Progranulin, sICAM-1, and sVCAM-1 May Predict an Increased Risk for Ventricular Arrhythmias in Patients with Systemic Sclerosis
Abstract
1. Introduction
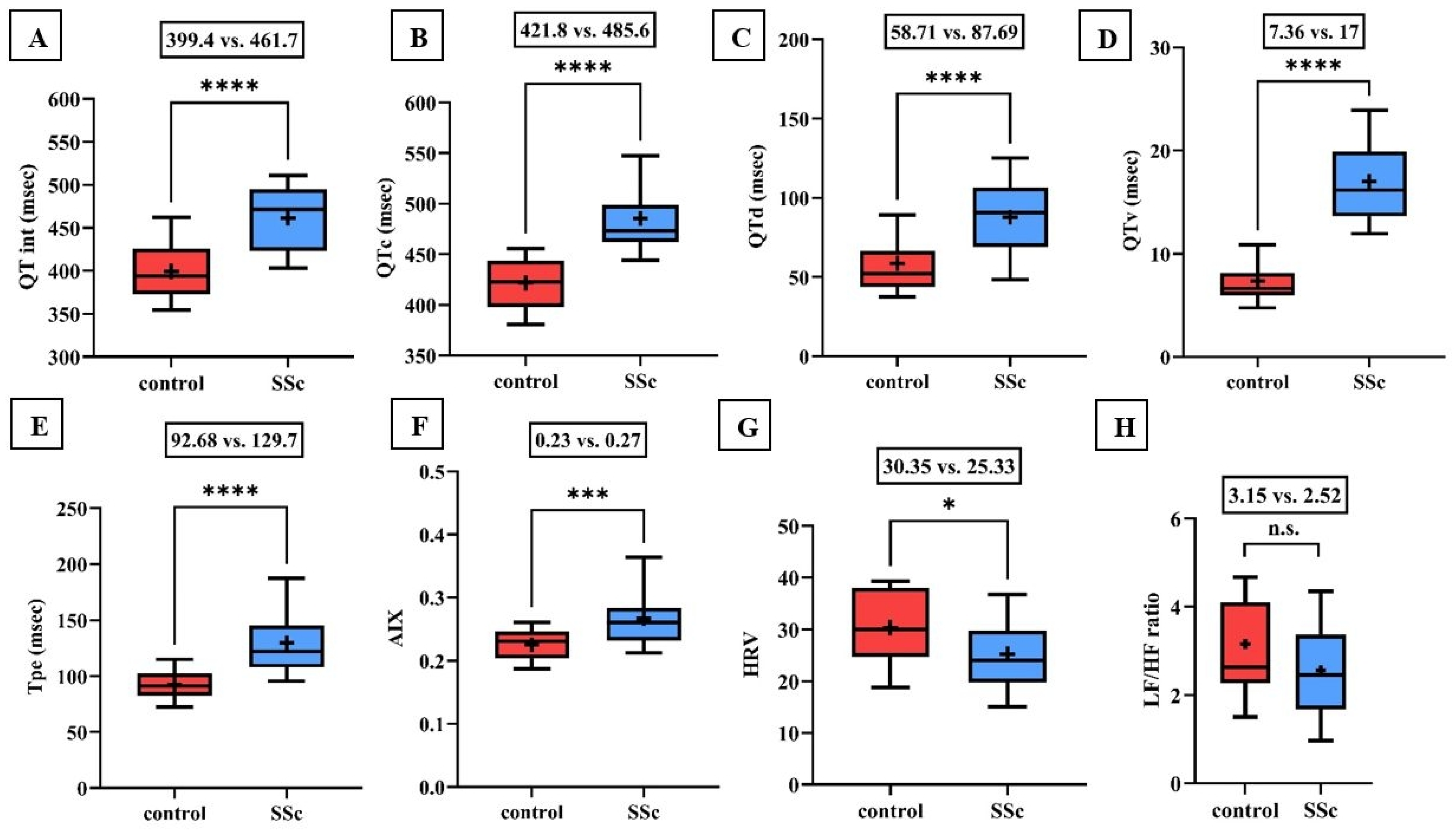
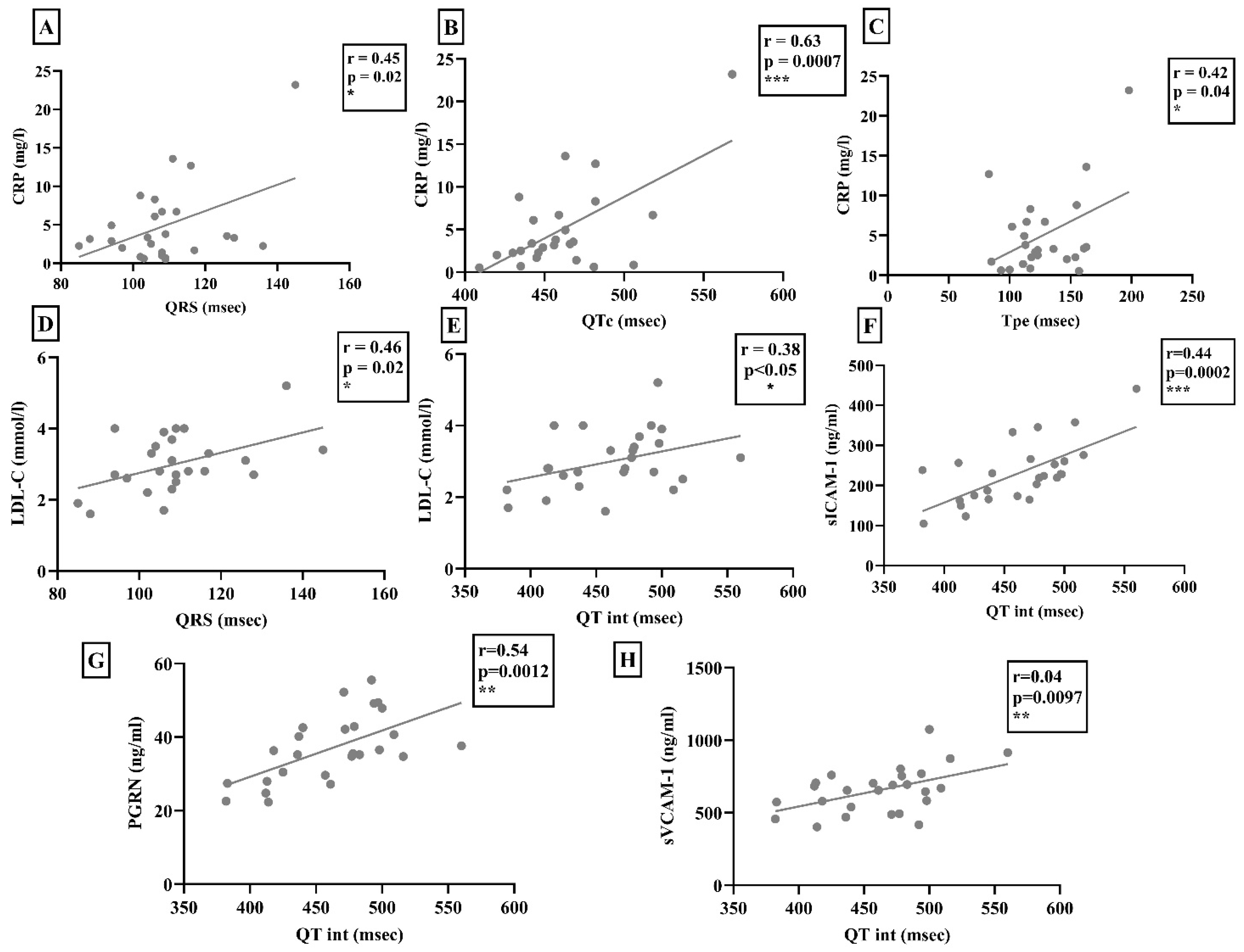
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Protocol
4.2. Echocardiography
4.3. Electrocardiography
4.4. 24-h Holter-ECG Monitoring
4.5. Laboratory Parameters
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, M.; Muller-Ladner, U. The pathogenesis of systemic sclerosis revisited. Clin. Rev. Allergy Immunol. 2011, 40, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.Y.; Wang, X.D.; Zhang, T.; Xue, J. Cardiac complications in systemic sclerosis: Early diagnosis and treatment. Chin. Med. J. 2019, 132, 2865–2871. [Google Scholar] [CrossRef]
- Ramalho, A.R.; Costa, S.; Silva, F.; Donato, P.; Franco, F.; Pego, G.M. Autoimmune myocarditis in systemic sclerosis: An unusual form of scleroderma heart disease presentation. ESC Heart Fail. 2017, 4, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Lambova, S. Cardiac manifestations in systemic sclerosis. World J. Cardiol. 2014, 6, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Tyndall, A.J.; Bannert, B.; Vonk, M.; Airò, P.; Cozzi, F.; Carreira, P.E.; Bancel, D.F.; Allanore, Y.; Müller-Ladner, U.; Distler, O.; et al. Causes and risk factors for death in systemic sclerosis: A study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann. Rheum. Dis. 2010, 69, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Bosello, S.L.; Canestrari, G.; Cavalli, G.; Dagna, L.; Ferraccioli, G. QTc interval prolongation in Systemic Sclerosis: Correlations with clinical variables and arrhythmic risk. Int. J. Cardiol. 2017, 239, 33. [Google Scholar] [CrossRef] [PubMed]
- Yayla, C.; Yayla, M.E.; Yayla, K.G.; Ilgen, U.; Akboga, M.K.; Duzgun, N. The Assessment of Tp-e Interval and Tp-e/QT Ratio in Patients With Systemic Sclerosis. Arch. Rheumatol. 2016, 31, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Vacca, A.; Meune, C.; Gordon, J.; Chung, L.; Proudman, S.; Assassi, S.; Nikpour, M.; Rodriguez-Reyna, T.S.; Khanna, D.; Lafyatis, R.; et al. Cardiac arrhythmias and conduction defects in systemic sclerosis. Rheumatology 2014, 53, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Bombace, S.; Monti, L. Heart Involvement in Systemic Sclerosis: The Role of Magnetic Resonance Imaging. Clin. Rev. Allergy Immunol. 2023, 64, 343–357. [Google Scholar] [CrossRef]
