Green Extraction of Reed Lignin: The Effect of the Deep Eutectic Solvent Composition on the UV-Shielding and Antioxidant Properties of Lignin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Spectral Properties of Lignins
2.2. Antioxidant Activity and the Total Phenolic Content of Lignins
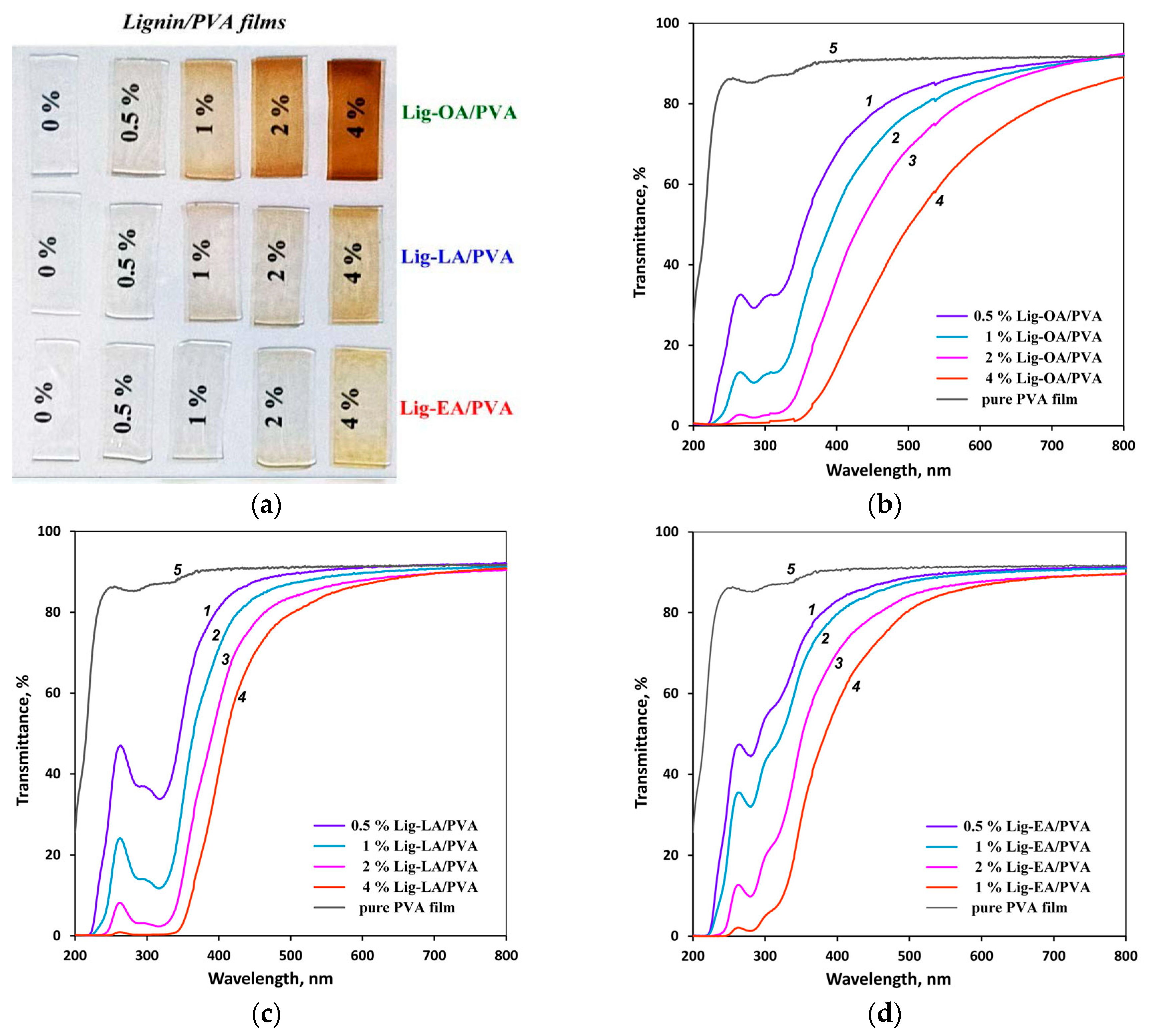
2.3. Optical and UV-Protective Properties of Polyvinyl Alcohol/Lignin Films
3. Materials and Methods
3.1. Materials
3.2. DESs Synthesis, Reed Straw Pretreatment, and Lignin Recovery
3.3. Preparation of Nanosized Lignin Particles (NPLig)
3.4. Preparation of Lig/PVA Composite Films
3.5. Characterization of Lignins
3.6. Determination of Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Usmani, Z.; Sharma, M.; Awasthi, A.K.; Lukk, T.; Tuohy, M.G.; Gong, L.; Nguyen-Tri, P.; Goddard, A.D.; Bill, R.M.; Nayak, S.C.; et al. Lignocellulosic biorefineries: The current state of challenges and strategies for efficient commercialization. Renew. Sustain. Energy Rev. 2021, 148, 111258. [Google Scholar] [CrossRef]
- Kumar, B.; Verma, P. Biomass-based biorefineries: An important architype towards a circular economy. Fuel 2021, 288, 119622. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Chowdhury, S.N.; Nahrin, M.; Rafa, N.; Chowdhury, A.T.; Nuzhat, S.; Ong, H.C. Pathways of lignocellulosic biomass deconstruction for biofuel and value-added products production. Fuel 2022, 318, 123618. [Google Scholar] [CrossRef]
- Brienza, F.; Cannella, D.; Montesdeoca, D.; Cybulska, I.; Debecker, D.P. A guide to lignin valorization in biorefineries: Traditional, recent, and forthcoming approaches to convert raw lignocellulose into valuable materials and chemicals. RSC Sustain. 2024, 2, 37–90. [Google Scholar] [CrossRef]
- Demuner, I.F.; Colodette, J.L.; Demuner, A.J.; Jardim, C.M. Biorefinery review: Wide-reaching products through kraft lignin. BioResources 2019, 14, 7543–7581. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of Lignocellulosic Fibers and Lignin in Bioplastics: A Review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Sethupathy, S.; Morales, G.M.; Gao, L.; Wang, H.; Yang, B.; Jiang, J.; Sun, J.; Zhu, D. Lignin valorization: Status, challenges and opportunities. Bioresour. Technol. 2022, 347, 126696. [Google Scholar] [CrossRef] [PubMed]
- Gujjala, L.K.S.; Kim, J.; Won, W. Technical lignin to hydrogels: An Eclectic review on suitability, synthesis, applications, challenges and future prospects. J. Clean. Prod. 2022, 363, 132585. [Google Scholar] [CrossRef]
