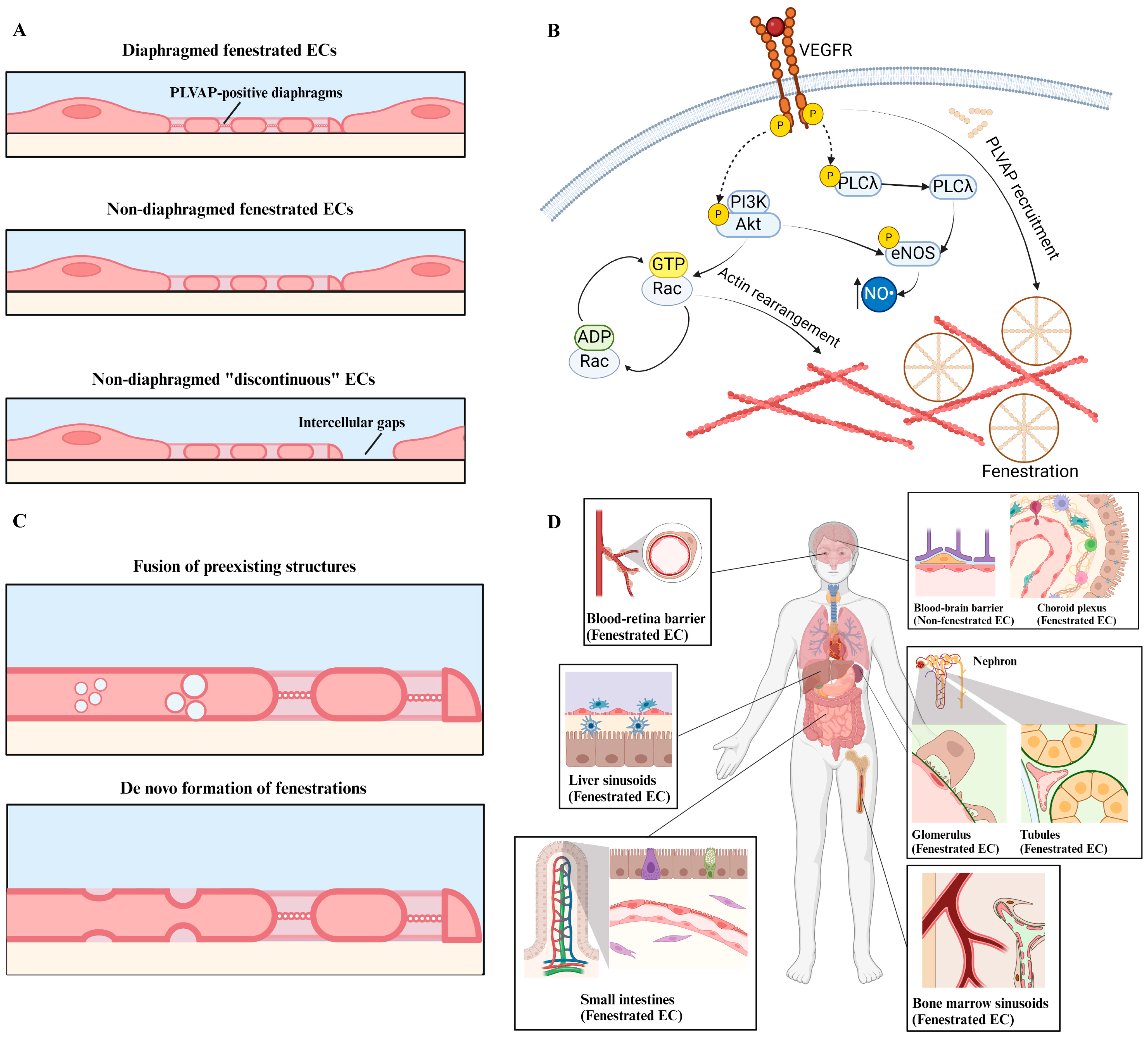
2. Endothelial Cell Fenestrations: From Development to Structure and Function
2.1. Structure and Function
2.2. Development
2.3. Fenestrations in Different Organs
2.3.1. Brain
2.3.2. Gastrointestinal Tract
2.3.3. Liver
2.3.4. Bone
2.3.5. Retina
2.3.6. Kidneys
| Organ/Tissue Type | Diaphragmed? | Discontinued? | Basement Membrane? | Primary Functions | References |
|---|---|---|---|---|---|
| Choroid plexus | Yes | No | Yes | Deliver water for cerebrospinal fluid production; Facilitate lymphocyte infiltration. | [36,37,38] |
| Small intestine | Yes | No | Yes | Nutrient transport; Facilitate immune cell-intestinal epithelial cell crosstalk. | [41,43] |
| Liver sinusoids | No | Yes | No | Molecular transport across live sinusoids to facilitate liver metabolism and homeostasis; Promote T cell interaction with hepatocytes; Trans-endothelium migration of monocytes. | [45,49,50] |
| Bone | No | Yes | Yes | Facilitate osteogenesis and hematopoiesis through trafficking of cells, delivery of oxygen and nutrients, and angiocrine factors to mediate regeneration | [60,61] |
| Retina | Yes | No | Yes | Regulates oxygen and nutrient supply to photoreceptors and glial cells of the retina, which maintains visual perception | [64,65,66] |
| Kidney glomerulus | No | No | Yes | Facilitate water and small solute transport across the glomerular filtration barrier. | [73,75] |
| Kidney peritubular capillaries | Yes | No | Yes | Regulation of water, protein, and small molecule transport in the interstitium. | [76,77] |
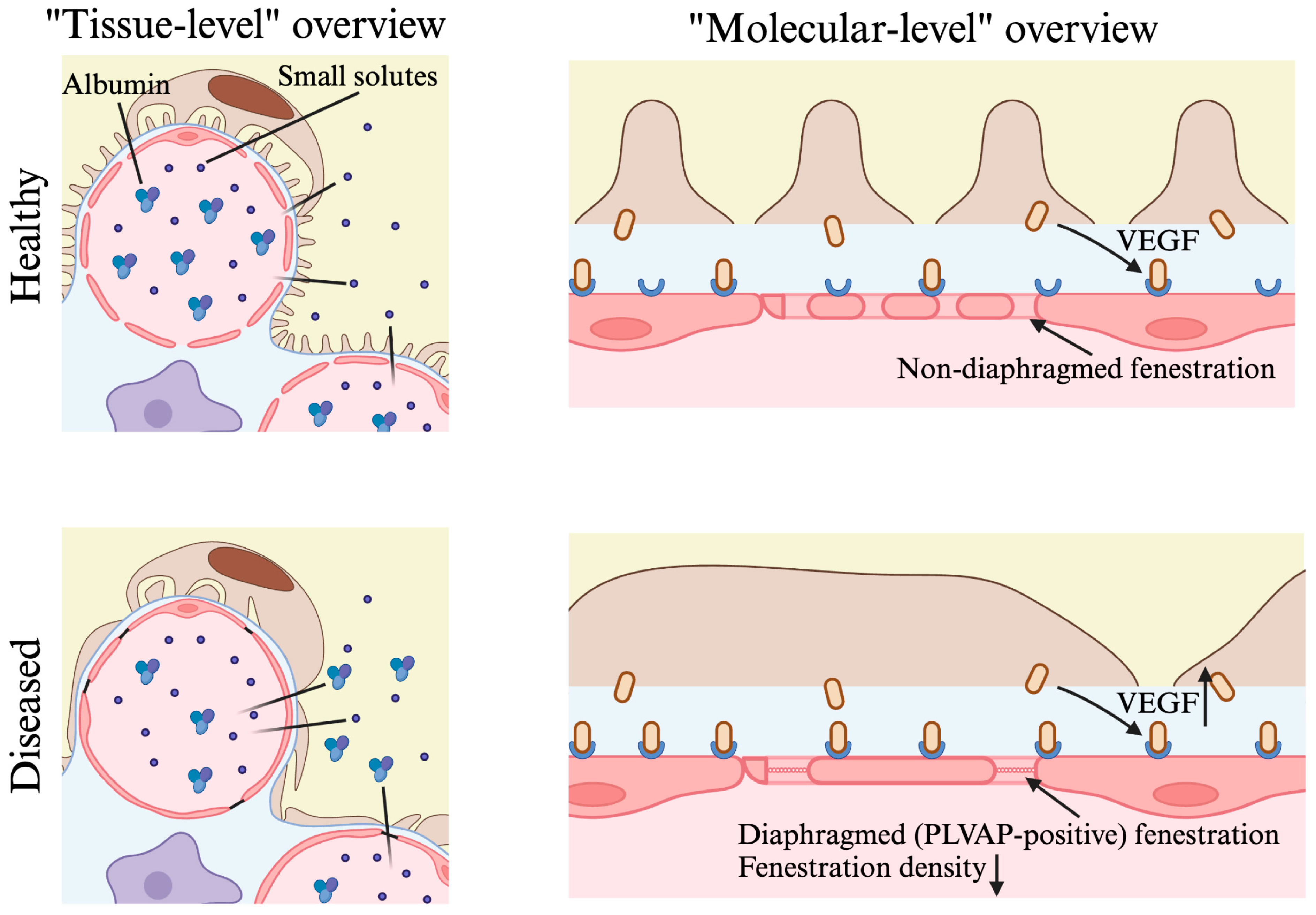
2.4. Implications for Kidney Disease
2.4.1. Diabetic Nephropathy
2.4.2. Focal Segmental Glomerulosclerosis
2.4.3. Alport Syndrome
2.4.4. Preeclampsia
3. Future Perspectives
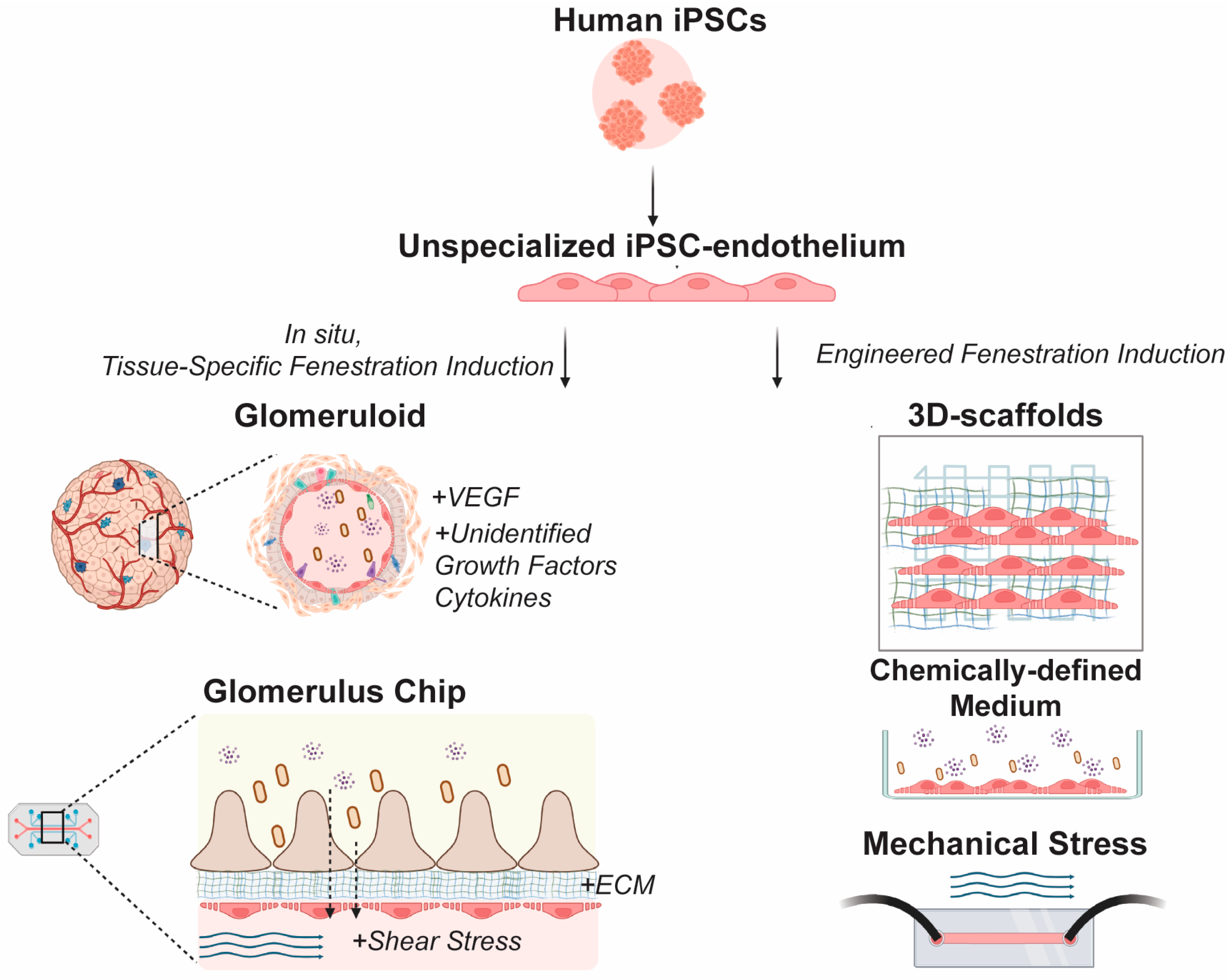
3.1. Leveraging Specialized EC Phenotypes for In Vitro Kidney Disease Modeling
3.2. Challenges in Modeling Kidney EC Fenestrations In Vitro
3.3. Emerging Microphysiological Models of Kidney EC Fenestrations
3.4. Towards hiPSC-Derived Models of Fenestrated Kidney ECs
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rafii, S.; Butler, J.M.; Ding, B.-S. Angiocrine functions of organ-specific endothelial cells. Nature 2016, 529, 316–325. [Google Scholar] [CrossRef]
- Marcu, R.; Choi, Y.J.; Xue, J.; Fortin, C.L.; Wang, Y.; Nagao, R.J.; Xu, J.; MacDonald, J.W.; Bammler, T.K.; Murry, C.E. Human organ-specific endothelial cell heterogeneity. IScience 2018, 4, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Kalucka, J.; de Rooij, L.P.; Goveia, J.; Rohlenova, K.; Dumas, S.J.; Meta, E.; Conchinha, N.V.; Taverna, F.; Teuwen, L.-A.; Veys, K. Single-cell transcriptome atlas of murine endothelial cells. Cell 2020, 180, 764–779. [Google Scholar] [CrossRef] [PubMed]
- Augustin, H.G.; Koh, G.Y. Organotypic vasculature: From descriptive heterogeneity to functional pathophysiology. Science 2017, 357, eaal2379. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Sancho, J.; Caparrós, E.; Fernández-Iglesias, A.; Francés, R. Role of liver sinusoidal endothelial cells in liver diseases. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 411–431. [Google Scholar] [CrossRef] [PubMed]
- Satchell, S.C.; Braet, F. Glomerular endothelial cell fenestrations: An integral component of the glomerular filtration barrier. Am. J. Physiol.-Ren. Physiol. 2009, 296, F947–F956. [Google Scholar] [CrossRef]
- Zapotoczny, B.; Braet, F.; Wisse, E.; Lekka, M.; Szymonski, M. Biophysical nanocharacterization of liver sinusoidal endothelial cells through atomic force microscopy. Biophys. Rev. 2020, 12, 625–636. [Google Scholar] [CrossRef]
- Finch, N.C.; Neal, C.R.; Welsh, G.I.; Foster, R.R.; Satchell, S.C. The unique structural and functional characteristics of glomerular endothelial cell fenestrations and their potential as a therapeutic target in kidney disease. Am. J. Physiol.-Ren. Physiol. 2023, 325, F465–F478. [Google Scholar] [CrossRef]
- Cogger, V.C.; Roessner, U.; Warren, A.; Fraser, R.; Le Couteur, D.G. A Sieve-Raft Hypothesis for the regulation of endothelial fenestrations. Comput. Struct. Biotechnol. J. 2013, 8, e201308003. [Google Scholar] [CrossRef][Green Version]
- Carloni, S.; Bertocchi, A.; Mancinelli, S.; Bellini, M.; Erreni, M.; Borreca, A.; Braga, D.; Giugliano, S.; Mozzarelli, A.M.; Manganaro, D. Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science 2021, 374, 439–448. [Google Scholar] [CrossRef]