- Mavrogeni, S.; Gargani, L.; Pepe, A.; Monti, L.; Markousis-Mavrogenis, G.; De Santis, M.; De Marchi, D.; Koutsogeorgopoulou, L.; Karabela, G.; Stavropoulos, E.; et al. Cardiac magnetic resonance predicts ventricular arrhythmias in scleroderma: The Scleroderma Arrhythmia Clinical Utility Study (SAnCtUS). Rheumatology 2020, 59, 1938–1948. [Google Scholar] [CrossRef]
- Butt, S.A.; Jeppesen, J.L.; Torp-Pedersen, C.; Sam, F.; Gislason, G.H.; Jacobsen, S.; Andersson, C. Cardiovascular Manifestations of Systemic Sclerosis: A Danish Nationwide Cohort Study. J. Am. Heart Assoc. 2019, 8, e013405. [Google Scholar] [CrossRef] [PubMed]
- Bissell, L.A.; Anderson, M.; Burgess, M.; Chakravarty, K.; Coghlan, G.; Dumitru, R.B.; Graham, L.; Ong, V.; Pauling, J.D.; Plein, S.; et al. Consensus best practice pathway of the UK Systemic Sclerosis Study group: Management of cardiac disease in systemic sclerosis. Rheumatology 2017, 56, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Muresan, L.; Petcu, A.; Pamfil, C.; Muresan, C.; Rinzis, M.; Mada, R.O.; Gusetu, G.N.; Pop, D.; Zdrenghea, D.; Rednic, S. Cardiovascular profiles of scleroderma patients with arrhythmias and conduction disorders. Acta Reumatol. Port. 2016, 41, 26–39. [Google Scholar] [PubMed]
- Bruni, C.; Buch, M.H.; Djokovic, A.; De Luca, G.; Dumitru, R.B.; Giollo, A.; Galetti, I.; Steelandt, A.; Bratis, K.; Suliman, Y.A.; et al. Consensus on the assessment of systemic sclerosis-associated primary heart involvement: World Scleroderma Foundation/Heart Failure Association guidance on screening, diagnosis, and follow-up assessment. J. Scleroderma Relat. Disord. 2023, 8, 169–182. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Matucci-Cerinic, M.; Mavrogeni, S.I. Diagnosis and management of primary heart involvement in systemic sclerosis. Curr. Opin. Rheumatol. 2024, 36, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.; Wang, M.; Steele, R.; Baron, M.; Fritzler, M.J.; Canadian Scleroderma Research Group; Hudson, M. NT-proBNP, hs-cTnT, and CRP predict the risk of cardiopulmonary outcomes in systemic sclerosis: Findings from the Canadian Scleroderma Research Group. J. Scleroderma Relat. Disord. 