- Zhou, M.; Fakayode, O.A.; Yagoub, A.E.A.; Ji, Q.; Zhou, C. Lignin fractionation from lignocellulosic biomass using deep eutectic solvents and its valorization. Renew. Sustain. Energy Rev. 2022, 156, 111986. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.L.; Chen, C.W.; Sun, P.P.; Patel, A.K.; Singhania, R.R.; Nargotra, P.; Dong, C.D. Deep eutectic solvents as promising pretreatment agents for sustainable lignocellulosic biorefineries: A review. Bioresour. Technol. 2022, 360, 127631. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.; Row, K.H. Development of deep eutectic solvents for sustainable chemistry. J. Mol. Liq. 2022, 362, 119654. [Google Scholar] [CrossRef]
- Bao, Y.; Wang, Y.; Yan, C.; Xue, Z. Deep eutectic solvents for fractionation and valorization of lignocellulose. Green Chem. Eng. 2024, in press. [CrossRef]
- Xiao, T.; Hou, M.; Guo, X.; Cao, X.; Li, C.; Zhang, Q.; Jia, W.; Sun, Y.; Guo, Y.; Shi, H. Recent progress in deep eutectic solvent (DES) fractionation of lignocellulosic component: A review. Renew. Sustain. Energy Rev. 2024, 192, 114243. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Munro, H.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Morozova, O.V.; Vasil’eva, I.S.; Shumakovich, G.P.; Zaitseva, E.A.; Yaropolov, A.I. Deep eutectic solvents for biotechnology applications. Biochem. Mosc. 2023, 88, S150–S175. [Google Scholar] [CrossRef] [PubMed]
- Jurić, T.; Uka, D.; Holló, B.B.; Jović, B.; Kordić, B.; Popović, B.M. Comprehensive physicochemical evaluation of choline chloride-based natural deep eutectic solvents. J. Mol. Liq. 2021, 343, 116968. [Google Scholar] [CrossRef]
- Duan, L.; Dou, L.-L.; Guo, L.; Li, P.; Liu, E.H. Comprehensive evaluation of deep eutectic solvents in extraction of bioactive natural products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. New natural and renewable low transition temperature mixtures (LTTMs): Screening as solvents for lignocellulosic biomass processing. Green Chem. 2012, 14, 2153–2157. [Google Scholar] [CrossRef]
- Lynam, J.G.; Kumar, N.; Wong, M.J. Deep eutectic solvents’ ability to solubilize lignin, cellulose, and hemicellulose; thermal stability; and density. Bioresour. Technol. 2017, 238, 684–689. [Google Scholar] [CrossRef]
