Relation of T Cell Profile with Vitamin D Receptor and Vitamin D-Binding Protein Gene Polymorphisms in Atopy
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Studied Subject
2.2. Distribution of Single Nucleotide Polymorphisms of VDR and GC Genes
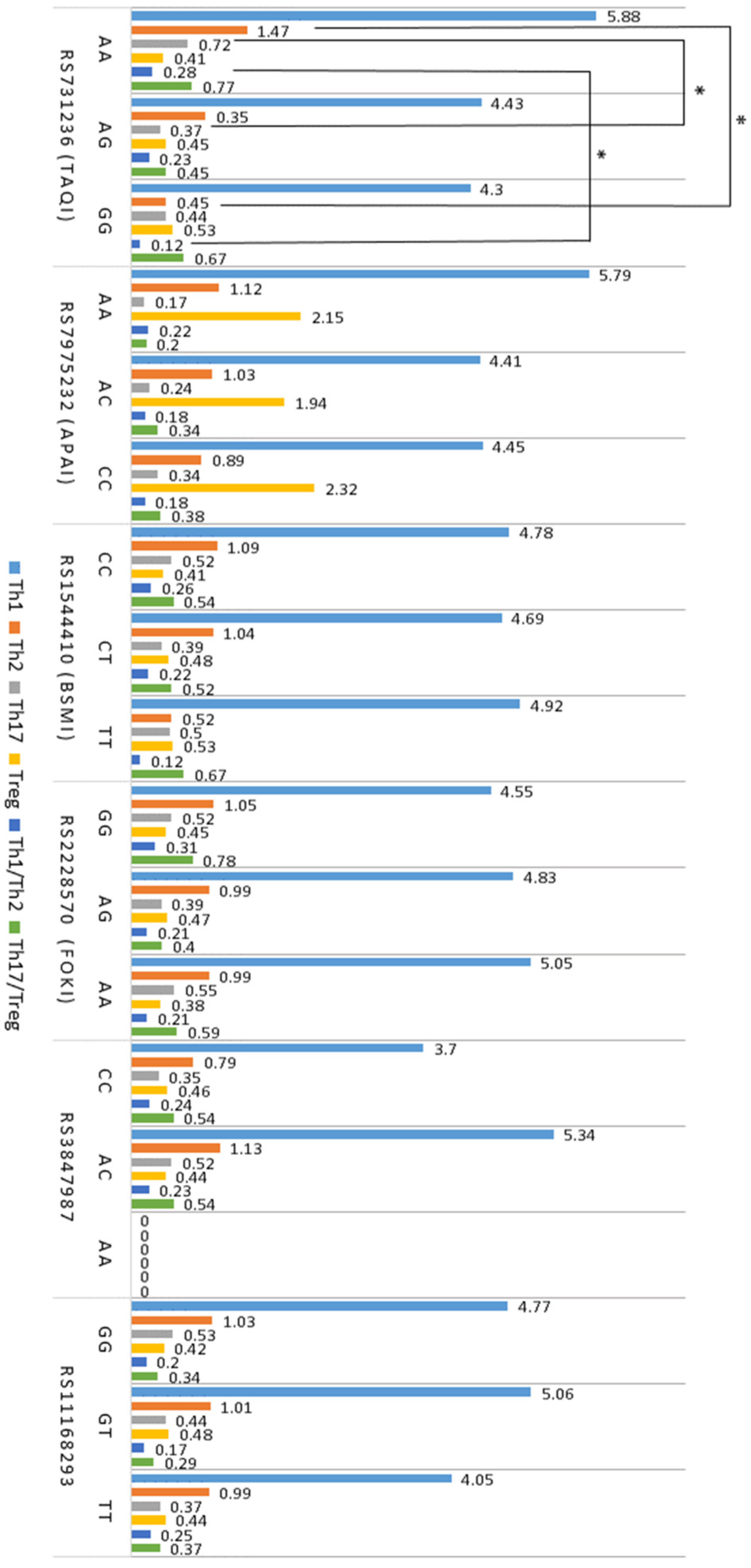
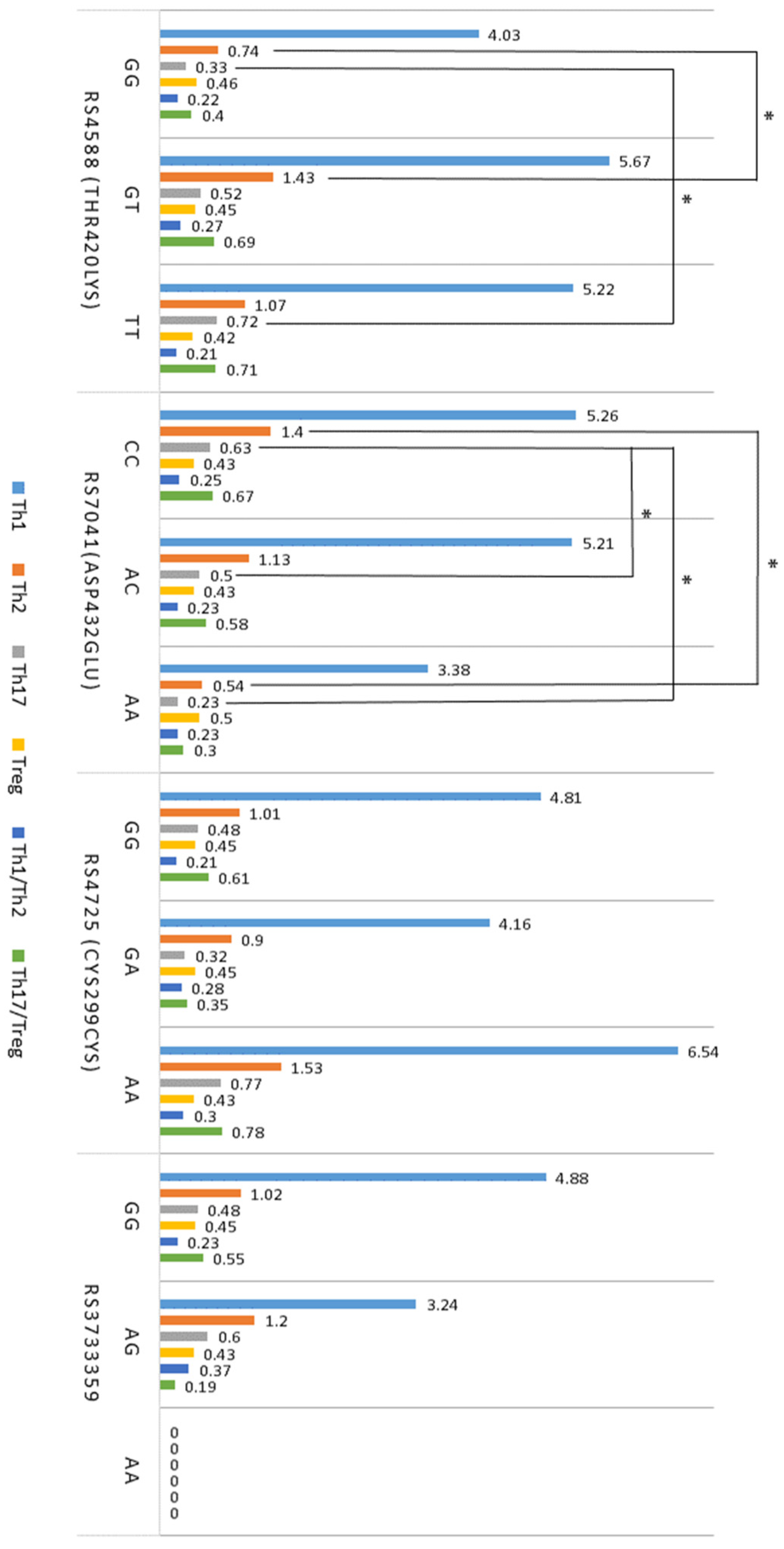
2.3. T Cell Profiles
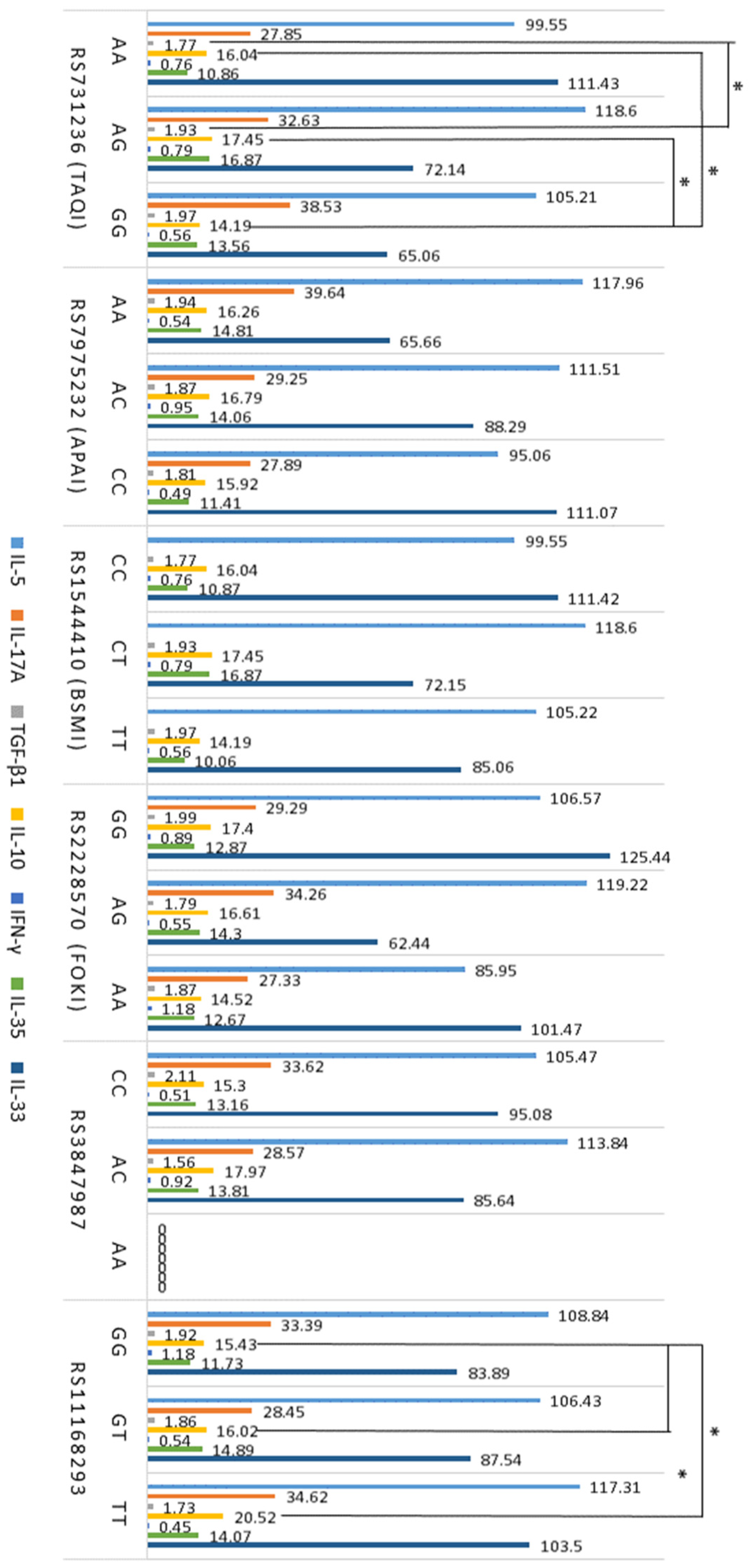
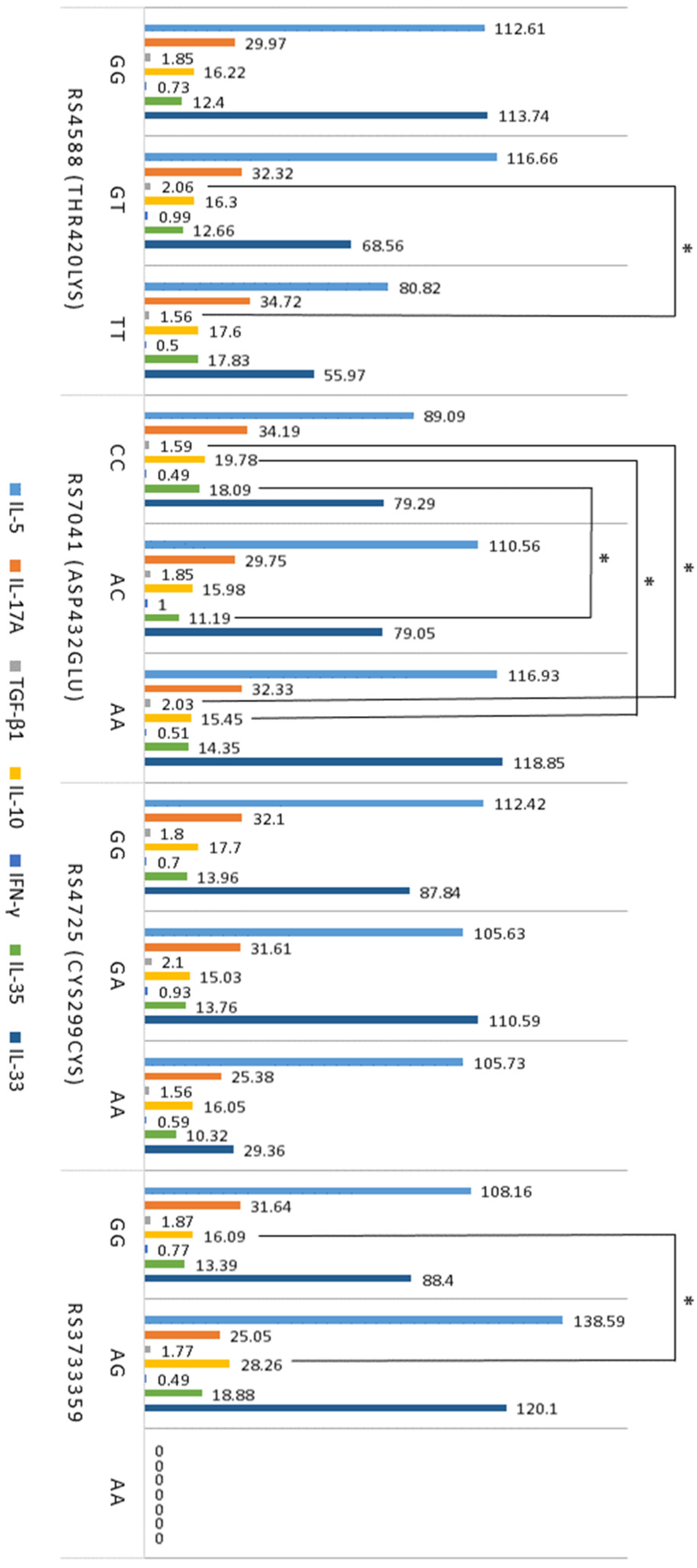
2.4. Levels of Pro-Inflammatory (IL-5, IL-17A, IL-33) and Anti-Inflammatory (TGF-β1, IL-10, IFN-γ, IL-35) Serum Cytokines
2.5. Association between T Cells, Cytokines, VDR, and GC SNPs in Atopy
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Sample Collection and Storage
4.3. Measurements of Total IgE, and Vitamin D
4.4. Evaluation of VDR and GC Gene Single Nucleotide Polymorphisms
4.5. Analysis of Peripheral Blood Cells and T Cell Profile
4.6. Measurements of Serum Cytokines
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CD4+ T cells | CD4 positive cells (T helper cells) |
| CYP11A1 | The cholesterol side-chain cleavage enzyme, known as cytochrome P450 side-chain cleavage enzyme |
| ELISA | Enzyme-linked immunosorbent assay |
| GC | Vitamin D-binding proteine (VDBP) |
| GINA | Global Initiative for Asthma |
| IFN-γ | Interferon-γ |
| IgE | Total immunoglobulin E |
| IL | Interleukin |
| PBMCs | Peripheral blood mononuclear cells |
| SEM | Standard error of mean |
| SNP | Single nucleotide polymorphism |
| TGF-β1 | Transforming growth factor-β1 |
| Th | T helper cells |
| Treg | T regulatory cells |
| VDR | Vitamin D receptor |
References
- Fyhrquist, N.; Werfel, T.; Bilò, M.B.; Mülleneisen, N.; Gerth van Wijk, R. The roadmap for the Allergology specialty and allergy care in Europe and adjacent countries. An EAACI position paper. Clin. Transl. Allergy 2019, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Dierick, B.J.H.; van der Molen, T.; Flokstra-de Blok, B.M.J.; Muraro, A.; Postma, M.J.; Kocks, J.W.H.; van Boven, J.F.M. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev. Pharmacoecon. Outcomes Res. 2020, 20, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Cosmi, L.; Maggi, L.; Mazzoni, A.; Liotta, F.; Annunziato, F. Biologicals targeting type 2 immunity: Lessons learned from asthma, chronic urticaria and atopic dermatitis. Eur. J. Immunol. 