2022, 7, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Reyna, T.S.; Rosales-Uvera, S.G.; Kimura-Hayama, E.; Hernández-Reyes, P.; Mercado-Velázquez, P.; Benavides-Suárez, S.A.; Esquinca-González, A.; Núñez-Álvarez, C.A. Myocardial fibrosis detected by magnetic resonance imaging, elevated U-CRP and higher mRSS are predictors of cardiovascular complications in systemic sclerosis (SSc) patients. Semin. Arthritis Rheum. 2019, 49, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Rabquer, B.J.; Hou, Y.; Del Galdo, F.; Haines 3rd, G.K.; Gerber, M.L.; Jimenez, S.A.; Seibold, J.R.; Koch, A.E. The proadhesive phenotype of systemic sclerosis skin promotes myeloid cell adhesion via ICAM-1 and VCAM-1. Rheumatology 2009, 48, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.P.; Bickerstaff, M.C.; Shiwen, X.; Carulli, M.T.; Haskard, D.O.; Dubois, R.M.; Black, C.M. Serial circulating adhesion molecule levels reflect disease severity in systemic sclerosis. Br. J. Rheumatol. 1995, 34, 1048–1054. [Google Scholar] [CrossRef]
- Lorincz, H.; Somodi, S.; Ratku, B.; Harangi, M.; Paragh, G. Crucial Regulatory Role of Organokines in Relation to Metabolic Changes in Non-Diabetic Obesity. Metabolites 2023, 13, 270. [Google Scholar] [CrossRef]
- Huang, G.; An, L.; Fan, M.; Zhang, M.; Chen, B.; Zhu, M.; Wu, J.; Liu, Y.; Wang, Y.; Huang, Q.; et al. Potential role of full-length and nonfull-length progranulin in affecting aortic valve calcification. J. Mol. Cell Cardiol. 2020, 141, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wu, Z.; Xie, L. Progranulin is essential for bone homeostasis and immunology. Ann. N. Y. Acad. Sci. 2022, 1518, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Li, G.; Hettinghouse, A.; Liu, C. Progranulin: A key player in autoimmune diseases. Cytokine 2018, 101, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.J.; Sam, N.B.; Cheng, M.H.; Pan, H.F.; Gao, J. Progranulin as a Potential Therapeutic Target in Immune-Mediated Diseases. J. Inflamm. Res. 2021, 14, 6543–6556. [Google Scholar] [CrossRef] [PubMed]
- Yoo, W.; Lee, J.; Noh, K.H.; Lee, S.; Jung, D.; Kabir, M.H.; Park, D.; Lee, C.; Kwon, K.-S.; Kim, J.-S.; et al. Progranulin attenuates liver fibrosis by downregulating the inflammatory response. Cell Death Dis. 2019, 10, 758. [Google Scholar] [CrossRef]
- Alyahya, A.M. The role of progranulin in ischemic heart disease and its related risk factors. Eur. J. Pharm. Sci. 2022, 175, 106215. [Google Scholar] [CrossRef]
- Klemm, P.; Assmann, G.; Preuss, K.-D.; Fadle, N.; Regitz, E.; Martin, T.; Pfreundschuh, M.; Thurner, L. Progranulin autoantibodies in systemic sclerosis and autoimmune connective tissue disorders: A preliminary study. Immun. Inflamm. Dis. 2019, 7, 271–275. [Google Scholar] [CrossRef]
