Vitamin C Supplementation in the Treatment of Autoimmune and Onco-Hematological Diseases: From Prophylaxis to Adjuvant Therapy
Abstract
1. Introduction
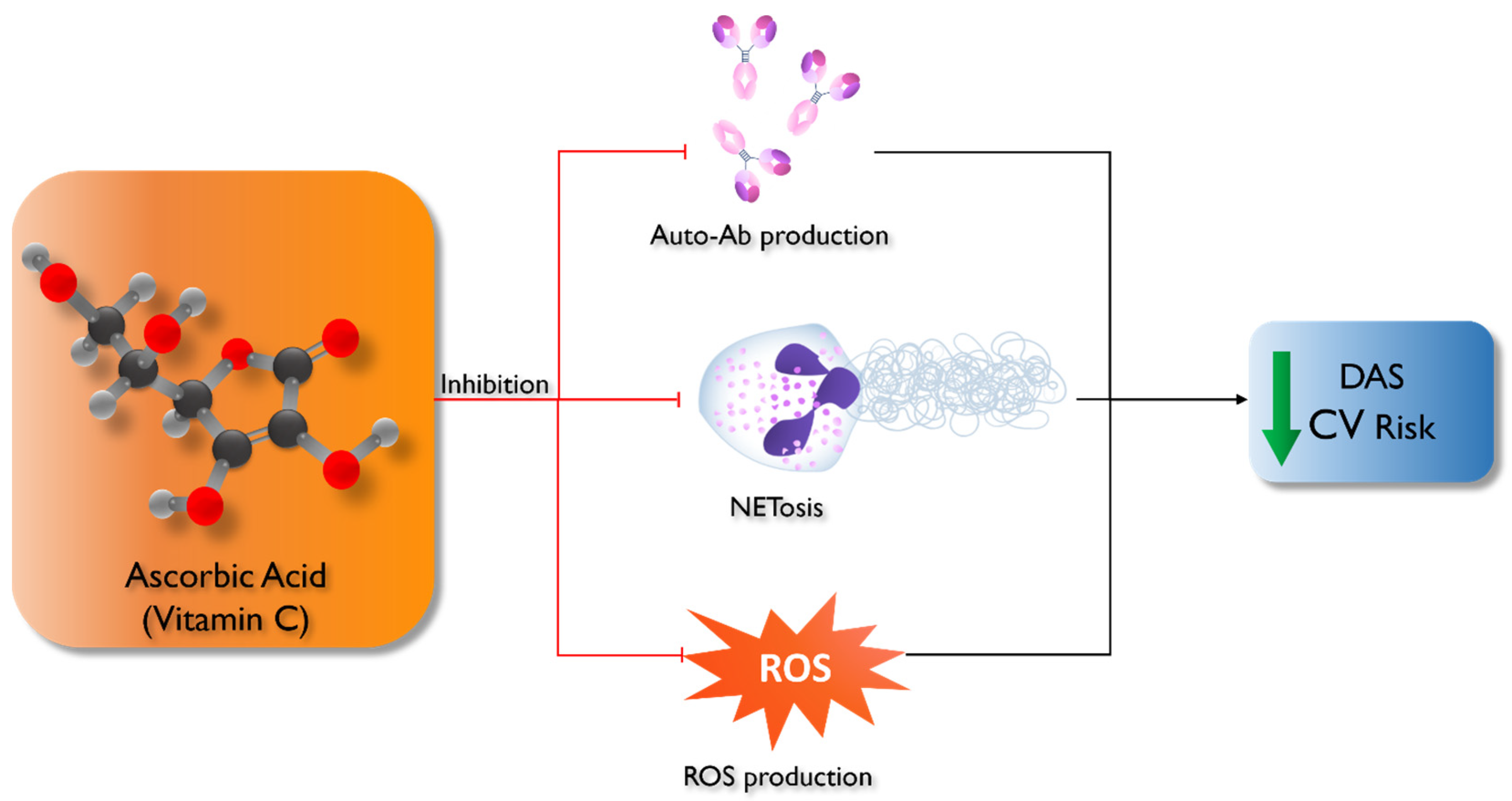
2. Vitamin C and Autoimmune Diseases
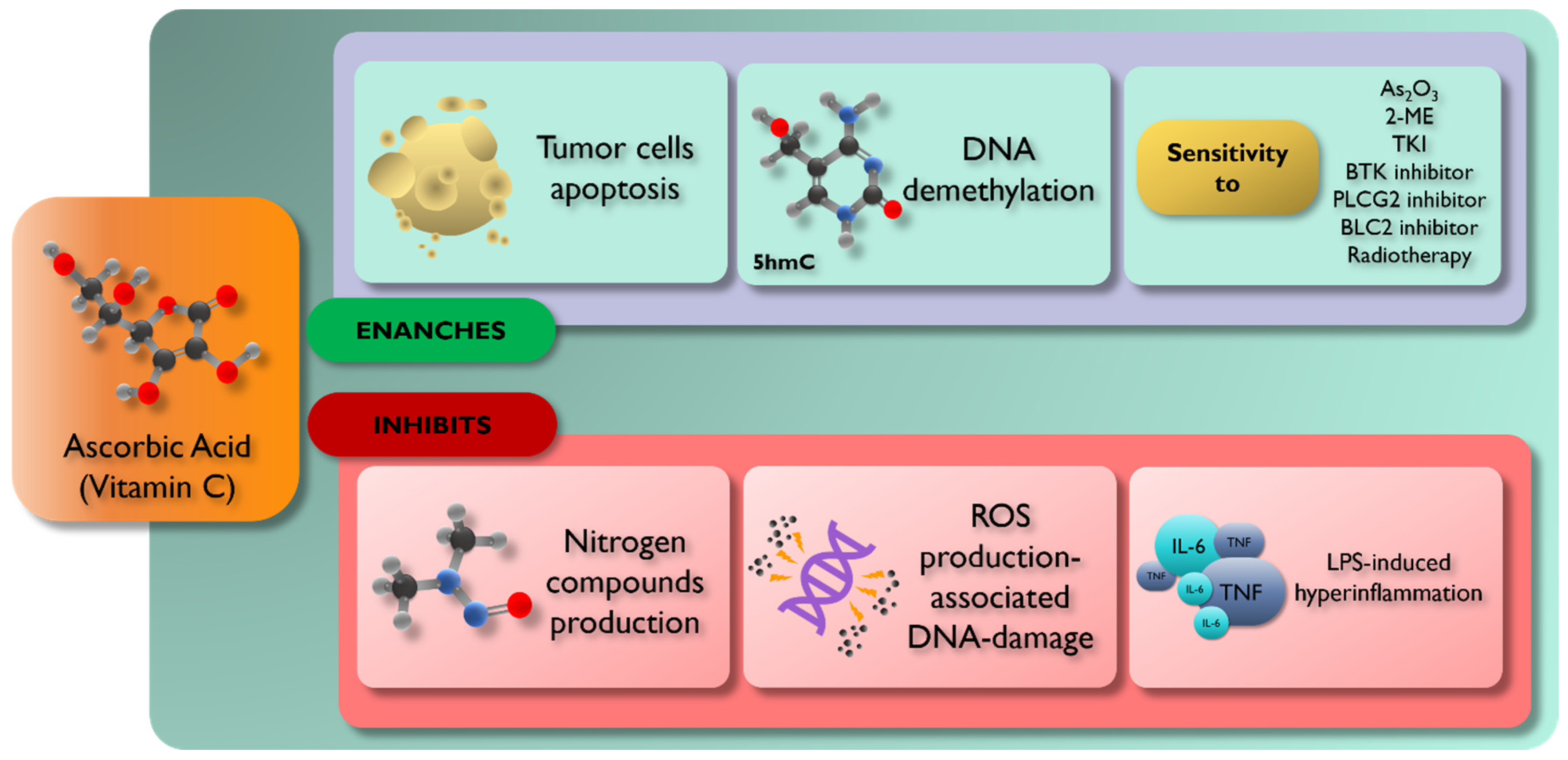
3. Vitamin C and Hematological Neoplastic Diseases
3.1. Vitamin C and Acute Leukemia
3.2. Vitamin C and Transplant
3.3. Vitamin C and Chronic Myeloid Leukemia
3.4. Vitamin C and Lymphoma
3.5. Vitamin C and Chronic Lymphocytic Leukemia
3.6. Vitamin C and Multiple Myeloma
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sauberlich, H.E. A History of Scurvy and Vitamin C; Marcel Dekker Inc.: New York, NY, USA, 1997; pp. 1–24. [Google Scholar]
- Camarena, V.; Wang, G. The epigenetic role of vitamin C in health and disease. Cell. Mol. Life Sci. 2016, 73, 1645–1658. [Google Scholar] [CrossRef]
- Liugan, M.; Carr, A.C. Vitamin C and Neutrophil Function: Findings from Randomized Controlled Trials. Nutrients 2019, 11, 2102. [Google Scholar] [CrossRef]
- Kasahara, H.; Kondo, T.; Nakatsukasa, H.; Chikuma, S.; Ito, M.; Ando, M.; Kurebayashi, Y.; Sekiya, T.; Yamada, T.; Okamoto, S.; et al. Generation of allo-antigen-specific induced Treg stabilized by vitamin C treatment and its application for prevention of acute graft versus host disease model. Int. Immunol. 2017, 29, 457–469. [Google Scholar] [CrossRef]
- Woo, A.; Kim, J.-H.; Jeong, Y.-J.; Maeng, H.G.; Lee, Y.-T.; Kang, J.S.; Lee, W.J.; Hwang, Y.-I. Vitamin C acts indirectly to modulate isotype switching in mouse B cells. Anat. Cell Biol. 2010, 43, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Ngo, B.; Van Riper, J.M.; Cantley, L.C.; Yun, J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat. Rev. Cancer 2019, 19, 271–282. [Google Scholar] [CrossRef]
- Magrì, A.; Germano, G.; Lorenzato, A.; Lamba, S.; Chilà, R.; Montone, M.; Amodio, V.; Ceruti, T.; Sassi, F.; Arena, S.; et al. High-dose vitamin C enhances cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaay8707. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, C.; Kong, J. Oxidative Stress in Neurodegenerative Diseases. Neural Regen. Res. 2012, 7, 376. [Google Scholar]
- Rochette, L.; Lorin, J.; Zeller, M.; Guilland, J.C.; Lorgis, L.; Cottin, Y.; Vergely, C. Nitric Oxide Synthase Inhibition and Oxidative Stress in Cardiovascular Diseases: Possible Therapeutic Targets? Pharmacol. Ther. 2013, 140, 239–257. [Google Scholar] [CrossRef]
- Dozor, A.J. The role of oxidative stress in the pathogenesis and treatment of asthma. Ann. N. Y. Acad. Sci. 2010, 1203, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Stamp, L.K.; Khalilova, I.; Tarr, J.M.; Senthilmohan, R.; Turner, R.; Haigh, R.C.; Winyard, P.G.; Kettle, A.J. Myeloperoxidase and oxidative stress in rheumatoid arthritis. Rheumatology 2012, 51, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, P.; Gęgotek, A.; Žarković, N.; Skrzydlewska, E. Oxidative Stress and Lipid Mediators Modulate Immune Cell Functions in Autoimmune Diseases. Int. J. Mol. Sci. 2021, 22, 723. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Nagafuchi, Y.; Fujio, K. Clinical and Immunological Biomarkers for Systemic Lupus Erythematosus. Biomolecules 2021, 11, 928. [Google Scholar] [CrossRef]
- Zucchi, D.; Elefante, E.; Schilirò, D.; Signorini, V.; Trentin, F.; Bortoluzzi, A.; Tani, C. One year in review 2022: Systemic lupus erythematosus. Clin. Exp. Rheumatol. 2022, 40, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Ameer, M.A.; Chaudhry, H.; Mushtaq, J.; Khan, O.S.; Babar, M.; Hashim, T.; Zeb, S.; Tariq, M.A.; Patlolla, S.R.; Ali, J.; et al. An Overview of Systemic Lupus Erythematosus (SLE) Pathogenesis, Classification, and Management. Cureus 2022, 14, e30330. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, Q.; Xia, Y. Oxidative Stress Contributes to Inflammatory and Cellular Damage in Systemic Lupus Erythematosus: Cellular Markers and Molecular Mechanism. J. Inflamm. Res. 2023, 16, 453–465. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Kronbichler, A.; Park, D.D.-Y.; Park, Y.; Moon, H.; Kim, H.; Choi, J.H.; Choi, Y.; Shim, S.; Lyu, I.S.; Yun, B.H.; et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review. Autoimmun. Rev. 2017, 16, 1160–1173. [Google Scholar] [CrossRef]
- Bozonet, S.M.; Carr, A.C. The Role of Physiological Vitamin C Concentrations on Key Functions of Neutrophils Isolated from Healthy Individuals. Nutrients 2019, 11, 1363. [Google Scholar] [CrossRef]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Farkas, D.; Brophy, D.F.; Fowler, A.A.; Natarajan, R. Vitamin C: A Novel Regulator of Neutrophil Extracellular Trap Formation. Nutrients 2013, 5, 3131–3150. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.-M.; Nita, I.E.; Olteanu, R.; Constantin, T.; Bucur, S.; Matei, C.; Raducan, A. Significance and impact of dietary factors on systemic lupus erythematosus pathogenesis (Review). Exp. Ther. Med. 2019, 17, 1085–1090. [Google Scholar] [CrossRef]
- Weimann, B.J.; Weiser, H. Effects of Antioxidant Vitamins C, E, and Beta-Carotene on Immune Functions in MRL/Lpr Mice and Rats. Ann. N. Y. Acad. Sci. 1992, 669, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Sasaki, T.; Arai, Y.; Kurisu, Y.; Hisamichi, S. Diet and systemic lupus erythematosus: A 4 year prospective study of Japanese patients. J. Rheumatol. 2003, 30, 747–754. [Google Scholar]
- Nuttall, S.L.; Heaton, S.; Piper, M.K.; Martin, U.; Gordon, C. Cardiovascular risk in systemic lupus erythematosus--evidence of increased oxidative stress and dyslipidaemia. Rheumatology 2003, 42, 758–762. [Google Scholar] [CrossRef]
- Tam, L.S.; Li, E.K.; Leung, V.Y.F.; Griffith, J.F.; Benzie, I.F.F.; Lim, P.L.; Whitney, B.; Lee, V.W.Y.; Lee, K.K.C.; Thomas, G.N.; et al. Effects of vitamins C and E on oxidative stress markers and endothelial function in patients with systemic lupus erythematosus: A double blind, placebo controlled pilot study. J. Rheumatol. 2005, 32, 275–282. [Google Scholar]