- Soares, B.; Tavares, D.J.P.; Amaral, J.L.; Silvestre, A.J.D.; Freire, C.S.R.; Coutinho, J.A.P. Enhanced solubility of lignin monomeric model compounds and technical lignins in aqueous solutions of deep eutectic solvents. ACS Sustain. Chem. Eng. 2017, 5, 4056–4065. [Google Scholar] [CrossRef]
- Xiao, B.; Sun, X.F.; Sun, R. Chemical, structural, and thermal characterizations of alkali-soluble lignins and hemicelluloses, and cellulose from maize stems, rye straw, and rice straw. Polym. Degrad. Stab. 2001, 74, 307–319. [Google Scholar] [CrossRef]
- Yang, L.; Wang, D.; Zhou, D.; Zhang, Y. Effect of different isolation methods on structure and properties of lignin from valonea of Quercus variabilis. Int. J. Biol. Macromol. 2016, 85, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lv, W.; Huang, D.; Zeng, C.; Wang, R. Physicochemical properties, thermal stability, and pyrolysis behavior of antioxidative lignin from water chestnut shell obtained with ternary deep eutectic solvents. Molecules 2023, 28, 4088. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Lu, G.; Pei, W.; Yan, W.; Li, Y.; Zhang, L.; Huang, C.; Jiang, Q. Understanding the relationship between the structural properties of lignin and their biological activities. Int. J. Biol. Macromol. 2021, 190, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Culebras, M.; Beaucamp, A.; Wang, Y.; Clauss, M.M.; Frank, E.; Collins, M.N. Biobased structurally compatible polymer blends based on lignin and thermoplastic elastomer polyurethane as carbon fiber precursors. ACS Sustain. Chem. Eng. 2018, 6, 8816–8825. [Google Scholar] [CrossRef]
- Zadeh, E.M.; O’Keefe, S.F.; Kim, Y.-T. Utilization of lignin in biopolymeric packaging films. ACS Omega 2018, 3, 7388–7398. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Collins, M.N.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; McLoughlin, A.; Stróżyk, M.A.; Culebras, M.; Teacă, C.-A. Valorization of lignin in polymer and composite systems for advanced engineering applications—A review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Robles, J.; Cárcamo-Martínez, Á.; Stewart, S.A.; Donnelly, R.F.; Larrañeta, E.; Borrega, M. Lignin for pharmaceutical and biomedical applications—Could this become a reality? Sustain. Chem. Pharm. 2020, 18, 100320. [Google Scholar] [CrossRef]
- Kaur, R.; Thakur, N.S.; Chandna, S.; Bhaumik, J. Sustainable lignin-based coatings doped with titanium dioxide nanocomposites exhibit synergistic microbicidal and UV-blocking performance toward personal protective equipment. ACS Sustain. Chem. Eng. 2021, 9, 11223–11237. [Google Scholar] [CrossRef]
