Photosystem I: A Paradigm for Understanding Biological Environmental Adaptation Mechanisms in Cyanobacteria and Algae
Abstract
1. Introduction
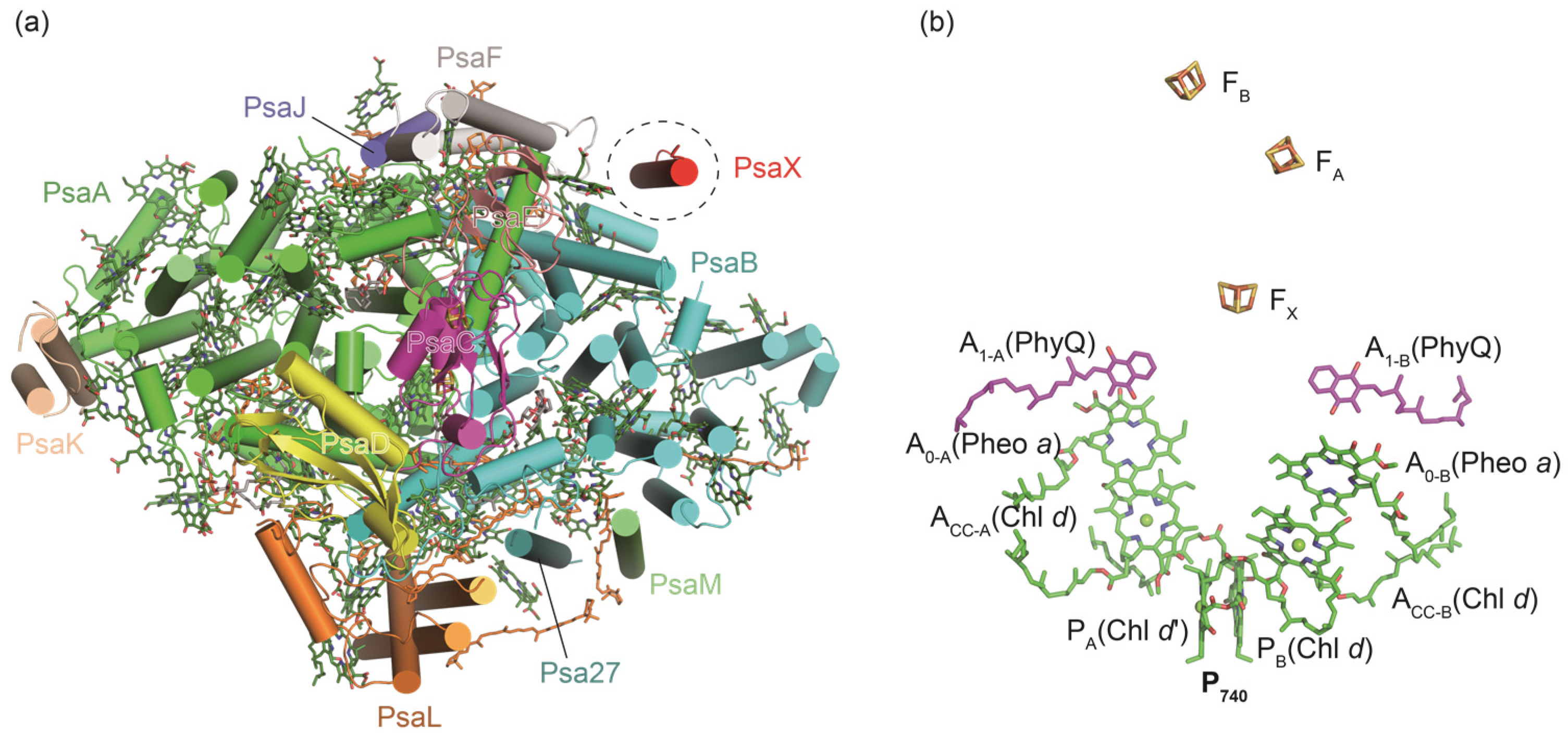
2. Structural Variations of PSI Complexes in Cyanobacteria
2.1. Oligomers of PSI Complexes in Cyanobacteria
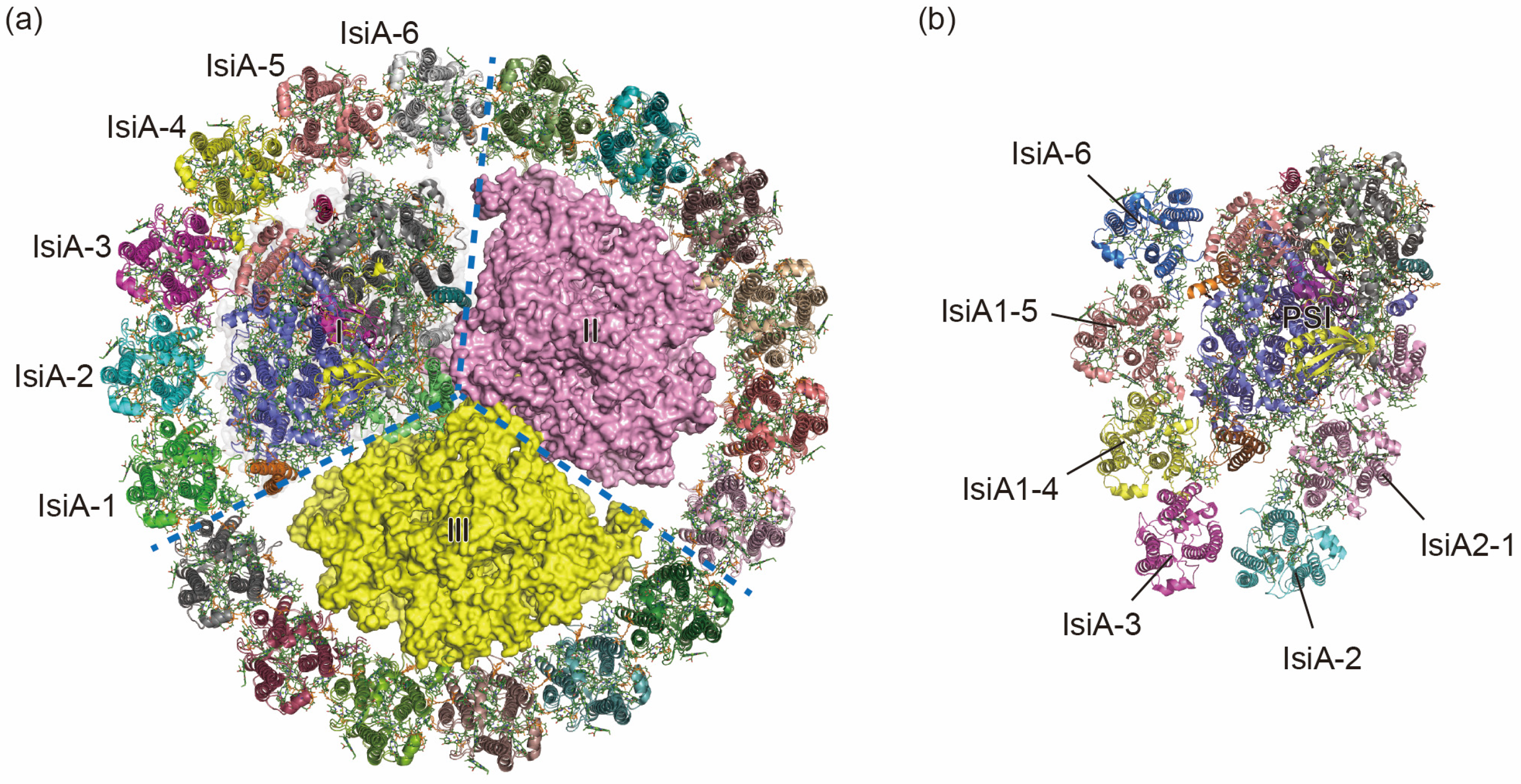
2.2. PSI-IsiA Complexes in Iron-Deficient Environment
2.3. PSI Complexes from Chls d/f-Containing Cyanobacteria
3. Structural Variations of Algal PSI–LHCI Complexes
3.1. PSI–LHCI Complexes of Chlamydomonas reinhardtii
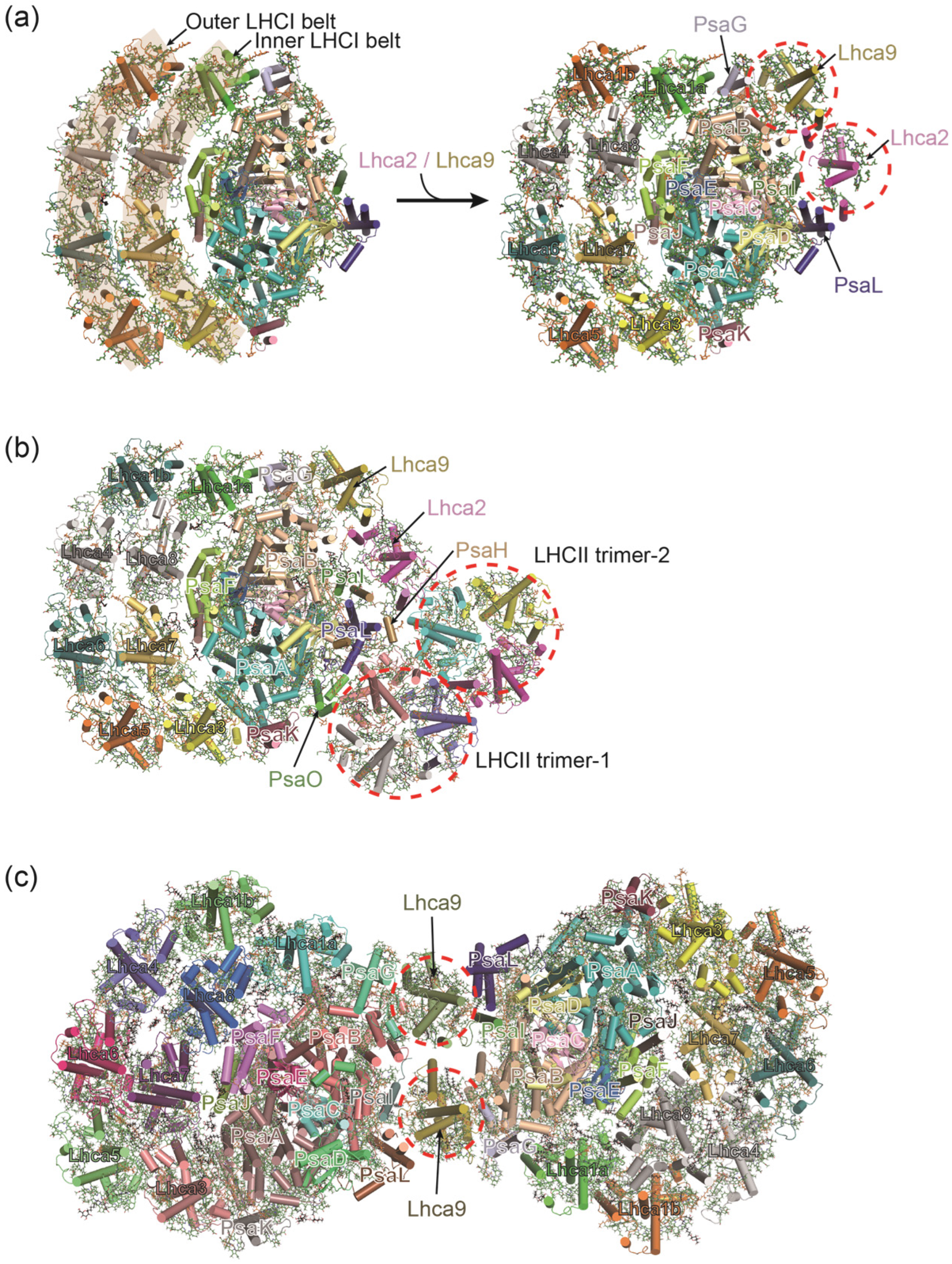
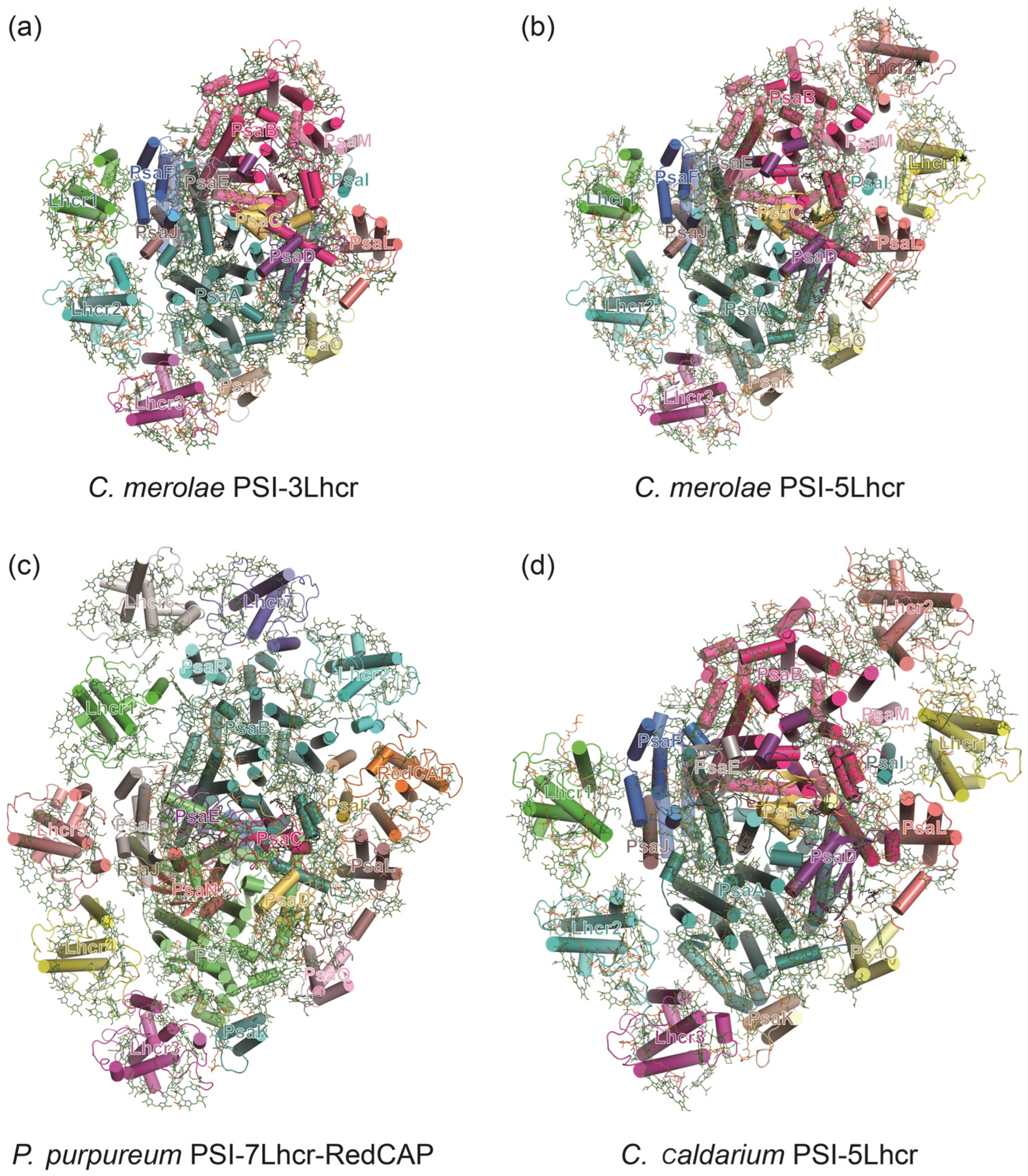
3.2. PSI–LHCI Complexes of Red Algae
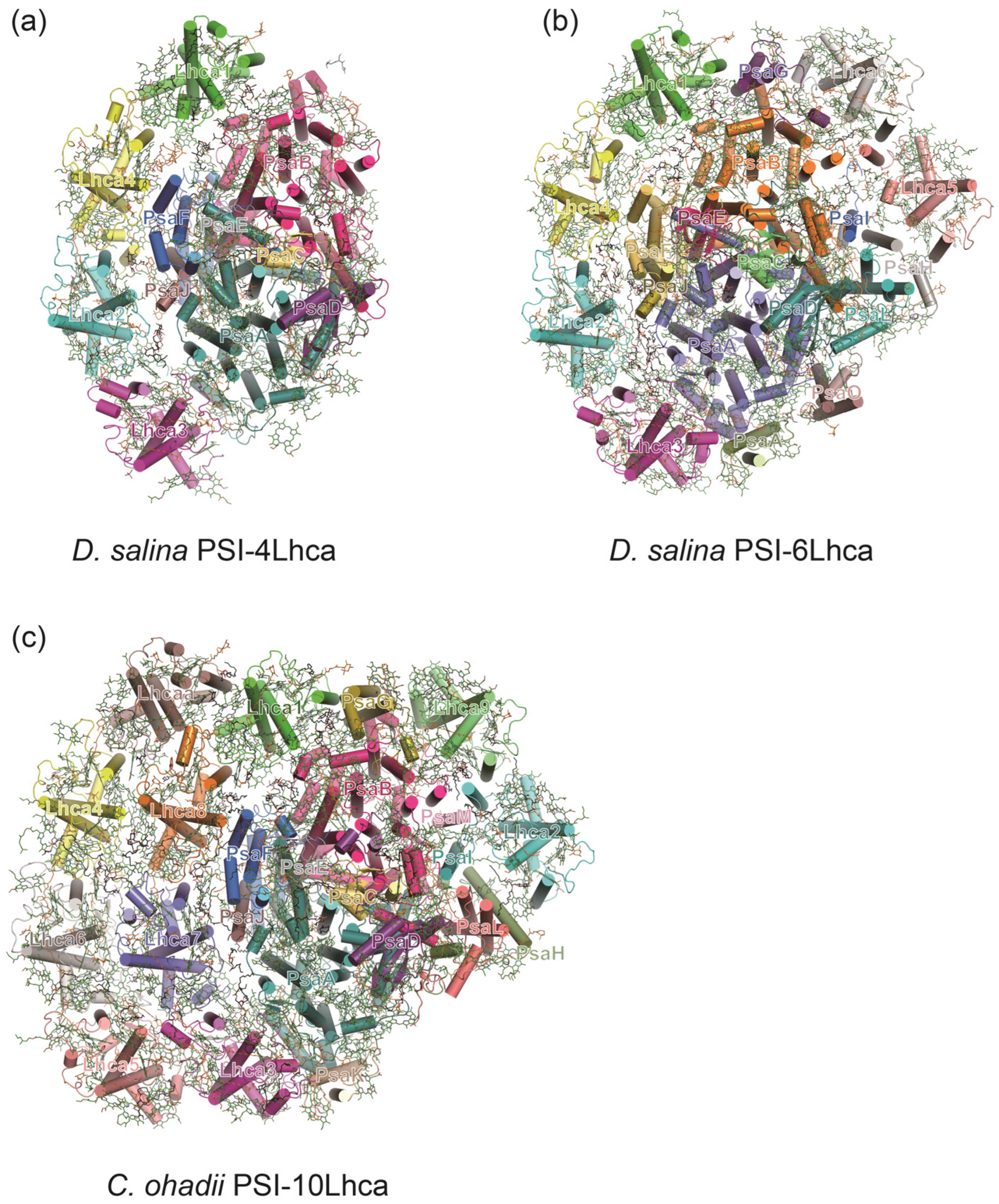
3.3. A Minimal PSI from Salt-Tolerant Green Alga Dunaliella salina
3.4. PSI–LHCI in Desert Algae Chlorella ohadii
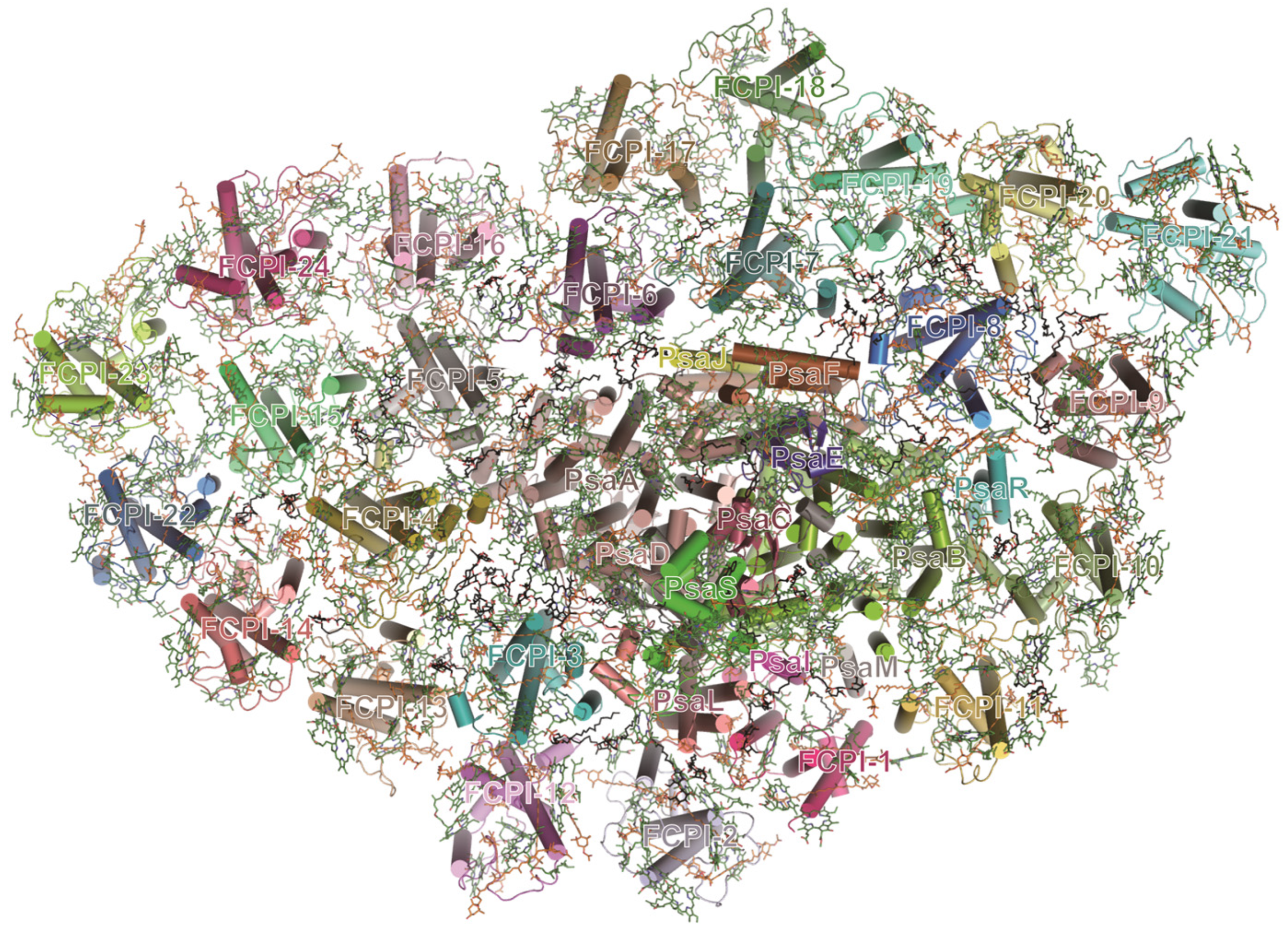
3.5. Diatom PSI-FCPI Complex
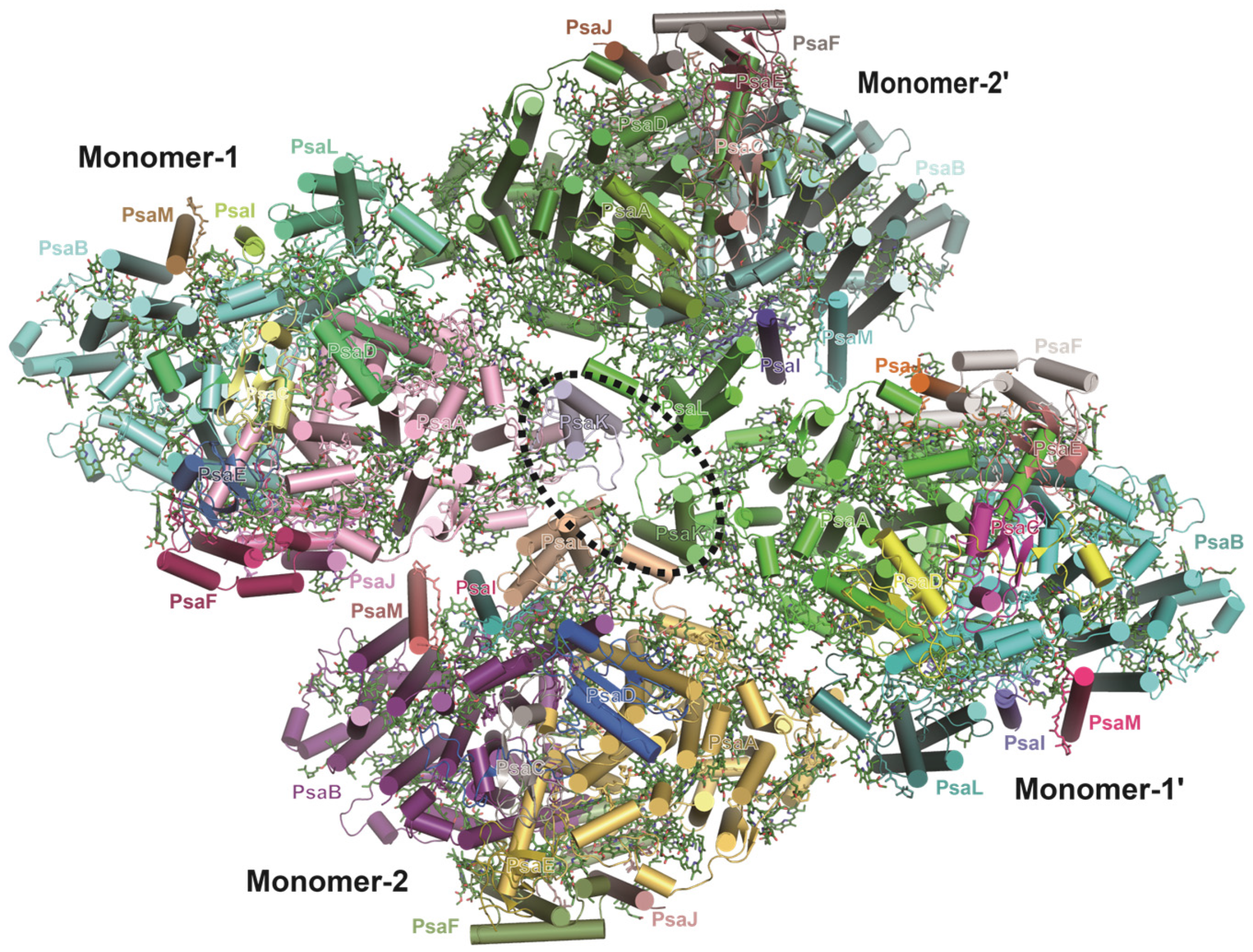
3.6. Tetrameric PSI from Glaucophyte Algae
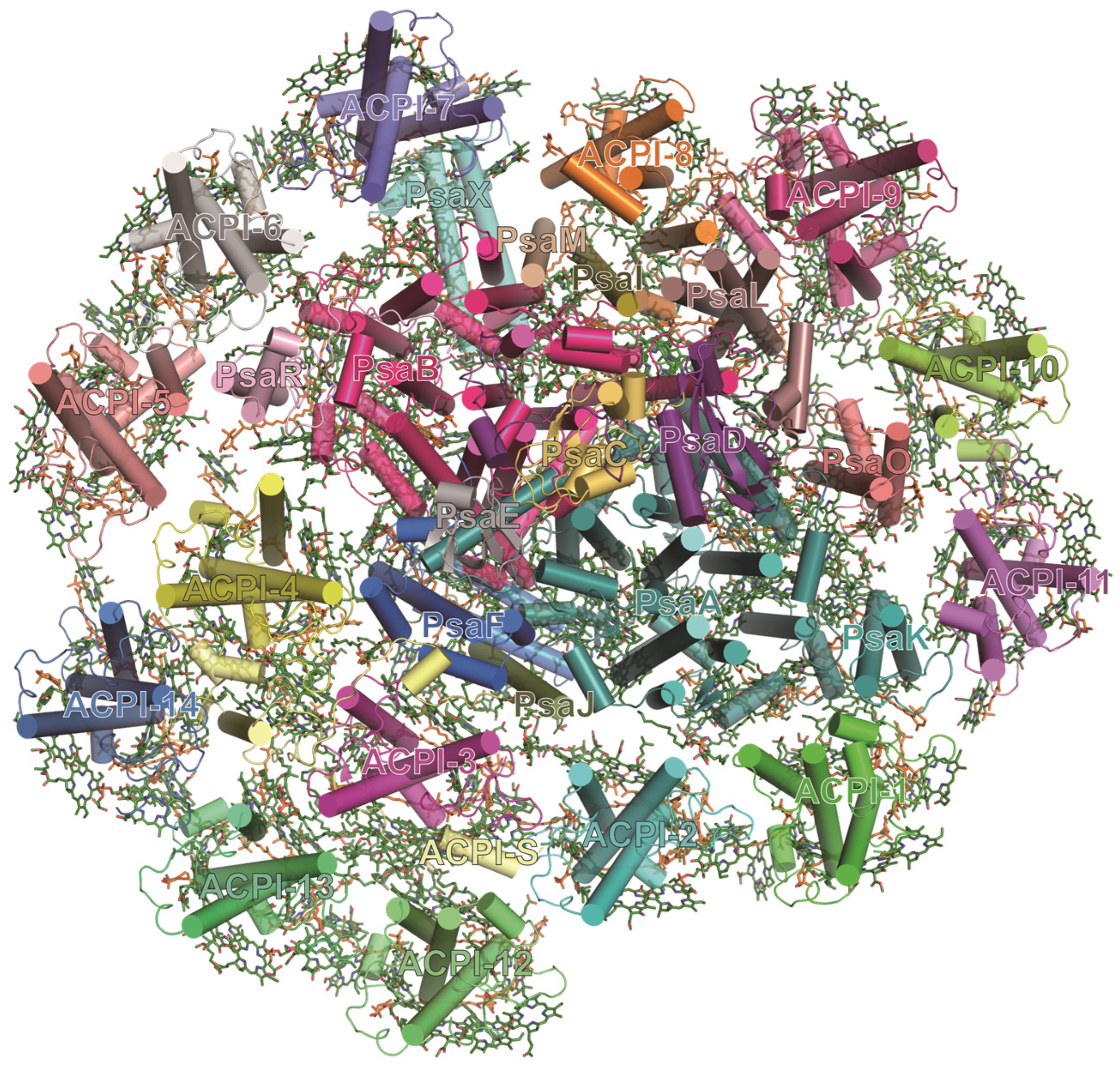
3.7. PSI-ACPI Complex in Cryptophytes
3.8. PSI-ACPPCI Complex in Symbiotic Dinoflagellates
4. Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nelson, N.; Ben-Shem, A. The complex architecture of oxygenic photosynthesis. Nat. Rev. Mol. Cell Biol. 2004, 5, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, P.R. Photosystem I: Function and physiology. Annu. Rev. Plant Biol. 2001, 52, 593–626. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.F. Photosynthesis of ATP-electrons, proton pumps, rotors, and poise. Cell 2002, 110, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, Y. Function and Structure of Cyanobacterial Photosystem I. In Photosynthesis: Structures, Mechanisms, and Applications; Hou, H., Najafpour, M., Moore, G., Allakhverdiev, S., Eds.; Springer: Cham, Switzerland, 2017; Chapter 7; pp. 111–168. ISBN 978-3-319-48873-8. [Google Scholar]
- Jordan, P.; Fromme, P.; Witt, H.T.; Klukas, O.; Saenger, W.; Krauss, N. Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature 2001, 411, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shem, A.; Frolow, F.; Nelson, N. Crystal structure of plant photosystem I. Nature 2003, 426, 630–635. [Google Scholar] [CrossRef]
- Amunts, A.; Drory, O.; Nelson, N. The structure of a plant photosystem I supercomplex at 3.4 Å resolution. Nature 2007, 447, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Suga, M.; Kuang, T.; Shen, J.-R. Structural basis for energy transfer pathways in the plant PSI-LHCI supercomplex. Science 2015, 348, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Mazor, Y.; Borovikova, A.; Caspy, I.; Nelson, N. Structure of the plant photosystem I supercomplex at 2.6 Å resolution. Nat. Plants 2017, 3, 17014. [Google Scholar] [CrossRef] [PubMed]
- Kuhlbrandt, W. Cryo-EM enters a new era. eLife 2014, 3, e03678. [Google Scholar] [CrossRef]
- Hippler, M.; Nelson, N. The plasticity of photosystem I. Plant Cell Physiol. 2021, 62, 1073–1081. [Google Scholar] [CrossRef]
- Çoruh, O.; Frank, A.; Tanaka, H.; Kawamoto, A.; El-Mohsnawy, E.; Kato, T.; Namba, K.; Gerle, C.; Nowaczyk, M.M.; Kurisu, G. Cryo-EM structure of a functional monomeric Photosystem I from Thermosynechococcus elongatus reveals red chlorophyll cluster. Commun. Biol. 2021, 4, 304. [Google Scholar] [CrossRef]
- Chen, M.; Liu, X.; He, Y.; Li, N.; He, J.; Zhang, Y. Diversity among cyanobacterial Photosystem I oligomers. Front. Microbiol. 2022, 12, 781826. [Google Scholar] [CrossRef]
- Bai, T.; Guo, L.; Xu, M.; Tian, L. Structural diversity of photosystem I and its light-harvesting system in eukaryotic algae and plants. Front. Plant Sci. 2021, 12, 781035. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.-R. Structure, function, and variations of the photosystem I-antenna supercomplex from different photosynthetic organisms. In Macromolecular Protein Complexes IV: Subcellular Biochemistry; Harris, J.R., Marles-Wright, J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; Volume 99, pp. 351–377. [Google Scholar]
