Exosomes Derived from Hypertrophic Scar Fibroblasts Suppress Melanogenesis in Normal Human Epidermal Melanocytes
Abstract
1. Introduction
2. Results
2.1. HTSF-Exosomes Inhibit Proliferation and Production of Melanin
2.2. HTSF-Exosomes Did Not Induce Apoptosis
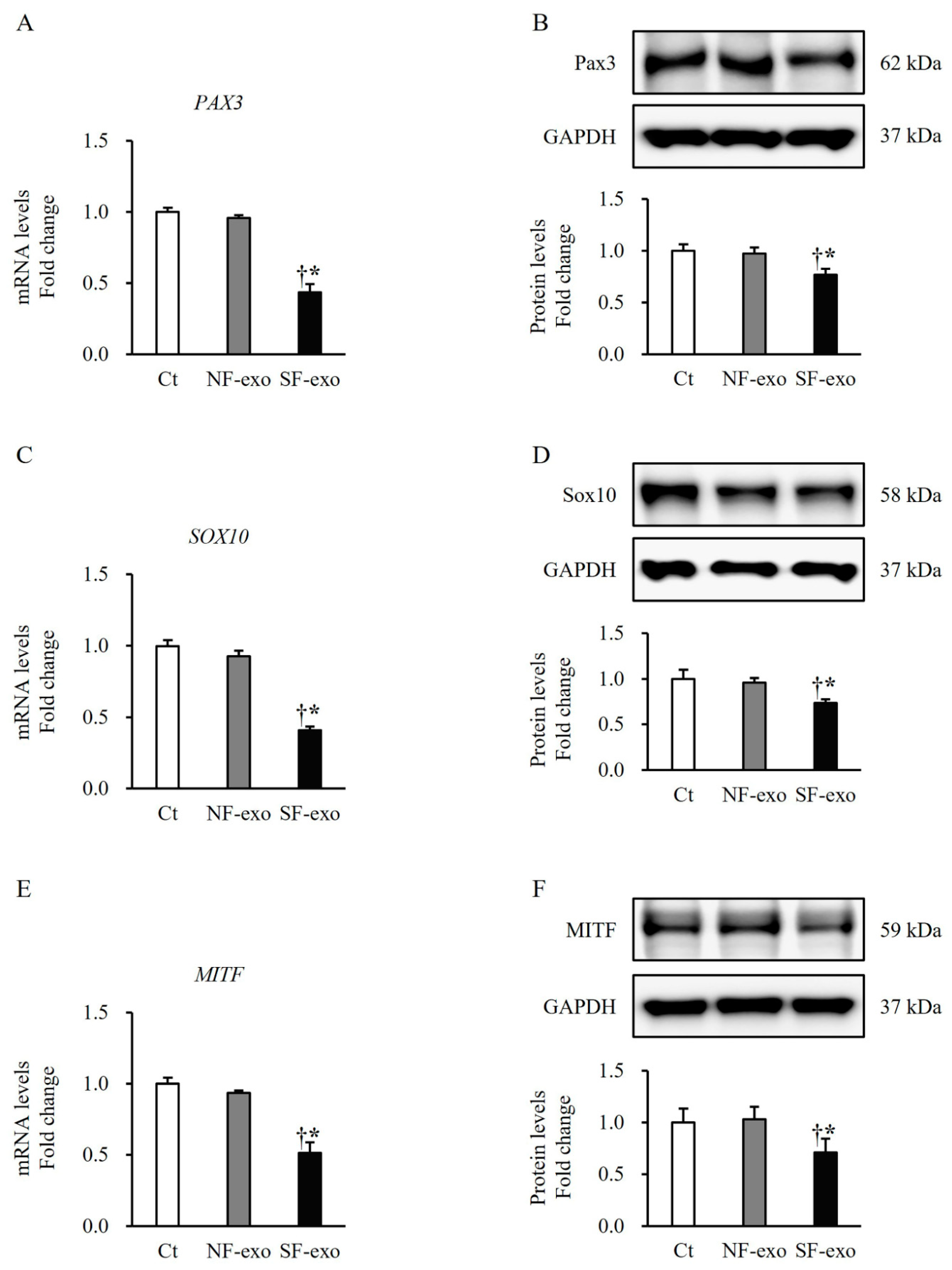
2.3. HTSF-Exosomes Decreased the Expression of Melanogenesis-Related Transcription Factors
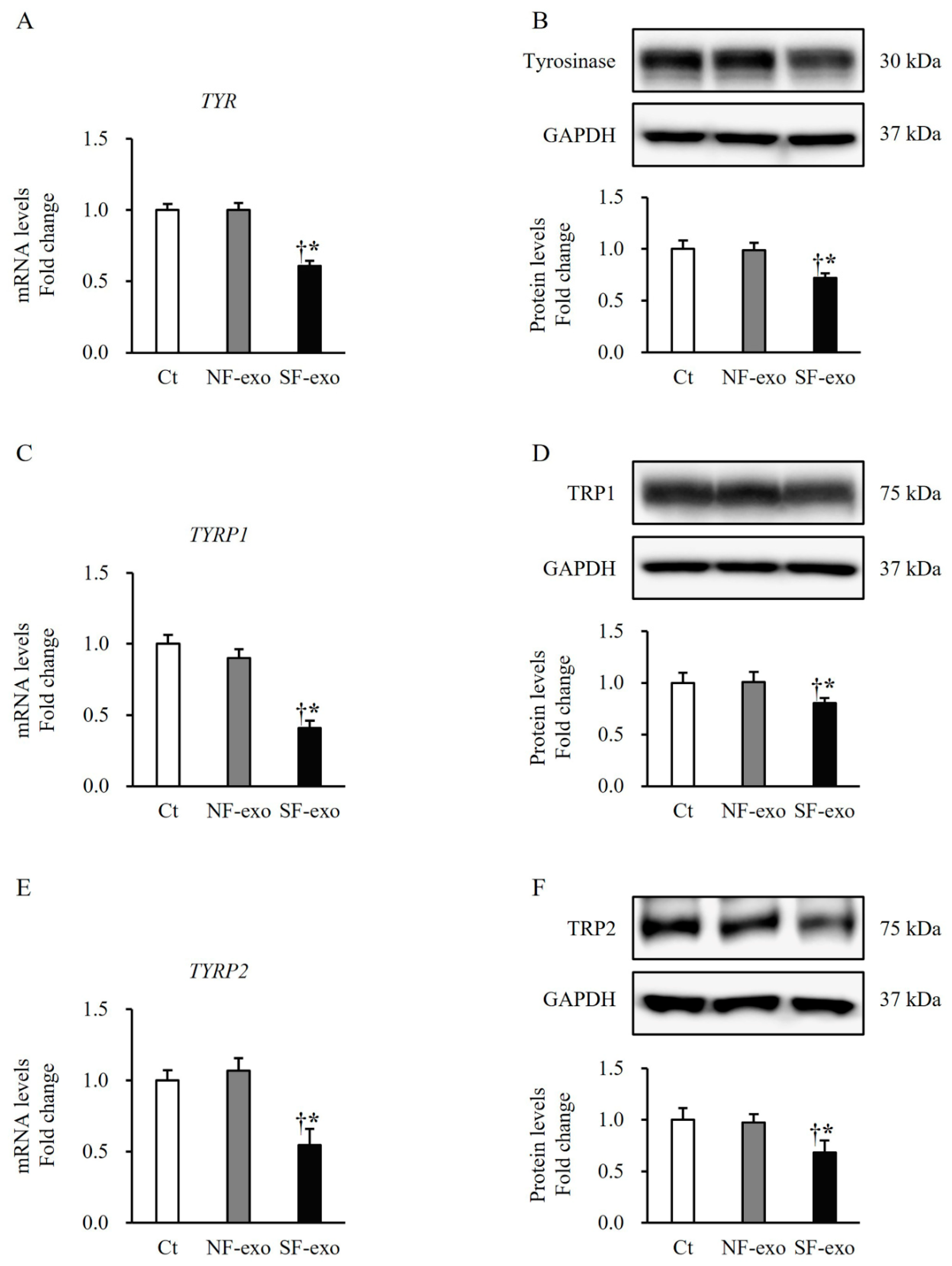
2.4. HTSF-Exosomes Decreased the Expression of Melanin Synthesis-Related Regulatory Enzymes
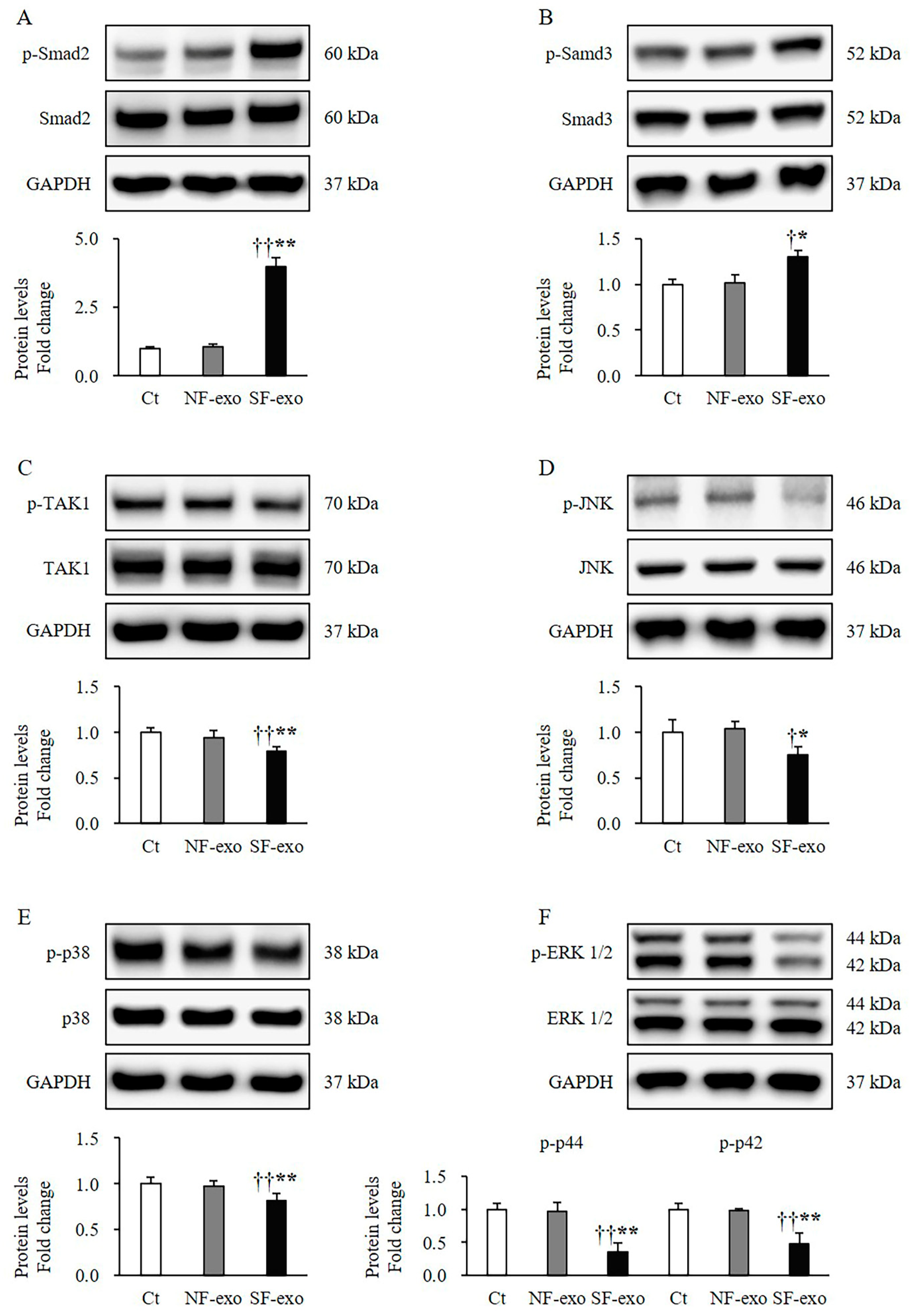
2.5. HTSF-Exosomes Induce Melanogenesis-Related Signaling Changes
2.6. HTSF-Exosomes Increase Phosphorylation of Transcription Factors
2.7. Expression of miRNAs in HTSFs-Exosomes
3. Discussion
4. Materials and Methods
4.1. Primary Fibroblast Culture
4.2. Exosome Purification
4.3. Human Melanocyte Culture and Exosome Treatment
4.4. Cell Proliferation Assay
4.5. Melanin Content Assay
4.6. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
4.7. Western Blot Analysis
4.8. MicroRNA Analysis