- Ullah, I.; Chen, Z.; Xie, Y.; Khan, S.S.; Singh, S.; Yu, C.; Cheng, G. Recent advances in biological activities of lignin and emerging biomedical applications: A short review. Int. J. Biol. Macromol. 2022, 208, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ochoa, F.; Vergara, P.; Wojtusik, M.; Gutiérrez, S.; Santos, V.E.; Ladero, M.; Villar, J.C. Multi-feedstock lignocellulosic biorefineries based on biological processes: An overview. Ind. Crops Prod. 2021, 172, 114062. [Google Scholar] [CrossRef]
- Semenova, M.V.; Vasil’eva, I.S.; Yaropolov, A.I.; Sinitsyn, A.P. Cane pretreatment by deep eutectic solvents to increase its reactivity during enzymatic hydrolysis with cellulases. Appl. Biochem. Microbiol. 2023, 59, 290–296. [Google Scholar] [CrossRef]
- Semenova, M.V.; Rozhkova, A.M.; Osipov, D.O.; Telitsin, V.D.; Rubtsova, E.A.; Kondrat`eva, E.G.; Vasil’eva, I.S.; Morozova, O.V.; Yaropolov, A.I.; Sinitsyn, A.P. Methods for pre-processing cane to obtain enzymative hydrolysates with high sugar content. Appl. Biochem. Microbiol. 2024, 60, 931–941. [Google Scholar]
- Zhong, L.; Wang, C.; Xu, M.; Ji, X.; Yang, G.; Chen, J.; Janaswamy, S.; Lyu, G. Alkali-Catalyzed Organosolv Pretreatment of Lignocellulose Enhances Enzymatic Hydrolysis and Results in Highly Antioxidative Lignin. Energy Fuels 2021, 35, 5039–5048. [Google Scholar] [CrossRef]
- Lybeer, B.; Koch, G. Lignin distribution in the tropical bamboo species Gigantochloa levis. IAWA J. 2005, 26, 443–456. [Google Scholar] [CrossRef]
- Sun, S.L.; Wen, J.L.; Ma, M.G.; Li, M.F.; Sun, R.C. Revealing the structural inhomogeneity of lignins from sweet sorghum stem by successive alkali extractions. J. Agric. Food Chem. 2013, 61, 4226–4235. [Google Scholar] [CrossRef] [PubMed]
- Vivekanand, V.; Chawade, A.; Larsson, M.; Larsson, A.; Olsson, O. Identification and qualitative characterization of high and low lignin lines from an oat TILLING population. Ind. Crops Prod. 2014, 59, 1–8. [Google Scholar] [CrossRef]