- Suga, M.; Shen, J.-R. Structural variations of photosystem I-antenna supercomplex in response to adaptations to different light environments. Curr. Opin. Struct. Biol. 2020, 63, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N. Investigating the balance between structural conservation and functional flexibility in Photosystem I. Int. J. Mol. Sci. 2024, 25, 5073. [Google Scholar] [CrossRef] [PubMed]
- Grotjohann, I.; Fromme, P. Structure of cyanobacterial photosystem I. Photosynth. Res. 2005, 85, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Rögner, M.; Mühlenhoff, U.; Boekema, E.J.; Witt, H.T. Mono-, di- and trimeric PS I reaction center complexes isolated from the thermophilic cyanobacterium Synechococcus sp.: Size, shape and activity. Biochim. Biophys. Acta 1990, 1015, 415–424. [Google Scholar] [CrossRef]
- Kato, K.; Nagao, R.; Jiang, T.Y.; Ueno, Y.; Yokono, M.; Chan, S.K.; Watanabe, M.; Ikeuchi, M.; Shen, J.R.; Akimoto, S.; et al. Structure of a cyanobacterial photosystem I tetramer revealed by cryo-electron microscopy. Nat. Commun. 2019, 10, 4929. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, Y.; Li, X.; Zhong, Q.; Li, N.; Zhang, K.; Zhang, Y.; Chu, H.; Ma, C.; Li, G.; et al. Structural and functional insights into the tetrameric photosystem I from heterocyst-forming cyanobacteria. Nat. Plants 2019, 5, 1087–1097. [Google Scholar] [CrossRef]
- Chen, M.; Perez-Boerema, A.; Zhang, L.; Li, Y.; Yang, M.; Li, S.; Amunts, A. Distinct structural modulation of photosystem I and lipid environment stabilizes its tetrameric assembly. Nat. Plants 2020, 6, 314–320. [Google Scholar] [CrossRef]
- Watanabe, M.; Kubota, H.; Wada, H.; Narikawa, R.; Ikeuchi, M. Novel supercomplex organization of photosystem I in Anabaena and Cyanophora paradoxa. Plant Cell Physiol. 2011, 52, 162–168. [Google Scholar] [CrossRef]
- Li, M.; Semchonok, D.A.; Boekema, E.J.; Bruce, B.D. Characterization and evolution of tetrameric photosystem I from the thermophilic cyanobacterium Chroococcidiopsis sp TS-821. Plant Cell. 2014, 26, 1230–1245. [Google Scholar] [CrossRef]
- Li, M.; Calteau, A.; Semchonok, D.A.; Witt, T.A.; Nguyen, J.T.; Sassoon, N.; Boekema, E.J.; Whitelegge, J.; Gugger, M.; Bruce, B.D. Physiological and evolutionary implications of tetrameric photosystem I in cyanobacteria. Nat. Plants 2019, 5, 1309–1319. [Google Scholar] [CrossRef]
- Semchonok, D.A.; Mondal, J.; Cooper, C.J.; Schlum, K.; Li, M.; Amin, M.; Sorzano, C.O.S.; Ramírez-Aportela, E.; Kastritis, P.L.; Boekema, E.J.; et al. Cryo-EM structure of a tetrameric photosystem I from Chroococcidiopsis TS-821, a thermophilic, unicellular, non-heterocyst-forming cyanobacterium. Plant Commun. 2022, 3, 100248. [Google Scholar] [CrossRef]
- Netzer-El, S.Y.; Caspy, I.; Nelson, N. Crystal structure of Photosystem I monomer from Synechocystis PCC 6803. Front. Plant Sci. 2019, 9, 1865. [Google Scholar] [CrossRef]
- Malavath, T.; Caspy, I.; Netzer-El, S.Y.; Klaiman, D.; Nelson, N. Structure and function of wild-type and subunit-depleted photosystem I in Synechocystis. Biochim. Biophys. Acta 2018, 1859, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, V.P.; Chitnis, P.R. PsaL subunit is required for the formation of Photosystem I trimers in the cyanobacterium Synechocystis sp. PCC 6803. FEBS Lett. 1993, 336, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Kłodawska, K.; Kovács, L.; Vladkova, R.; Rzaska, A.; Gombos, Z.; Laczkó-Dobos, H.; Malec, P. Trimeric organization of photosystem I is required to maintain the balanced photosynthetic electron flow in cyanobacterium Synechocystis sp. PCC 6803. Photosynth. Res. 2020, 143, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Cao, D.; Si, L.; Su, X.; Tian, L.; Chang, W.; Liu, Z.; Zhang, X.; Li, M. Structural basis for energy and electron transfer of the photosystem I–IsiA–flavodoxin supercomplex. Nat. Plants 2020, 6, 167–176. [Google Scholar] [CrossRef]