- Stan, R.-V. Endothelial stomatal and fenestral diaphragms in normal vessels and angiogenesis. J. Cell. Mol. Med. 2007, 11, 621–643. [Google Scholar] [CrossRef] [PubMed]
- Hennigs, J.K.; Matuszcak, C.; Trepel, M.; Körbelin, J. Vascular endothelial cells: Heterogeneity and targeting approaches. Cells 2021, 10, 2712. [Google Scholar] [CrossRef] [PubMed]
- Urbanczyk, M.; Zbinden, A.; Schenke-Layland, K. Organ-specific endothelial cell heterogenicity and its impact on regenerative medicine and biomedical engineering applications. Adv. Drug Deliv. Rev. 2022, 186, 114323. [Google Scholar] [CrossRef]
- Pérez-Gutiérrez, L.; Ferrara, N. Biology and therapeutic targeting of vascular endothelial growth factor A. Nat. Rev. Mol. Cell Biol. 2023, 24, 816–834. [Google Scholar] [CrossRef]
- Esser, S.; Wolburg, K.; Wolburg, H.; Breier, G.; Kurzchalia, T.; Risau, W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J. Cell Biol. 1998, 140, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Breier, G.; Albrecht, U.; Sterrer, S.; Risau, W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development 1992, 114, 521–532. [Google Scholar] [CrossRef]
- Roberts, W.G.; Palade, G.E. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J. Cell Sci. 1995, 108, 2369–2379. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, J. PV1: Gatekeeper of endothelial permeability. Am. J. Respir. Cell Mol. Biol. 2020, 63, 413–414. [Google Scholar] [CrossRef]
- Strickland, L.A.; Jubb, A.M.; Hongo, J.A.; Zhong, F.; Burwick, J.; Fu, L.; Frantz, G.D.; Koeppen, H. Plasmalemmal vesicle-associated protein (PLVAP) is expressed by tumour endothelium and is upregulated by vascular endothelial growth factor-A (VEGF). J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2005, 206, 466–475. [Google Scholar] [CrossRef]
- Reeves, W.H.; Kanwar, Y.S.; Farquhar, M.G. Assembly of the glomerular filtration surface. Differentiation of anionic sites in glomerular capillaries of newborn rat kidney. J. Cell Biol. 1980, 85, 735–753. [Google Scholar] [CrossRef]
- Eriksson, A.; Cao, R.; Roy, J.; Tritsaris, K.; Wahlestedt, C.; Dissing, S.; Thyberg, J.; Cao, Y. Small GTP-binding protein Rac is an essential mediator of vascular endothelial growth factor-induced endothelial fenestrations and vascular permeability. Circulation 2003, 107, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Westwick, J.K.; Lambert, Q.T.; Clark, G.J.; Symons, M.; Van Aelst, L.; Pestell, R.G.; Der, C.J. Rac regulation of transformation, gene expression, and actin organization by multiple, PAK-independent pathways. Mol. Cell Biol. 1997, 17, 1324–1335. [Google Scholar] [CrossRef]
- Machesky, L.M.; Hall, A. Role of Actin Polymerization and Adhesion to Extracellular Matrix in Rac- and Rho-induced Cytoskeletal Reorganization. J. Cell Biol. 1997, 138, 913–926. [Google Scholar] [CrossRef]
- Nakakura, T.; Tanaka, H.; Suzuki, T. Regulation of fenestra formation via actin-dynamin2 interaction in rat pituitary endothelial cells. Cell Tissue Res. 2022, 390, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, K.; Stan, R.V.; Kurihara, H.; Sakai, T. Glomerular endothelial cells form diaphragms during development and pathologic conditions. J. Am. Soc. Nephrol. 2008, 19, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. The enhanced permeability and retention (EPR) effect: The significance of the concept and methods to enhance its application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.K. Chapter 9–Application Potential of Engineered Liposomes in Tumor Targeting. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, NL, USA, 2017; pp. 171–191. [Google Scholar]
- Roberts, W.G.; Palade, G.E. Neovasculature induced by vascular endothelial growth factor is fenestrated. Cancer Res. 1997, 57, 765–772. [Google Scholar]
- Cao, R.; Eriksson, A.; Kubo, H.; Alitalo, K.; Cao, Y.; Thyberg, J. Comparative evaluation of FGF-2–, VEGF-A–, and VEGF-C–induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ. Res. 2004, 94, 664–670. [Google Scholar] [CrossRef]
- Grunstein, J.; Roberts, W.G.; Mathieu-Costello, O.; Hanahan, D.; Johnson, R.S. Tumor-derived expression of vascular endothelial growth factor is a critical factor in tumor expansion and vascular function. Cancer Res. 1999, 59, 1592–1598. [Google Scholar] [PubMed]
- Sun, R.; Xiang, J.; Zhou, Q.; Piao, Y.; Tang, J.; Shao, S.; Zhou, Z.; Bae, Y.H.; Shen, Y. The tumor EPR effect for cancer drug delivery: Current status, limitations, and alternatives. Adv. Drug Deliv. Rev. 2022, 191, 114614. [Google Scholar] [CrossRef]
