Morphology-Dependent Photocatalytic Activity of Nanostructured Titanium Dioxide Coatings with Silver Nanoparticles
Abstract
1. Introduction
2. Results
2.1. Titanium Dioxide Coating Characterization
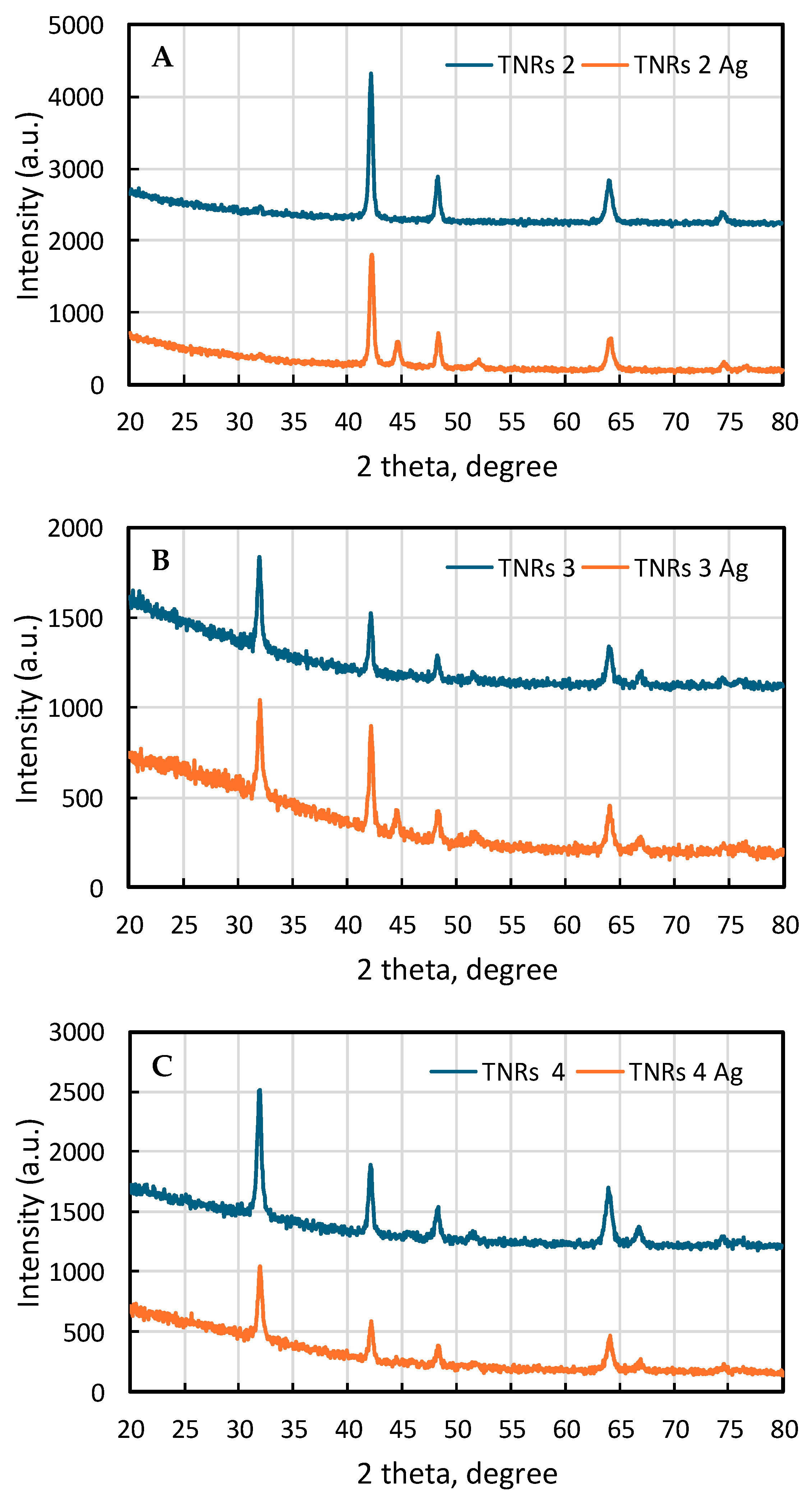
2.1.1. X-ray Diffraction (XRD)
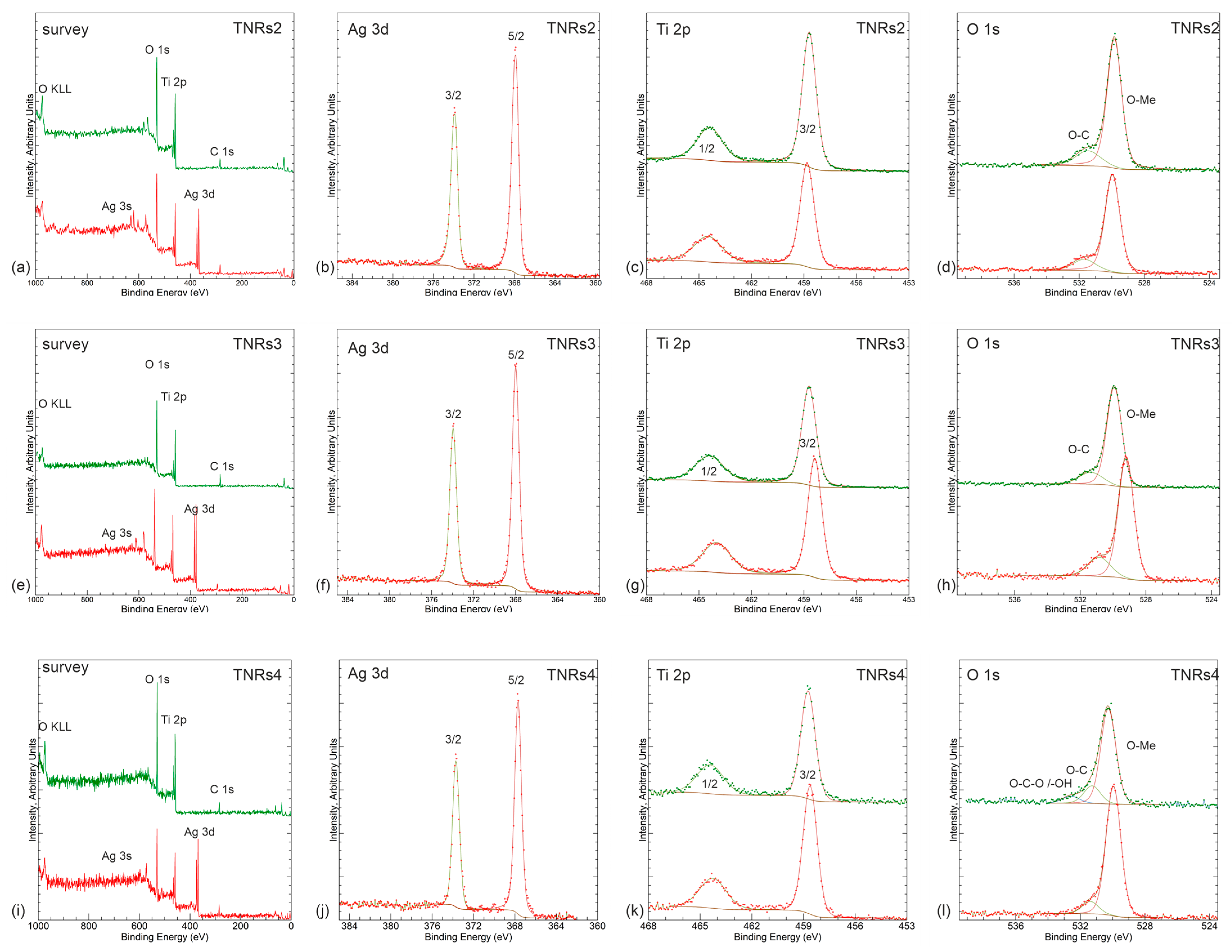
2.1.2. X-ray Photoelectron Spectroscopy (XPS)
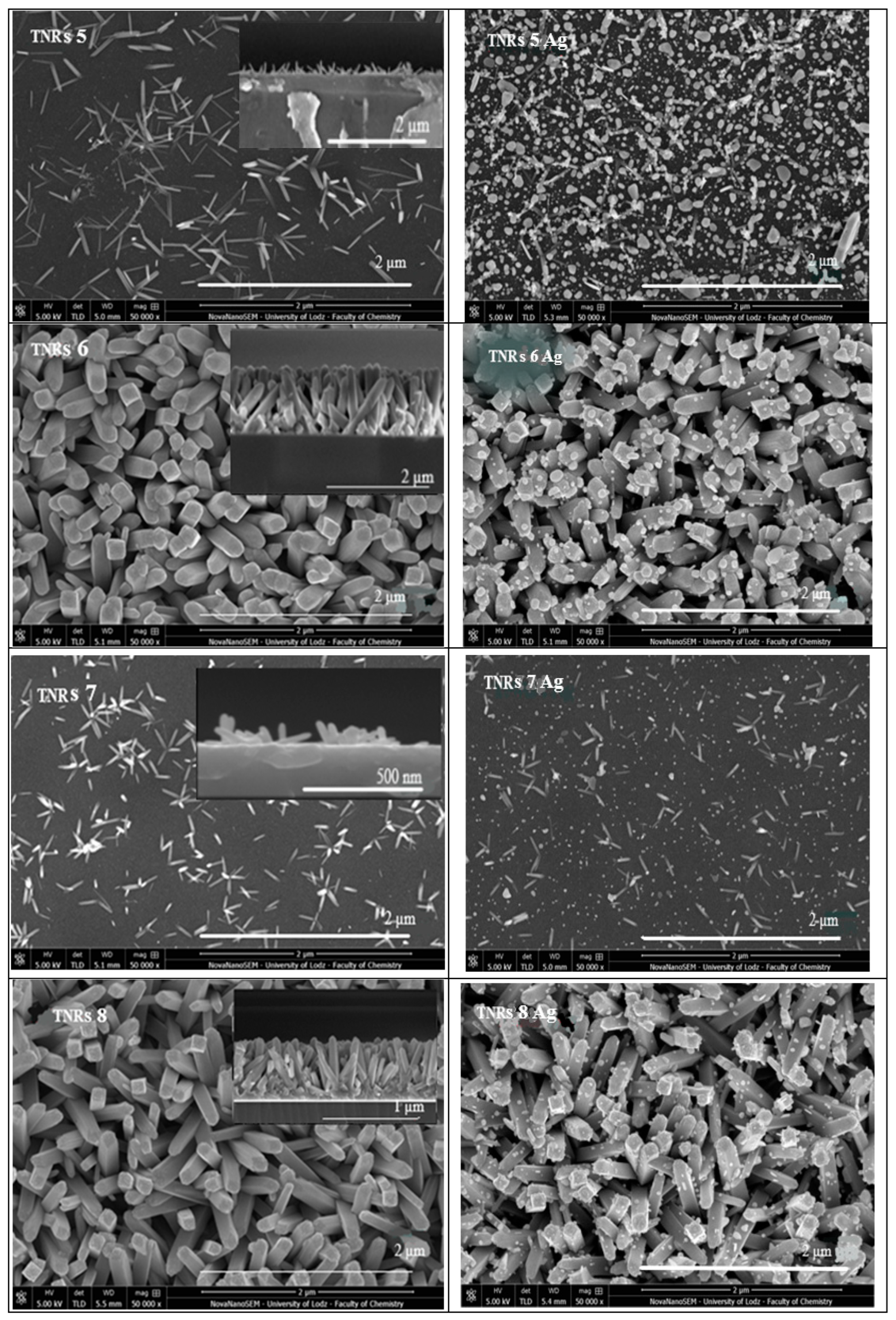
2.1.3. Scanning Electron Microscope (SEM)
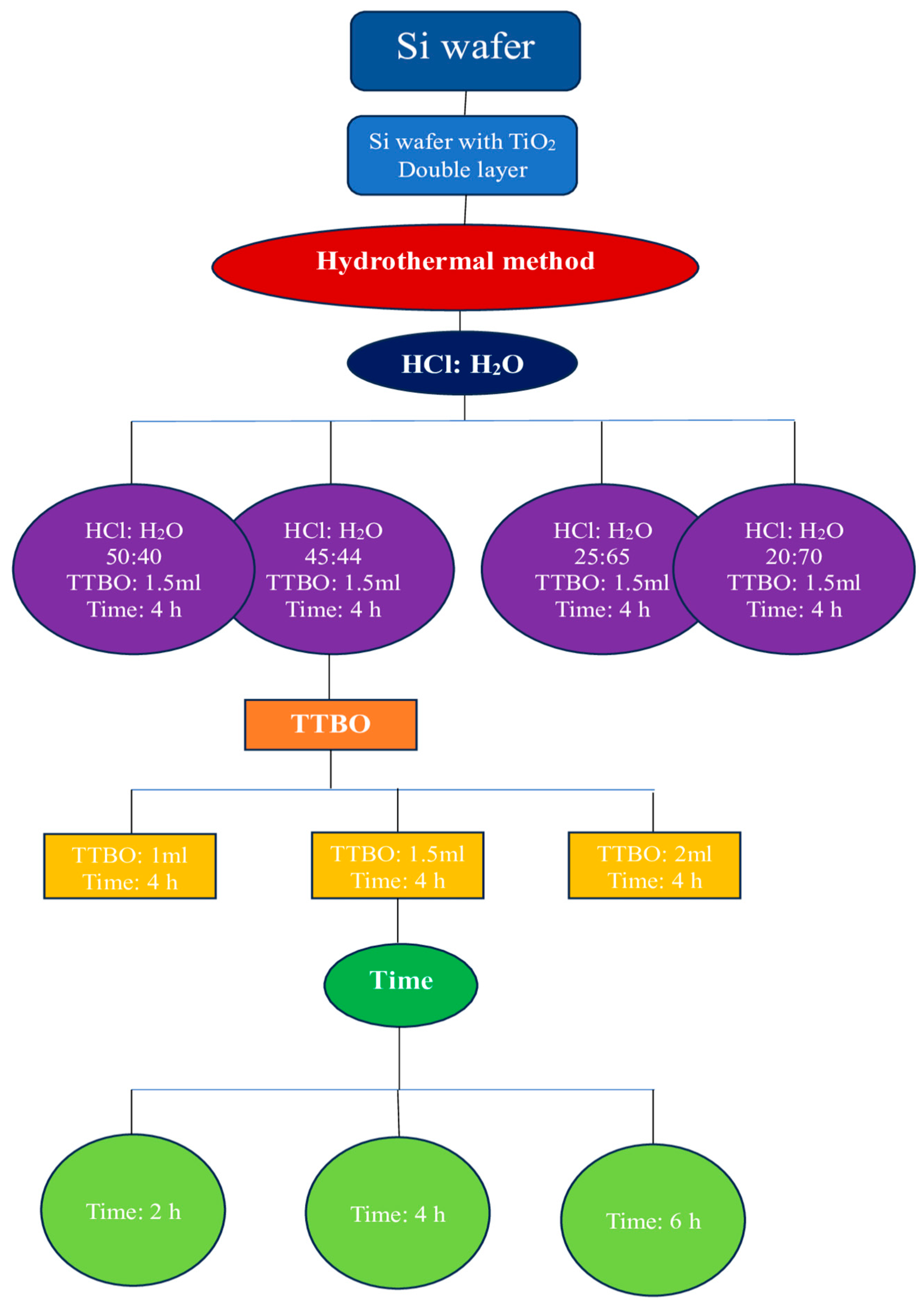
2.2. Synthesis of TiO2 Nanorods on Si/TiO2 Substrate
2.2.1. Effect of the Acid:Water Ratio
2.2.2. Effect of the Precursor Amount
2.2.3. Effect of the Reaction Time
2.2.4. Effect of the Morphology on Ag NPs’ Size
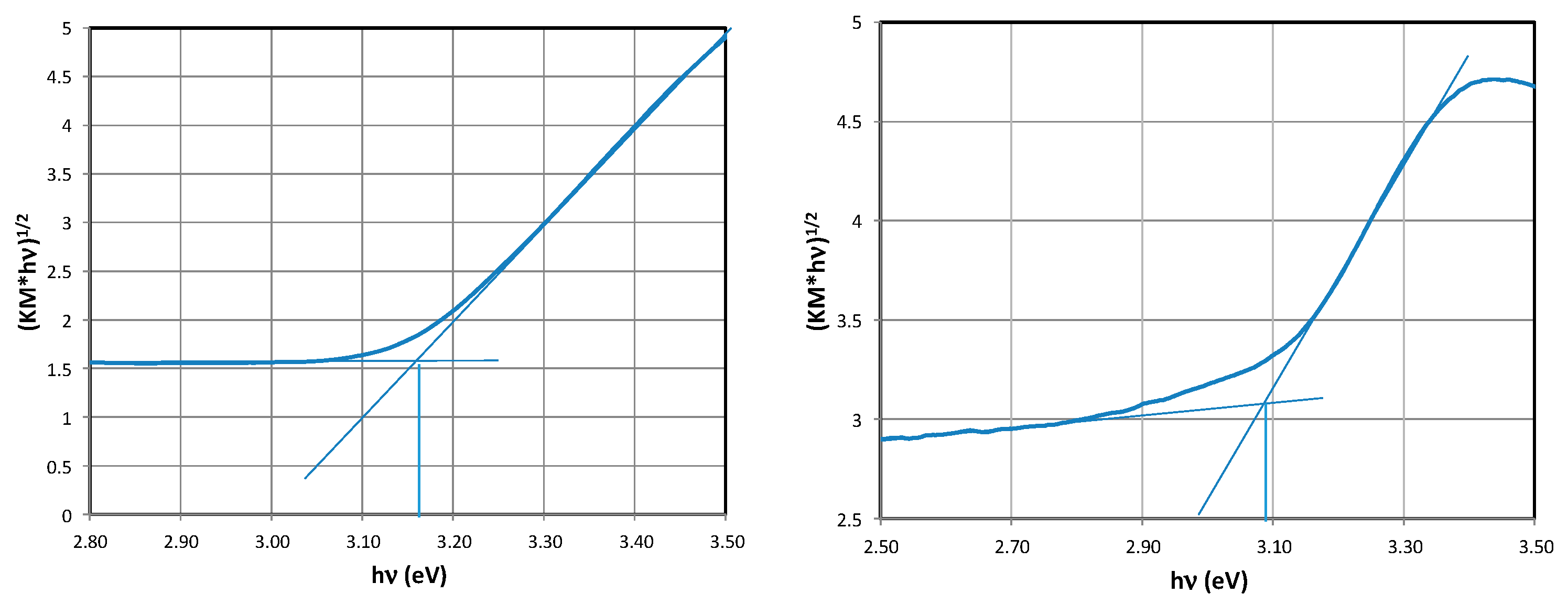
2.3. Determination of the Band Gap Eg
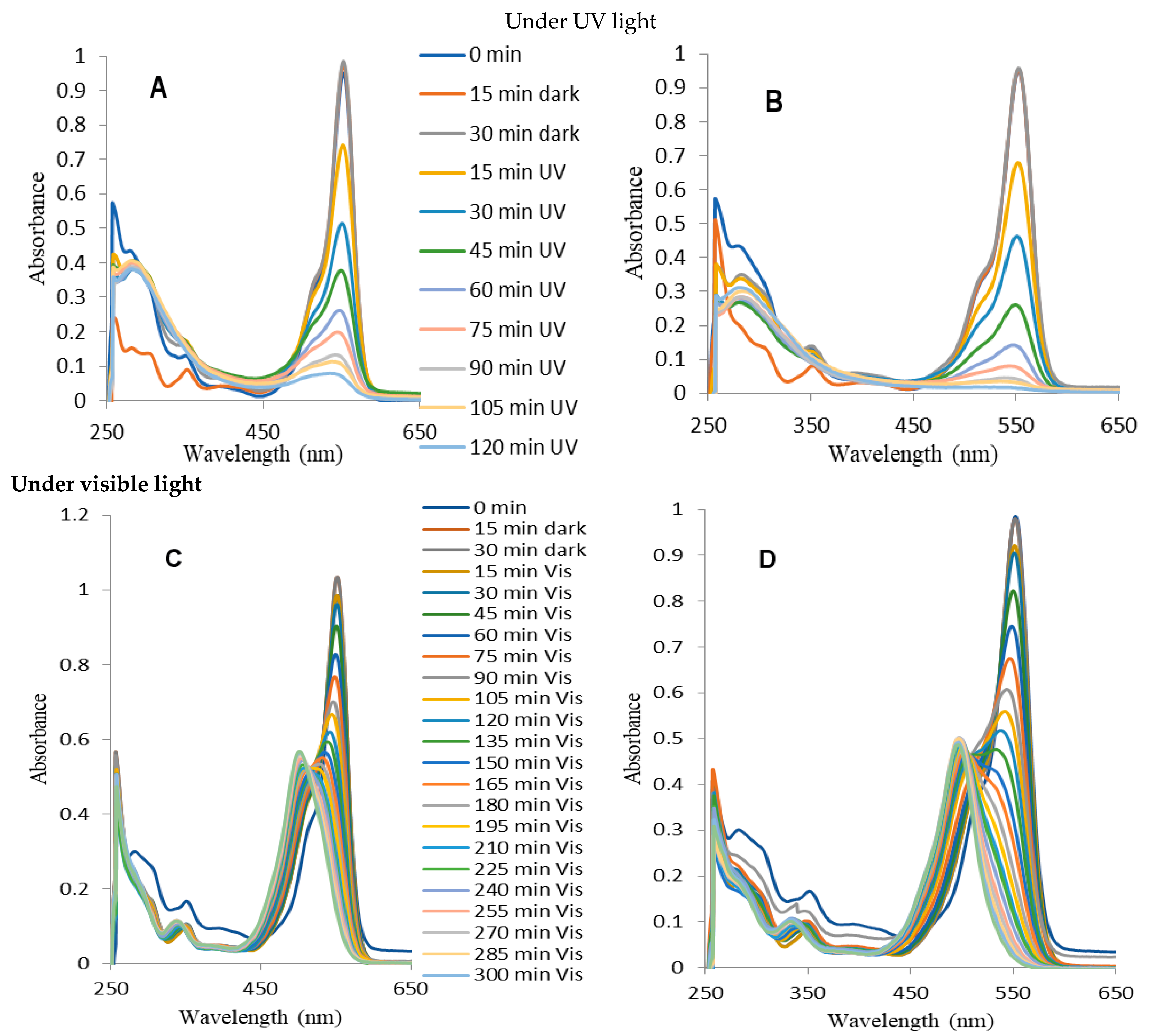
2.4. Photocatalytic Decomposition of Rhodamine B (RhB)
3. Discussion
3.1. Transformations of RhB under Visible Light
3.2. Decompositions of RhB under UV Irradiation
4. Materials and Methods
4.1. Materials