- Toporik, H.; Li, J.; Williams, D.; Chiu, P.-L.; Mazor, Y. The structure of the stress-induced photosystem I–IsiA antenna supercomplex. Nat. Struct. Mol. Biol. 2019, 26, 443–449. [Google Scholar] [CrossRef]
- Nagao, R.; Yokono, M.; Ueno, Y.; Suzuki, T.; Kato, K.; Kato, K.; Tsuboshita, N.; Jiang, T.; Dohmae, N.; Shen, J.-R.; et al. Molecular organizations and function of iron-stress-induced-A protein family in Anabaena sp. PCC 7120. Biochim. Biophys. Acta 2021, 862, 148327. [Google Scholar] [CrossRef] [PubMed]
- Nagao, R.; Kato, K.; Hamaguchi, T.; Ueno, Y.; Tsuboshita, N.; Shimizu, S.; Furutani, M.; Ehira, S.; Nakajima, Y.; Kawakami, K.; et al. Structure of a monomeric photosystem I core associated with iron-stress-induced-A proteins from Anabaena sp. PCC 7120. Nat. Commun. 2023, 14, 920. [Google Scholar] [CrossRef] [PubMed]
- Akita, F.; Nagao, R.; Kato, K.; Nakajima, Y.; Yokono, M.; Ueno, Y.; Suzuki, T.; Dohmae, N.; Shen, J.-R.; Akimoto, S.; et al. Structure of a cyanobacterial photosystem I surrounded by octadecameric isiA antenna proteins. Commun. Biol. 2020, 3, 232. [Google Scholar] [CrossRef]
- Chen, H.-Y.S.; Bandyopadhyay, A.; Pakrasi, H.B. Function, regulation and distribution of IsiA, a membrane-bound chlorophyll a-antenna protein in cyanobacteria. Photosynthetica 2018, 56, 322–333. [Google Scholar] [CrossRef]
- Adir, N.; Bar-Zvi, S.; Harris, D. The amazing phycobilisome. Biochim. Biophys. Acta 2020, 1861, 148047. [Google Scholar] [CrossRef]
- Harris, D.; Toporik, H.; Schlau-Cohen, G.S.; Mazor, Y. Energetic robustness to large scale structural fluctuations in a photosynthetic supercomplex. Nat. Commun. 2023, 14, 4650. [Google Scholar] [CrossRef] [PubMed]
- Umena, Y.; Kawakami, K.; Shen, J.-R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef]
- La Roche, J.; Van der Staay, G.W.M.; Partensky, F.; Ducret, A.; Aebersold, R.; Li, R.; Golden, S.S.; Hiller, R. G.; Wrench, P. M.; Larkum, A.W.; et al. Independent evolution of the prochlorophyte and green plant chlorophyll a/b light-harvesting proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 15244–15248. [Google Scholar] [CrossRef]
- Watanabe, M.; Semchonok, D.A.; Webber-Birungi, M.T.; Ehira, S.; Kondo, K.; Narikawa, R.; Ohmori, M.; Boekema, E.J.; Ikeuchi, M. Attachment of phycobilisomes in an antenna–photosystem I supercomplex of cyanobacteria. Proc. Natl. Acad. Sci. USA 2014, 111, 2512–2517. [Google Scholar] [CrossRef]
- You, X.; Zhang, X.; Cheng, J.; Xiao, Y.; Ma, J.; Sun, S.; Zhang, X.; Wang, H.W.; Sui, S.F. In situ structure of the red algal phycobilisome–PSII–PSI–LHC megacomplex. Nature 2023, 616, 199–206. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Liu, D.; Qin, S.; Sun, S.; Zhao, J.; Sui, S.-F. Structure of phycobilisome from the red alga Griffitshia pacifica. Nature 2017, 551, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Boekema, E.J.; Hifney, A.; Yakushevska, A.E.; Piotrowski, M.; Keegstra, W.; Berry, S.; Michel, K.P.; Pistorius, E.K.; Kruip, J. A giant chlorophyll-protein complex induced by iron deficiency in cyanobacteria. Nature 2001, 412, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Bibby, T.S.; Nield, J.; Barber, J. Iron defciency induces the formation of an antenna ring around trimeric photosystem I in cyanobacteria. Nature 2001, 412, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Melkozernov, A.N.; Bibby, T.S.; Lin, S.; Barber, J.; Blankenship, R.E. Time-resolved absorption and emission show that the CP43′ antenna ring of iron-stressed Synechocystis sp. PCC6803 is efficiently coupled to the Photosystem I reaction center core. Biochemistry 2003, 42, 3893–3903. [Google Scholar] [CrossRef] [PubMed]