- Faix, O. Classification of Lignins from Different Botanical Origins by FT-IR Spectroscopy. Holzforschung 1991, 45, 21–28. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.A.; van Dam, J.E.G. Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind. Crops Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, J.; Yang, Q.; Hu, J.; Zhou, Q.; Wang, L.; Liu, Z.; Hui, L. Solid acid facilitated deep eutectic solvents extraction of high-purity and antioxidative lignin production from poplar wood. Int. J. Biol. Macromol. 2021, 193, 64–70. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Wang, G.; Jia, H.; Liu, C.; Sui, W.; Si, C. Fractionation of enzymatic hydrolysis lignin by sequential extraction for enhancing antioxidant performance. Int. J. Biol. Macromol. 2017, 99, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Fu, Y.; Wang, P.; Zhang, Y.; Qin, M.; Li, X.; Zhang, F. Modification of the aspen lignin structure during integrated fractionation process of autohydrolysis and formic acid delignification. Int. J. Biol. Macromol. 2020, 165, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Xu, M.; Wang, C.; Shao, L.; Mao, J.; Jiang, W.; Ji, X.; Yang, G.; Chen, J.; Lyu, G.; et al. Pretreatment of willow using the alkaline-catalyzed sulfolane/water solution for high-purity and antioxidative lignin production. Int. J. Biol. Macromol. 2020, 159, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, L.; Ren, J.; He, B. Effect of Ternary Deep Eutectic Solvents on Bagasse Cellulose and Lignin Structure in Low-Temperature Pretreatment. Processes 2022, 10, 778. [Google Scholar] [CrossRef]
- Oh, Y.; Park, S.; Jung, D.; Oh, K.K.; Lee, S.H. Effect of hydrogen bond donor on the choline chloride-based deep eutectic solvent-mediated extraction of lignin from pine wood. Int. J. Biol. Macromol. 2020, 165, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Xing, D.; Lia, J. FTIR Studies of the Changes in Wood Chemistry from Wood Forming Tissue under Inclined Treatment. Energy Procedia 2012, 16, 758–762. [Google Scholar] [CrossRef]
- Robert, D. Carbon-13 Nuclear Magnetic Resonance Spectrometry. In Methods in Lignin Chemistry; Lin, S.Y., Dence, C.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 250–273. [Google Scholar] [CrossRef]
- Günther, H. NMR Spectroscopy: Basic Principles, Concepts and Applications in Chemistry, 3rd ed.; Wiley: Weinheim, Germany, 2013; 734p. [Google Scholar]
- Claridge, T.D.W. Chapter 4—One-Dimensional Techniques. In High-Resolution NMR Techniques in Organic Chemistry, 3rd ed.; Claridge, T.D.W., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 133–169. [Google Scholar] [CrossRef]