2019, 49, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Thiriou, D.; Morianos, I.; Xanthou, G.; Samitas, K. Innate immunity as the orchestrator of allergic airway inflammation and resolution in asthma. Int. Immunopharmacol. 2017, 48, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Haarala, A.K.; Sinikumpu, S.-P.; Jokelainen, J.; Pekkanen, J.; Huilaja, L. Associative factors for atopic dermatitis and other atopic diseases in middle-aged adults: A population-based birth cohort study among 5373 subjects. Health Sci. Rep. 2023, 6, e1015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gang, X.; Yang, S.; Cui, M.; Sun, L.; Li, Z.; Wang, G. The Alterations in and the Role of the Th17/Treg Balance in Metabolic Diseases. Front. Immunol. 2021, 12, 678355. [Google Scholar] [CrossRef] [PubMed]
- Agua-Doce, A.; Graca, L. Regulatory T Cells and the Control of the Allergic Response. J. Allergy 2012, 2012, e948901. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Domvri, K.; Porpodis, K.; Tzimagiorgis, G.; Chatzopoulou, F.; Kontakiotis, T.; Kyriazis, G.; Papakosta, D. Th2/Th17 cytokine profile in phenotyped Greek asthmatics and relationship to biomarkers of inflammation. Respir. Med. 2019, 151, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Jenmalm, M.C.; Van Snick, J.; Cormont, F.; Salman, B. Allergen-induced Th1 and Th2 cytokine secretion in relation to specific allergen sensitization and atopic symptoms in children. Clin. Exp. Allergy 2001, 31, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Chen, Z.; Zhang, T.; Feng, D.; Li, H.; Yang, H. Th17/Treg homeostasis, but not Th1/Th2 homeostasis, is implicated in exacerbation of human bronchial asthma. Ther. Clin. Risk Manag. 2018, 14, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Tamasauskiene, L.; Golubickaite, I.; Ugenskiene, R.; Sjakste, N.; Paramonova, N.; Wu, L.S.-H.; Wang, L.S.-J.-Y.; Sitkauskiene, B. Vitamin D receptor gene polymorphisms in atopy. Immun. Inflamm. Dis. 2021, 9, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.-J.; Gysemans, C.; Verstuyf, A.; Mathieu, C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.-K.; Janjetovic, Z.; Slominski, R.M.; Li, W.; Jetten, A.M.; Indra, A.K.; Mason, R.S.; Tuckey, R.C. Biological Effects of CYP11A1-Derived Vitamin D and Lumisterol Metabolites in the Skin. J. Investig. Dermatol. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Agliardi, C.; Guerini, F.R.; Bolognesi, E.; Zanzottera, M.; Clerici, M. VDR Gene Single Nucleotide Polymorphisms and Autoimmunity: A Narrative Review. Biology 2023, 12, 916. [Google Scholar] [CrossRef] [PubMed]