- Snarskaya, E.S.; Vasileva, K.D. Localized scleroderma: Actual insights and new biomarkers. Int. J. Dermatol. 2022, 61, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Furst, D.E.; Clements, P.J.; Allanore, Y.; Baron, M.; Czirjak, L.; Distler, O.; Foeldvari, I.; Kuwana, M.; Matucci-Cerinic, M.; et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J. Scleroderma Relat. Disord. 2017, 2, 11–18. [Google Scholar] [CrossRef]
- D'Andrea, A.; Caso, P.; Cuomo, S.; di Uccio, F.S.; Scarafile, R.; Salerno, G.; Romano, S.; Stisi, S.; Scherillo, M.; Calabrò, R. Myocardial and vascular dysfunction in systemic sclerosis: The potential role of noninvasive assessment in asymptomatic patients. Int. J. Cardiol. 2007, 121, 298–301. [Google Scholar] [CrossRef]
- Bernardo, P.; Conforti, M.L.; Bellando-Randone, S.; Pieragnoli, P.; Blagojevic, J.; Kaloudi, O.; Guiducci, S.; Porta, F.; Padeletti, L.; Gensini, G.F.; et al. Implantable cardioverter defibrillator prevents sudden cardiac death in systemic sclerosis. J. Rheumatol. 2011, 38, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Fairley, J.L.; Ross, L.; Quinlivan, A.; Hansen, D.; Paratz, E.; Stevens, W.; Kistler, P.M.; McLellan, A.; La Gerche, A.; Nikpour, M. Sudden cardiac death, arrhythmias and abnormal electrocardiography in systemic sclerosis: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2023, 62, 152229. [Google Scholar] [CrossRef] [PubMed]
- Draeger, H.T.; Assassi, S.; Sharif, R.; Gonzalez, E.B.; Harper, B.E.; Arnett, F.C.; Manzoor, A.; Lange, R.A.; Mayes, M.D. Right bundle branch block: A predictor of mortality in early systemic sclerosis. PLoS ONE 2013, 8, e78808. [Google Scholar] [CrossRef] [PubMed]
- Bayar, N.; Çay, H.F.; Erkal, Z.; Sezer, I.; Arslan, S.; Çağırcı, G.; Çay, S.; Yüksel, I.Ö.; Köklü, E. The importance of fragmented QRS in the early detection of cardiac involvement in patients with systemic sclerosis. Anatol. J. Cardiol. 2015, 15, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Tigen, K.; Sunbul, M.; Ozen, G.; Durmus, E.; Kivrak, T.; Cincin, A.; Ozben, B.; Atas, H.; Direskeneli, H.; Basaran, Y. Regional myocardial dysfunction assessed by two-dimensional speckle tracking echocardiography in systemic sclerosis patients with fragmented QRS complexes. J. Electrocardiol. 