- Maeda, H. The 35th anniversary of the discovery of EPR effect: A new wave of nanomedicines for tumor-targeted drug delivery—Personal remarks and future prospects. J. Pers. Med. 2021, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Ghalehbandi, S.; Yuzugulen, J.; Pranjol, M.Z.I.; Pourgholami, M.H. The role of VEGF in cancer-induced angiogenesis and research progress of drugs targeting VEGF. Eur. J. Pharmacol. 2023, 949, 175586. [Google Scholar] [CrossRef] [PubMed]
- Hunt, N.J.; Lockwood, G.P.; Warren, A.; Mao, H.; McCourt, P.A.; Le Couteur, D.G.; Cogger, V.C. Manipulating fenestrations in young and old liver sinusoidal endothelial cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2019, 316, G144–G154. [Google Scholar] [CrossRef] [PubMed]
- Finch, N.C.; Fawaz, S.S.; Neal, C.R.; Butler, M.J.; Lee, V.K.; Salmon, A.J.; Lay, A.C.; Stevens, M.; Dayalan, L.; Band, H.; et al. Reduced Glomerular Filtration in Diabetes Is Attributable to Loss of Density and Increased Resistance of Glomerular Endothelial Cell Fenestrations. J. Am. Soc. Nephrol. 2022, 33, 1120–1136. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Javed, K.; Reddy, V.; Lui, F. Neuroanatomy, Choroid Plexus; StatPearls: St. Petersburg, FL, USA, 2019. [Google Scholar]
- Carrithers, M.D.; Visintin, I.; Viret, C.; Janeway, C.A., Jr. Role of genetic background in P selectin-dependent immune surveillance of the central nervous system. J. Neuroimmunol. 2002, 129, 51–57. [Google Scholar] [CrossRef]
- Parab, S.; Quick, R.E.; Matsuoka, R.L. Endothelial cell-type-specific molecular requirements for angiogenesis drive fenestrated vessel development in the brain. Elife 2021, 10, e64295. [Google Scholar] [CrossRef]
- Kamba, T.; Tam, B.Y.; Hashizume, H.; Haskell, A.; Sennino, B.; Mancuso, M.R.; Norberg, S.M.; O’Brien, S.M.; Davis, R.B.; Gowen, L.C. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am. J. Physiol.-Heart Circ. Physiol. 2006, 290, H560–H576. [Google Scholar] [CrossRef]
- Bernier-Latmani, J.; Mauri, C.; Marcone, R.; Renevey, F.; Durot, S.; He, L.; Vanlandewijck, M.; Maclachlan, C.; Davanture, S.; Zamboni, N. ADAMTS18+ villus tip telocytes maintain a polarized VEGFA signaling domain and fenestrations in nutrient-absorbing intestinal blood vessels. Nat. Commun. 2022, 13, 3983. [Google Scholar] [CrossRef]
- Carloni, S.; Rescigno, M. Unveiling the gut-brain axis: Structural and functional analogies between the gut and the choroid plexus vascular and immune barriers. Proc. Semin. Immunopathol. 2022, 44, 869–882. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Kaissi, S.; Homaidan, F.R.; Naim, H.Y.; El-Sabban, M.E. Cross-talk between intestinal epithelial cells and immune cells in inflammatory bowel disease. Sci. Rep. 2016, 6, 29783. [Google Scholar] [CrossRef]
- Spadoni, I.; Zagato, E.; Bertocchi, A.; Paolinelli, R.; Hot, E.; Di Sabatino, A.; Caprioli, F.; Bottiglieri, L.; Oldani, A.; Viale, G. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 2015, 350, 830–834. [Google Scholar] [CrossRef]
- Lorente, S.; Hautefeuille, M.; Sanchez-Cedillo, A. The liver, a functionalized vascular structure. Sci. Rep. 2020, 10, 16194. [Google Scholar] [CrossRef] [PubMed]
- Wisse, E. An electron microscopic study of the fenestrated endothelial lining of rat liver sinusoids. J. Ultrastruct. Res. 1970, 31, 125–150. [Google Scholar] [CrossRef] [PubMed]
- Szafranska, K.; Neuman, T.; Baster, Z.; Rajfur, Z.; Szelest, O.; Holte, C.; Kubisiak, A.; Kus, E.; Wolfson, D.L.; Chlopicki, S. From fixed-dried to wet-fixed to live–comparative super-resolution microscopy of liver sinusoidal endothelial cell fenestrations. Nanophotonics 2022, 11, 2253–2270. [Google Scholar] [CrossRef]
- Strauss, O.; Phillips, A.; Ruggiero, K.; Bartlett, A.; Dunbar, P.R. Immunofluorescence identifies distinct subsets of endothelial cells in the human liver. Sci. Rep. 2017, 7, 44356. [Google Scholar] [CrossRef]
- Bernardi, M.; Angeli, P.; Claria, J.; Moreau, R.; Gines, P.; Jalan, R.; Caraceni, P.; Fernandez, J.; Gerbes, A.L.; O’Brien, A.J. Albumin in decompensated cirrhosis: New concepts and perspectives. Gut 2020, 69, 1127–1138. [Google Scholar] [CrossRef]
- Shetty, S.; Lalor, P.F.; Adams, D.H. Liver sinusoidal endothelial cells—gatekeepers of hepatic immunity. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Liaskou, E.; Zimmermann, H.W.; Li, K.K.; Oo, Y.H.; Suresh, S.; Stamataki, Z.; Qureshi, O.; Lalor, P.F.; Shaw, J.; Syn, W.k. Monocyte subsets in human liver disease show distinct phenotypic and functional characteristics. Hepatology 2013, 57, 385–398. [Google Scholar] [CrossRef]