- Ahmadi, M.; Gharibi, T.; Dolati, S.; Rostamzadeh, D.; Aslani, S.; Baradaran, B.; Younesi, V.; Yousefi, M. Epigenetic modifications and epigenetic based medication implementations of autoimmune diseases. Biomed. Pharmacother. 2017, 87, 596–608. [Google Scholar] [CrossRef]
- Yue, X.; Trifari, S.; Äijö, T.; Tsagaratou, A.; Pastor, W.A.; Zepeda-Martínez, J.A.; Lio, C.-W.J.; Li, X.; Huang, Y.; Vijayanand, P.; et al. Control of Foxp3 stability through modulation of TET activity. J. Exp. Med. 2016, 213, 377–397. [Google Scholar] [CrossRef]
- Klack, K.; Bonfa, E.; Neto, E.F.B. Diet and nutritional aspects in systemic lupus erythematosus. Rev. Bras. Reumatol. 2012, 52, 384–408. [Google Scholar]
- Kono, M.; Nagafuchi, Y.; Shoda, H.; Fujio, K. The Impact of Obesity and a High-Fat Diet on Clinical and Immunological Features in Systemic Lupus Erythematosus. Nutrients 2021, 13, 504. [Google Scholar] [CrossRef]
- Islam, A.; Khandker, S.S.; Kotyla, P.J.; Hassan, R. Immunomodulatory Effects of Diet and Nutrients in Systemic Lupus Erythematosus (SLE): A Systematic Review. Front. Immunol. 2020, 11, 1477. [Google Scholar] [CrossRef]
- Ollier, W.E.R.; Harrison, B.; Symmons, D. What Is the Natural History of Rheumatoid Arthritis? Best Pract. Res. Clin. Rheumatol. 2001, 15, 27–48. [Google Scholar] [CrossRef]
- Romão, V.C.; Fonseca, J.E. Etiology and Risk Factors for Rheumatoid Arthritis: A State-of-the-Art Review. Front. Med. 2021, 8, 689698. [Google Scholar] [CrossRef]
- Dadoun, S.; Zeboulon-Ktorza, N.; Combescure, C.; Elhai, M.; Rozenberg, S.; Gossec, L.; Fautrel, B. Mortality in rheumatoid arthritis over the last fifty years: Systematic review and meta-analysis. Jt. Bone Spine 2012, 80, 29–33. [Google Scholar] [CrossRef]
- Cutolo, M.; Kitas, G.D.; van Riel, P.L. Burden of disease in treated rheumatoid arthritis patients: Going beyond the joint. Semin. Arthritis Rheum. 2014, 43, 479–488. [Google Scholar] [CrossRef]
- Malm, K.; Bremander, A.; Arvidsson, B.; Andersson, M.L.E.; Bergman, S.; Larsson, I. The influence of lifestyle habits on quality of life in patients with established rheumatoid arthritis—A constant balancing between ideality and reality. Int. J. Qual. Stud. Health Well-Being 2016, 11, 30534. [Google Scholar] [CrossRef]
- Radu, A.-F.; Bungau, S.G. Management of Rheumatoid Arthritis: An Overview. Cells 2021, 10, 2857. [Google Scholar] [CrossRef]
- Ozkan, Y.; Yardým-Akaydýn, S.; Sepici, A.; Keskin, E.; Sepici, V.; Simsek, B. Oxidative status in rheumatoid arthritis. Clin. Rheumatol. 2006, 26, 64–68. [Google Scholar] [CrossRef]
- Karatas, F.; Ozates, I.; Canatan, H.; Halifeoglu, I.; Karatepe, M.; Colakt, R. Antioxidant status & lipid peroxidation in patients with rheumatoid arthritis. Indian J. Med. Res. 2003, 118, 178–181. [Google Scholar] [PubMed]
- Pattison, D.J.; Silman, A.J.; Goodson, N.J.; Lunt, M.; Bunn, D.; Luben, R.; Welch, A.; Bingham, S.; Khaw, K.-T.; Day, N.; et al. Vitamin C and the risk of developing inflammatory polyarthritis: Prospective nested case-control study. Ann. Rheum. Dis. 2004, 63, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Stripp, C.; Klarlund, M.; Olsen, S.F.; Tjønneland, A.M.; Frisch, M. Diet and risk of rheumatoid arthritis in a prospective cohort. J. Rheumatol. 2005, 32, 1249–1252. [Google Scholar] [PubMed]
- Arablou, T.; Aryaeian, N.; Djalali, M.; Shahram, F.; Rasouli, L. Association between dietary intake of some antioxidant micronutrients with some inflammatory and antioxidant markers in active Rheumatoid Arthritis patients. Int. J. Vitam. Nutr. Res. 2019, 89, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Jalili, M.; Kolahi, S.; Aref-Hosseini, S.-R.; Mamegani, M.E.; Hekmatdoost, A. Beneficial Role of Antioxidants on Clinical Outcomes and Erythrocyte Antioxidant Parameters in Rheumatoid Arthritis Patients. Int. J. Prev. Med. 2014, 5, 835–840. [Google Scholar]
- Gomathi, A.; Chenthamarai, G.; Manvizhi, S.; Gowrithilagam, T.G. Effects of Vitamin C and Vitamin E in Rheumatoid Arthritis-A Randomized, Open Label, and Comparative Study in a Tertiary Care Hospital. Natl. J. Physiol. Pharm. Pharmacol. 2022, 12, 1463. [Google Scholar]
- Nourmohamm, I.; Athari-Nik, S.; Vafa, M.; Bidari, A.; Jazayeri, S.; Hoshyarrad, A.; Hoseini, F.; Fasihi-Rad, M. Effects of Antioxidant Supplementations on Oxidative Stress in Rheumatoid Arthritis Patients. J. Biol. Sci. 2009, 10, 63–66. [Google Scholar] [CrossRef][Green Version]
- Moertel, C.G.; Fleming, T.R.; Creagan, E.T.; Rubin, J.; O’Connell, M.J.; Ames, M.M. High-Dose Vitamin C versus Placebo in the Treatment of Patients with Advanced Cancer Who Have Had No Prior Chemotherapy: A randomized double-blind comparison. N. Engl. J. Med. 1985, 312, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Block, G. Vitamin C and cancer prevention: The epidemiologic evidence. Am. J. Clin. Nutr. 1991, 53, 270S–282S. [Google Scholar] [CrossRef]
- Block, G. Epidemiologic evidence regarding vitamin C and cancer. Am. J. Clin. Nutr. 1991, 54, 1310S–1314S. [Google Scholar] [CrossRef]
- Dachs, G.U.; Gandhi, J.; Wohlrab, C.; Carr, A.C.; Morrin, H.R.; Pullar, J.M.; Bayer, S.B.; Eglinton, T.W.; Robinson, B.A.; Vissers, M.C.M. Vitamin C Administration by Intravenous Infusion Increases Tumor Ascorbate Content in Patients With Colon Cancer: A Clinical Intervention Study. Front. Oncol. 2021, 10, 600715. [Google Scholar] [CrossRef]
- Rychtarcikova, Z.; Lettlova, S.; Tomkova, V.; Korenková, V.; Langerová, L.; Simonova, E.; Zjablovskaja, P.; Alberich-Jorda, M.; Neuzil, J.; Truksa, J. Tumor-initiating cells of breast and prostate origin show alterations in the expression of genes related to iron metabolism. Oncotarget 2017, 8, 6376–6398. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Pooput, C.; Kirk, K.L.; Krishna, M.C.; Khosh, D.B.; Drisko, J.; Levine, M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 11105–11109. [Google Scholar] [CrossRef]