- Dizhbite, T.; Telysheva, G.; Jurkjane, V.; Viesturs, U. Characterization of the radical scavenging activity of lignins—Natural antioxidants. Bioresour. Technol. 2004, 95, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Xiong, L.; Yoo, C.G.; Dong, C.Y.; Meng, X.Z.; Dai, J.; Ragauskas, A.; Yu, J.; Chen, X. Correlations of the physicochemical properties of organosolv lignins from Broussonetia papyrifera with their antioxidant activities. Sustain. Energy Fuels 2020, 4, 5114–5119. [Google Scholar] [CrossRef]
- Li, P.; Lu, Y.; Long, G.; Li, S.; Li, K.; Jiang, B.; Wu, W. Structural characterization of acid des-modified alkaline lignin and evaluation of antioxidant properties. Forests 2023, 14, 550. [Google Scholar] [CrossRef]
- Abed, N.E.; Kaabi, B.; Smaali, M.I.; Chabbouh, M.; Habibi, K.; Mejri, M.; Marzouki, M.N.; Ahmed, S.B.H. Chemical Composition, Antioxidant and Antimicrobial Activities of Thymus capitata Essential Oil with Its Preservative Effect against Listeria monocytogenes Inoculated in Minced Beef Meat. eCAM 2014, 2014, 152487. [Google Scholar] [CrossRef]
- Arshanitsa, A.; Ponomarenko, J.; Dizhbite, T.; Andersone, A.; Gosselink, R.J.A.; van der Putten, J.; Lauberts, M.; Telysheva, G. Fractionation of technical lignins as a tool for improvement of their antioxidant properties. J. Anal. Appl. Pyrolysis 2013, 103, 78–85. [Google Scholar] [CrossRef]
- Michelin, M.; Liebentritt, S.; Vicente, A.A.; Teixeira, J.A. Lignin from an integrated process consisting of liquid hot water and ethanol organosolv: Physicochemical and antioxidant properties. Int. J. Biol. Macromol. 2018, 120, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Gordobil, O.; Herrera, R.; Yahyaoui, M.; İlk, S.; Kaya, M.; Labidi, J. Potential use of kraft and organosolv lignins as a natural additive for healthcare products. RSC Adv. 2018, 8, 24525–24533. [Google Scholar] [CrossRef]
- Tavares, D.; Cavali, M.; Tanobe, V.d.O.A.; Torres, L.A.Z.; Rozendo, A.S.; Zandoná Filho, A.; Soccol, C.R.; Woiciechowski, A.L. Lignin from Residual Sawdust of Eucalyptus spp.—Isolation, Characterization, and Evaluation of the Antioxidant Properties. Biomass 2022, 2, 195–208. [Google Scholar] [CrossRef]