- Andrizhiyevskaya, E.G.; Frolov, D.; Van Grondelle, R.; Dekker, J.P. Energy transfer and trapping in the Photosystem I complex of Synechococcus PCC 7942 and in its supercomplex with IsiA. Biochim. Biophys. Acta 2004, 1656, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Ryan-Keogh, T.J.; Macey, A.I.; Cockshutt, A.M.; Moore, C.M.; Bibby, T.S. The cyanobacterial chlorophyll-binding-protein isiA acts to increase the in vivo effective absorption cross-section of PSI under iron limitation. J. Phycol. 2011, 48, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Sandström, S.; Park, Y.-I.; Öquist, G.; Gustafsson, P. CP43′, the IsiA gene product, functions as an excitation energy dissipator in the cyanobacterium Synechococcus Sp. PCC 7942. Photochem. Photobiol. 2007, 74, 431–437. [Google Scholar] [CrossRef]
- Ihalainen, J.A.; D’Haene, S.; Yeremenko, N.; van Roon, H.; Arteni, A.A.; Boekema, E.J.; van Grondelle, R.; Matthijs, H.C.P.; Dekker, J.P. Aggregates of the chlorophyll-binding protein IsiA (CP43′) dissipate energy in cyanobacteria. Biochemistry 2005, 44, 10846–10853. [Google Scholar] [CrossRef] [PubMed]
- Yeremenko, N.; Kouřil, R.; Ihalainen, J.A.; D’haene, S.; Van Oosterwijk, N.; Andrizhiyevskaya, E.G.; Keegstra, W.; Dekker, H.L.; Hagemann, M.; Boekema, E.J.; et al. Supramolecular organization and dual function of the isia chlorophyll-binding protein in cyanobacteria. Biochemistry 2004, 43, 10308–10313. [Google Scholar] [CrossRef]
- Goñi, G.; Herguedas, B.; Hervás, M.; Peregrina, J.R.; De La Rosa, M.A.; Gómez-Moreno, C.; Navarro, J.A.; Hermoso, J.A.; Martínez-Júlvez, M.; Medina, M. Flavodoxin: A compromise between efficiency and versatility in the electron transfer from Photosystem I to Ferredoxin-NADP+ reductase. Biochim. Biophys. Acta 2009, 1787, 144–154. [Google Scholar] [CrossRef]
- Kato, K.; Shinoda, T.; Nagao, R.; Akimoto, S.; Suzuki, T.; Dohmae, N.; Chen, M.; Allakhverdiev, S.I.; Shen, J.-R.; Akita, F.; et al. Structural basis for the adaptation and function of chlorophyll f in photosystem I. Nat. Commun. 2020, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Schliep, M.; Willows, R.D.; Cai, Z.L.; Neilan, B.A.; Scheer, H. A red-shifted chlorophyll. Science 2010, 329, 1318–1319. [Google Scholar] [CrossRef] [PubMed]
- Melkozernov, A.N.; Blankenship, R.E. Photosynthetic Functions of Chlorophylls. In Chlorophylls and Bacteriochlorophylls; Grimm, B., Porra, R.J., Rüdiger, W., Scheer, H., Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2006; pp. 397–412. ISBN 978-1-4020-4516-5. [Google Scholar]
- Motten, A.F. Diversity of Photosynthetic Pigments. In Tested Studies for Laboratory Teaching, Proceedings of the 16th Workshop/Conference of the Association for Biology Laboratory Education (ABLE), Atlanta, Georgia, 7–11 June 1994; Goldman, C.A., Ed.; Yale University, Department of Biology: New Haven, CT, USA, 1995; Volume 16, pp. 81–98. [Google Scholar]
- Gisriel, C.J. Recent structural discoveries of photosystems I and II acclimated to absorb far-red light. Biochim. Biophys. Acta 2024, 1865, 149032. [Google Scholar] [CrossRef] [PubMed]
- Büchel, C. Light harvesting complexes in chlorophyll c-containing algae. Biochim. Biophys. Acta 2020, 1861, 148027. [Google Scholar] [CrossRef] [PubMed]
- Croce, R.; van Amerongen, H. Light harvesting in oxygenic photosynthesis: Structural biology meets spectroscopy. Science 2020, 369, eaay2058. [Google Scholar] [CrossRef] [PubMed]
- Gan, F.; Zhang, S.; Rockwell, N.C.; Martin, S.S.; Lagarias, J.C.; Bryant, D.A. Extensive remodeling of a cyanobacterial photosynthetic apparatus in far-red light. Science 2014, 345, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Airs, R.L.; Temperton, B.; Sambles, C.; Farnham, G.; Skill, S.C.; Llewellyn, C.A. Chlorophyll f and chlorophyll d are produced in the cyanobacterium Chlorogloeopsis fritschii when cultured under natural light and near–infrared radiation. FEBS Lett. 2014, 588, 3770–3777. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, L.; Brejnrod, A.; Schliep, M.; Sørensen, S.J.; Larkum, A.W.; Kühl, M. Chlorophyll f-driven photosynthesis in a cavernous cyanobacterium. ISME J. 2015, 9, 2108–2111. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, H.; Ikemoto, H.; Kurano, N.; Adachi, K.; Chihara, M.; Miyachi, S. Chlorophyll d as a major pigment. Nature 1996, 383, 402. [Google Scholar] [CrossRef]