- Rawal, M.; Schroeder, S.R.; Wagner, B.A.; Cushing, C.M.; Welsh, J.L.; Button, A.M.; Du, J.; Sibenaller, Z.A.; Buettner, G.R.; Cullen, J.J. Manganoporphyrins Increase Ascorbate-Induced Cytotoxicity by Enhancing H2O2 Generation. Cancer Res. 2013, 73, 5232–5241. [Google Scholar] [CrossRef]
- Bowie, A.; O’Neill, L.A.J. Vitamin C Inhibits NF Kappa B Activation in Endothelial Cells. Biochem. Soc. Trans. 1997, 25, 131S. [Google Scholar] [CrossRef]
- Peng, D.; Ge, G.; Gong, Y.; Zhan, Y.; He, S.; Guan, B.; Li, Y.; Xu, Z.; Hao, H.; He, Z.; et al. Vitamin C increases 5-hydroxymethylcytosine level and inhibits the growth of bladder cancer. Clin. Epigenet. 2018, 10, 94. [Google Scholar] [CrossRef]
- Tauler, P.; Aguiló, A.; Gimeno, I.; Noguera, A.; Agustí, A.; Tur, J.A.; Pons, A. Differential response of lymphocytes and neutrophils to high intensity physical activity and to vitamin C diet supplementation. Free Radic. Res. 2003, 37, 931–938. [Google Scholar] [CrossRef]
- Kizhina, A.; Pechorina, E.; Mikheeva, V. Effect of vitamin C supplementation on some leukocyte parameters in American mink (Neovison vison) with abnormal granulogenesis. Tissue Cell 2022, 77, 101870. [Google Scholar] [CrossRef]
- Huijskens, M.J.A.J.; Walczak, M.; Koller, N.; Briedé, J.J.; Senden-Gijsbers, B.L.M.G.; Schnijderberg, M.C.; Bos, G.M.J.; Germeraad, W.T.V. Technical Advance: Ascorbic Acid Induces Development of Double-Positive T Cells from Human Hematopoietic Stem Cells in the Absence of Stromal Cells. J. Leukoc. Biol. 2014, 96, 1165–1175. [Google Scholar] [CrossRef]
- Huijskens, M.J.; Walczak, M.; Sarkar, S.; Atrafi, F.; Senden-Gijsbers, B.L.; Tilanus, M.G.; Bos, G.M.; Wieten, L.; Germeraad, W.T. Ascorbic acid promotes proliferation of natural killer cell populations in culture systems applicable for natural killer cell therapy. Cytotherapy 2015, 17, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, L.; Dolgalev, I.; Wang, Y.; Yoshimi, A.; Martin, G.H.; Wang, J.; Ng, V.; Xia, B.; Witkowski, M.T.; Mitchell-Flack, M.; et al. Restoration of TET2 Function Blocks Aberrant Self-Renewal and Leukemia Progression. Cell 2017, 170, 1079–1095.e20. [Google Scholar] [CrossRef]
- Foster, M.N.; Carr, A.C.; Antony, A.; Peng, S.; Fitzpatrick, M.G. Intravenous Vitamin C Administration Improved Blood Cell Counts and Health-Related Quality of Life of Patient with History of Relapsed Acute Myeloid Leukaemia. Antioxidants 2018, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Portugal, C.C.; da Encarnação, T.G.; Sagrillo, M.A.; Pereira, M.R.; Relvas, J.B.; Socodato, R.; Paes-De-Carvalho, R. Activation of adenosine A3 receptors regulates vitamin C transport and redox balance in neurons. Free Radic. Biol. Med. 2020, 163, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.R.; Kadowaki, M. Effect and Proposed Mechanism of Vitamin C Modulating Amino Acid Regulation of Autophagic Proteolysis. Biochimie 2017, 142, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Rothe, K.; Porter, V.; Jiang, X. Current Outlook on Autophagy in Human Leukemia: Foe in Cancer Stem Cells and Drug Resistance, Friend in New Therapeutic Interventions. Int. J. Mol. Sci. 2019, 20, 461. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M.; Davies, S.M.; Jacobs, D.R.; Folsom, A.R.; Potter, J.D. Diet and risk of leukemia in the Iowa Women’s Health Study. Cancer Epidemiol. Biomark. Prev. 2002, 11, 777–781. [Google Scholar]
- Rice, J.M.; Rehm, S.; Donovan, P.J.; Perantoni, A.O. Comparative transplacental carcinogenesis by directly acting and metabolism-dependent alkylating agents in rodents and nonhuman primates. IARC Sci. Publ. 1989, 96, 17–34. [Google Scholar]
- Tannenbaum, S.R.; Mergens, W. Reaction of nitrite with vitamins C and E. Ann. N. Y. Acad. Sci. 1980, 355, 267–277. [Google Scholar] [CrossRef]
- Blot, W.J.; Henderson, B.E.; Boice, J.D. Childhood Cancer in Relation to Cured Meat Intake: Review of the Epidemiological Evidence. Nutr. Cancer 1999, 34, 111–118. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.-H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an Antioxidant: Evaluation of Its Role in Disease Prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.L.; Block, G.; Selvin, S.; Month, S.; Buffler, P.A. Food Consumption by Children and the Risk of Childhood Acute Leukemia. Am. J. Epidemiol. 2004, 160, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.M.; Preston-Martin, S.; London, S.J.; Bowthan, J.D.; Buckley, J.D.; Thomas, D.C. Processed meats and risk of childhood leukemia (California, USA). Cancer Causes Control 1994, 5, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y. Risk factors and remaining challenges in the treatment of acute promyelocytic leukemia. Int. J. Hematol. 2024, 1–8. [Google Scholar] [CrossRef]
- Grad, J.M.; Bahlis, N.J.; Reis, I.; Oshiro, M.M.; Dalton, W.S.; Boise, L.H. Ascorbic acid enhances arsenic trioxide–induced cytotoxicity in multiple myeloma cells. Blood 2001, 98, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Yedjou, C.G.; Brown, E.; Rogers, C.; Tchounwou, P.B. Ascorbic acid—Modulation of arsenic trioxide toxicity: Implication for the clinical treatment of acute promyelocytic leukemia. In Metal Ions in Biology and Medicine: Proceedings of the … International Symposium on Metal Ions in Biology and Medicine held … = Les Ions Metalliques en Biologie et en Medecine: … Symposium International sur les Ions Metalliques …; NIH Public Access: Washington, DC, USA, 2008; Volume 10, p. 413. [Google Scholar]
- Biological Nature of the Effect of Ascorbic Acids on the Growth of Human Leukemic Cells1|Cancer Research|American Association for Cancer Research. Available online: https://aacrjournals.org/cancerres/article/45/8/3969/489885/Biological-Nature-of-the-Effect-of-Ascorbic-Acids (accessed on 3 April 2024).