2014, 47, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Mathai, S.C.; Sibley, C.T.; Forfia, P.R.; Mudd, J.O.; Fisher, M.R.; Tedford, R.J.; Lechtzin, N.; Boyce, D.; Hummers, L.K.; Housten, T.; et al. Tricuspid annular plane systolic excursion is a robust outcome measure in systemic sclerosis-associated pulmonary arterial hypertension. J. Rheumatol. 2011, 38, 2410–2418. [Google Scholar] [CrossRef] [PubMed]
- Rietzschel, E. and M. De Buyzere, High-sensitive C-reactive protein: Universal prognostic and causative biomarker in heart disease? Biomark. Med. 2012, 6, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Pacholczak-Madej, R.; Kuszmiersz, P.; Bazan-Socha, S.; Kosałka-Wêgiel, J.; Iwaniec, T.; Zarêba, L.; Kielczewski, S.; Rams, A.; Walocha, J.A.; Musiał, J.; et al. Endothelial dysfunction in patients with systemic sclerosis. Postepy Dermatol. Alergol. 2020, 37, 495–502. [Google Scholar] [CrossRef]
- Mathew, D.T.; Peigh, G.; Lima, J.A.C.; Bielinski, S.J.; Larson, N.B.; Allison, M.A.; Shah, S.J.; Patel, R.B. Associations of circulating Vascular Cell Adhesion Molecule-1 and Intercellular Adhesion Molecule-1 with long-term cardiac function. J. Am. Heart Assoc. 2024, 13, e032213. [Google Scholar] [CrossRef]
- Tonjes, A.; Scholz, M.; Krüger, J.; Krause, K.; Schleinitz, D.; Kirsten, H.; Gebhardt, C.; Marzi, C.; Grallert, H.; Ladenvall, C.; et al. Genome-wide meta-analysis identifies novel determinants of circulating serum progranulin. Hum. Mol. Genet. 2018, 27, 546–558. [Google Scholar] [CrossRef]
- Kurnellas, M.; Mitra, A.; Schwabe, T.; Paul, R.; Arrant, A.E.; Roberson, E.D.; Ward, M.; Yeh, F.; Long, H.; Rosenthal, A. Latozinemab, a novel progranulin-elevating therapy for frontotemporal dementia. J. Transl. Med. 2023, 21, 387. [Google Scholar] [CrossRef]
- Kaur, J.; Mukheja, S.; Varma, S.; Kalra, H.S.; Khosa, B.S.; Vohra, K. Serum progranulin/tumor necrosis factor-alpha ratio as independent predictor of systolic blood pressure in overweight hypertensive patients: A cross-sectional study. Egypt. Heart J. 2020, 72, 25. [Google Scholar] [CrossRef]
- Ahmed, Z.; Mackenzie, I.R.A.; Hutton, M.L.; Dickson, D.W. Progranulin in frontotemporal lobar degeneration and neuroinflammation. J. Neuroinflammation 2007, 4, 7. [Google Scholar] [CrossRef]
- Alyahya, A.M.; Al-Masri, A.; Hersi, A.; El Eter, E.; Husain, S.; Lateef, R.; Mawlana, O.H. The Effects of Progranulin in a Rat Model of Acute Myocardial Ischemia/Reperfusion are Mediated by Activation of the P13K/Akt Signaling Pathway. Med. Sci. Monit. Basic. Res. 2019, 25, 229–237. [Google Scholar] [CrossRef]