- Miyashita, H.; Ohkubo, S.; Komatsu, H.; Sorimachi, Y.; Fukayama, D.; Fujinuma, D.; Akutsu, S.; Kobayashi, M. Discovery of chlorophyll d in Acaryochloris marina and chlorophyll f in a unicellular cyanobacterium, Strain KC1, isolated from Lake Biwa. J. Phys. Chem. Biophys. 2014, 4, 149. [Google Scholar] [CrossRef]
- Loughlin, P.; Lin, Y.; Chen, M. Chlorophyll d and Acaryochloris marina: Current status. Photosynth. Res. 2013, 116, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Kawakami, K.; Shinzawa-Itoh, K.; Inoue-Kashino, N.; Itoh, S.; Ifuku, K.; Yamashita, E.; Maeda, K.; Yonekura, K.; Kashino, Y. Structure of the far-red light utilizing photosystem I of Acaryochloris marina. Nat. Commun. 2021, 12, 2333. [Google Scholar] [CrossRef]
- Xu, C.; Zhu, Q.; Chen, J.; Shen, L.; Yi, X.; Huang, Z.; Wang, W.; Chen, M.; Kuang, T.; Shen, J.; et al. A unique photosystem I reaction center from a chlorophyll d-containing cyanobacterium Acaryochloris marina. J. Integr. Plant Biol. 2021, 63, 1740–1752. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, Y.; Birch, D.; Willows, R.D. A cyanobacterium that contains chlorophyll f–a red-absorbing photopigment. FEBS Lett. 2012, 586, 3249–3254. [Google Scholar] [CrossRef] [PubMed]
- Kühl, M.; Trampe, E.; Mosshammer, M.; Johnson, M.; Larkum, A.W.; Frigaard, N.U.; Koren, K. Substantial near-infrared radiation-driven photosynthesis of chlorophyll f-containing cyanobacteria in a natural habitat. eLife 2020, 9, e50871. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Canniffe, D.P.; Ho, M.Y.; Kurashov, V.; van der Est, A.; Golbeck, J.H.; Bryant, D.A. Characterization of chlorophyll f synthase heterologously produced in Synechococcus sp. PCC 7002. Photosynth. Res. 2019, 140, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Nürnberg, D.J.; Morton, J.; Santabarbara, S.; Telfer, A.; Joliot, P.; Antonaru, L.A.; Ruban, A.V.; Cardona, T.; Krausz, E.; Boussac, A.; et al. Photochemistry beyond the red limit in chlorophyll f-containing photosystems. Science 2018, 360, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Gisriel, C.; Shen, G.; Kurashov, V.; Ho, M.-Y.; Zhang, S.; Williams, D.; Golbeck, J.H.; Fromme, P.; Bryant, D.A. The structure of Photosystem I acclimated to far-red light illuminates an ecologically important acclimation process in photosynthesis. Sci. Adv. 2020, 6, eaay6415. [Google Scholar] [CrossRef]
- Ho, M.-Y.; Shen, G.; Canniffe, D.P.; Zhao, C.; Bryant, D.A. Light-dependent chlorophyll f synthase is a highly divergent paralog of PsbA of photosystem II. Science 2016, 353, aaf9178. [Google Scholar]
- Zhao, C.; Gan, F.; Shen, G.; Bryant, D.A. RfpA, RfpB, and RfpC are the master control elements of far-red light photoacclimation (FaRLiP). Front. Microbiol. 2015, 6, 1303. [Google Scholar]
- Chen, M.; Hernandez-Prieto, M.A.; Loughlin, P.C.; Li, Y.; Willows, R.D. Genome and proteome of the chlorophyll f-producing cyanobacterium Halomicronema hongdechloris: Adaptative proteomic shifts under different light conditions. BMC Genom. 2019, 20, 207. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, S.; Merchant, S.S. Chlamydomonas reinhardtii: A model for photosynthesis and so much more. Nat. Methods 2023, 20, 1441–1442. [Google Scholar] [CrossRef]
- Suga, M.; Ozawa, S.-I.; Yoshida-Motomura, K.; Akita, F.; Miyazaki, N.; Takahashi, Y. Structure of the green algal photosystem I supercomplex with a decameric light-harvesting complex I. Nat. Plants 2019, 5, 626–636. [Google Scholar] [CrossRef]
- Mullineaux, C.W. State transitions: An example of acclimation to low-light stress. J. Exp. Bot. 2004, 56, 389–393. [Google Scholar] [CrossRef]
- Allen, J.F. Protein phosphorylation in regulation of photosynthesis. Biochim. Biophys. Acta 1992, 1098, 275–335. [Google Scholar] [CrossRef]
- Nawrocki, W.J.; Santabarbara, S.; Mosebach, L.; Wollman, F.-A.; Rappaport, F. State transitions redistribute rather than dissipate energy between the two photosystems in Chlamydomonas. Nat. Plants 2016, 2, 16031. [Google Scholar] [CrossRef] [PubMed]