- Chen, Y.-C.; Lin-Shiau, S.-Y.; Lin, J.-K. Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis. J. Cell. Physiol. 1998, 177, 324–333. [Google Scholar] [CrossRef]
- Jing, Y.; Dai, J.; Chalmers-Redman, R.M.E.; Tatton, W.G.; Waxman, S. Arsenic Trioxide Selectively Induces Acute Promyelocytic Leukemia Cell Apoptosis via a Hydrogen Peroxide-Dependent Pathway. Blood 1999, 94, 2102–2111. [Google Scholar] [CrossRef] [PubMed]
- Retsky, K.L.; Frei, B. Vitamin C prevents metal ion-dependent initiation and propagation of lipid peroxidation in human low-density lipoprotein. Biochim. Biophys. Acta Lipids Lipid Metab. 1995, 1257, 279–287. [Google Scholar] [CrossRef]
- Huijskens, M.J.; Wodzig, W.K.; Walczak, M.; Germeraad, W.T.; Bos, G.M. Ascorbic acid serum levels are reduced in patients with hematological malignancies. Results Immunol. 2016, 6, 8–10. [Google Scholar] [CrossRef]
- Carr, A.C.; Cook, J. Intravenous Vitamin C for Cancer Therapy—Identifying the Current Gaps in Our Knowledge. Front. Physiol. 2018, 9, 1182. [Google Scholar] [CrossRef]
- Rasheed, M.; Simmons, G.; Fisher, B.; Leslie, K.; Reed, J.; Roberts, C.; Natarajan, R.; Fowler, A.; Toor, A. Reduced plasma ascorbic acid levels in recipients of myeloablative conditioning and hematopoietic cell transplantation. Eur. J. Haematol. 2019, 103, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Spencer, E.; Das, A.; Meijer, N.; Lauren, C.; MacPherson, S.; Chambers, S.T. Patients Undergoing Myeloablative Chemotherapy and Hematopoietic Stem Cell Transplantation Exhibit Depleted Vitamin C Status in Association with Febrile Neutropenia. Nutrients 2020, 12, 1879. [Google Scholar] [CrossRef]
- Bruemmer, B.; Patterson, R.E.; Cheney, C.; Aker, S.N.; Witherspoon, R.P. The association between vitamin C and vitamin E supplement use before hematopoietic stem cell transplant and outcomes to two years. J. Am. Diet. Assoc. 2003, 103, 982–990. [Google Scholar] [CrossRef]
- Urbalejo-Ceniceros, V.I.; Rocha-González, H.I.; Acosta-Maldonado, B.L.; Valero-Saldaña, L.M.; Hernández-Alcantara, A.E.; Pérez-Camargo, D.A. Effect of vitamin C on immune reconstitution after bone marrow transplantation. Int. J. Clin. Pharmacol. Ther. 2022, 60, 384–391. [Google Scholar] [CrossRef]
- Raoufinejad, K.; Shamshiri, A.R.; Pezeshki, S.; Chahardouli, B.; Hadjibabaie, M.; Jahangard-Rafsanjani, Z.; Gholami, K.; Rajabi, M.; Vaezi, M. Oral calcitriol in hematopoietic recovery and survival after autologous stem cell transplantation: A randomized clinical trial. DARU J. Pharm. Sci. 2019, 27, 709–720. [Google Scholar] [CrossRef]
- Rubenstein, E.B.; Peterson, D.E.; Schubert, M.; Keefe, D.; McGuire, D.; Epstein, J.; Elting, L.S.; Fox, P.C.; Cooksley, C.; Sonis, S.T.; et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 2004, 100, 2026–2046. [Google Scholar] [CrossRef]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B. Perspectives on Cancer Therapy-Induced Mucosal Injury: Pathogenesis, Measurement, Epidemiology, and Consequences for Patients. Cancer 2004, 100, 1995–2025. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, N.C.; Muthukrishnan, A.; Babu, D.B.G.; Kumari, C.S.; Lakshmi, M.A.; Palat, G.; Alam, K.S. Role of Vitamin E and Vitamin A in Oral Mucositis Induced by Cancer Chemo/Radiotherapy—A Meta-Analysis. J. Clin. Diagn. Res. 2017, 11, ZE06–ZE09. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, M.; Roberts, C.H.; Gupta, G.; Fisher, B.J.; Leslie, K.; Simmons, G.L.; Wiedl, C.M.; McCarty, J.M.; Clark, W.B.; Chung, H.M.; et al. Low Plasma Vitamin C Levels in Patients Undergoing Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2017, 23, S286–S287. [Google Scholar] [CrossRef]
- Kletzel, M.; Powers, K.; Hayes, M. Scurvy: A new problem for patients with chronic GVHD involving mucous membranes; an easy problem to resolve. Pediatr. Transplant. 2014, 18, 524–526. [Google Scholar] [CrossRef]
- Ma, Y.; Chapman, J.; Levine, M.; Polireddy, K.; Drisko, J.; Chen, Q. High-Dose Parenteral Ascorbate Enhanced Chemosensitivity of Ovarian Cancer and Reduced Toxicity of Chemotherapy. Sci. Transl. Med. 2014, 6, 222ra18. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Vissers, M.C.M.; Cook, J.S. The Effect of Intravenous Vitamin C on Cancer- and Chemotherapy-Related Fatigue and Quality of Life. Front. Oncol. 2014, 4, 283. [Google Scholar] [CrossRef] [PubMed]
- Lugo, T.G.; Pendergast, A.M.; Muller, A.J.; Witte, O.N. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science 1990, 247, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Aldapt, M.B.; Al-Mashdali, A.F.; Obeidat, K.; Chandra, P.; Yassin, M. Viral infections and incidence of reactivations in chronic myeloid leukemia patients. Oncology 2023, 102, 380–388. [Google Scholar] [CrossRef]
- Pires, D.A.; Brandão-Rangel, M.A.R.; Silva-Reis, A.; Olímpio, F.R.S.; Aimbire, F.; Oliveira, C.R.; Mateus-Silva, J.R.; Zamarioli, L.S.; Bachi, A.L.L.; Bella, Y.F.; et al. Vitamin C Inhibits Lipopolysaccharide-Induced Hyperinflammatory State of Chronic Myeloid Leukemia Cells through Purinergic Signaling and Autophagy. Nutrients 2024, 16, 383. [Google Scholar] [CrossRef]
- Mueck, A.; Seeger, H. 2-Methoxyestradiol—Biology and mechanism of action. Steroids 2010, 75, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Leung, K. 2-[Methyl-11C]Methoxyestradiol; Molecular Imaging and Contrast Agent Database (MICAD): Bethesda, MD, USA, 2007. [Google Scholar]
- Li, D.-L.; Zhang, J.; Zhang, W.-J.; Pan, L.; Wang, Z.-W. Effects of 2-methoxyestradiol on the expression of caspase-3 and survivin in chronic myelocytic leukemia K562 cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2009, 17, 335–339. [Google Scholar]
- She, M.-R.; Li, J.-G.; Guo, K.-Y.; Lin, W.; Du, X.; Niu, X.-Q. Requirement of reactive oxygen species generation in apoptosis of leukemia cells induced by 2-methoxyestradiol. Acta Pharmacol. Sin. 2007, 28, 1037–1044. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, H.; Li, J.; Fan, J.; Chen, J. 2-Methoxyestradiol Combined with Ascorbic Acid Facilitates the Apoptosis of Chronic Myeloid Leukemia Cells via the MicroRNA-223/Fms-like Tyrosine Kinase 3/Phosphatidylinositol-3 Kinase/Protein Kinase B Axis. Bioengineered 2022, 13, 3470. [Google Scholar] [CrossRef]
- Druker, B.J.; Tamura, S.; Buchdunger, E.; Ohno, S.; Segal, G.M.; Fanning, S.; Zimmermann, J.; Lydon, N.B. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr–Abl positive cells. Nat. Med. 1996, 2, 561–566. [Google Scholar] [CrossRef]
- Jiang, X.; Saw, K.M.; Eaves, A.; Eaves, C. Instability of BCR-ABL Gene in Primary and Cultured Chronic Myeloid Leukemia Stem Cells. JNCI J. Natl. Cancer Inst. 2007, 99, 680–693. [Google Scholar] [CrossRef]
- Shah, N.P.; Nicoll, J.M.; Nagar, B.; Gorre, M.E.; Paquette, R.L.; Kuriyan, J.; Sawyers, C.L. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell 2002, 2, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Behrend, L.; Henderson, G.; Zwacka, R.M. Reactive oxygen species in oncogenic transformation. Biochem. Soc. Trans. 2003, 31, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-S. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006, 25, 695–705. [Google Scholar] [CrossRef]
- Sun, Y.; Oberley, L.W.; Elwell, J.H.; Sierra-Rivera, E. Antioxidant enzyme activities in normal and transformed mouse liver cells. Int. J. Cancer 1989, 44, 1028–1033. [Google Scholar] [CrossRef]
- Verrax, J.; Cadrobbi, J.; Marques, C.; Taper, H.; Habraken, Y.; Piette, J.; Calderon, P.B. Ascorbate potentiates the cytotoxicity of menadione leading to an oxidative stress that kills cancer cells by a non-apoptotic caspase-3 independent form of cell death. Apoptosis 2004, 9, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Verrax, J.; Vanbever, S.; Stockis, J.; Taper, H.; Calderon, P.B. Role of glycolysis inhibition and poly(ADP-ribose) polymerase activation in necrotic-like cell death caused by ascorbate/menadione-induced oxidative stress in K562 human chronic myelogenous leukemic cells. Int. J. Cancer 2006, 120, 1192–1197. [Google Scholar] [CrossRef]
- Dejeans, N.; Tajeddine, N.; Beck, R.; Verrax, J.; Taper, H.; Gailly, P.; Calderon, P.B. Endoplasmic reticulum calcium release potentiates the ER stress and cell death caused by an oxidative stress in MCF-7 cells. Biochem. Pharmacol. 2010, 79, 1221–1230. [Google Scholar] [CrossRef]
- Beck, R.; Verrax, J.; Gonze, T.; Zappone, M.; Pedrosa, R.C.; Taper, H.; Feron, O.; Calderon, P.B. Hsp90 cleavage by an oxidative stress leads to its client proteins degradation and cancer cell death. Biochem. Pharmacol. 2009, 77, 375–383. [Google Scholar] [CrossRef]
- Verrax, J.; Pedrosa, R.C.; Beck, R.; Dejeans, N.; Taper, H.; Calderon, P.B. In situ modulation of oxidative stress: A novel and efficient strategy to kill cancer cells. Curr. Med. Chem. 2009, 16, 1821–1830. [Google Scholar] [CrossRef]
- Beck, R.; Pedrosa, R.C.; Dejeans, N.; Glorieux, C.; Levêque, P.; Gallez, B.; Taper, H.; Eeckhoudt, S.; Knoops, L.; Calderon, P.B.; et al. Ascorbate/menadione-induced oxidative stress kills cancer cells that express normal or mutated forms of the oncogenic protein Bcr-Abl. An in vitro and in vivo mechanistic study. Investig. New Drugs 2010, 29, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C.; Stern, A. Human red cells scavenge extracellular hydrogen peroxide and inhibit formation of hypochlorous acid and hydroxyl radical. J. Clin. Investig. 1987, 80, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- The Heat Shock Protein 90 Antagonist Geldanamycin Alters Chaperone Association with P210bcr-Abl and v-Src Proteins before Their Degradation by the Proteasome|Cell Growth & Differentiation|American Association for Cancer Research. Available online: https://aacrjournals.org/cgd/article/11/7/355/705455/The-Heat-Shock-Protein-90-Antagonist-Geldanamycin (accessed on 4 April 2024).