- Zimmermann, H.W.; Bruns, T.; Weston, C.J.; Curbishley, S.M.; Liaskou, E.; Li, K.K.; Resheq, Y.J.; Badenhorst, P.W.; Adams, D.H. Bidirectional transendothelial migration of monocytes across hepatic sinusoidal endothelium shapes monocyte differentiation and regulates the balance between immunity and tolerance in liver. Hepatology 2016, 63, 233–246. [Google Scholar] [CrossRef]
- Baiocchini, A.; Del Nonno, F.; Taibi, C.; Visco-Comandini, U.; D’Offizi, G.; Piacentini, M.; Falasca, L. Liver sinusoidal endothelial cells (LSECs) modifications in patients with chronic hepatitis C. Sci. Rep. 2019, 9, 8760. [Google Scholar] [CrossRef] [PubMed]
- Szafranska, K.; Kruse, L.D.; Holte, C.F.; McCourt, P.; Zapotoczny, B. The wHole story about fenestrations in LSEC. Front. Physiol. 2021, 12, 735573. [Google Scholar] [CrossRef] [PubMed]
- Hunt, N.J.; Lockwood, G.P.; Kang, S.W.; Pulpitel, T.; Clark, X.; Mao, H.; McCourt, P.A.; Cooney, G.J.; Wali, J.A.; Le Couteur, F.H. The effects of metformin on age-related changes in the liver sinusoidal endothelial cell. J. Gerontol. Ser. A 2020, 75, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Pi, X.; Xie, L.; Patterson, C. Emerging roles of vascular endothelium in metabolic homeostasis. Circ. Res. 2018, 123, 477–494. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Cogger, V.C.; Markus, A.M.; Harvey, P.J.; Yin, Z.-L.; Ansselin, A.D.; McLean, A.J. Pseudocapillarization and associated energy limitation in the aged rat liver. Hepatology 2001, 33, 537–543. [Google Scholar] [CrossRef]
- McLachlan, A.J.; Pont, L.G. Drug metabolism in older people—A key consideration in achieving optimal outcomes with medicines. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2012, 67, 175–180. [Google Scholar] [CrossRef]
- Hilmer, S.N.; Cogger, V.C.; Muller, M.; Le Couteur, D.G. The hepatic pharmacokinetics of doxorubicin and liposomal doxorubicin. Drug Metab. Dispos. 2004, 32, 794–799. [Google Scholar] [CrossRef]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef]
- Bixel, M.G.; Kusumbe, A.P.; Ramasamy, S.K.; Sivaraj, K.K.; Butz, S.; Vestweber, D.; Adams, R.H. Flow dynamics and HSPC homing in bone marrow microvessels. Cell Rep. 2017, 18, 1804–1816. [Google Scholar] [CrossRef]
- Heil, J.; Olsavszky, V.; Busch, K.; Klapproth, K.; de la Torre, C.; Sticht, C.; Sandorski, K.; Hoffmann, J.; Schönhaber, H.; Zierow, J. Bone marrow sinusoidal endothelium controls terminal erythroid differentiation and reticulocyte maturation. Nat. Commun. 2021, 12, 6963. [Google Scholar] [CrossRef]
- Rohde, D.; Vandoorne, K.; Lee, I.-H.; Grune, J.; Zhang, S.; McAlpine, C.S.; Schloss, M.J.; Nayar, R.; Courties, G.; Frodermann, V. Bone marrow endothelial dysfunction promotes myeloid cell expansion in cardiovascular disease. Nat. Cardiovasc. Res. 2022, 1, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; Humphries, P. The blood-retina barrier: Tight junctions and barrier modulation. In Biology and Regulation of Blood-Tissue Barriers; Springer: Berlin, Germany, 2013; pp. 70–84. [Google Scholar]
- Mulfaul, K.; Russell, J.F.; Voigt, A.P.; Stone, E.M.; Tucker, B.A.; Mullins, R.F. The essential role of the choriocapillaris in vision: Novel insights from imaging and molecular biology. Annu. Rev. Vis. Sci. 2022, 8, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Kim, S.J.; Choi, Y.A.; Yoon, H.-J.; Kim, A.; Lee, J. Retinal VEGFA maintains the ultrastructure and function of choriocapillaris by preserving the endothelial PLVAP. Biochem. Biophys. Res. Commun. 2020, 522, 240–246. [Google Scholar] [CrossRef]
- Iwagawa, T.; Saita, K.; Sagara, H.; Watanabe, S. Downregulation of VEGF in the retinal pigment epithelium followed by choriocapillaris atrophy after NaIO3 treatment in mice. Exp. Eye Res. 2023, 234, 109598. [Google Scholar] [CrossRef]
- Ida, H.; Ishibashi, K.; Reiser, K.; Hjelmeland, L.M.; Handa, J.T. Ultrastructural Aging of the RPE–Bruch’s Membrane–Choriocapillaris Complex in the d-Galactose–Treated Mouse. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Farber, G.; Hurtado, R.; Loh, S.; Monette, S.; Mtui, J.; Kopan, R.; Quaggin, S.; Meyer-Schwesinger, C.; Herzlinger, D.; Scott, R.P. Glomerular endothelial cell maturation depends on ADAM10, a key regulator of Notch signaling. Angiogenesis 2018, 21, 335–347. [Google Scholar] [CrossRef]
- Barry, D.M.; McMillan, E.A.; Kunar, B.; Lis, R.; Zhang, T.; Lu, T.; Daniel, E.; Yokoyama, M.; Gomez-Salinero, J.M.; Sureshbabu, A. Molecular determinants of nephron vascular specialization in the kidney. Nat. Commun. 2019, 10, 5705. [Google Scholar] [CrossRef]