4.2. Preparation of Titanium Dioxide Coatings
4.3. Hydrothermal Growth of TiO2 Nanorods
4.3.1. Pre-Treatment of the Substrate
4.3.2. Synthesis of TiO2 Nanorod
4.4. Surface Modification of TiO2 Nanorods with Silver Nanoparticles (AgNPs)
5. Characterization
5.1. XPS Measurements
5.2. Scanning Electron Microscope Imaging
5.3. Band Gap Determination—Diffuse Reflectance Measurements
5.4. Photocatalytic Measurements
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.B.; Linic, S. Water splitting on composite plasmonic-metal/semiconductor photoelectrodes: Evidence for selective plasmon-induced formation of charge carriers near the semiconductor surface. J. Am. Chem. Soc. 2011, 133, 5202–5205. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Schutte-Smith, M.; Erasmus, E.; Mogale, R.; Marogoa, N.; Jayiya, A.; Visser, H.G. Using visible light to activate antiviral and antimicrobial properties of TiO2 nanoparticles in paints and coatings: Focus on new developments for frequent-touch surfaces in hospitals. J. Coatings Technol. Res. 2023, 20, 789–817. [Google Scholar] [CrossRef] [PubMed]
- Hazra, A.; Bhattacharyya, P. Tailoring of the gas sensing performance of TiO2 nanotubes by 1-D vertical electron transport technique. IEEE Trans. Electron Devices 2014, 61, 3483–3489. [Google Scholar] [CrossRef]
- Liu, B.; Aydil, E.S. Growth of oriented single-crystalline rutile TiO2 nanorods on transparent conducting substrates for dye-sensitized solar cells. J. Am. Chem. Soc. 2009, 131, 3985–3990. [Google Scholar] [CrossRef]
- Cao, C.; Hu, C.; Wang, X.; Wang, S.; Tian, Y.; Zhang, H. UV sensor based on TiO2 nanorod arrays on FTO thin film. Sens. Actuators B Chem. 2011, 156, 114–119. [Google Scholar] [CrossRef]
- Mu, Q.; Li, Y.; Wang, H.; Zhang, Q. Self-organized TiO2 nanorod arrays on glass substrate for self-cleaning antireflection coatings. J. Colloid Interface Sci. 2012, 365, 308–313. [Google Scholar] [CrossRef]
- Tang, D.; Cheng, K.; Weng, W.; Song, C.; Du, P.; Shen, G.; Han, G. TiO2 nanorod films grown on Si wafers by a nanodot-assisted hydrothermal growth. Thin Solid Films 2011, 519, 7644–7649. [Google Scholar] [CrossRef]