- Kim, K.; Lee, M.; Park, H.; Kim, J.-H.; Kim, S.; Chung, H.; Choi, K.; Kim, I.-S.; Seong, B.L.; Kwon, I.C. Cell-Permeable and Biocompatible Polymeric Nanoparticles for Apoptosis Imaging. J. Am. Chem. Soc. 2006, 128, 3490–3491. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B.; Orrenius, S.; Zhivotovsky, B. The most unkindest cut of all: On the multiple roles of mammalian caspases. Leukemia 2000, 14, 1514–1525. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boeneman, K.; Mei, B.C.; Dennis, A.M.; Bao, G.; Deschamps, J.R.; Mattoussi, H.; Medintz, I.L. Sensing Caspase 3 Activity with Quantum Dot–Fluorescent Protein Assemblies. J. Am. Chem. Soc. 2009, 131, 3828–3829. [Google Scholar] [CrossRef]
- Cohen, G.M. Caspases: The executioners of apoptosis. Biochem. J. 1997, 326, 1–16. [Google Scholar] [CrossRef]
- Allegra, A.; Petrarca, C.; Di Gioacchino, M.; Mirabile, G.; Gangemi, S. Electrochemical Biosensors in the Diagnosis of Acute and Chronic Leukemias. Cancers 2022, 15, 146. [Google Scholar] [CrossRef]
- Zhou, S.; Kong, Y.; Shen, Q.; Ren, X.; Zhang, J.-R.; Zhu, J.-J. Chronic Myeloid Leukemia Drug Evaluation Using a Multisignal Amplified Photoelectrochemical Sensing Platform. Anal. Chem. 2014, 86, 11680–11689. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, Y.; Zhao, M.; Jiang, L.P.; Zhu, J.J. CdSeTe@CdS@ZnS Quantum-Dot-Sensitized Macroporous Tio2 Film: A Multisignal-Amplified Photoelectrochemical Platform. Chemphyschem 2015, 16, 2826–2835. [Google Scholar] [CrossRef]
- Kelemen, L.E.; Cerhan, J.R.; Lim, U.; Davis, S.; Cozen, W.; Schenk, M.; Colt, J.; Hartge, P.; Ward, M.H. Vegetables, Fruit, and Antioxidant-Related Nutrients and Risk of Non-Hodgkin Lymphoma: A National Cancer Institute-Surveillance, Epidemiology, and End Results Population-Based Case-Control Study. Am. J. Clin. Nutr. 2006, 83, 1401–1410. [Google Scholar] [CrossRef]
- Chang, E.T.; Bälter, K.M.; Torrång, A.; Smedby, K.E.; Melbye, M.; Sundström, C.; Glimelius, B.; Adami, H.-O. Nutrient Intake and Risk of Non-Hodgkin’s Lymphoma. Am. J. Epidemiol. 2006, 164, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Purdue, M.P.; Bassani, D.G.; Klar, N.S.; Sloan, M.; Kreiger, N.; Paulse, B.; Dewar, R.; Dryer, D.; Kliewer, E.; Robson, D.; et al. Dietary Factors and Risk of Non-Hodgkin Lymphoma by Histologic Subtype: A Case-Control Analysis. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1665–1676. [Google Scholar] [CrossRef]
- Talamini, R.; Polesel, J.; Montella, M.; Dal Maso, L.; Crovatto, M.; Crispo, A.; Spina, M.; Canzonieri, V.; La Vecchia, C.; Franceschi, S. Food Groups and Risk of Non-Hodgkin Lymphoma: A Multicenter, Case-Control Study in Italy. Int. J. Cancer 2006, 118, 2871–2876. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Holford, T.R.; Leaderer, B.; Zhang, Y.; Zahm, S.H.; Flynn, S.; Tallini, G.; Zhang, B.; Zhou, K.; Owens, P.H.; et al. Diet and Nutrient Intakes and Risk of Non-Hodgkin’s Lymphoma in Connecticut Women. Am. J. Epidemiol. 2004, 159, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Kabat, G.C.; Kim, M.Y.; Wactawski-Wende, J.; Shikany, J.M.; Vitolins, M.Z.; Rohan, T.E. Intake of Antioxidant Nutrients and Risk of Non-Hodgkin’s Lymphoma in the Women’s Health Initiative. Nutr. Cancer 2012, 64, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Zhang, X.; Ji, C.; Liu, S.-M.; Wang, J. Transcriptomic and functional pathways analysis of ascorbate-induced cytotoxicity and resistance of Burkitt lymphoma. Oncotarget 2016, 7, 63950–63959. [Google Scholar] [CrossRef]
- Allegra, A.; Caserta, S.; Mirabile, G.; Gangemi, S. Aging and Age-Related Epigenetic Drift in the Pathogenesis of Leukemia and Lymphomas: New Therapeutic Targets. Cells 2023, 12, 2392. [Google Scholar] [CrossRef]
- Musolino, C.; Sant’antonio, E.; Penna, G.; Alonci, A.; Russo, S.; Granata, A.; Allegra, A. Epigenetic therapy in myelodysplastic syndromes. Eur. J. Haematol. 2010, 84, 463–473. [Google Scholar] [CrossRef]
- De, S.; Shaknovich, R.; Riester, M.; Elemento, O.; Geng, H.; Kormaksson, M.; Jiang, Y.; Woolcock, B.; Johnson, N.; Polo, J.M.; et al. Aberration in DNA Methylation in B-Cell Lymphomas Has a Complex Origin and Increases with Disease Severity. PLoS Genet. 2013, 9, e1003137. [Google Scholar] [CrossRef]
- Clozel, T.; Yang, S.; Elstrom, R.L.; Tam, W.; Martin, P.; Kormaksson, M.; Banerjee, S.; Vasanthakumar, A.; Culjkovic, B.; Scott, D.W.; et al. Mechanism-Based Epigenetic Chemosensitization Therapy of Diffuse Large B-Cell Lymphoma. Cancer Discov. 2013, 3, 1002–1019. [Google Scholar] [CrossRef]
- Shenoy, N.; Bhagat, T.; Nieves, E.; Stenson, M.; Lawson, J.; Choudhary, G.S.; Habermann, T.; Nowakowski, G.; Singh, R.; Wu, X.; et al. Upregulation of TET activity with ascorbic acid induces epigenetic modulation of lymphoma cells. Blood Cancer J. 2017, 7, e587. [Google Scholar] [CrossRef]
- Cohen, J.I. Epstein-Barr Virus Infection. N. Engl. J. Med. 2000, 343, 481–492. [Google Scholar] [CrossRef]
- Cohen, J.I.; Bollard, C.M.; Khanna, R.; Pittaluga, S. Current understanding of the role of Epstein–Barr virus in lymphomagenesis and therapeutic approaches to EBV-associated lymphomas. Leuk. Lymphoma 2008, 49, 27–34. [Google Scholar] [CrossRef]
- Osipova-Goldberg, H.I.; Turchanowa, L.V.; Adler, B.; Pfeilschifter, J.M. H2O2 inhibits BCR-dependent immediate early induction of EBV genes in Burkitt’s lymphoma cells. Free Radic. Biol. Med. 2009, 47, 1120–1129. [Google Scholar] [CrossRef]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef]
- Verrax, J.; Calderon, P.B. Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Radic. Biol. Med. 2009, 47, 32–40. [Google Scholar] [CrossRef]
- Shatzer, A.N.; Espey, M.G.; Chavez, M.; Tu, H.; Levine, M.; Cohen, J.I. Ascorbic Acid Kills Epstein-Barr Virus Positive Burkitt Lymphoma Cells and Epstein-Barr Virus Transformed B-Cells in Vitro, but Not in Vivo. Leuk. Lymphoma 2013, 54, 1069–1078. [Google Scholar] [CrossRef]
- Li, C.; Thompson, M.A.; Tamayo, A.T.; Zuo, Z.; Lee, J.; Vega, F.; Ford, R.J.; Pham, L.V. Over-expression of Thioredoxin-1 mediates growth, survival, and chemoresistance and is a druggable target in diffuse large B-cell lymphoma. Oncotarget 2012, 3, 314–326. [Google Scholar] [CrossRef]
- Trzeciecka, A.; Klossowski, S.; Bajor, M.; Zagozdzon, R.; Gaj, P.; Muchowicz, A.; Malinowska, A.; Czerwoniec, A.; Barankiewicz, J.; Domagala, A.; et al. Dimeric peroxiredoxins are druggable targets in human Burkitt lymphoma. Oncotarget 2015, 7, 1717–1731. [Google Scholar] [CrossRef]
- Zhang, W.; Trachootham, D.; Liu, J.; Chen, G.; Pelicano, H.; Garcia-Prieto, C.; Lu, W.; Burger, J.A.; Croce, C.M.; Plunkett, W.; et al. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nature 2012, 14, 276–286. [Google Scholar] [CrossRef]
- Hoffer, L.J.; Levine, M.; Assouline, S.; Melnychuk, D.; Padayatty, S.J.; Rosadiuk, K.; Rousseau, C.; Robitaille, L.; Miller, W.H. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann. Oncol. 2008, 19, 1969–1974. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Højgaard, M.; Andersen, J.T.; Jørgensen, N.R.; Zerahn, B.; Kristensen, B.; Henriksen, T.; Lykkesfeldt, J.; Mikines, K.J.; Poulsen, H.E. Weekly ascorbic acid infusion in castration-resistant prostate cancer patients: A single-arm phase II trial. Transl. Androl. Urol. 2017, 6, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Galán, P.; Dreyling, M.; Wiestner, A. Mantle cell lymphoma: Biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood 2011, 117, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-H.; Shen, Y.-L.; Jing, Y.-K.; Cai, X.; Jia, P.-M.; Huang, Y.; Tang, W.; Shi, G.-Y.; Sun, Y.-P.; Dai, J.; et al. Apoptosis and Growth Inhibition in Malignant Lymphocytes After Treatment With Arsenic Trioxide at Clinically Achievable Concentrations. JNCI. J. Natl. Cancer Inst. 1999, 91, 772–778. [Google Scholar] [CrossRef]