- Wei, X.; Liu, Y.; Luo, Y.; Shen, Z.; Wang, S.; Li, M.; Zhang, L. Effect of organosolv extraction on the structure and antioxidant activity of eucalyptus kraft lignin. Int. J. Biol. Macromol. 2021, 187, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Wörmeyer, K.; Ingram, T.; Saake, B.; Brunner, G.; Smirnova, I. Comparison of different pretreatment methods for lignocellulosic materials. Part II: Influence of pretreatment on the properties of rye straw lignin. Bioresour. Technol. 2011, 102, 4157–4164. [Google Scholar] [CrossRef] [PubMed]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.-L.; Hult, E.L.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setäläb, H.; Österberg, M. A simple process for lignin nanoparticle preparation. Green Chem. 2016, 18, 1416–1422. [Google Scholar] [CrossRef]
- Tang, Q.; Qian, Y.; Yang, D.; Qiu, X.; Qin, Y.; Zhou, M. Lignin-Based Nanoparticles: A Review on Their Preparations and Applications. Polymers 2020, 12, 2471. [Google Scholar] [CrossRef] [PubMed]
- Hussin, M.H.; Appaturi, J.N.; Poh, N.E.; Latif, N.H.A.; Brosse, N.; Ziegler-Devin, I.; Vahabi, H.; Syamani, F.A.; Fatriasari, W.; Solihat, N.N.; et al. A recent advancement on preparation, characterization and application of nanolignin. Int. J. Biol. Macromol. 2022, 200, 303–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, T.; Song, G.; Sun, R. Advanced and Versatile Lignin-Derived Biodegradable Composite Film Materials Toward a Sustainable World. Green Chem. 2021, 23, 3790–3817. [Google Scholar] [CrossRef]
- Tian, D.; Hu, J.; Bao, J.; Chandra, R.P.; Saddler, J.N.; Lu, C. Lignin valorization: Lignin nanoparticles as high-value bio-additive for multifunctional nanocomposites. Biotechnol. Biofuels 2017, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Suhr, J.; Seo, H.W.; Sun, H.; Kim, S.; Park, I.K.; Kim, S.H.; Lee, Y.; Kim, K.J.; Nam, J.D. All Biomass and UV Protective Composite Composed of Compatibilized Lignin and Poly (Lactic-acid). Sci. Rep. 2017, 7, 43596. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Ren, X. Transparent and ultra-tough PVA/alkaline lignin films with UV shielding and antibacterial functions. Int. J. Biol. Macromol. 2022, 216, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Zdanowicz, M.; Sałasińska, K.; Lewandowski, K.; Skórczewska, K. Thermoplastic Starch/Ternary Deep Eutectic Solvent/Lignin Materials: Study of Physicochemical Properties and Fire Behavior. ACS Sustain. Chem. Eng. 2022, 10, 4579–4587. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, H.; Zhao, Q.S.; Zhao, B. Effect of DES lignin incorporation on physicochemical, antioxidant and antimicrobial properties of carboxymethyl cellulose-based films. Int. J. Biol. Macromol. 2024, 263, 130294. [Google Scholar] [CrossRef] [PubMed]