- George, M.; Rainey, M.A.; Naramura, M.; Foster, K.W.; Holzapfel, M.S.; Willoughby, L.L.; Ying, G.; Goswami, R.M.; Gurumurthy, C.B.; Band, V. Renal thrombotic microangiopathy in mice with combined deletion of endocytic recycling regulators EHD3 and EHD4. PLoS ONE 2011, 6, e17838. [Google Scholar] [CrossRef]
- Tavasoli, M.; Al-Momany, A.; Wang, X.; Li, L.; Edwards, J.C.; Ballermann, B.J. Both CLIC4 and CLIC5A activate ERM proteins in glomerular endothelium. Am. J. Physiol.-Ren. Physiol. 2016, 311, F945–F957. [Google Scholar] [CrossRef]
- Satchell, S.C. The glomerular endothelium emerges as a key player in diabetic nephropathy. Kidney Int. 2012, 82, 949–951. [Google Scholar] [CrossRef]
- Haraldsson, B.; Nyström, J.; Deen, W.M. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol. Rev. 2008, 88, 451–487. [Google Scholar] [CrossRef]
- Menon, M.C.; Chuang, P.Y.; He, C.J. The glomerular filtration barrier: Components and crosstalk. Int. J. Nephrol. 2012, 2012, 749010. [Google Scholar] [CrossRef] [PubMed]
- Gaupp, C.; Schmid, B.; Tripal, P.; Edwards, A.; Daniel, C.; Zimmermann, S.; Goppelt-Struebe, M.; Willam, C.; Rosen, S.; Schley, G. Reconfiguration and loss of peritubular capillaries in chronic kidney disease. Sci. Rep. 2023, 13, 19660. [Google Scholar] [CrossRef] [PubMed]
- Kida, Y. Peritubular capillary rarefaction: An underappreciated regulator of CKD progression. Int. J. Mol. Sci. 2020, 21, 8255. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Rottoli, D.; Mahmoud, R.; Abbate, M.; Corna, D.; Cerullo, D.; Tomasoni, S.; Remuzzi, G.; Zoja, C.; Benigni, A.; et al. Endothelial Glycocalyx of Peritubular Capillaries in Experimental Diabetic Nephropathy: A Target of ACE Inhibitor-Induced Kidney Microvascular Protection. Int. J. Mol. Sci. 2023, 24, 16543. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, G.; Nagao, R.J.; Xue, J.; Choi, Y.J.; Xu, J.; Ren, S.; Aburatani, T.; Anderson, S.K.; MacDonald, J.W.; Bammler, T.K. A novel three–dimensional human peritubular microvascular system. J. Am. Soc. Nephrol. 2016, 27, 2370–2381. [Google Scholar] [CrossRef]
- Martínez-Salgado, C.; Sánchez-Juanes, F.; López-Hernández, F.J.; Muñoz-Félix, J.M. Endothelial activin receptor-like kinase 1 (ALK1) regulates myofibroblast emergence and peritubular capillary stability in the early stages of kidney fibrosis. Front. Pharmacol. 2022, 13, 843732. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, C.; Yang, Y.; Hu, X.; Ni, H.; Li, L.; Cheng, Z.; Huang, J.; Chen, P. Identifying key genes related to the peritubular capillary rarefaction in renal interstitial fibrosis by bioinformatics. Sci. Rep. 2023, 13, 19611. [Google Scholar] [CrossRef]
- Chen, Y.; Zee, J.; Janowczyk, A.R.; Rubin, J.; Toro, P.; Lafata, K.J.; Mariani, L.H.; Holzman, L.B.; Hodgin, J.B.; Madabhushi, A. Clinical relevance of computationally derived attributes of peritubular capillaries from kidney biopsies. Kidney360 2023, 4, 648–658. [Google Scholar] [CrossRef]
- Noronha, I.L.; Santa-Catharina, G.P.; Andrade, L.; Coelho, V.A.; Jacob-Filho, W.; Elias, R.M. Glomerular filtration in the aging population. Front. Med. 2022, 9, 769329. [Google Scholar] [CrossRef]
- Lv, J.-C.; Zhang, L.-X. Prevalence and disease burden of chronic kidney disease. In Renal Fibrosis: Mechanisms and Therapies; Springer: Berlin, Germany, 2019; pp. 3–15. [Google Scholar]
- Charles, C.; Ferris, A.H. Chronic kidney disease. Prim. Care Clin. Off. Pract. 2020, 47, 585–595. [Google Scholar] [CrossRef]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.-W. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Sun, Y.B.; Qu, X.; Zhang, X.; Caruana, G.; Bertram, J.F.; Li, J. Glomerular endothelial cell injury and damage precedes that of podocytes in adriamycin-induced nephropathy. PLoS ONE 2013, 8, e55027. [Google Scholar] [CrossRef]
- Sol, M.; Kamps, J.A.; van den Born, J.; van den Heuvel, M.C.; van der Vlag, J.; Krenning, G.; Hillebrands, J.-L. Glomerular endothelial cells as instigators of glomerular sclerotic diseases. Front. Pharmacol. 2020, 11, 573557. [Google Scholar] [CrossRef]
- Samsu, N. Diabetic nephropathy: Challenges in pathogenesis, diagnosis, and treatment. BioMed Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef] [PubMed]
- Selby, N.M.; Taal, M.W. An updated overview of diabetic nephropathy: Diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes. Metab. 2020, 22, 3–15. [Google Scholar] [CrossRef]
- Sagoo, M.K.; Gnudi, L. Diabetic nephropathy: An overview. In Diabetic Nephropathy: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2020; pp. 3–7. [Google Scholar]