- Gill, H.; Au, W.Y.; Cheung, W.W.; Lee, E.Y.; Kwong, Y.L. Oral arsenic trioxide-based regimen as salvage treatment for relapsed or refractory mantle cell lymphoma. Ann. Oncol. 2014, 25, 1391–1397. [Google Scholar] [CrossRef]
- Chang, J.E.; Voorhees, P.M.; Kolesar, J.M.; Ahuja, H.G.; Sanchez, F.A.; Rodriguez, G.A.; Kim, K.; Werndli, J.; Bailey, H.H.; Kahl, B.S. Phase II Study of Arsenic Trioxide and Ascorbic Acid for Relapsed or Refractory Lymphoid Malignancies: A Wisconsin Oncology Network Study. Hematol. Oncol. 2009, 27, 11–13. [Google Scholar] [CrossRef]
- Heaney, M.L.; Gardner, J.R.; Karasavvas, N.; Golde, D.W.; Scheinberg, D.A.; Smith, E.A.; O’Connor, O.A. Vitamin C Antagonizes the Cytotoxic Effects of Antineoplastic Drugs. Cancer Res. 2008, 68, 8031–8038. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.G.; Mannino, F.; Innao, V.; Musolino, C.; Allegra, A. Radioprotective Agents and Enhancers Factors. Preventive and Therapeutic Strategies for Oxidative Induced Radiotherapy Damages in Hematological Malignancies. Antioxidants 2020, 9, 1116. [Google Scholar] [CrossRef] [PubMed]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef]
- Shankar, B.; Kumar, S.S.; Sainis, K.B. Generation of Reactive Oxygen Species and Radiation Response in Lymphocytes and Tumor Cells. Radiat. Res. 2003, 160, 478–487. [Google Scholar] [CrossRef]
- Mane, S.D.; Kamatham, A.N. Ascorbyl stearate and ionizing radiation potentiate apoptosis through intracellular thiols and oxidative stress in murine T lymphoma cells. Chem. Interact. 2018, 281, 37–50. [Google Scholar] [CrossRef]
- McGirt, L.Y.; Jia, P.; Baerenwald, D.A.; Duszynski, R.J.; Dahlman, K.B.; Zic, J.A.; Zwerner, J.P.; Hucks, D.; Dave, U.; Zhao, Z.; et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood 2015, 126, 508–519. [Google Scholar] [CrossRef]
- Jawed, S.I.; Myskowski, P.L.; Horwitz, S.; Moskowitz, A.; Querfeld, C. Primary Cutaneous T-Cell Lymphoma (Mycosis Fungoides and Sézary Syndrome): Part I. Diagnosis: Clinical and Histopathologic Features and New Molecular and Biologic Markers. J. Am. Acad. Dermatol. 2014, 70, 205.e1–2005.e16. [Google Scholar] [CrossRef]
- Wildman, R.E.C. Isoprenoids, Health and Disease. In Handbook of Nutraceuticals and Functional Foods; CRC Press: Boca Raton, FL, USA, 2002; pp. 53–76. [Google Scholar]
- Chen, T.C.; Da Fonseca, C.O.; Schönthal, A.H. Preclinical development and clinical use of perillyl alcohol for chemoprevention and cancer therapy. Am. J. Cancer Res. 2015, 5, 1580–1593. [Google Scholar]
- Silva-Hirschberg, C.; Hartman, H.; Stack, S.; Swenson, S.; Minea, R.O.; Davitz, M.A.; Chen, T.C.; Schönthal, A.H. Cytotoxic Impact of a Perillyl Alcohol-Temozolomide Conjugate, NEO212, on Cutaneous T-Cell Lymphoma in Vitro. Ther. Adv. Med. Oncol. 2019, 11, 1758835919891567. [Google Scholar] [CrossRef]
- Smith, A.; Howell, D.; Patmore, R.; Jack, A.; Roman, E. Incidence of haematological malignancy by sub-type: A report from the Haematological Malignancy Research Network. Br. J. Cancer 2011, 105, 1684–1692. [Google Scholar] [CrossRef]
- Chang, E.T.; Smedby, K.E.; Zhang, S.M.; Hjalgrim, H.; Melbye, M.; Öst, A.; Glimelius, B.; Wolk, A.; Adami, H.-O. Dietary Factors and Risk of Non-Hodgkin Lymphoma in Men and Women. Cancer Epidemiol. Biomark. Prev. 2005, 14, 512–520. [Google Scholar] [CrossRef]
- Rohrmann, S.; Becker, N.; Linseisen, J.; Nieters, A.; Rüdiger, T.; Raaschou-Nielsen, O.; Tjønneland, A.; Johnsen, H.E.; Overvad, K.; Kaaks, R.; et al. Fruit and vegetable consumption and lymphoma risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Causes Control 2007, 18, 537–549. [Google Scholar] [CrossRef]
- Tsai, H.-T.; Cross, A.J.; Graubard, B.I.; Oken, M.; Schatzkin, A.; Caporaso, N.E. Dietary Factors and Risk of Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma: A Pooled Analysis of Two Prospective Studies. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2680–2684. [Google Scholar] [CrossRef][Green Version]
- Chiu, B.C.-H.; Kwon, S.; Evens, A.M.; Surawicz, T.; Smith, S.M.; Weisenburger, D.D. Dietary intake of fruit and vegetables and risk of non-Hodgkin lymphoma. Cancer Causes Control 2011, 22, 1183–1195. [Google Scholar] [CrossRef]
- Holtan, S.G.; O’Connor, H.M.; Fredericksen, Z.S.; Liebow, M.; Thompson, C.A.; Macon, W.R.; Micallef, I.N.; Wang, A.H.; Slager, S.L.; Habermann, T.M.; et al. Food-Frequency Questionnaire-Based Estimates of Total Antioxidant Capacity and Risk of Non-Hodgkin Lymphoma. Int. J. Cancer 2012, 131, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Skibola, C.F.; Bracci, P.M.; Halperin, E.; Nieters, A.; Hubbard, A.; Paynter, R.A.; Skibola, D.R.; Agana, L.; Becker, N.; Tressler, P.; et al. Polymorphisms in the Estrogen Receptor 1 and Vitamin C and Matrix Metalloproteinase Gene Families Are Associated with Susceptibility to Lymphoma. PLoS ONE 2008, 3, e2816. [Google Scholar] [CrossRef] [PubMed]
- Chaigne, B.; Dartigeas, C.; Benboubker, L.; Chaumier, F.; Ertault, M.; Lissandre, S.; Stacoffe, M.; Maillot, F.; Blasco, H.; Vourc’h, P.; et al. Could a Citrus Keep the Haematologist Away? Br. J. Haematol. 2014, 166, 298–300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, J.; Okumura, H.; Ohtake, S.; Nakamura, S.; Nakao, S. Arsenic Trioxide Induces Apoptosis in Leukemia/Lymphoma Cell Lines via the CD95/CD95L System. Oncol. Rep. 2003, 10, 705–709. [Google Scholar] [PubMed]
- Farber, C.M.; Liebes, L.F.; Kanganis, D.N.; Silber, R. Human B lymphocytes show greater susceptibility to H2O2 toxicity than T lymphocytes. J. Immunol. 1984, 132, 2543–2546. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Zhao, X.; Mone, A.P.; Mo, X.; Vargo, M.; Jarjoura, D.; Byrd, J.C.; Muthusamy, N. Arsenic trioxide and ascorbic acid demonstrate promising activity against primary human CLL cells in vitro. Leuk. Res. 2010, 34, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Yosifov, D.Y.; Wolf, C.; Stilgenbauer, S.; Mertens, D. From Biology to Therapy: The CLL Success Story. HemaSphere 2019, 3, e175. [Google Scholar] [CrossRef] [PubMed]
- Darwiche, W.; Gomila, C.; Ouled-Haddou, H.; Naudot, M.; Doualle, C.; Morel, P.; Nguyen-Khac, F.; Garçon, L.; Marolleau, J.-P.; Ghamlouch, H. Ascorbic acid (vitamin C) synergistically enhances the therapeutic effect of targeted therapy in chronic lymphocytic leukemia. J. Exp. Clin. Cancer Res. 2020, 39, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Zhang, H.; Zhang, W.; Feng, L.; Du, M.; Zhou, Y.; Chen, Z.; Pelicano, H.; Plunkett, W.; Wierda, W.G.; et al. Effective elimination of fludarabine-resistant CLL cells by PEITC through a redox-mediated mechanism. Blood 2008, 112, 1912–1922. [Google Scholar] [CrossRef]
- Chapman, C.M.; Sun, X.; Roschewski, M.; Aue, G.; Farooqui, M.; Stennett, L.; Gibellini, F.; Arthur, D.; Pérez-Galán, P.; Wiestner, A. ON 01910.Na Is Selectively Cytotoxic for Chronic Lymphocytic Leukemia Cells through a Dual Mechanism of Action Involving PI3K/AKT Inhibition and Induction of Oxidative Stress. Clin. Cancer Res. 2012, 18, 1979–1991. [Google Scholar] [CrossRef]