- McElhanon, K.E.; Huff, T.C.; Hirenallur-Shanthappa, D.; Miller, R.A.; Christoforou, N. Increased circulating progranulin is not sufficient to induce cardiac dysfunction or supraventricular arrhythmia. Sci. Rep. 2023, 13, 21541. [Google Scholar] [CrossRef]
- Hwang, H.J.; Jung, T.W.; Hong, H.C.; Choi, H.Y.; Seo, J.-A.; Kim, S.G.; Kim, N.H.; Choi, K.M.; Choi, D.S.; Baik, S.H.; et al. Progranulin protects vascular endothelium against atherosclerotic inflammatory reaction via Akt/eNOS and nuclear factor-kappaB pathways. PLoS ONE 2013, 8, e76679. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, C.; Orosz, A.; Hegyi, P.; Komka, Z.; Udvardy, A.; Bosnyák, E.; Trájer, E.; Pavlik, G.; Tóth, M.; Wittmann, T.; et al. Increased short-term variability of the QT interval in professional soccer players: Possible implications for arrhythmia prediction. PLoS ONE 2011, 6, e18751. [Google Scholar] [CrossRef] [PubMed]
- Sebestyen, V.; Szűcs, G.; Páll, D.; Ujvárosy, D.; Ötvös, T.; Csige, I.; Pataki, T.; Lőrincz, I.; Szabó, Z. Electrocardiographic markers for the prediction of ventricular arrhythmias in patients with systemic sclerosis. Rheumatology 2020, 59, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Kors, J.A.; van Eck, H.J.R.; van Herpen, G. The meaning of the Tp-Te interval and its diagnostic value. J. Electrocardiol. 2008, 41, 575–580. [Google Scholar] [CrossRef]
- Salvi, V.; Karnad, D.R.; Panicker, G.K.; Natekar, M.; Hingorani, P.; Kerkar, V.; Ramasamy, A.; de Vries, M.; Zumbrunnen, T.; Kothari, S.; et al. Comparison of 5 methods of QT interval measurements on electrocardiograms from a thorough QT/QTc study: Effect on assay sensitivity and categorical outliers. J. Electrocardiol. 2011, 44, 96–104. [Google Scholar] [CrossRef]
- Gupta, P.; Patel, C.; Patel, H.; Narayanaswamy, S.; Malhotra, B.; Green, J.T.; Yan, G.-X. T(p-e)/QT ratio as an index of arrhythmogenesis. J. Electrocardiol. 2008, 41, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Varro, A.; Baczko, I. Possible mechanisms of sudden cardiac death in top athletes: A basic cardiac electrophysiological point of view. Pflugers Arch. 2010, 460, 31–40. [Google Scholar] [CrossRef] [PubMed]
| SSc Patients | Control Group | |
|---|---|---|
| n = 26 | n = 36 | |
| Gender, M/F (n) | 19/26 | 25/36 |