- Bellafiore, S.; Barneche, F.; Peltier, G.; Rochaix, J.-D. State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 2005, 433, 892–895. [Google Scholar] [CrossRef]
- Wollman, F.A. State-transitions-reveal-the-dynamics-and-flexibility-of-the-photosynthetic-apparatus. EMBO J. 2001, 20, 3623–3630. [Google Scholar] [CrossRef]
- Wu, J.; Chen, S.; Wang, C.; Lin, W.; Huang, C.; Fan, C.; Han, D.L.D.; Xu, X.; Sui, S.; Zhang, L. Regulatory dynamics of the higher-plant PSI–LHCI supercomplex during state transitions. Mol. Plant 2023, 16, 1937–1950. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Li, M.; Pan, X. Dynamic regulation of the light-harvesting system through state transitions in land plants and green algae. Plants 2023, 12, 1173. [Google Scholar] [CrossRef]
- Huang, Z.; Shen, L.; Wang, W.; Mao, Z.; Yi, X.; Kuang, T.; Shen, J.-R.; Zhang, X.; Han, G. Structure of photosystem I-LHCI-LHCII from the green alga Chlamydomonas reinhardtii in State 2. Nat. Commun. 2021, 12, 1100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tang, K.; Yan, Q.; Li, X.; Shen, L.; Wang, W.; He, Y.-K.; Kuang, T.; Han, G.; Shen, J.R.; et al. Structural insights into a unique PSI–LHCI–LHCII–Lhcb9 supercomplex from moss Physcomitrium patens. Nat. Plants 2023, 9, 832–846. [Google Scholar] [CrossRef] [PubMed]
- Lima-Melo, Y.; Kiliç, M.; Aro, E.-M.; Gollan, P.J. Photosystem I inhibition, protection and signalling: Knowns and unknowns. Front. Plant Sci. 2021, 12, 791124. [Google Scholar] [CrossRef] [PubMed]
- Zavafer, A.; Mancilla, C. Concepts of photochemical damage of Photosystem II and the role of excessive excitation. J Photoch. Photobio. C. 2021, 47, 100421. [Google Scholar] [CrossRef]
- Su, J.; Jiao, Q.; Jia, T.; Hu, X. The photosystem-II repair cycle: Updates and open questions. Planta 2023, 259, 20. [Google Scholar] [CrossRef] [PubMed]
- Naschberger, A.; Mosebach, L.; Tobiasson, V.; Kuhlgert, S.; Scholz, M.; Perez-Boerema, A.; Ho, T.T.H.; Vidal-Meireles, A.; Takahashi, Y.; Hippler, M.; et al. Algal photosystem I dimer and high-resolution model of PSI-plastocyanin complex. Nat. Plants 2022, 8, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Ma, J.; Pan, X.; Zhao, X.; Chang, W.; Liu, Z.; Zhang, X.; Li, M. Antenna arrangement and energy transfer pathways of a green algal photosystem-I–LHCI supercomplex. Nat. Plants 2019, 5, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Pi, X.; Tian, L.; Dai, H.-E.; Qin, X.; Cheng, L.; Kuang, T.; Sui, S.-F.; Shen, J.-R. Unique organization of photosystem I–light-harvesting supercomplex revealed by Cryo-EM from a red alga. Proc. Natl. Acad. Sci. USA 2018, 115, 4423–4428. [Google Scholar] [CrossRef]
- Chang, L.; Tian, L.; Ma, F.; Mao, Z.; Liu, X.; Han, G.; Wang, W.; Yang, Y.; Kuang, T.; Pan, J.; et al. Regulation of photosystem I-light-harvesting complex I from a red alga Cyanidioschyzon Merolae in response to light intensities. Photosynth. Res. 2020, 146, 287–297. [Google Scholar] [CrossRef]
- Qin, X.; Pi, X.; Wang, W.; Han, G.; Zhu, L.; Liu, M.; Cheng, L.; Shen, J.-R.; Kuang, T.; Sui, S.-F. Structure of a green algal photosystem I in complex with a large number of light-harvesting complex I subunits. Nat. Plants 2019, 5, 263–272. [Google Scholar] [CrossRef]
- Perez-Boerema, A.; Klaiman, D.; Caspy, I.; Netzer-El, S.Y.; Amunts, A.; Nelson, N. Structure of a minimal photosystem I from the green alga Dunaliella salina. Nat. Plants 2020, 6, 321–327. [Google Scholar] [CrossRef]
- Treves, H.; Raanan, H.; Kedem, I.; Murik, O.; Keren, N.; Zer, H.; Berkowicz, S.M.; Giordano, M.; Norici, A.; Shotland, Y.; et al. The mechanisms whereby the green alga Chlorella ohadii, isolated from desert soil crust, exhibits unparalleled photodamage resistance. New Phytol. 2016, 210, 1229–1243. [Google Scholar] [CrossRef]