- Longo, P.G.; Laurenti, L.; Gobessi, S.; Sica, S.; Leone, G.; Efremov, D.G. The Akt/Mcl-1 pathway plays a prominent role in mediating antiapoptotic signals downstream of the B-cell receptor in chronic lymphocytic leukemia B cells. Blood 2008, 111, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Klingelhoeffer, C.; Kämmerer, U.; Koospal, M.; Mühling, B.; Schneider, M.; Kapp, M.; Kübler, A.; Germer, C.-T.; Otto, C. Natural resistance to ascorbic acid induced oxidative stress is mainly mediated by catalase activity in human cancer cells and catalase-silencing sensitizes to oxidative stress. BMC Complement. Altern. Med. 2012, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Doskey, C.M.; Buranasudja, V.; Wagner, B.A.; Wilkes, J.G.; Du, J.; Cullen, J.J.; Buettner, G.R. Tumor cells have decreased ability to metabolize H2O2: Implications for pharmacological ascorbate in cancer therapy. Redox Biol. 2016, 10, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, C.; Chignola, R.; Dando, I.; Perbellini, O.; Mimiola, E.; Lovato, O.; Laudanna, C.; Pizzolo, G.; Donadelli, M.; Scupoli, M.T. Low catalase expression confers redox hypersensitivity and identifies an indolent clinical behavior in CLL. Blood 2018, 131, 1942–1954. [Google Scholar] [CrossRef] [PubMed]
- Linley, A.; Valle-Argos, B.; Steele, A.J.; Stevenson, F.K.; Forconi, F.; Packham, G. Higher levels of reactive oxygen species are associated with anergy in chronic lymphocytic leukemia. Haematologica 2015, 100, e265–e268. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Innao, V.; Gerace, D.; Vaddinelli, D.; Musolino, C. Adoptive immunotherapy for hematological malignancies: Current status and new insights in chimeric antigen receptor T cells. Blood Cells Mol. Dis. 2016, 62, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tripathi, M.; Satyam, A.; Kumar, L. Study of antioxidant levels in patients with multiple myeloma. Leuk. Lymphoma 2009, 50, 809–815. [Google Scholar] [CrossRef]
- Xia, J.; Xu, H.; Zhang, X.; Allamargot, C.; Coleman, K.L.; Nessler, R.; Frech, I.; Tricot, G.; Zhan, F. Multiple Myeloma Tumor Cells are Selectively Killed by Pharmacologically-dosed Ascorbic Acid. eBioMedicine 2017, 18, 41–49. [Google Scholar] [CrossRef]
- Rousselot, P.; Arnulf, B.; Poupon, J.; Larghero, J.; Royer, B.; Hermine, O.; Dombret, H.; Brouet, J.C.; Fermand, J.P. Phase I/II Study of Arsenic Trioxide in the Treatment of Refractory Multiple Myeloma. Blood 2003, 102, 380B–381B. [Google Scholar]
- Berenson, J.R.; Matous, J.; Swift, R.A.; Mapes, R.; Morrison, B.; Yeh, H.S. A Phase I/II Study of Arsenic Trioxide/Bortezomib/Ascorbic Acid Combination Therapy for the Treatment of Relapsed or Refractory Multiple Myeloma. Clin. Cancer Res. 2007, 13, 1762–1768. [Google Scholar] [CrossRef]
- Berenson, J.R.; Yellin, O.; Woytowitz, D.; Flam, M.S.; Cartmell, A.; Patel, R.; Duvivier, H.; Nassir, Y.; Eades, B.; Abaya, C.D.; et al. Bortezomib, ascorbic acid and melphalan (BAM) therapy for patients with newly diagnosed multiple myeloma: An effective and well-tolerated frontline regimen. Eur. J. Haematol. 2009, 82, 433–439. [Google Scholar] [CrossRef]
- Qian, W.; Wang, L.; Li, P.; Hu, Y.; Wang, Q.; Yi, K.; Wu, M.; Xu, Y.; Song, J.; Chen, P.; et al. Efficiency and Tolerability of Induction and Consolidation Therapy with Arsenic Trioxide/Bortezomib/Ascorbic Acid/Dexamethasone (ABCD) Regimen Compared to Bortezomib/Dexamethasone (BD) Regimen in Newly Diagnosed Myeloma Patients. Cancer Manag. Res. 2020, 12, 431–441. [Google Scholar] [CrossRef]
- Held, L.A.; Rizzieri, D.; Long, G.D.; Gockerman, J.P.; Diehl, L.F.; De Castro, C.M.; Moore, J.O.; Horwitz, M.E.; Chao, N.J.; Gasparetto, C. A Phase I Study of Arsenic Trioxide (Trisenox), Ascorbic Acid, and Bortezomib (Velcade) Combination Therapy in Patients with Relapsed/Refractory Multiple Myeloma. Cancer Investig. 2013, 31, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, W.-J. The clinical activity of arsenic trioxide, ascorbic acid, ifosfamide and prednisone combination therapy in patients with relapsed and refractory multiple myeloma. OncoTargets Ther. 2015, 8, 775–781. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mitsiades, C.S.; Mitsiades, N.; McMullan, C.J.; Poulaki, V.; Shringarpure, R.; Hideshima, T.; Chauhan, D.; Treon, S.P.; Richardson, P.G.; Munshi, N.C. The Proteasome Inhibitor Bortezomib (PS-341) Is Active against Waldenstrom’s Macroglobulinemia (WM). In Blood; American Society of Hematology: Washington, DC, USA, 2003; Volume 102, p. 181A. [Google Scholar]
- May, J.M.; Qu, Z.-C.; Li, X. Requirement for GSH in recycling of ascorbic acid in endothelial cells. Biochem. Pharmacol. 2001, 62, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.H.; Yang, H.H.; Parker, K.; Manyak, S.; Friedman, J.M.; Altamirano, C.; Wu, Z.-Q.; Borad, M.J.; Frantzen, M.; Roussos, E.; et al. The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin. Cancer Res. 2003, 9, 1136–1144. [Google Scholar] [PubMed]
- Karasavvas, N.; Cárcamo, J.M.; Stratis, G.; Golde, D.W. Vitamin C protects HL60 and U266 cells from arsenic toxicity. Blood 2005, 105, 4004–4012. [Google Scholar] [CrossRef]
- Innao, V.; Rizzo, V.; Allegra, A.G.; Musolino, C.; Allegra, A. Promising Anti-Mitochondrial Agents for Overcoming Acquired Drug Resistance in Multiple Myeloma. Cells 2021, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Bolaman, A.Z.; Turgutkaya, A.; Küçükdiler, H.E.; Selim, C.; Yavaşoğlu, İ. Pharmacological dose ascorbic acid administration in relapsed refractory multiple myeloma patients. Leuk. Res. Rep. 2021, 16, 100281. [Google Scholar] [CrossRef]
- Allegra, A.; Rizzo, V.; Innao, V.; Alibrandi, A.; Mazzeo, A.; Leanza, R.; Terranova, C.; Gentile, L.; Girlanda, P.; Allegra, A.G.; et al. Diagnostic utility of Sudoscan for detecting bortezomib-induced painful neuropathy: A study on 18 patients with multiple myeloma. Arch. Med. Sci. 2021, 18, 696–703. [Google Scholar] [CrossRef]
- Nakano, A.; Abe, M.; Oda, A.; Amou, H.; Hiasa, M.; Nakamura, S.; Miki, H.; Harada, T.; Fujii, S.; Kagawa, K.; et al. Delayed treatment with vitamin C and N-acetyl-l-cysteine protects Schwann cells without compromising the anti-myeloma activity of bortezomib. Int. J. Hematol. 2011, 93, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Cook, M.; Cockwell, P. Current Trends of Renal Impairment in Multiple Myeloma. Kidney Dis. 2015, 1, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Parrow, N.L.; Leshin, J.A.; Levine, M. Parenteral Ascorbate As a Cancer Therapeutic: A Reassessment Based on Pharmacokinetics. Antioxid. Redox Signal. 2013, 19, 2141–2156. [Google Scholar] [CrossRef] [PubMed]
- Mussa, A.; Idris, R.A.M.; Ahmed, N.; Ahmad, S.; Murtadha, A.H.; Din, T.A.D.A.A.T.; Yean, C.Y.; Rahman, W.F.W.A.; Lazim, N.M.; Uskoković, V.; et al. High-Dose Vitamin C for Cancer Therapy. Pharmaceuticals 2022, 15, 711. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.; Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: Reevaluation of prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA 1978, 75, 4538–4542. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-KappaB Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C.; Chang, J.H.; Jin, J. Regulation of Nuclear Factor-ΚB in Autoimmunity. Trends Immunol. 2013, 34, 282–289. [Google Scholar] [CrossRef]
- van Gorkom, G.N.Y.; Lookermans, E.L.; Van Elssen, C.H.M.J.; Bos, G.M.J. The Effect of Vitamin C (Ascorbic Acid) in the Treatment of Patients with Cancer: A Systematic Review. Nutrients 2019, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Thyagarajan, A.; Sahu, R.P. Potential Contributions of Antioxidants to Cancer Therapy: Immunomodulation and Radiosensitization. Integr. Cancer Ther. 2018, 17, 210–216. [Google Scholar] [CrossRef]