- Davis, K.N.; Hines, A.E.; Schaefer, M.C.; Naseman, K.W. Protecting the kidneys: Update on therapies to treat diabetic nephropathy. Clin. Diabetes 2022, 40, 305–311. [Google Scholar] [CrossRef]
- van Bommel, E.J.; Muskiet, M.H.; Tonneijck, L.; Kramer, M.H.; Nieuwdorp, M.; van Raalte, D.H. SGLT2 inhibition in the diabetic kidney—From mechanisms to clinical outcome. Clin. J. Am. Soc. Nephrol. 2017, 12, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Zoja, C.; Conti, S.; Cerullo, D.; Corna, D.; Rottoli, D.; Zanchi, C.; Tomasoni, S.; Remuzzi, G.; Benigni, A. Empagliflozin protects glomerular endothelial cell architecture in experimental diabetes through the VEGF-A/caveolin-1/PV-1 signaling pathway. J. Pathol. 2022, 256, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, M.; Najafian, B.; Kim, Y.; Caramori, M.L.; Mauer, M. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes 2007, 56, 2155–2160. [Google Scholar] [CrossRef] [PubMed]
- Weil, E.J.; Lemley, K.V.; Mason, C.C.; Yee, B.; Jones, L.I.; Blouch, K.; Lovato, T.; Richardson, M.; Myers, B.D.; Nelson, R.G. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int. 2012, 82, 1010–1017. [Google Scholar] [CrossRef]
- Onions, K.L.; Gamez, M.; Buckner, N.R.; Baker, S.L.; Betteridge, K.B.; Desideri, S.; Dallyn, B.P.; Ramnath, R.D.; Neal, C.R.; Farmer, L.K.; et al. VEGFC Reduces Glomerular Albumin Permeability and Protects Against Alterations in VEGF Receptor Expression in Diabetic Nephropathy. Diabetes 2019, 68, 172–187. [Google Scholar] [CrossRef]
- Fogo, A.B. Causes and pathogenesis of focal segmental glomerulosclerosis. Nat. Rev. Nephrol. 2015, 11, 76–87. [Google Scholar] [CrossRef]
- Tampe, B.; Schridde, L.; Hakroush, S. MO087 Plasmalemmal Vesicle-Associated Protein-1 (Plvap) Indicates the Formation of Diaphragm-Bridged Fenestrations of Glomerular Endothelial Cells in Kidney Disease. Nephrol. Dial. Transplant. 2021, 36, gfab078-0023. [Google Scholar] [CrossRef]
- Morita, M.; Mii, A.; Yasuda, F.; Arakawa, Y.; Kashiwagi, T.; Shimizu, A. Diverse alterations of glomerular capillary networks in focal segmental glomerular sclerosis. Kidney Int. Rep. 2022, 7, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Lassén, E.; Daehn, I.S. Clues to Glomerular Cell Chatter in Focal Segmental Glomerulosclerosis: Via Endothelin-1/ETAR. Kidney Int. Rep. 2021, 6, 1758–1760. [Google Scholar] [CrossRef]
- Kruegel, J.; Rubel, D.; Gross, O. Alport syndrome—Insights from basic and clinical research. Nat. Rev. Nephrol. 2013, 9, 170–178. [Google Scholar] [CrossRef]
- Sedrakyan, S.; Villani, V.; Da Sacco, S.; Tripuraneni, N.; Porta, S.; Achena, A.; Lavarreda-Pearce, M.; Petrosyan, A.; Soloyan, H.; Filippo, R.D. Amniotic fluid stem cell-derived vesicles protect from VEGF-induced endothelial damage. Sci. Rep. 2017, 7, 16875. [Google Scholar] [CrossRef]
- Gross, O.; Weber, M.; Fries, J.W.; Müller, G.-A. Living donor kidney transplantation from relatives with mild urinary abnormalities in Alport syndrome: Long-term risk, benefit and outcome. Nephrol. Dial. Transplant. 2009, 24, 1626–1630. [Google Scholar] [CrossRef] [PubMed]
- Kashtan, C.E.; Ding, J.; Gregory, M.; Gross, O.; Heidet, L.; Knebelmann, B.; Rheault, M.; Licht, C. Clinical practice recommendations for the treatment of Alport syndrome: A statement of the Alport Syndrome Research Collaborative. Pediatr. Nephrol. 2013, 28, 5–11. [Google Scholar] [CrossRef]
- Akihisa, T.; Sato, M.; Wakayama, Y.; Taneda, S.; Horita, S.; Hirose, O.; Makabe, S.; Kataoka, H.; Mori, T.; Sohara, E.; et al. Glomerular Basement Membrane Protein Expression and the Diagnosis and Prognosis of Autosomal Dominant Alport Syndrome. Kidney Med. 2019, 1, 391–396. [Google Scholar] [CrossRef]
- Mat Tamizi, N.F.; Abdullah, S.; Ab Hamid, S.A.; Jummaat, F.; Asyikeen Wan Adnan, W.N. Preeclampsia severity and associated factors in Kelantan, Malaysia. Gulhane Med. J. 2024, 66, 17–22. [Google Scholar] [CrossRef]
- Lafayette, R.A.; Druzin, M.; Sibley, R.; Derby, G.; Malik, T.; Huie, P.; Polhemus, C.; Deen, W.M.; Myers, B.D. Nature of glomerular dysfunction in pre-eclampsia. Kidney Int. 1998, 54, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Pippias, M.; Skinner, L.; Noordzij, M.; Reisæter, A.V.; Abramowicz, D.; Stel, V.S.; Jager, K.J. Pregnancy after living kidney donation, a systematic review of the available evidence, and a review of the current guidance. Am. J. Transplant. 2022, 22, 2360–2380. [Google Scholar] [CrossRef] [PubMed]