- Iordanidou, M.; Paraskakis, E.; Giasari, G.; Manolopoulos, V.; Chatzimichael, A. VDR and VDBP polymorphisms are associated with 25(OH)D3 levels in asthmatic children. Eur. Respir. J. 2014, 44, 4211. [Google Scholar]
- Chang, S.H.; Chung, Y.; Dong, C. Vitamin D Suppresses Th17 Cytokine Production by Inducing C/EBP Homologous Protein (CHOP) Expression. J. Biol. Chem. 2010, 285, 38751–38755. [Google Scholar] [CrossRef] [PubMed]
- Urry, Z.; Xystrakis, E.; Richards, D.F.; McDonald, J.; Sattar, Z.; Cousins, D.J.; Corrigan, C.J.; Hickman, E.; Brown, Z.; Hawrylowicz, C.M. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1alpha,25-dihydroxyvitamin D3 abrogates regulatory function. J. Clin. Investig. 2009, 119, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Muthian, G.; Raikwar, H.P.; Rajasingh, J.; Bright, J.J. 1,25 Dihydroxyvitamin-D3 modulates JAK-STAT pathway in IL-12/IFNgamma axis leading to Th1 response in experimental allergic encephalomyelitis. J. Neurosci. Res. 2006, 83, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Batmaz, S.B.; Arıkoğlu, T.; Tamer, L.; Eskandari, G.; Kuyucu, S. Seasonal variation of asthma control, lung function tests and allergic inflammation in relation to vitamin D levels: A prospective annual study. Postep. Dermatol. Alergol. 2018, 35, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, W.; Yao, J.; Cheng, H.; Sun, X.; Li, L. Correlation of Blood FoxP3+ Regulatory T Cells and Disease Activity of Atopic Dermatitis. J. Immunol. Res. 2019, 2019, 1820182. [Google Scholar] [CrossRef] [PubMed]
- Wong, Q.Y.A.; Lim, J.J.; Ng, J.Y.; Malipeddi, P.; Lim, Y.Y.E.; Sio, Y.Y.; Chew, F.T. An updated prevalence of asthma, its phenotypes, and the identification of the potential asthma risk factors among young Chinese adults recruited in Singapore. World Allergy Organ. J. 2023, 16, 100757. [Google Scholar] [CrossRef]
- Tokura, Y.; Phadungsaksawasdi, P.; Ito, T. Atopic dermatitis as Th2 disease revisited. J. Cutan. Immunol. Allergy 2018, 1, 158–164. [Google Scholar] [CrossRef]