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finnerty, C.C.; Jeschke, M.G.; Branski, L.K.; Barret, J.P.; Dziewulski, P.; Herndon, D.N. Hypertrophic scarring: The greatest unmet challenge after burn injury. Lancet 2016, 388, 1427–1436. [Google Scholar] [CrossRef]
- Koike, S.; Yamasaki, K. Melanogenesis connection with innate immunity and toll-like receptors. Int. J. Mol. Sci. 2020, 21, 9769. [Google Scholar] [CrossRef]
- Carney, B.C.; McKesey, J.P.; Rosenthal, D.S.; Shupp, J.W. Treatment strategies for hypopigmentation in the context of burn hypertrophic scars. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1642. [Google Scholar] [CrossRef]
- Armour, A.; Scott, P.G.; Tredget, E.E. Cellular and molecular pathology of HTS: Basis for treatment. Wound. Repair Regen. 2007, 15 (Suppl. 1), S6–S17. [Google Scholar] [CrossRef]
- Cui, H.S.; Hong, A.R.; Kim, J.B.; Yu, J.H.; Cho, Y.S.; Joo, S.Y.; Seo, C.H. Extracorporeal shock wave therapy alters the expression of fibrosis-related molecules in fibroblast derived from human hypertrophic scar. Int. J. Mol. Sci. 2018, 19, 124. [Google Scholar] [CrossRef]