- Hage, R.E.; Brosse, N.; Chrusciel, L.; Sanchez, C.; Sannigrahi, P.; Ragauskas, A. Characterization of milled wood lignin and ethanol organosolv lignin from miscanthus. Polym. Degrad. Stab. 2009, 94, 1632–1638. [Google Scholar] [CrossRef]
- Chertkov, V.A.; Shestakova, A.K.; Davydov, D.V. Regioselective N-arylation of nitroazoles. Determination of the structure of N-arylnitroazoles on the basis of NMR spectroscopic data and quantum-chemical calculations. Chem. Heterocycl. Compd. 2011, 47, 45–54. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
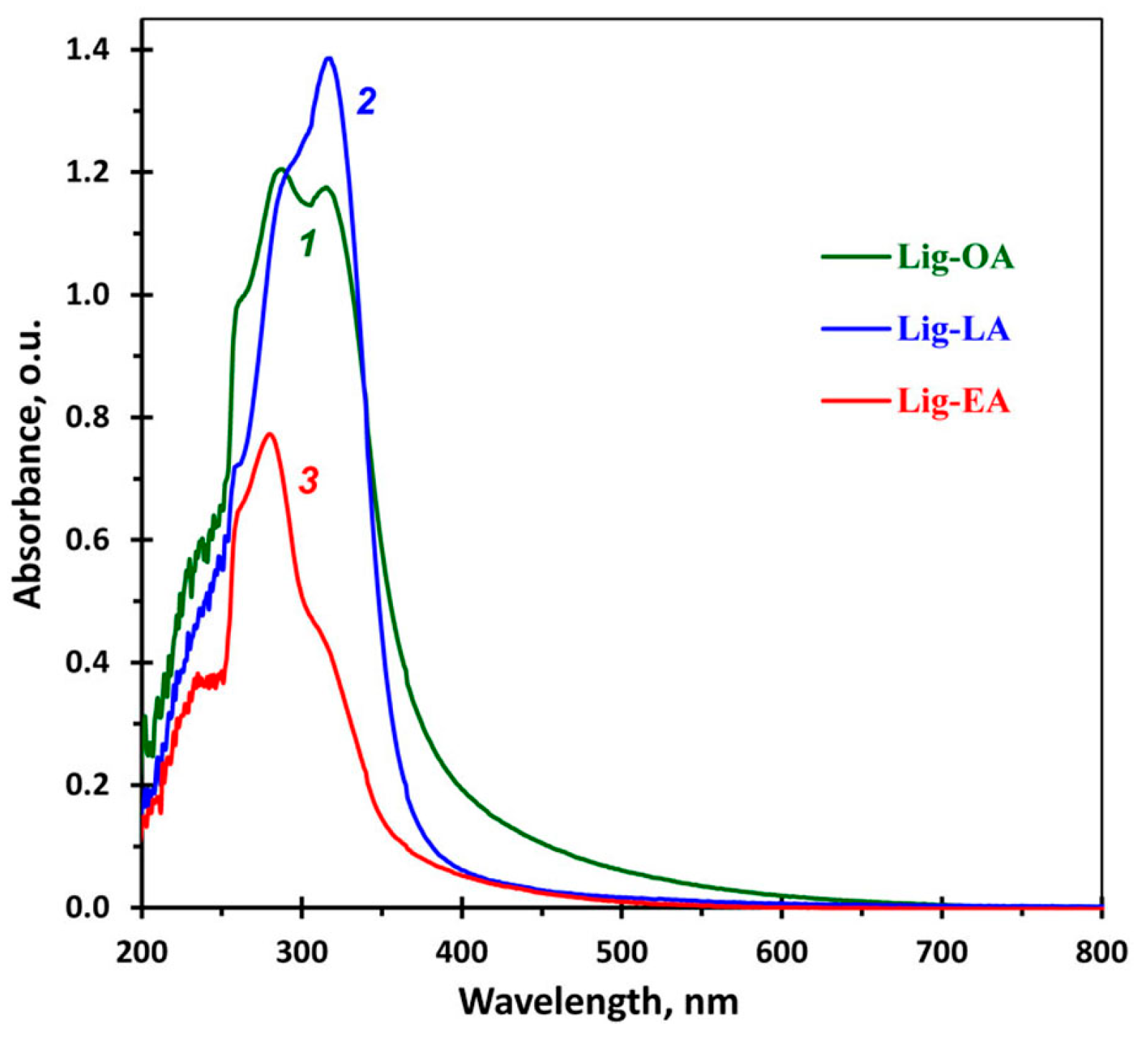
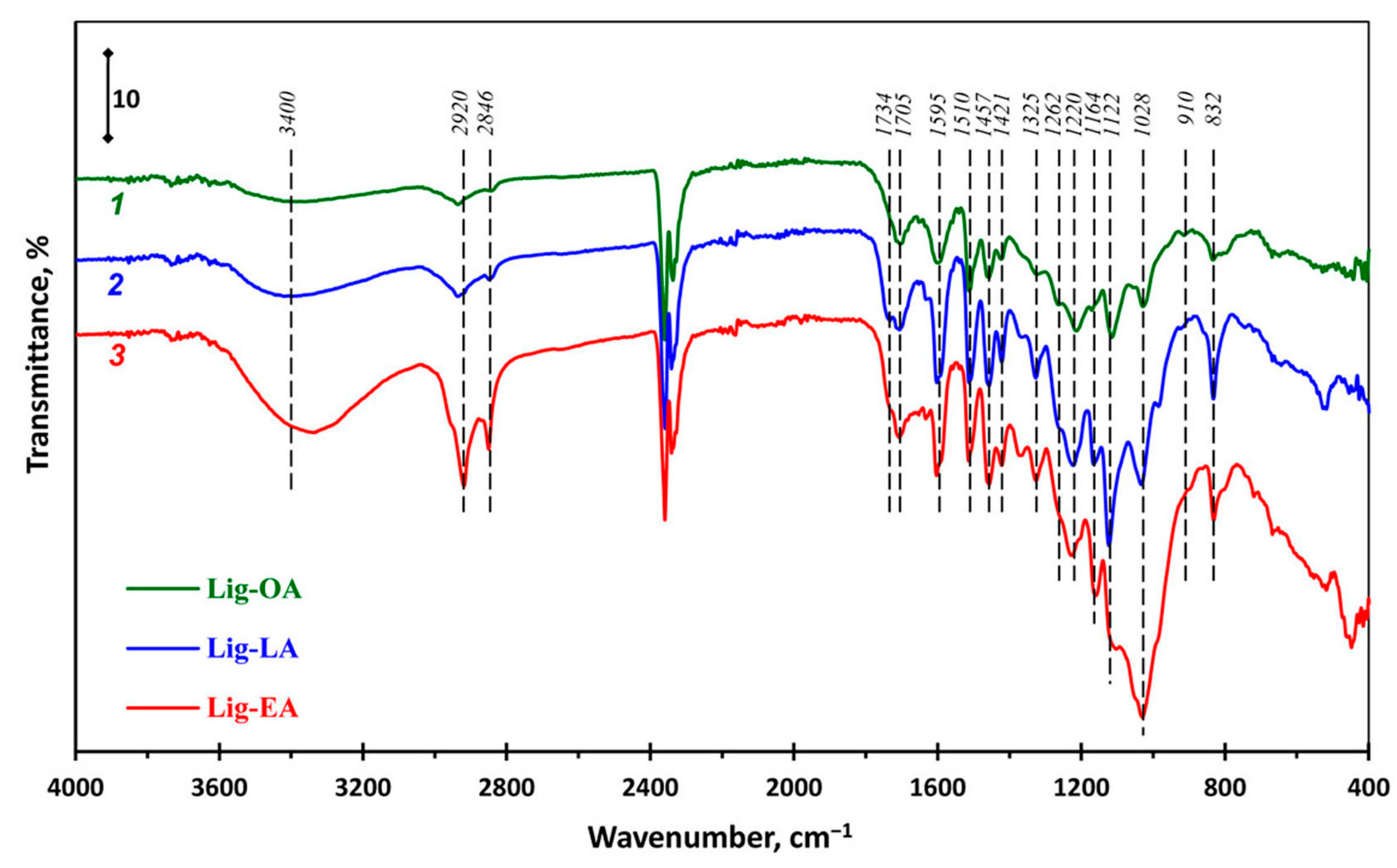
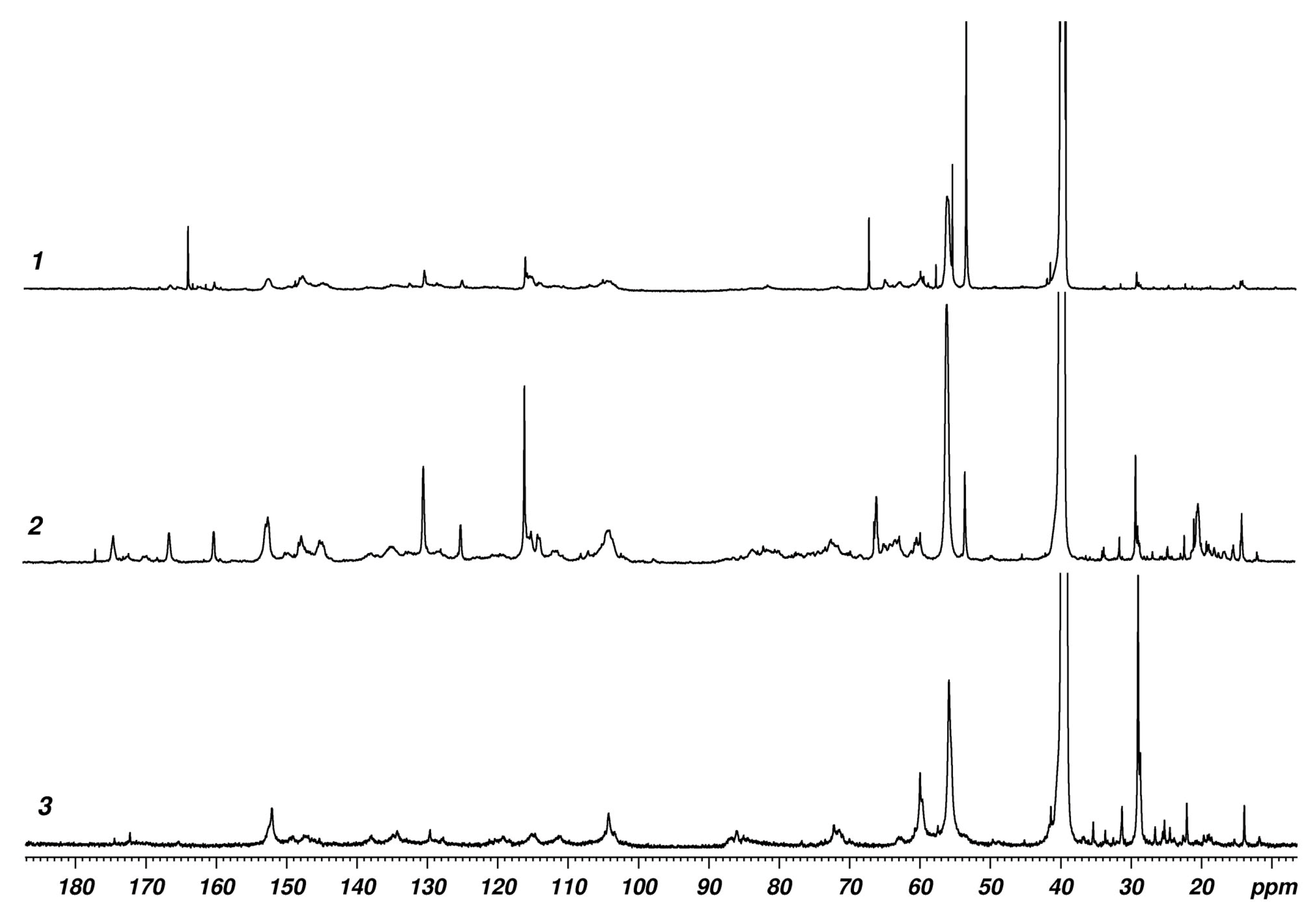
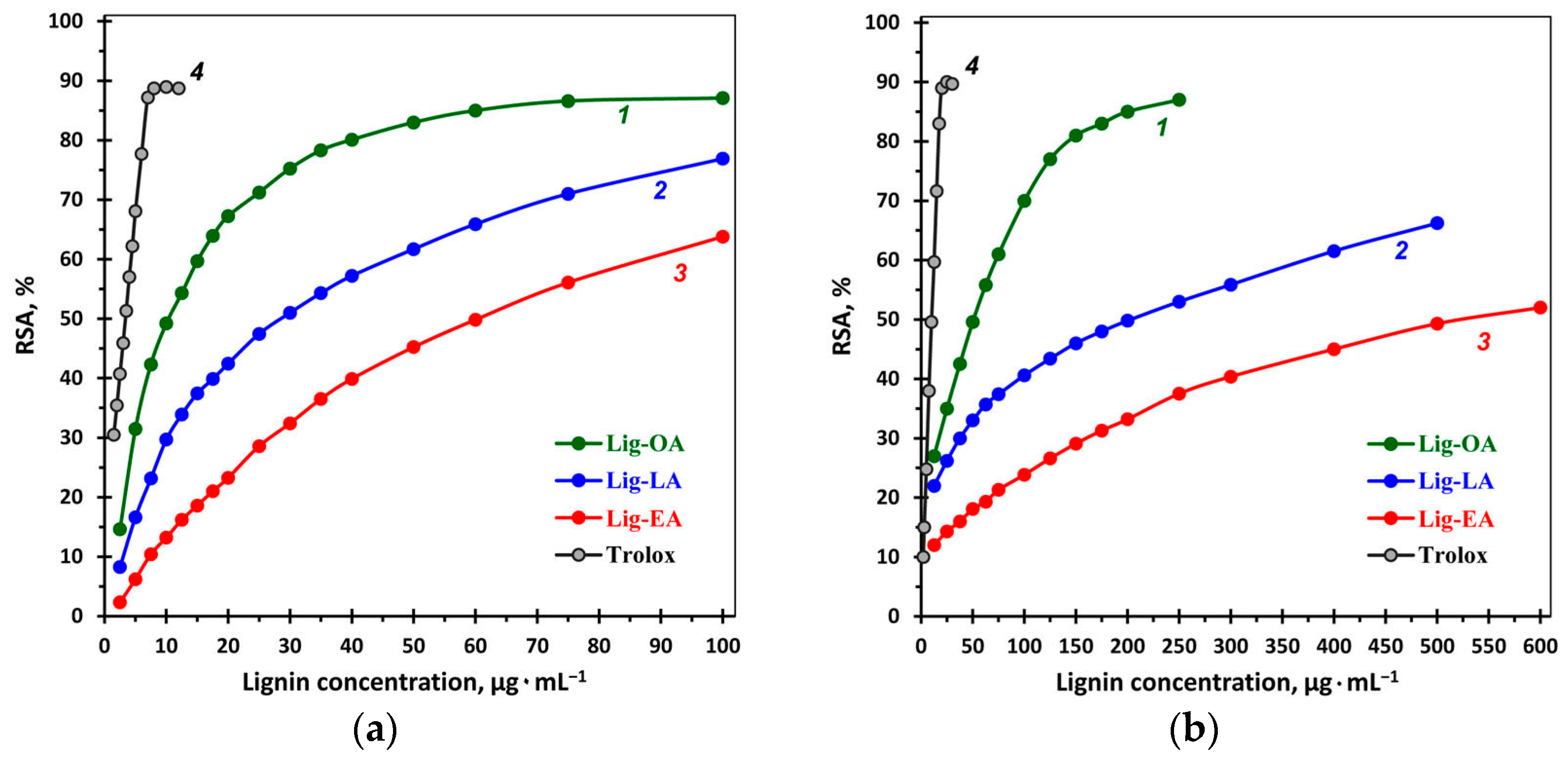
| Sample | TPC 1 | ABTS 2 | DPPH 2 |
|---|---|---|---|
| Lig-OA | 169.67 ± 1.45 | 10 | 50 |
| Lig-LA | 77.17 ± 1.48 | 30 | 200 |
| Lig-EA | 23.11 ± 0.73 | 60 | 500 |
| Trolox | – | 3 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morozova, O.; Vasil’eva, I.; Shumakovich, G.; Khlupova, M.; Chertkov, V.; Shestakova, A.; Yaropolov, A. Green Extraction of Reed Lignin: The Effect of the Deep Eutectic Solvent Composition on the UV-Shielding and Antioxidant Properties of Lignin. Int. J. Mol. Sci. 2024, 25, 8277. https://doi.org/10.3390/ijms25158277
Morozova O, Vasil’eva I, Shumakovich G, Khlupova M, Chertkov V, Shestakova A, Yaropolov A. Green Extraction of Reed Lignin: The Effect of the Deep Eutectic Solvent Composition on the UV-Shielding and Antioxidant Properties of Lignin. International Journal of Molecular Sciences. 2024; 25(15):8277. https://doi.org/10.3390/ijms25158277
Chicago/Turabian StyleMorozova, Olga, Irina Vasil’eva, Galina Shumakovich, Maria Khlupova, Vyacheslav Chertkov, Alla Shestakova, and Alexander Yaropolov. 2024. "Green Extraction of Reed Lignin: The Effect of the Deep Eutectic Solvent Composition on the UV-Shielding and Antioxidant Properties of Lignin" International Journal of Molecular Sciences 25, no. 15: 8277. https://doi.org/10.3390/ijms25158277
APA StyleMorozova, O., Vasil’eva, I., Shumakovich, G., Khlupova, M., Chertkov, V., Shestakova, A., & Yaropolov, A. (2024). Green Extraction of Reed Lignin: The Effect of the Deep Eutectic Solvent Composition on the UV-Shielding and Antioxidant Properties of Lignin. International Journal of Molecular Sciences, 25(15), 8277. https://doi.org/10.3390/ijms25158277