- Harker, J.A.; Lloyd, C.M. T helper 2 cells in asthma. J. Exp. Med. 2023, 220, e20221094. [Google Scholar] [CrossRef]
- Gurram, R.K.; Zhu, J. Orchestration between ILC2s and Th2 cells in shaping type 2 immune responses. Cell. Mol. Immunol. 2019, 16, 225–235. [Google Scholar] [CrossRef]
- Vercelli, D.; Jabara, H.H.; Lauener, R.P.; Geha, R.S. IL-4 inhibits the synthesis of IFN-gamma and induces the synthesis of IgE in human mixed lymphocyte cultures. J. Immunol. 1990, 144, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Boguniewicz, M.; Jaffe, H.S.; Izu, A.; Sullivan, M.J.; York, D.; Geha, R.S.; Leung, D.Y. Recombinant gamma interferon in treatment of patients with atopic dermatitis and elevated IgE levels. Am. J. Med. 1990, 88, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, U.; Kukel, S.; Brzoska, J.; Kreysel, H.W. Systemic interferon gamma treatment in severe atopic dermatitis. J. Am. Acad. Dermatol. 1993, 29, 58–63. [Google Scholar] [CrossRef]
- Noval Rivas, M.; Chatila, T.A. Regulatory T cells in allergic diseases. J. Allergy Clin. Immunol. 2016, 138, 639–652. [Google Scholar] [CrossRef]
- Abdel-Gadir, A.; Massoud, A.H.; Chatila, T.A. Antigen-specific Treg cells in immunological tolerance: Implications for allergic diseases. F1000Research 2018, 7, 38. [Google Scholar] [CrossRef]
- Moaaz, M.; Youssry, S.; Elfatatry, A.; El Rahman, M.A. Th17/Treg cells imbalance and their related cytokines (IL-17, IL-10 and TGF-β) in children with autism spectrum disorder. J. Neuroimmunol. 2019, 337, 577071. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Wan, Y.Y. Intricacies of TGF-β signaling in Treg and Th17 cell biology. Cell. Mol. Immunol. 2023, 20, 1002–1022. [Google Scholar] [CrossRef]
- Weissler, K.A.; Frischmeyer-Guerrerio, P.A. Genetic evidence for the role of transforming growth factor-β in atopic phenotypes. Curr. Opin. Immunol. 2019, 60, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Shi, G.; Wan, H.; Jiang, L.; Ai, X.; Zhu, H.; Tang, W.; Ma, J.; Jin, X.; Zhang, B. Coexistence of Th1/Th2 and Th17/Treg imbalances in patients with allergic asthma. Chin. Med. J. 2011, 124, 1951–1956. [Google Scholar]
- Slominski, A.T.; Kim, T.-K.; Takeda, Y.; Janjetovic, Z.; Brozyna, A.A.; Skobowiat, C.; Wang, J.; Postlethwaite, A.; Li, W.; Tuckey, R.C.; et al. RORα and ROR γ are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014, 28, 2775–2789. [Google Scholar] [CrossRef]