- Chakraborty, A.; Jana, N.R. Vitamin C-Conjugated Nanoparticle Protects Cells from Oxidative Stress at Low Doses but Induces Oxidative Stress and Cell Death at High Doses. ACS Appl. Mater. Interfaces 2017, 9, 41807–41817. [Google Scholar] [CrossRef]
- Gajbhiye, K.R.; Soni, V. An In vivo Investigation of Ascorbic Acid Tethered Polymeric Nanoparticles for Effectual Brain Transport of Rivastigmine. Curr. Drug Deliv. 2023, 20, 961–977. [Google Scholar] [CrossRef] [PubMed]
- Baldini, N.; Torreggiani, E.; Roncuzzi, L.; Perut, F.; Zini, N.; Avnet, S. Exosome-like Nanovesicles Isolated from Citrus limon L. Exert Antioxidative Effect. Curr. Pharm. Biotechnol. 2018, 19, 877–885. [Google Scholar] [CrossRef] [PubMed]
| NCT | Study Title | Intervention | Status | Study Type |
|---|---|---|---|---|
| NCT00329498 | L-Ascorbic Acid Depletion to Treat Acute Myeloid Leukemia and Myelodysplastic Syndromes | L-Ascorbic Acid | Suspended | Interventional |
| NCT00184054 | Trial of Arsenic Trioxide With Ascorbic Acid in the Treatment of Adult Non-Acute Promyelocytic Leukemia (APL) Acute Myelogenous Leukemia | Arsenic Trioxide; Vitamin C | Terminated with results | Interventional |
| NCT02877277 | Epigenetics, Vitamin C and Abnormal Hematopoiesis—Pilot Study | Dietary Supplement: Vitamin C Dietary Supplement: Placebo | Completed | Interventional |
| NCT03999723 | Combining Active and Passive DNA Hypomethylation | Dietary Supplement: vitamin C Dietary Supplement: Placebo | Recruiting | Interventional |
| NCT03526666 | Ascorbic Acid Levels in MDS, AML, and CMML Patients | Peripheral blood collection | Completed | Observational |
| NCT03397173 | TET2 Mutations in Myelodysplastic Syndromes and Acute Myeloid Leukemia with Azacitidine + vitamin C | Azacitidine and vitamin C | Completed | Interventional |
| NCT03624270 | Oral Arsenic Trioxide for Newly Diagnosed Acute Promyelocytic Leukemia | Oral arsenic Trioxide, ATRA and vitamin C | Recruiting | Interventional |
| NCT00671697 | Decitabine, Arsenic Trioxide and vitamin C for Myelodysplastic Syndromes and Acute Myeloid Leukemia | Arsenic Trioxide Decitabine | Completed | Interventional |
| NCT | Study Title | Intervention | Status | Study Type |
|---|---|---|---|---|
| NCT00274820 | Arsenic Trioxide, Ascorbic Acid, Dexamethasone, and Thalidomide in Myelofibrosis/Myeloproliferative Disorder | Vitamin C; Arsenic trioxid; dexamethasone | Completed | Interventional |
| NCT01670084 | Nilotinib and Combination Chemotherapy in Treating Patients with Newly Diagnosed Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia or Blastic Phase Chronic Myelogenous Leukemia | Nilotinib; rituximab; cyclophosphamide | Withdrawn | Interventional |
| NCT00003619 | Combination Chemotherapy Followed by Peripheral Stem Cell Transplantation or Isotretinoin in Treating Patients with Acute Myeloid Leukemia, Myelodysplastic Syndrome, or Acute Lymphocytic Leukemia | Filgrastim; dietary Supplement: vitamin E; busulfan | Completed | Interventional |
| NCT00005641 | Removal of T Cells to Prevent Graft-Versus-Host Disease in Patients Undergoing Bone Marrow Transplantation | Anti-thymocyte globulin; busulfan; cyclophosphamide | Terminated | Interventional |
| NCT | Study Title | Intervention | Status | Study Type |
|---|---|---|---|---|
| NCT03602235 | High Dose Ascorbic Acid for Plasma Cell Disorders | Ascorbate: Melphalan | Recruiting | Interventional |
| NCT03964688 | Effect of Vitamin C in Autologous Stem Cell Transplantations | Vitamin C; Placebos | Completed | Interventional |
| NCT00590603 | Trisenox, Ascorbic Acid and Bortezomib in Patients with Relapsed/Refractory Multiple Myeloma | Arsenic Trioxide, vitamin C and Bortezomib | Terminated | Interventional |
| NCT00661544 | Arsenic Trioxide with Ascorbic Acid and Melphalan for Myeloma Patients | Arsenic Trioxide; Melphalan; vitamin C | Terminated with results | Interventional |
| NCT00227682 | Arsenic Trioxide, Thalidomide, Dexamethasone, and Ascorbic Acid in Treating Patients with Relapsed or Refractory Multiple Myeloma | Dietary Supplement: vitamin C; arsenic trioxid; dexamethasone | Terminated | Interventional |
| NCT00317811 | Bortezomib, Ascorbic Acid, and Melphalan in Treating Patients with Newly Diagnosed Multiple Myeloma | Vitamin C; bortezomib; melphalan | Completed | Interventional |
| NCT00258245 | Arsenic Trioxide and vitamin C combined with Bortezomib, Thalidomide, and Dexamethasone in Treating Patients with Relapsed or Refractory Multiple Myeloma or Plasma Cell Leukemia | Vitamin C; arsenic trioxid; bortezomib | Completed | Interventional |
| NCT00469209 | Velcade, Trisenox, Vitamin C and Melphalan for Myeloma Patients | Arsenic Trioxide; Bortezomib; Melphalan | Completed with results | Interventional |
| NCT00112879 | Arsenic Trioxide, vitamin C, Dexamethasone, and Thalidomide in Treating Patients with Multiple Myeloma | arsenic trioxide; vitamin C; dexamethasone | Withdrawn | Interventional |
| NCT00085345 | Melphalan, Arsenic Trioxide, and Ascorbic Acid in Treating Patients with Relapsed or Refractory Multiple Myeloma | Arsenic trioxide; vitamin C; melphalan | Withdrawn | Interventional |
| NCT00006021 | Arsenic Trioxide Plus Vitamin C in Treating Patients with Recurrent or Refractory Multiple Myeloma | Vitamin C; arsenic trioxide | Completed | Interventional |
| NCT01125449 | Study of High Dose Intravenous (IV) Ascorbic Acid in Measurable Solid Tumor Disease | Vitamin C | Suspended | Interventional |
| NCT02206425 | Ixazomib as a Replacement for Carfilzomib and Bortezomib for |Multiple Myeloma Patients | Melphalan; Prednison; Cyclophosphamide | Completed | Interventional |
| NCT02931942 | Changing Over Time of Ascorbic Acid After Chemotherapy | Blood withdrawn | Active not recruiting | Observational |
| NCT03276481 | Prospective Evaluation of Taste Function in Multiple Myeloma Patients Undergoing Autologous Hematopoietic Cell Transplantation | Diagnostic Tests: Oral microbiota assessment; Comprehensive chemical gustometry; Measurement of salivary flow | Completed | Observational |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isola, S.; Gammeri, L.; Furci, F.; Gangemi, S.; Pioggia, G.; Allegra, A. Vitamin C Supplementation in the Treatment of Autoimmune and Onco-Hematological Diseases: From Prophylaxis to Adjuvant Therapy. Int. J. Mol. Sci. 2024, 25, 7284. https://doi.org/10.3390/ijms25137284
Isola S, Gammeri L, Furci F, Gangemi S, Pioggia G, Allegra A. Vitamin C Supplementation in the Treatment of Autoimmune and Onco-Hematological Diseases: From Prophylaxis to Adjuvant Therapy. International Journal of Molecular Sciences. 2024; 25(13):7284. https://doi.org/10.3390/ijms25137284
Chicago/Turabian StyleIsola, Stefania, Luca Gammeri, Fabiana Furci, Sebastiano Gangemi, Giovanni Pioggia, and Alessandro Allegra. 2024. "Vitamin C Supplementation in the Treatment of Autoimmune and Onco-Hematological Diseases: From Prophylaxis to Adjuvant Therapy" International Journal of Molecular Sciences 25, no. 13: 7284. https://doi.org/10.3390/ijms25137284
APA StyleIsola, S., Gammeri, L., Furci, F., Gangemi, S., Pioggia, G., & Allegra, A. (2024). Vitamin C Supplementation in the Treatment of Autoimmune and Onco-Hematological Diseases: From Prophylaxis to Adjuvant Therapy. International Journal of Molecular Sciences, 25(13), 7284. https://doi.org/10.3390/ijms25137284