- Rahmani, N.; Dariani, R.S.; Rajabi, M. A proposed mechanism for investigating the effect of porous silicon buffer layer on TiO2 nanorods growth. Appl. Surf. Sci. 2016, 366, 359–364. [Google Scholar] [CrossRef]
- Chandra, A.; Ghosh, S.; Sarkar, R.; Sarkar, S.; Chattopadhyay, K.K. TiO2 nanorods decorated Si nanowire hierarchical structures for UV light activated photocatalytic application. Chemosphere 2024, 352, 141249. [Google Scholar] [CrossRef] [PubMed]
- Andoshe, D.M.; Choi, S.; Shim, Y.S.; Lee, S.H.; Kim, Y.; Moon, C.W.; Jang, H.W. A wafer-scale antireflective protection layer of solution-processed TiO2 nanorods for high performance silicon-based water splitting photocathodes. J. Mater. Chem. 2016, 4, 9477–9485. [Google Scholar] [CrossRef]
- Wagner, C.D.; Naumkin, A.V.; Kraut-Vass, A.; Allison, J.W.; Powell, C.J.; Rumble, J.R., Jr. NIST Standard Reference Database 20, Version 3.4 (Web Version). 2003. Available online: https://srdata.nist.gov/xps/ (accessed on 1 July 2024).