- Zhao, R.; Zhang, W.; Ma, C.; Zhao, Y.; Xiong, R.; Wang, H.; Chen, W.; Zheng, S.G. Immunomodulatory Function of Vitamin D and Its Role in Autoimmune Thyroid Disease. Front. Immunol. 2021, 12, 574967. [Google Scholar] [CrossRef]
- Wei, R.; Christakos, S. Mechanisms Underlying the Regulation of Innate and Adaptive Immunity by Vitamin D. Nutrients 2015, 7, 8251–8260. [Google Scholar] [CrossRef]
- Slominski, A.T.; Li, W.; Kim, T.-K.; Semak, I.; Wang, J.; Zjawiony, J.K.; Tuckey, R.C. Novel activities of CYP11A1 and their potential physiological significance. J. Steroid. Biochem. Mol. Biol. 2015, 151, 25–37. [Google Scholar] [CrossRef]
- Mailhot, G.; White, J.H. Vitamin D and Immunity in Infants and Children. Nutrients 2020, 12, 1233. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Kim, S.H.; Lee, N.; Lee, W.-W.; Hwang, K.-A.; Shin, M.S.; Lee, S.-H.; Kim, W.-U.; Kang, I. 1,25-Dihyroxyvitamin D3 Promotes FOXP3 Expression via Binding to Vitamin D Response Elements in Its Conserved Noncoding Sequence Region. J. Immunol. 2012, 188, 5276–5282. [Google Scholar] [CrossRef]
- Kuti, B.P.; Kuti, D.K. Relationship between serum 25-hydroxyvitamin D and inflammatory cytokines in Nigerian children with asthma. J. Asthma 2021, 58, 604–613. [Google Scholar] [CrossRef]
- Looman, K.I.M.; Jansen, M.A.E.; Voortman, T.; van den Heuvel, D.; Jaddoe, V.W.V.; Franco, O.H.; van Zelm, M.C.; Moll, H.A. The role of vitamin D on circulating memory T cells in children: The Generation R study. Pediatr. Allergy Immunol. 2017, 28, 579–587. [Google Scholar] [CrossRef]
- Hutchinson, K.; Kerley, C.P.; Faul, J.; Greally, P.; Coghlan, D.; Louw, M.; Elnazir, B.; Rochev, Y. Vitamin D receptor variants and uncontrolled asthma. Eur. Ann. Allergy Clin. Immunol. 2018, 50, 108–116. [Google Scholar] [CrossRef]
- Bastyte, D.; Tamasauskiene, L.; Stakaitiene, I.; Ugenskiene, R.; Gradauskiene (Sitkauskiene), B. The Association of Vitamin D Receptor Gene Polymorphisms with Vitamin D, Total IgE, and Blood Eosinophils in Patients with Atopy. Biomolecules 2024, 14, 212. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Arora, J. Two lineages of immune cells that differentially express the vitamin D receptor. J. Steroid. Biochem. Mol. Biol. 2023, 228, 106253. [Google Scholar] [CrossRef]