- Dupont, V.; Berg, A.H.; Yamashita, M.; Huang, C.; Covarrubias, A.E.; Ali, S.; Stotland, A.; Van Eyk, J.E.; Jim, B.; Thadhani, R. Impaired renal reserve contributes to preeclampsia via the kynurenine and soluble fms–like tyrosine kinase 1 pathway. J. Clin. Investig. 2022, 132, e158346. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057. [Google Scholar] [CrossRef]
- Musah, S.; Bhattacharya, R.; Himmelfarb, J. Kidney Disease Modeling with Organoids and Organs-on-Chips. Annu. Rev. Biomed. Eng. 2024, 26, 383–414. [Google Scholar] [CrossRef]
- Rederer, A.; Rose, V.; Krüger, R.; Schmittutz, L.; Swierzy, I.; Fischer, L.; Thievessen, I.; Bauer, J.; Friedrich, O.; Schiffer, M.; et al. Partner, Neighbor, Housekeeper and Dimension: 3D versus 2D Glomerular Co-Cultures Reveal Drawbacks of Currently Used Cell Culture Models. Int. J. Mol. Sci. 2023, 24, 10384. [Google Scholar] [CrossRef]
- Maggiore, J.C.; LeGraw, R.; Przepiorski, A.; Velazquez, J.; Chaney, C.; Streeter, E.; Silva-Barbosa, A.; Franks, J.; Hislop, J.; Hill, A. Genetically engineering endothelial niche in human kidney organoids enables multilineage maturation, vascularization and de novo cell types. bioRxiv 2023. [Google Scholar] [CrossRef]
- Basagiannis, D.; Christoforidis, S. Constitutive endocytosis of VEGFR2 protects the receptor against shedding. J. Biol. Chem. 2016, 291, 16892–16903. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bove, A.M.; Simone, G.; Ma, B. Molecular bases of VEGFR-2-mediated physiological function and pathological role. Front. Cell Dev. Biol. 2020, 8, 599281. [Google Scholar] [CrossRef]
- Paeng, J.; Park, J.; Um, J.E.; Nam, B.Y.; Kang, H.-Y.; Kim, S.; Oh, H.J.; Park, J.T.; Han, S.H.; Ryu, D.-R. The locally activated renin-angiotensin system is involved in albumin permeability in glomerular endothelial cells under high glucose conditions. Nephrol. Dial. Transplant. 2017, 32, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Satchell, S.; Tasman, C.; Singh, A.; Ni, L.; Geelen, J.; Von Ruhland, C.; O’hare, M.; Saleem, M.; Van Den Heuvel, L.; Mathieson, P. Conditionally immortalized human glomerular endothelial cells expressing fenestrations in response to VEGF. Kidney Int. 2006, 69, 1633–1640. [Google Scholar] [CrossRef]
- Mujagic, E.; Gianni-Barrera, R.; Trani, M.; Patel, A.; Gürke, L.; Heberer, M.; Wolff, T.; Banfi, A. Induction of aberrant vascular growth, but not of normal angiogenesis, by cell-based expression of different doses of human and mouse VEGF is species-dependent. Hum. Gene Ther. Methods 2013, 24, 28–37. [Google Scholar] [CrossRef]
- Mou, X.; Shah, J.; Roye, Y.; Du, C.; Musah, S. An ultrathin membrane mediates tissue-specific morphogenesis and barrier function in a human kidney chip. Sci. Adv. 2024, 10, eadn2689. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Qian, J.-Y.; Cheng, H.; Li, X.-M. Effects of shear stress on differentiation of stem cells into endothelial cells. World J. Stem Cells 2021, 13, 894. [Google Scholar] [CrossRef] [PubMed]
- Bevan, H.S.; Slater, S.C.; Clarke, H.; Cahill, P.A.; Mathieson, P.W.; Welsh, G.I.; Satchell, S.C. Acute laminar shear stress reversibly increases human glomerular endothelial cell permeability via activation of endothelial nitric oxide synthase. Am. J. Physiol.-Ren. Physiol. 2011, 301, F733–F742. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Hosoyamada, Y.; Miyaki, T.; Yamaguchi, J.; Kakuta, S.; Sakai, T.; Ichimura, K. Three-dimensional architecture of glomerular endothelial cells revealed by FIB-SEM tomography. Front. Cell Dev. Biol. 2021, 9, 653472. [Google Scholar] [CrossRef]
- Xu, C.; Wu, X.; Hack, B.K.; Bao, L.; Cunningham, P.N. TNF causes changes in glomerular endothelial permeability and morphology through a Rho and myosin light chain kinase-dependent mechanism. Physiol. Rep. 2015, 3, e12636. [Google Scholar] [CrossRef]
- Xu, C.; Chang, A.; Hack, B.K.; Eadon, M.T.; Alper, S.L.; Cunningham, P.N. TNF-mediated damage to glomerular endothelium is an important determinant of acute kidney injury in sepsis. Kidney Int. 2014, 85, 72–81. [Google Scholar] [CrossRef]
- Zhang, T.; Lih, D.; Nagao, R.J.; Xue, J.; Berthier, E.; Himmelfarb, J.; Zheng, Y.; Theberge, A.B. Open microfluidic coculture reveals paracrine signaling from human kidney epithelial cells promotes kidney specificity of endothelial cells. Am. J. Physiol. -Ren. Physiol. 2020, 319, F41–F51. [Google Scholar] [CrossRef] [PubMed]