- Gaarenstroom, S.W.; Winograd, N. Initial and final state effects in the ESCA spectra of cadmium and silver oxides. J. Chem. Phys. 1977, 67, 3500–3506. [Google Scholar] [CrossRef]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase characterization of TiO2 powder by XRD and TEM. Agric. Nat. Resour. 2008, 42, 357–361. [Google Scholar]
- Wang, D.; Yu, B.; Zhou, F.; Wang, C.; Liu, W. Synthesis and characterization of anatase TiO2 nanotubes and their use in dye-sensitized solar cells. Mater. Chem. Phys. 2009, 113, 602–606. [Google Scholar] [CrossRef]
- Muniandy, S.S.; Kaus, N.H.M.; Jiang, Z.T.; Altarawneh, M.; Lee, H.L. Green synthesis of mesoporous anatase TiO2 nanoparticles and their photocatalytic activities. RSC Adv. 2017, 7, 48083–48094. [Google Scholar] [CrossRef]
- Aziz, W.J.; Sabry, R.S.; Jarallah, A.K. Effect shapes of nanorods, nanowires and nanobelts TiO2 on the degradation of MB photo catalyst. J. Phys. Conf. Ser. 2018, 1032, 012021. [Google Scholar] [CrossRef]
- Reyes, C.D.; Rodríguez, G.G.; Espinosa, P.M.E.; Cab, C.; De Coss, R.D.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef] [PubMed]
- Prathan, A.; Sanglao, J.; Wang, T.; Bhoomanee, C.; Ruankham, P.; Gardchareon, A.; Wongratanaphisan, D. Controlled structure and growth mechanism behind hydrothermal growth of TiO2 nanorods. Sci. Rep. 2020, 10, 8065. [Google Scholar] [CrossRef]
- Nayak, J.; Prabakar, K.; Park, J.W.; Kim, H. Effect of synthesis temperature on structure, optical and photovoltaic properties of TiO2 nanorod thin films. Electrochim. Acta 2012, 65, 44–49. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Rajan, R. Removal of rhodamine B from a water medium using hydroxyl and sulphate radicals generated by iron loaded activated carbon. RSC Adv. 2016, 6, 5330–5340. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Thomas, M.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Study on UV-LED/TiO2 process for degradation of Rhodamine B dye. J. Chem. Eng. 2011, 169, 126–134. [Google Scholar] [CrossRef]
- Kazemifard, S.; Naji, L.; Taromi, F.A. Enhancing the photovoltaic performance of bulk heterojunction polymer solar cells by adding Rhodamine B laser dye as co-sensitizer. J. Colloid Interface Sci. 2018, 515, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Tyona, M.D.; Jambure, S.B.; Lokhande, C.D.; Banpurkar, A.G.; Osuji, R.U.; Ezema, F.I. Dye-sensitized solar cells based on Al-doped ZnO photoelectrodes sensitized with rhodamine. Mater. Lett. 2018, 220, 281–284. [Google Scholar] [CrossRef]
- Hsiao, Y.C.; Wu, T.F.; Wang, Y.S.; Hu, C.C.; Huang, C. Evaluating the sensitizing effect on the photocatalytic decoloration of dyes using anatase-TiO2. Appl. Catal. B Environ. 2014, 148, 250–257. [Google Scholar] [CrossRef]
- Zaleska-Medynska, A. Metal Oxide-Based Photocatalysis: Fundamentals and Prospects for Application; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Qi, H.P.; Wang, H.L.; Zhao, D.Y.; Jiang, W.F. Preparation and photocatalytic activity of Ag-modified GO-TiO2 mesocrystals under visible light irradiation. Appl. Surf. Sci. 2019, 480, 105–114. [Google Scholar] [CrossRef]
- Zhang, L.; Ni, C.; Jiu, H.; Xie, C.; Yan, J.; Qi, G. One-pot synthesis of Ag-TiO2/reduced graphene oxide nanocomposite for high performance of adsorption and photocatalysis. Ceram. Int. 2017, 43, 5450–5456. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Jiu, H.; Ni, C.; Zhang, X.; Xu, M. Graphene-based hollow TiO2 composites with enhanced photocatalytic activity for removal of polltants. J. Phys. Chem. Solids 2015, 86, 82–89. [Google Scholar] [CrossRef]