| Age (years) | 56.82 ± 2.27 | 53.21 ± 6.31 |
| BMI (kg/m2) | 25 | 28 |
| Medication | ||
| Antihypertensives | ACE-Is/ARBs (10/26), NDHP type Ca-channel blocker (7/26) | ACE-Is/ARBs (6/36), NDHP type Ca-channel blocker (2/36) |
| Beta-blockers | 12/26 | 7/36 |
| DHP type Ca-channel blockers | 7/26 | 0/36 |
| Pentoxifylline | 19/26 | 0/36 |
| Diuretics | 4/26 | 3/36 |
| Immunomodulatory therapy | MMF (4/26), MTX (7/26), cyclophosphamid (1/26), Medrol (4/26), permanently do not take any (13/26) | 0/36 |
| comorbidities | ||
| Hypertension | 13/26 | 12/36 |
| GERD | 8/26 | 7/36 |
| Hypothyroidism | 2/26 | 4/36 |
| Autoantibodies | Occurrence of Positivity |
| Antinuclear antibody (ANA) | 26/26 |
| anti-Ro52 | 6/26 |
| Anti-topoisomerase I antibody (Scl-70) | 11/26 |
| anti-Ku | 11/26 |
| RNA polymerase III antibody (RNSP III) | 3/26 |
| Anti-citrullinated protein antibodies (ACPA) | 3/26 |
| Anti-centromere antibody (ACA) | 1/26 |
| Organ involvement | Frequency |
| Lung | |
| Interstitial lung disease | 17/26 |
| Pulmonary arterial hypertension (PAH) | 3/26 |
| Cardiac | |
| Diastolic dysfunction | 21/26 |
| Cardiomyopathy | 4/26 |
| Gastrointestinal | |
| Esophagus dysmotility | 12/26 |
| Gastrointestinal reflux (GERD) | 8/26 |
| Esophageal achalasia | 5/26 |
| Other | |
| Arthritis | 6/26 |
| Digital ulcers | 6/26 |
| SSc Patients | Control Group | p Values | |
|---|---|---|---|
| Na (mmol/L) | 141.15 ± 3.52 | 140.39 ± 2.33 | 0.31 n.s. |
| K (mmol/L) | 4.46 ± 0.36 | 4.22 ± 0.37 | 0.015 * |
| tCa (mmol/L) | 2.34 ± 0.1 | 2.41 ± 0.09 | 0.0086 ** |
| iCa (mmol/L) | 1.25 ± 0.03 | 1.27 ± 0.04 | 0.056 n.s. |
| CRP (mg/L) | 5.04 ± 1.07 | 2.69 ± 0.96 | 0.03 * |
| cTnT (ng/L) | 17.8 (9.5–27.75) | 8 (5–10) | 0.01 ** |
| CK (U/L) | 101.65 ± 50.2 | 157.31 ± 85.7 | 0.03 * |
| NT-proBNP (ng/L) | 120 (80–374) | 43 (17–70) | 0.0001 **** |
| Uric acid (μmol/L) | 281.42 ± 69.8 | 251 ± 81.65 | 0.31 n.s. |
| sTSH (mU/L) | 1.67 ± 0.43 | 1.97 ± 0.74 | 0.27 n.s. |
| fT3 (pmol/L) | 4.78 ± 0.4 | 5.12 ± 1.51 | 0.28 n.s. |
| fT4 (pmol/L) | 15.78 ± 2.62 | 15.47 ± 2 | 0.57 n.s. |
| eGFR (ml/min/1.73 m2) | 79.12 ± 16.92 | 80.31 ± 9.59 | 0.75 n.s. |
| TG (mmol/L) | 1.47 ± 0.8 | 1.85 ± 1 | 0.08 n.s. |
| TC (mmol/L) | 4.86 ± 0.95 | 5.59 ± 1.07 | 0.004 ** |
| LDL-C (mmol/L) | 2.99 ± 0.62 | 3.43 ± 0.77 | 0.037 * |
| HDL-C (mmol/L) | 1.34 ± 0.29 | 1.39 ± 0.34 | 0.55 n.s. |
| ApoA-1 (g/L) | 1.41 ± 0.2 | 1.52 ± 0.22 | 0.045 * |