- Zhu, Z.; Ding, J.; Tredget, E.E. The molecular basis of hypertrophic scars. Burns Trauma 2016, 4, 2. [Google Scholar] [CrossRef]
- Tredget, E.E.; Shankowsky, H.A.; Pannu, R.; Nedelec, B.; Iwashina, T.; Ghahary, A.; Taerum, T.V.; Scott, P.G. Transforming growth factor-beta in thermally injured patients with hypertrophic scars: Effects of interferon alpha-2b. Plast. Reconstr. Surg. 1998, 102, 1317–1328; discussion 1329–1330. [Google Scholar] [CrossRef]
- Wang, R.; Ghahary, A.; Shen, Q.; Scott, P.G.; Roy, K.; Tredget, E.E. Hypertrophic scar tissues and fibroblasts produce more transforming growth factor-beta1 mRNA and protein than normal skin and cells. Wound Repair Regen. 2000, 8, 128–137. [Google Scholar] [CrossRef]
- Colwell, A.S.; Phan, T.T.; Kong, W.; Longaker, M.T.; Lorenz, P.H. Hypertrophic scar fibroblasts have increased connective tissue growth factor expression after transforming growth factor-beta stimulation. Plast. Reconstr. Surg. 2005, 116, 1387–1390; discussion 1391–1132. [Google Scholar] [CrossRef]