- Fang, H.; Pan, Y.; Yin, M.; Pan, C. Enhanced photocatalytic activity and mechanism of Ti3C2–OH/Bi2WO6: Yb3+, Tm3+ towards degradation of RhB under visible and near infrared light irradiation. Mater. Res. Bull. 2020, 121, 110618. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A. ZnO conjugated graphene: An efficient sunlight driven photocatalyst for degradation of organic dyes. Mater. Res. Bull. 2020, 129, 110911. [Google Scholar] [CrossRef]
- Kusior, A.; Michalec, K.; Jelen, P.; Radecka, M. Shaped Fe2O3 nanoparticles–synthesis and enhanced photocatalytic degradation towards RhB. Appl. Surf. Sci. 2019, 476, 342–352. [Google Scholar] [CrossRef]
- Liang, H.; Jia, Z.; Zhang, H.; Wang, X.; Wang, J. Photocatalysis oxidation activity regulation of Ag/TiO2 composites evaluated by the selective oxidation of Rhodamine B. Appl. Surf. Sci. 2017, 422, 1–10. [Google Scholar] [CrossRef]
- Wu, T.; Liu, G.; Zhao, J.; Hidaka, H.; Serpone, N. Photoassisted degradation of dye pollutants. V. Self-photosensitized oxidative transformation of rhodamine B under visible light irradiation in aqueous TiO2 dispersions. J. Phys. Chem. B 1998, 102, 5845–5851. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, J.; Hidaka, H. Highly selective deethylation of rhodamine B: Adsorption and photooxidation pathways of the dye on the TiO2/SiO2 composite photocatalyst. Int. J. Photoenergy 2003, 5, 209–217. [Google Scholar] [CrossRef]
- Spilarewicz-Stanek, K.; Jakimińska, A.; Kisielewska, A.; Batory, D.; Piwoński, I. Understanding the role of silver nanostructures and graphene oxide applied as surface modification of TiO2 in photocatalytic transformations of rhodamine B under UV and vis irradiation. Materials 2020, 13, 4653. [Google Scholar] [CrossRef] [PubMed]
- Merka, O.; Yarovyi, V.; Bahnemann, D.W.; Wark, M. pH-control of the photocatalytic degradation mechanism of rhodamine B over Pb3Nb4O13. J. Phys. Chem. C 2011, 115, 8014–8023. [Google Scholar] [CrossRef]
- Rasalingam, S.; Wu, C.M.; Koodali, R.T. Modulation of pore sizes of titanium dioxide photocatalysts by a facile template free hydrothermal synthesis method: Implications for photocatalytic degradation of rhodamine B. ACS Appl. Mater. Interfaces 2015, 7, 4368–4380. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Gao, H.; Wang, H.; Lu, X.; Xu, G.; Wang, D.; Wu, Y. Controlled deposition and enhanced visible light photocatalytic performance of Pt-modified TiO2 nanotube arrays. Appl. Surf. Sci. 2015, 351, 225–231. [Google Scholar] [CrossRef]
- Zhuang, J.; Dai, W.; Tian, Q.; Li, Z.; Xie, L.; Wang, J.; Wang, D. Photocatalytic degradation of RhB over TiO2 bilayer films: Effect of defects and their location. Langmuir 2010, 26, 9686–9694. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, S.; Xu, T.; Zhu, Y.; Chen, J. Photocatalytic degradation of RhB by fluorinated Bi2WO6 and distributions of the intermediate products. Environ. Sci. Technol. 2008, 42, 2085–2091. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, C.; Gu, F.L.; Wu, H.; Guo, Q. Significant visible-light photocatalytic enhancement in Rhodamine B degradation of silver orthophosphate via the hybridization of N-doped graphene and poly (3-hexylthiophene). J. Hazard. Mater. 2016, 315, 23–34. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Y.; Zhang, Z.; Liu, X.; Jia, H.; Xu, B. Synthesis of spherical Ag/ZnO heterostructural composites with excellent photocatalytic activity under visible light and UV irradiation. Appl. Surf. Sci. 2015, 355, 644–652. [Google Scholar] [CrossRef]