- Arora, J.; Wang, J.; Weaver, V.; Zhang, Y.; Cantorna, M.T. Novel insight into the role of the vitamin D receptor in the development and function of the immune system. J. Steroid. Biochem. Mol. Biol. 2022, 219, 106084. [Google Scholar] [CrossRef]
- Mehrani, Y.; Morovati, S.; Tieu, S.; Karimi, N.; Javadi, H.; Vanderkamp, S.; Sarmadi, S.; Tajik, T.; Kakish, J.E.; Bridle, B.W.; et al. Vitamin D Influences the Activity of Mast Cells in Allergic Manifestations and Potentiates Their Effector Functions against Pathogens. Cells 2023, 12, 2271. [Google Scholar] [CrossRef] [PubMed]
- Bendix, M.; Dige, A.; Deleuran, B.; Dahlerup, J.F.; Jørgensen, S.P.; Bartels, L.E.; Husted, L.B.; Harsløf, T.; Langdahl, B.; Agnholt, J. Flow cytometry detection of vitamin D receptor changes during vitamin D treatment in Crohn’s disease. Clin. Exp. Immunol. 2015, 181, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Kongsbak, M.; von Essen, M.R.; Boding, L.; Levring, T.B.; Schjerling, P.; Lauritsen, J.P.H.; Woetmann, A.; Ødum, N.; Bonefeld, C.M.; Geisler, C. Vitamin D up-regulates the vitamin D receptor by protecting it from proteasomal degradation in human CD4+ T cells. PLoS ONE 2014, 9, e96695. [Google Scholar] [CrossRef]
- Jeffery, L.E.; Wood, A.M.; Qureshi, O.S.; Hou, T.Z.; Gardner, D.; Briggs, Z.; Kaur, S.; Raza, K.; Sansom, D.M. Availability of 25-hydroxyvitamin D(3) to APCs controls the balance between regulatory and inflammatory T cell responses. J. Immunol. 2012, 189, 5155–5164. [Google Scholar] [CrossRef]
- Chun, R.F.; Lauridsen, A.L.; Suon, L.; Zella, L.A.; Pike, J.W.; Modlin, R.L.; Martineau, A.R.; Wilkinson, R.J.; Adams, J.; Hewison, M. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J. Clin. Endocrinol. Metab. 2010, 95, 3368–3376. [Google Scholar] [CrossRef]
- Kato, S. The function of vitamin D receptor in vitamin D action. J. Biochem. 2000, 127, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Front. Endocrinol. 2020, 10, 910. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.G. Plasma vitamin D-binding protein (Gc-globulin): Multiple tasks. J. Steroid. Biochem. Mol. Biol. 1995, 53, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Böhme, M.; Svensson, Å.; Kull, I.; Wahlgren, C.-F. Hanifin’s and Rajka’s minor criteria for atopic dermatitis: Which do 2-year-olds exhibit? J. Am. Acad. Dermatol. 2000, 43, 785–792. [Google Scholar] [CrossRef]