- Pajoumshariati, R.; Ewart, L.; Kujala, V.; Luc, R.; Peel, S.; Corrigan, A.; Weber, H.; Nugraha, B.; Hansen, P.B.; Williams, J. Physiological replication of the human glomerulus using a triple culture microphysiological system. Adv. Sci. 2023, 10, 2303131. [Google Scholar] [CrossRef] [PubMed]
- Radisic, M.; Loskill, P. Beyond PDMS and membranes: New materials for organ-on-a-chip devices. ACS Biomater. Sci. Eng. 2021, 7, 2861–2863. [Google Scholar] [CrossRef] [PubMed]
- Nagao, R.J.; Marcu, R.; Shin, Y.J.; Lih, D.; Xue, J.; Arang, N.; Wei, L.; Akilesh, S.; Kaushansky, A.; Himmelfarb, J. Cyclosporine induces fenestra-associated injury in human renal microvessels in vitro. ACS Biomater. Sci. Eng. 2021, 8, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Wattanapanitch, M.; Chailangkarn, T.; Miranda, H.C.; Muotri, A.R. Advances in iPSC technology for disease modeling and therapeutic applications. Front. Cell Dev. Biol. 2023, 11, 1261279. [Google Scholar] [CrossRef]
- Meijer, E.; van Dijk, C.; Giles, R.; Gijsen, K.; Chrifi, I.; Verhaar, M.; Cheng, C. Induction of Fenestrae in hiPSC-Derived Endothelial Cells for Disease Modeling. Tissue Eng. Part. A 2023, 30, 168–180. [Google Scholar] [CrossRef]
- Ciampi, O.; Bonandrini, B.; Derosas, M.; Conti, S.; Rizzo, P.; Benedetti, V.; Figliuzzi, M.; Remuzzi, A.; Benigni, A.; Remuzzi, G. Engineering the vasculature of decellularized rat kidney scaffolds using human induced pluripotent stem cell-derived endothelial cells. Sci. Rep. 2019, 9, 8001. [Google Scholar] [CrossRef]
- van den Berg, C.W.; Ritsma, L.; Avramut, M.C.; Wiersma, L.E.; van den Berg, B.M.; Leuning, D.G.; Lievers, E.; Koning, M.; Vanslambrouck, J.M.; Koster, A.J. Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Rep. 2018, 10, 751–765. [Google Scholar] [CrossRef]
- McMahon, A.P. Development of the mammalian kidney. Curr. Top. Dev. Biol. 2016, 117, 31–64. [Google Scholar]
- Wu, T.-C.; Chang, C.-C.; Leu, H.-B.; Huang, P.-H.; Lin, S.-J.; Chen, J.-W. Phorbol ester-induced angiogenesis of endothelial progenitor cells: The role of NADPH oxidase-mediated, redox-related matrix metalloproteinase pathways. PLoS ONE 2019, 14, e0209426. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.V.; Gkouzioti, V.; Maass, C.; Verhaar, M.C.; Vernooij, R.W.; van Balkom, B.W. A systematic review of kidney-on-a-chip-based models to study human renal (patho-) physiology. Dis. Models Mech. 2023, 16, dmm050113. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; An, F.; Luo, Y.; Lu, Y.; Liu, T.; Zhao, W.; Lin, B. A nephron model for study of drug-induced acute kidney injury and assessment of drug-induced nephrotoxicity. Biomaterials 2018, 155, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Mahler, G.J. Modelling renal filtration and reabsorption processes in a human glomerulus and proximal tubule microphysiological system. Micromachines 2021, 12, 983. [Google Scholar] [CrossRef]
- Lee, H.N.; Choi, Y.Y.; Kim, J.W.; Lee, Y.S.; Choi, J.W.; Kang, T.; Kim, Y.K.; Chung, B.G. Effect of biochemical and biomechanical factors on vascularization of kidney organoid-on-a-chip. Nano Converg. 2021, 8, 35. [Google Scholar] [CrossRef]
- Homan, K.A.; Gupta, N.; Kroll, K.T.; Kolesky, D.B.; Skylar-Scott, M.; Miyoshi, T.; Mau, D.; Valerius, M.T.; Ferrante, T.; Bonventre, J.V. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 2019, 16, 255–262. [Google Scholar] [CrossRef]
- Chen, S.J.; Lv, L.L.; Liu, B.C.; Tang, R.N. Crosstalk between tubular epithelial cells and glomerular endothelial cells in diabetic kidney disease. Cell Prolif. 2020, 53, e12763. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mou, X.; Leeman, S.M.; Roye, Y.; Miller, C.; Musah, S. Fenestrated Endothelial Cells across Organs: Insights into Kidney Function and Disease. Int. J. Mol. Sci. 2024, 25, 9107. https://doi.org/10.3390/ijms25169107
Mou X, Leeman SM, Roye Y, Miller C, Musah S. Fenestrated Endothelial Cells across Organs: Insights into Kidney Function and Disease. International Journal of Molecular Sciences. 2024; 25(16):9107. https://doi.org/10.3390/ijms25169107
Chicago/Turabian StyleMou, Xingrui, Sophia M. Leeman, Yasmin Roye, Carmen Miller, and Samira Musah. 2024. "Fenestrated Endothelial Cells across Organs: Insights into Kidney Function and Disease" International Journal of Molecular Sciences 25, no. 16: 9107. https://doi.org/10.3390/ijms25169107
APA StyleMou, X., Leeman, S. M., Roye, Y., Miller, C., & Musah, S. (2024). Fenestrated Endothelial Cells across Organs: Insights into Kidney Function and Disease. International Journal of Molecular Sciences, 25(16), 9107. https://doi.org/10.3390/ijms25169107