- Li, W.; Hua, F.; Yue, J.; Li, J. Ag@AgCl plasmon-induced sensitized ZnO particle for high-efficiency photocatalytic property under visible light. Appl. Surf. Sci. 2013, 285, 490–497. [Google Scholar] [CrossRef]
- Perera, S.D.; Mariano, R.G.; Vu, K.; Nour, N.; Seitz, O.; Chabal, Y.; Balkus, K.J., Jr. Hydrothermal synthesis of graphene-TiO2 nanotube composites with enhanced photocatalytic activity. ACS Catal. 2012, 2, 949–956. [Google Scholar] [CrossRef]
- Park, H.; Kim, H.I.; Moon, G.H.; Choi, W. Photoinduced charge transfer processes in solar photocatalysis based on modified TiO2. Energy Environ. Sci. 2016, 9, 411–433. [Google Scholar] [CrossRef]
- Lei, P.; Chen, C.; Yang, J.; Ma, W.; Zhao, J.; Zang, L. Degradation of dye pollutants by immobilized polyoxometalate with H2O2 under visible-light irradiation. Environ. Sci. Technol. 2005, 39, 8466–8474. [Google Scholar] [CrossRef]
- Seddigi, Z.S.; Gondal, M.A.; Rashid, S.G.; Abdulaziz, M.A.; Ahmed, S.A. Facile synthesis and catalytic performance of nanosheet–nanorods g-C3N4-Bi2WO6 heterojunction catalyst and effect of silver nanoparticles loading on bare Bi2WO6 and g-C3N4-Bi2WO6 for N-deethylation process. J. Mol. Catal. A Chem. 2016, 420, 167–177. [Google Scholar] [CrossRef]
- Piwoński, I.; Kądzioła, K.; Kisielewska, A.; Soliwoda, K.; Wolszczak, M.; Lisowska, K.; Wrońska, N.; Felczak, A. The effect of the deposition parameters on size, distribution and antimicrobial properties of photoinduced silver nanoparticles on titania coatings. Appl. Surf. Sci. 2011, 257, 7076–7082. [Google Scholar] [CrossRef]
- Kądzioła, K.; Piwoński, I.; Kisielewska, A.; Szczukocki, D.; Krawczyk, B.; Sielski, J. The photoactivity of titanium dioxide coatings with silver nanoparticles prepared by sol–gel and reactive magnetron sputtering methods–comparative studies. Appl. Surf. Sci. 2014, 288, 503–512. [Google Scholar] [CrossRef]
- Lindau, I.; Pianetta, P.; Yu, K.Y.; Spicer, W.E. Photoemission of gold in the energy range 30–300 eV using synchrotron radiation. Phys. Rev. B 1976, 13, 492–495. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. Reliable determination of chemical state in x-ray photoelectron spectroscopy based on sample-work-function referencing to adventitious carbon: Resolving the myth of apparent constant binding energy of the C 1s peak. Appl. Surf. Sci. 2018, 451, 99–103. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]

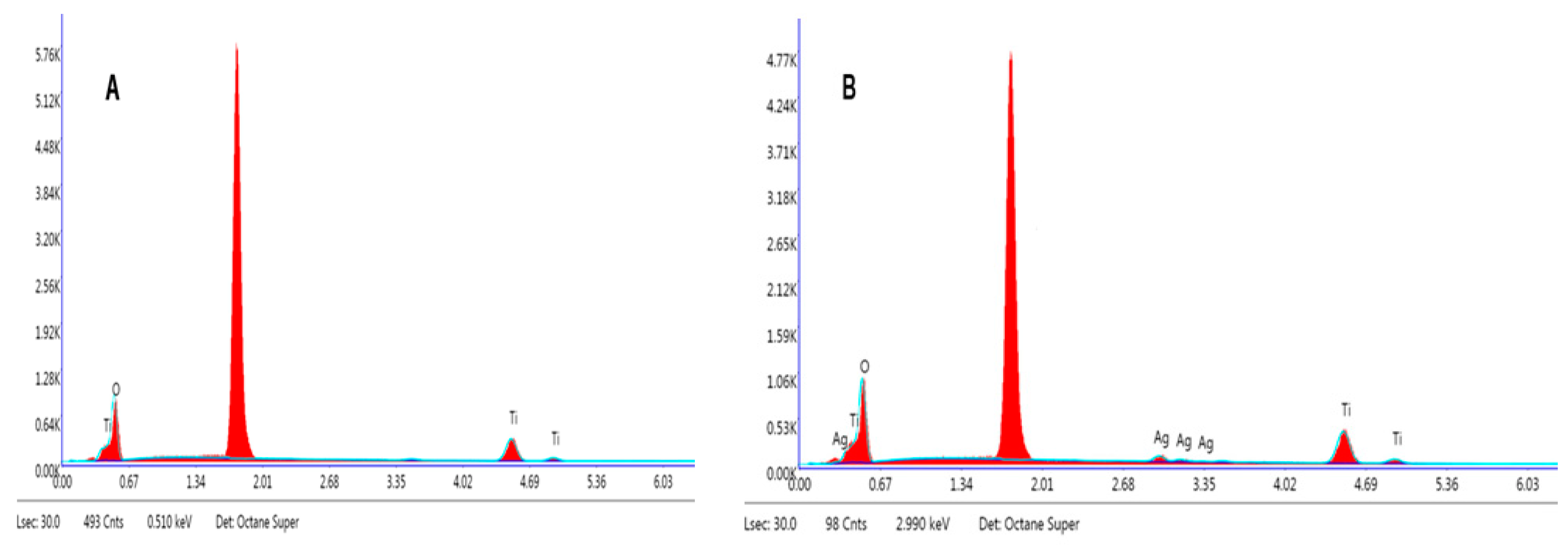

| Samples | Weight% | Atomic% | ||||
|---|---|---|---|---|---|---|
| Ti | O | Ag | Ti | O | Ag | |
| TNRs 1 | 2.72 | 2.28 | ---- | 3.98 | 1.59 | ---- |
| TNRs 1 Ag | 32.84 | 42.70 | 24.45 | 19.14 | 74.53 | 6.33 |
| TNRs 2 | 51.91 | 48.89 | ---- | 26.50 | 73.50 | ---- |
| TNRs 2 Ag | 50.10 | 42.95 | 6.97 | 27.26 | 70.74 | 1.70 |
| TNRs 3 | 61.47 | 38.60 | ---- | 34.70 | 65.30 | ---- |
| TNRs 3 Ag | 57.3 | 31.61 | 11.06 | 36.55 | 60.32 | 3.13 |
| TNRs 4 | 67.03 | 32.97 | ---- | 40.45 | 59.55 | ---- |
| TNRs 4 Ag | 77.51 | 9.07 | 13.41 | 70.06 | 24.55 | 5.38 |
| TNRs 5 | 2.73 | 3.56 | ---- | 4.08 | 1.56 | ---- |
| TNRs 5 Ag | 34.62 | 40.26 | 25.12 | 21.76 | 71.76 | 6.48 |
| TNRs 6 | 62.12 | 37.88 | ---- | 35.39 | 64.61 | ---- |
| TNRs 6 Ag | 60.80 | 35.88 | 3.31 | 35.83 | 63.30 | 0.87 |
| TNRs 7 | 46.33 | 53.67 | ---- | 22.38 | 77.62 | ---- |
| TNRs 7 Ag | 32.76 | 43.16 | 24.08 | 18.98 | 74.83 | 6.19 |
| TNRs 8 | 62.33 | 37.67 | ---- | 35.59 | 64.41 | ---- |
| TNRs 8 Ag | 59.71 | 35.25 | 5.04 | 35.65 | 63.01 | 1.34 |
| Type of Nanostructure | Thickness (from Cross-Section) [nm] | Length [nm] | Diameter [nm] | Diameter of Ag Particles [nm] |