| ApoB (g/L) | 0.99 ± 0.25 | 1.06 ± 0.25 | 0.19 n.s. |
| Lp(a) (mg/L) | 157.6 ± 34.52 | 218.3 ± 72.26 | 0.39 n.s. |
| Fe (μmol/L) | 14.29 ± 7.02 | 13.25 ± 4.44 | 0.35 n.s. |
| Ferritin (μg/L) | 120.35 ± 95.73 | 171.24 ± 42.9 | 0.11 n.s. |
| Transferrin (g/L) | 2.53 ± 0.47 | 2.68 ± 0.34 | 0.17 n.s. |
| sTrfR (mg/L) | 2.83 ± 1.7 | 1.39 ± 0.29 | 0.23 n.s. |
| Hgb (g/L) | 131.88 ± 14.3 | 140 ± 12.22 | 0.16 n.s. |
| RBC count (T/L) | 4.64 ± 0.45 | 4.7 ± 0.39 | 0.55 n.s. |
| Reticulocyte (G/L) | 61.77 ± 23.96 | 71.58 ± 31.5 | 0.19 n.s. |
| WBC count (G/L) | 6.17 ± 1.71 | 7.28 ± 1.79 | 0.0029 ** |
| Neut% | 65.66 ± 9.73 | 59.56 ± 7.76 | 0.008 ** |
| Lymph% | 23.87 ± 7.45 | 29.4 ± 7.04 | 0.004 ** |
| PLT (G/L) | 222.38 ± 46.29 | 254.42 ± 55.94 | 0.02 * |
| sICAM-1 (ng/mL) | 230.2 ± 76.4 | 186.4 ± 32.06 | 0.0098 ** |
| sVCAM-1 (ng/mL) | 655.7 ± 109.14 | 586.2 ± 98.12 | 0.046 * |
| PGRN (ng/mL) | 37 ± 9.09 | 36.3 ± 6.25 | 0.73 n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebestyén, V.; Ratku, B.; Ujvárosy, D.; Lőrincz, H.; Tari, D.; Végh, L.; Majai, G.; Somodi, S.; Páll, D.; Szűcs, G.; et al. Progranulin, sICAM-1, and sVCAM-1 May Predict an Increased Risk for Ventricular Arrhythmias in Patients with Systemic Sclerosis. Int. J. Mol. Sci. 2024, 25, 7380. https://doi.org/10.3390/ijms25137380
Sebestyén V, Ratku B, Ujvárosy D, Lőrincz H, Tari D, Végh L, Majai G, Somodi S, Páll D, Szűcs G, et al. Progranulin, sICAM-1, and sVCAM-1 May Predict an Increased Risk for Ventricular Arrhythmias in Patients with Systemic Sclerosis. International Journal of Molecular Sciences. 2024; 25(13):7380. https://doi.org/10.3390/ijms25137380
Chicago/Turabian StyleSebestyén, Veronika, Balázs Ratku, Dóra Ujvárosy, Hajnalka Lőrincz, Dóra Tari, Lilla Végh, Gyöngyike Majai, Sándor Somodi, Dénes Páll, Gabriella Szűcs, and et al. 2024. "Progranulin, sICAM-1, and sVCAM-1 May Predict an Increased Risk for Ventricular Arrhythmias in Patients with Systemic Sclerosis" International Journal of Molecular Sciences 25, no. 13: 7380. https://doi.org/10.3390/ijms25137380
APA StyleSebestyén, V., Ratku, B., Ujvárosy, D., Lőrincz, H., Tari, D., Végh, L., Majai, G., Somodi, S., Páll, D., Szűcs, G., Harangi, M., & Szabó, Z. (2024). Progranulin, sICAM-1, and sVCAM-1 May Predict an Increased Risk for Ventricular Arrhythmias in Patients with Systemic Sclerosis. International Journal of Molecular Sciences, 25(13), 7380. https://doi.org/10.3390/ijms25137380