- Kedem, I.; Milrad, Y.; Kaplan, A.; Yacoby, I. Juggling lightning: How Chlorella ohadii handles extreme energy inputs without damage. Photosynth. Res. 2021, 147, 329–344. [Google Scholar] [CrossRef]
- Caspy, I.; Neumann, E.; Fadeeva, M.; Liveanu, V.; Savitsky, A.; Frank, A.; Kalisman, Y.L.; Shkolnisky, Y.; Murik, O.; Treves, H.; et al. Cryo-EM photosystem I structure reveals adaptation mechanisms to extreme high light in Chlorella ohadii. Nat. Plants 2021, 7, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Malviya, S.; Scalco, E.; Audic, S.; Vincent, F.; Veluchamy, A.; Poulain, J.; Wincker, P.; Iudicone, D.; De Vargas, C.; Bittner, L.; et al. Insights into global diatom distribution and diversity in the world’s ocean. Proc. Natl. Acad. Sci. USA 2016, 113, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Büchel, C. Light-Harvesting Complexes of Diatoms: Fucoxanthin-Chlorophyll Proteins. In Photosynthesis in Algae: Biochemical and Physiological Mechanisms. Advances in Photosynthesis and Respiration; Larkum, A., Grossman, A., Raven, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 441–457. ISBN 978-3-030-33397-3. [Google Scholar]
- Xu, C.; Pi, X.; Huang, Y.; Han, G.; Chen, X.; Qin, X.; Huang, G.; Zhao, S.; Yang, Y.; Kuang, T.; et al. Structural basis for energy transfer in a huge diatom PSI-FCPI supercomplex. Nat. Commun. 2020, 11, 5081. [Google Scholar] [CrossRef] [PubMed]
- Löffelhardt, W.; Bohnert, H.J.; Bryant, D.A.; Hagemann, R. The cyanelles of Cyanophora paradoxa. Crit. Rev. Plant Sci. 1997, 16, 393–413. [Google Scholar] [CrossRef]
- Kato, K.; Nagao, R.; Ueno, Y.; Yokono, M.; Suzuki, T.; Jiang, T.-Y.; Dohmae, N.; Akita, F.; Akimoto, S.; Miyazaki, N.; et al. Structure of a tetrameric photosystem I from a glaucophyte alga Cyanophora paradoxa. Nat. Commun. 2022, 13, 1679. [Google Scholar] [CrossRef]
- Stiller, J.W.; Schreiber, J.; Yue, J.; Guo, H.; Ding, Q.; Huang, J. The evolution of photosynthesis in chromist algae through serial endosymbioses. Nat. Commun. 2014, 5, 5764. [Google Scholar] [CrossRef]
- Zimorski, V.; Ku, C.; Martin, W.F.; Gould, S.B. Endosymbiotic theory for organelle origins. Curr. Opin. Microbiol. 2014, 22, 38–48. [Google Scholar] [CrossRef]
- Kim, J.I.; Moore, C.E.; Archibald, J.M.; Bhattacharya, D.; Yi, G.; Yoon, H.S.; Shin, W. Evolutionary dynamics of cryptophyte plastid genomes. Genome Biol. Evol. 2017, 9, 1859–1872. [Google Scholar] [CrossRef]
- Hoffman, G.E.; Sanchez-Puerta, M.V.; Delwiche, C.F. Evolution of light harvesting complex proteins from Chl c-containing algae. BMC Evol. Biol. 2011, 11, 101. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, P.; Li, K.; Zhang, Q.; He, F.; Li, C.; Su, H.; Chen, X.; Liu, L.; Zhang, Y. Structural basis and evolution of the photosystem I–light-harvesting supercomplex of cryptophyte algae. Plant Cell 2023, 35, 2449–2463. [Google Scholar] [CrossRef]
- Cohen, N.R.; McIlvin, M.R.; Moran, D.M.; Held, N.A.; Saunders, J.K.; Hawco, N.J.; Brosnahan, M.; DiTullio, G.R.; Lamborg, C.; McCrow, J.P.; et al. Dinoflagellates alter their carbon and nutrient metabolic strategies across environmental gradients in the central Pacific Ocean. Nat. Microbiol. 2021, 6, 173–186. [Google Scholar] [CrossRef]
- Stephens, T.G.; Ragan, M.A.; Bhattacharya, D.; Chan, C.X. Core genes in diverse dinoflagellate lineages include a wealth of conserved dark genes with unknown functions. Sci. Rep. 2018, 8, 17175. [Google Scholar] [CrossRef] [PubMed]
- Janouškovec, J.; Gavelis, G.S.; Burki, F.; Dinh, D.; Bachvaroff, T.R.; Gornik, S.G.; Bright, K.J.; Imanian, B.; Strom, S.L.; Delwiche, C.F.; et al. Major transitions in dinoflagellate evolution unveiled by phylotranscriptomics. Proc. Natl. Acad. Sci. USA 2017, 114, 171–180. [Google Scholar] [CrossRef]
- Aranda, M.; Li, Y.; Liew, Y.J.; Baumgarten, S.; Simakov, O.; Wilson, M.C.; Piel, J.; Ashoor, H.; Bougouffa, S.; Bajic, V.B.; et al. Genomes of coral dinoflagellate symbionts highlight evolutionary adaptations conducive to a symbiotic lifestyle. Sci. Rep. 2016, 6, 39734. [Google Scholar] [CrossRef] [PubMed]