|---|---|---|---|---|
| TNRs 1 | ------- | 83 ± 20 | 13 ± 5 | 48 ± 23 |
| TNRs 2 | 827 ± 34 | 863 ± 50 | 97 ± 22 | 34 ± 14 |
| TNRs 3 | 612 ± 14 | 634 ± 57 | 285 ± 44 | 35 ± 20 |
| TNRs 4 | 19,043 ± 72 | 2064 ± 142 | 4279 ± 416 | 50 ± 36 |
| TNRs 5 | 226 ± 65 | 236 ± 33 | 23 ± 9 | 26 ± 17 |
| TNRs 6 | 920 ± 50 | 929 ± 74 | 130 ± 18 | 33 ± 20 |
| TNRs 7 | 103 ± 27 | 117 ± 30 | 20 ± 6 | 16 ± 11 |
| TNRs 8 | 1056 ± 22 | 1133 ± 124 | 104 ± 21 | 24 ± 13 |
| Acid:Water Ratio | Amount of Precursor | Time of Reaction | |||
|---|---|---|---|---|---|
| Material | Eg [eV] | Material | Eg [eV] | Material | Eg [eV] |
| TNRs 1 | 3.34 | TNRs 5 | 3.38 | TNRs 7 | 3.34 |
| TNRs 1 Ag | 3.30 | TNRs 5 Ag | 3.30 | TNRs 7 Ag | 3.30 |
| TNRs 2 | 3.15 | TNRs 2 | 3.15 | TNRs 2 | 3.15 |
| TNRs 2 Ag | 3.08 | TNRs 2 Ag | 3.08 | TNRs 2 Ag | 3.08 |
| TNRs 3 | 3.01 | TNRs 6 | 3.07 | TNRs 8 | 3.11 |
| TNRs 3 Ag | 1.98 | TNRs 6 Ag | 3.06 | TNRs 8 Ag | 3.08 |
| TNRs 4 | 2.96 | - | - | - | - |
| TNRs 4 Ag | 2.99 | - | - | - | - |
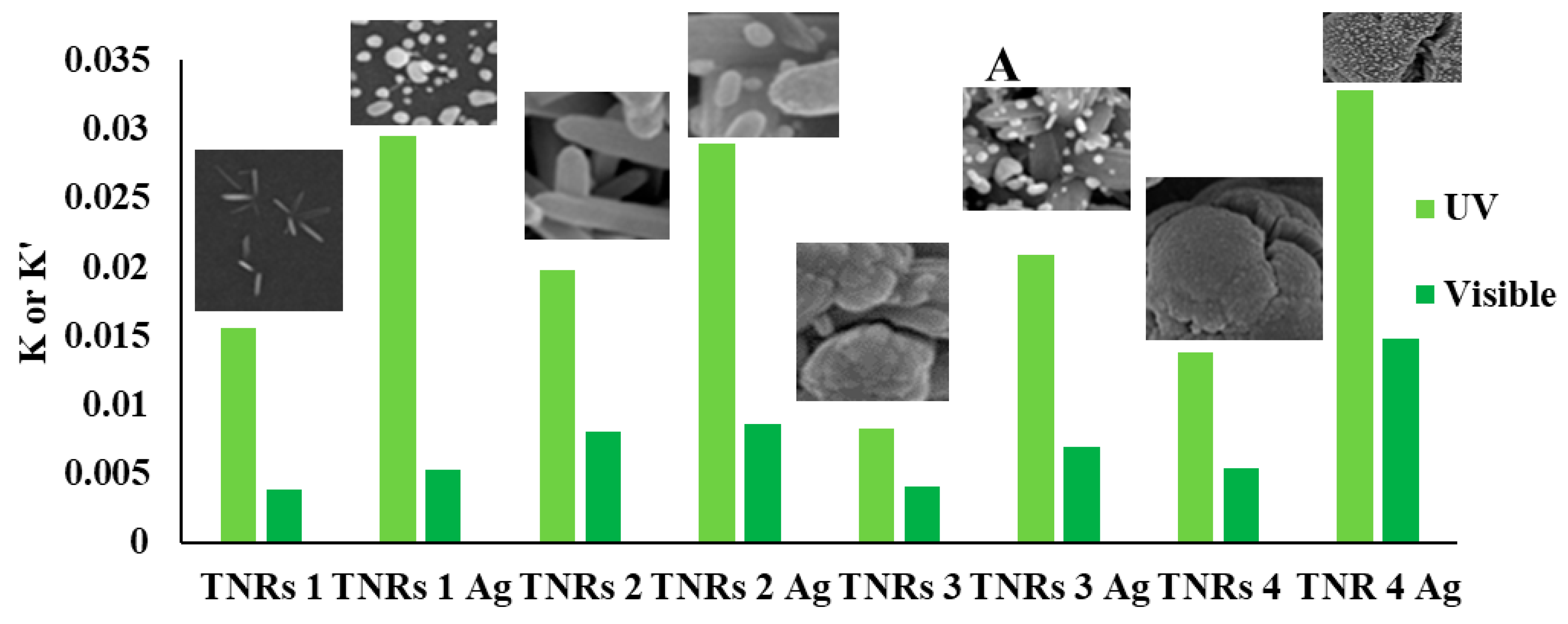
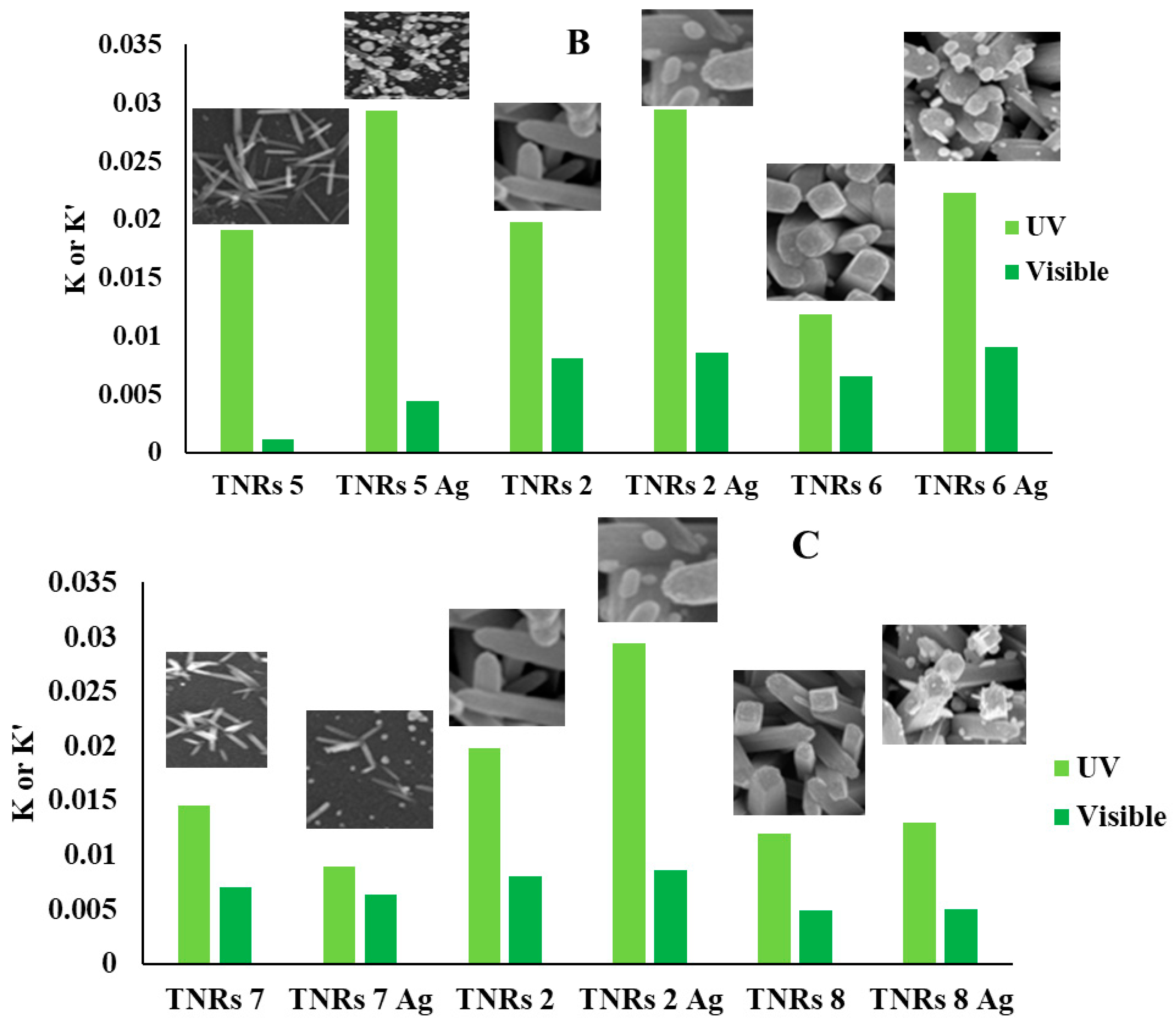
| Sample | UV | Visible | ||
|---|---|---|---|---|
| k [min−1] | k′ [min−1] | Wc% | Wr | |
| TNRs 1 | 0.016 | 0.004 | 68 | 32 |
| TNRs 1 Ag | 0.030 | 0.005 | 60 | 40 |
| TNRs 2 | 0.020 | 0.008 | 61 | 39 |
| TNRs 2 Ag | 0.029 | 0.009 | 50 | 50 |
| TNRs 3 | 0.008 | 0.004 | 70 | 30 |
| TNRs 3 Ag | 0.021 | 0.007 | 67 | 33 |
| TNRs 4 | 0.014 | 0.005 | 65 | 35 |
| TNRs 4 Ag | 0.033 | 0.015 | 50 | 50 |
| TNRs 5 | 0.019 | 0.001 | 93 | 07 |
| TNRs 5 Ag | 0.029 | 0.004 | 78 | 22 |
| TNRs 6 | 0.012 | 0.007 | 60 | 40 |
| TNRs 6 Ag | 0.022 | 0.009 | 48 | 52 |
| TNRs 7 | 0.015 | 0.007 | 67 | 33 |
| TNRs 7 Ag | 0.009 | 0.006 | 65 | 35 |
| TNRs 8 | 0.012 | 0.005 | 46 | 54 |
| TNRs 8 Ag | 0.013 | 0.005 | 46 | 54 |
| Samples | HCl:H2O (mL) | TTBO (mL) | Time (hours) | Morphology |
|---|---|---|---|---|
| TNRs 1 | 50:40 | 1.5 | 4 | Needle-like nanorods |
| TNRs 2 | 45:45 | 1.5 | 4 | Nanorods |
| TNRs 3 | 25:65 | 1.5 | 4 | Nanoflowers |
| TNRs 4 | 20:70 | 1.5 | 4 | Nanoballs |
| TNRs 5 | 45:45 | 1 | 4 | Needle-like nanorods |
| TNRs 6 | 45:45 | 2 | 4 | Dense nanorods |
| TNRs 7 | 45:45 | 1.5 | 2 | Needle-like nanorods |
| TNRs 8 | 45:45 | 1.5 | 6 | Dense nanorods |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakeel, N.; Piwoński, I.; Kisielewska, A.; Krzywiecki, M.; Batory, D.; Cichomski, M. Morphology-Dependent Photocatalytic Activity of Nanostructured Titanium Dioxide Coatings with Silver Nanoparticles. Int. J. Mol. Sci. 2024, 25, 8824. https://doi.org/10.3390/ijms25168824
Shakeel N, Piwoński I, Kisielewska A, Krzywiecki M, Batory D, Cichomski M. Morphology-Dependent Photocatalytic Activity of Nanostructured Titanium Dioxide Coatings with Silver Nanoparticles. International Journal of Molecular Sciences. 2024; 25(16):8824. https://doi.org/10.3390/ijms25168824
Chicago/Turabian StyleShakeel, Nasir, Ireneusz Piwoński, Aneta Kisielewska, Maciej Krzywiecki, Damian Batory, and Michał Cichomski. 2024. "Morphology-Dependent Photocatalytic Activity of Nanostructured Titanium Dioxide Coatings with Silver Nanoparticles" International Journal of Molecular Sciences 25, no. 16: 8824. https://doi.org/10.3390/ijms25168824
APA StyleShakeel, N., Piwoński, I., Kisielewska, A., Krzywiecki, M., Batory, D., & Cichomski, M. (2024). Morphology-Dependent Photocatalytic Activity of Nanostructured Titanium Dioxide Coatings with Silver Nanoparticles. International Journal of Molecular Sciences, 25(16), 8824. https://doi.org/10.3390/ijms25168824