- Levy, M.L.; Bacharier, L.B.; Bateman, E.; Boulet, L.-P.; Brightling, C.; Buhl, R.; Brusselle, G.; Cruz, A.A.; Drazen, J.M.; Duijts, L.; et al. Key recommendations for primary care from the 2022 Global Initiative for Asthma (GINA) update. NPJ Prim. Care Respir. Med. 2023, 33, 7. [Google Scholar] [CrossRef] [PubMed]
| Atopic Dermatitis (n = 100) | Allergic Asthma (n = 85) | Control Group (n = 81) | |
|---|---|---|---|
| Male/female, n | 31/69 | 30/54 | 32/49 |
| Age (years) | 29.51 ± 1 | 40.36 ± 1.5 | 35.57 ± 1.45 |
| Total IgE, kU/L | 919.83 ± 213 ** | 692.72 ± 170 * | 24.99 ± 5.7 |
| Blood eosinophils × 109/L | 0.40 ± 0.04 ** | 0.33 ± 0.06 ** | 0.06 ± 0.01 |
| Vitamin D level, ng/mL | 24.11 ± 0.94 *ʃ | 18.37 ± 0.83 ** | 27.23 ± 1.21 |
| Gene | SNP | Group | Genotype Frequency (%) | MAF | ||
|---|---|---|---|---|---|---|
| VDR | rs731236 (TaqI) A > G | AA | AG | GG | G | |
| Atopy | 33.1 | 55.2 | 11.6 | 39.2 | ||
| Control | 26.0 | 62.3 | 11.7 | 42.8 | ||
| rs7975232 (ApaI) A > C | AA | AC | CC | C | ||
| Atopy | 26.5 | 48.6 | 24.9 | 49.2 | ||
| Control | 25.9 | 46.9 | 27.2 | 49.4 | ||
| rs1544410 (BsmI) C > T | CC | TC | TT | T | ||
| Atopy | 42.2 | 45.9 | 11.9 | 34.9 | ||
| Control | 39.2 | 48.1 | 12.7 | 36.7 | ||
| rs2228570 (FokI) G > A | GG | GA | AA | A | ||
| Atopy | 31.4 | 50.3 | 18.4 | 43.4 | ||
| Control | 27.2 | 53.1 | 19.8 | 46.2 | ||
| rs3847987 C > A | CC | CA | AA | A | ||
| Atopy | 61.1 | 36.2 | 2.7 | 20.8 | ||
| Control | 55.6 | 43.2 | 1.2 | 22.6 | ||
| rs11168293 G > T | GG | GT | TT | T | ||
| Atopy | 42.7 | 40.5 | 16.8 | 37.1 | ||
| Control | 42.0 | 43.2 | 14.8 | 36.4 | ||
| GC | rs4588 (Thr420Lys) G > T | GG | GT | TT | T | |
| Atopy | 52.7 | 33.5 | 13.7 * | 30.5 | ||
| Control | 53.2 | 43.0 | 3.8 | 25.3 | ||
| rs7041(Asp432Glu) C > A | CC | AC | AA | A | ||
| Atopy | 35.0 | 48.3 | 16.7 | 40.9 | ||
| Control | 31.3 | 58.8 | 10.0 | 39.4 | ||
| rs4725 (Cys299Cys) G > A | GG | GA | AA | A | ||
| Atopy | 45.0 | 49.7 | 5.3 | 30.2 | ||
| Control | 38.2 | 59.2 | 2.6 | 32.2 | ||
| rs3733359 G > A | GG | GA | AA | A | ||
| Atopy | 95.1 | 4.4 | 0.5 | 2.7 | ||
| Control | 93.7 | 6.3 | 0 | 1.1 | ||
| T Cell Subset, % from PBMC | Atopic Dermatitis (n = 32) | Allergic Asthma (n = 29) | Control Group (n = 25) |
|---|---|---|---|
| Th1 (CD4+IFN-γ+) | 5.78 ± 0.61 * | 5.88 ± 0.82 * | 3.39 ± 0.56 |