- Jacobovitz, M.R.; Hambleton, E.A.; Guse, A. Unlocking the complex cell biology of coral–dinoflagellate symbiosis: A model systems approach. Annu. Rev. Genet. 2023, 57, 411–434. [Google Scholar] [CrossRef] [PubMed]
- Hehenberger, E.; Burki, F.; Kolisko, M.; Keeling, P.J. Functional relationship between a dinoflagellate host and its diatom endosymbiont. Mol. Biol. Evol. 2016, 33, 2376–2390. [Google Scholar] [CrossRef]
- Lin, S.; Wu, S.; He, J.; Wang, X.; Grossman, A.R. Shining light on dinoflagellate photosystem I. Nat. Commun. 2024, 15, 3337. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Wang, F.; Zhao, S.; Xu, C.; Mao, Z.; Duan, J.; Feng, Y.; Yang, Y.; Shen, L.; et al. Structures and organizations of PSI–AcpPCI supercomplexes from red tidal and coral symbiotic photosynthetic dinoflagellates. Proc. Natl. Acad. Sci. USA 2024, 121, e2315476121. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, N.; Li, K.; Li, C.; Guo, J.; He, F.; Liu, G.; Chen, X.; Gao, J.; Liu, L.; et al. Architecture of symbiotic dinoflagellate photosystem I–light-harvesting supercomplex in Symbiodinium. Nat. Commun. 2024, 15, 2392. [Google Scholar] [CrossRef] [PubMed]
- Sejima, T.; Takagi, D.; Fukayama, H.; Makino, A.; Miyake, C. Repetitive short-pulse light mainly inactivates photosystem I in sunflower leaves. Plant Cell Physiol. 2014, 55, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yamamoto, H.; Shikanai, T. Distinct contribution of two cyclic electron transport pathways to P700 oxidation. Plant Physiol. 2023, 192, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Shikanai, T. PGR5-dependent cyclic electron flow protects PSI under fluctuating light at donor and acceptor sides. Plant Physiol. 2019, 179, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Shikanai, T. Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annu. Rev. Plant Biol. 2016, 67, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Chaux, F.; Peltier, G.; Johnson, X. A security network in PSI photoprotection: Regulation of photosynthetic control, NPQ and O2 photoreduction by cyclic electron flow. Front. Plant Sci. 2015, 6, 875. [Google Scholar] [CrossRef]
- Shimakawa, G.; Miyake, C. Oxidation of P700 Ensures Robust Photosynthesis. Front. Plant Sci. 2018, 9, 1617. [Google Scholar] [CrossRef]
- Gisriel, C.; Sarrou, I.; Ferlez, B.; Golbeck, J.H.; Redding, K.E.; Fromme, R. Structure of a symmetric photosynthetic reaction center–photosystem. Science 2017, 357, 1021–1025. [Google Scholar] [CrossRef]
- Chen, J.-H.; Wu, H.; Xu, C.; Liu, X.-C.; Huang, Z.; Chang, S.; Wang, W.; Han, G.; Kuang, T.; Shen, J.-R.; et al. Architecture of the photosynthetic complex from a green sulfur bacterium. Science 2020, 370, eabb6350. [Google Scholar] [CrossRef]
- Dong, S.; Huang, G.; Wang, C.; Wang, J.; Sui, S.-F.; Qin, X. Structure of the Acidobacteria homodimeric reaction center bound with cytochrome c. Nat. Commun. 2022, 13, 7745. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, L.-R.; Chen, J.-H. Photosystem I: A Paradigm for Understanding Biological Environmental Adaptation Mechanisms in Cyanobacteria and Algae. Int. J. Mol. Sci. 2024, 25, 8767. https://doi.org/10.3390/ijms25168767
Tian L-R, Chen J-H. Photosystem I: A Paradigm for Understanding Biological Environmental Adaptation Mechanisms in Cyanobacteria and Algae. International Journal of Molecular Sciences. 2024; 25(16):8767. https://doi.org/10.3390/ijms25168767
Chicago/Turabian StyleTian, Li-Rong, and Jing-Hua Chen. 2024. "Photosystem I: A Paradigm for Understanding Biological Environmental Adaptation Mechanisms in Cyanobacteria and Algae" International Journal of Molecular Sciences 25, no. 16: 8767. https://doi.org/10.3390/ijms25168767
APA StyleTian, L.-R., & Chen, J.-H. (2024). Photosystem I: A Paradigm for Understanding Biological Environmental Adaptation Mechanisms in Cyanobacteria and Algae. International Journal of Molecular Sciences, 25(16), 8767. https://doi.org/10.3390/ijms25168767