| Th2 (CD4+IL-4+) | 1.51 ± 0.25 ** | 1.60 ± 0.85 ** | 0.57 ± 0.09 |
| Th17 (CD4+IL17-A+) | 0.53 ± 0.25 * | 0.58 ± 0.13 * | 0.25 ± 0.04 |
| Treg (CD4+CD25+FoxP3+) | 0.48 ± 0.03 ** | 0.41 ± 0.02 ** | 0.65 ± 0.04 |
| Th1/Th2 ratio | 0.21 ± 0.03 | 0.26 ± 0.02 | 0.19 ± 0.02 |
| Th17/Treg ratio | 0.54 ± 0.06 * | 0.65 ± 0.13 * | 0.32 ± 0.03 |
| Serum Cytokine | Atopic Dermatitis | Allergic Asthma | Control Groupe |
|---|---|---|---|
| n = 98 | n = 43 | n = 34 | |
| IL-5, pg/mL | 110.8 ± 8.55 ** | 104.1 ± 12.2 ** | 46.9 ± 5.32 |
| IL-17A, pg/mL | 31.0 ± 2.81 * | 32.82 ± 5.56 * | 14.3 ± 1.34 |
| IL-10, pg/mL | 15.48 ± 0.87 ** | 19.28 ± 1.18 * | 25.10 ± 2.41 |
| TGF-β1, ng/mL | 18.8 ± 0.07 * | 18.2 ± 0.12 ** | 12.9 ± 0.09 |
| n = 36 | n = 29 | n = 21 | |
| IFN-γ, pg/mL | 0.74 ± 0.19 | 0.81 ± 0.29 | 0.71 ± 0.22 |
| IL-35, pg/mL | 13.26 ± 1.56 | 15.18 ± 3.51 | 11.12 ± 0.44 |
| IL-33, pg/ mL | 103.15 ± 31.21 | 81.76 ± 16.20 | 36.21 ± 10.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastyte, D.; Tamasauskiene, L.; Stakaitiene, I.; Briede, K.; Ugenskiene, R.; Valiukeviciene, S.; Gradauskiene, B. Relation of T Cell Profile with Vitamin D Receptor and Vitamin D-Binding Protein Gene Polymorphisms in Atopy. Int. J. Mol. Sci. 2024, 25, 9021. https://doi.org/10.3390/ijms25169021
Bastyte D, Tamasauskiene L, Stakaitiene I, Briede K, Ugenskiene R, Valiukeviciene S, Gradauskiene B. Relation of T Cell Profile with Vitamin D Receptor and Vitamin D-Binding Protein Gene Polymorphisms in Atopy. International Journal of Molecular Sciences. 2024; 25(16):9021. https://doi.org/10.3390/ijms25169021
Chicago/Turabian StyleBastyte, Daina, Laura Tamasauskiene, Ieva Stakaitiene, Kamilija Briede, Rasa Ugenskiene, Skaidra Valiukeviciene, and Brigita Gradauskiene. 2024. "Relation of T Cell Profile with Vitamin D Receptor and Vitamin D-Binding Protein Gene Polymorphisms in Atopy" International Journal of Molecular Sciences 25, no. 16: 9021. https://doi.org/10.3390/ijms25169021
APA StyleBastyte, D., Tamasauskiene, L., Stakaitiene, I., Briede, K., Ugenskiene, R., Valiukeviciene, S., & Gradauskiene, B. (2024). Relation of T Cell Profile with Vitamin D Receptor and Vitamin D-Binding Protein Gene Polymorphisms in Atopy. International Journal of Molecular Sciences, 25(16), 9021. https://doi.org/10.3390/ijms25169021





.jpg)