- Wang, J.; Hori, K.; Ding, J.; Huang, Y.; Kwan, P.; Ladak, A.; Tredget, E.E. Toll-like receptors expressed by dermal fibroblasts contribute to hypertrophic scarring. J. Cell. Physiol. 2011, 226, 1265–1273. [Google Scholar] [CrossRef]
- Xue, H.; McCauley, R.L.; Zhang, W.; Martini, D.K. Altered interleukin-6 expression in fibroblasts from hypertrophic burn scars. J. Burn Care Rehabil. 2000, 21, 142–146. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Slominski, R.M.; Chen, J.Y.; Raman, C.; Slominski, A.T. Photo-neuro-immuno-endocrinology: How the ultraviolet radiation regulates the body, brain, and immune system. Proc. Natl. Acad. Sci. USA 2024, 121, e2308374121. [Google Scholar] [CrossRef]
- Upadhyay, P.R.; Ho, T.; Abdel-Malek, Z.A. Participation of keratinocyte- and fibroblast-derived factors in melanocyte homeostasis, the response to UV, and pigmentary disorders. Pigment Cell Melanoma Res. 2021, 34, 762–776. [Google Scholar] [CrossRef]
- Yang, G.; Li, Y.; Nishimura, E.K.; Xin, H.; Zhou, A.; Guo, Y.; Dong, L.; Denning, M.F.; Nickoloff, B.J.; Cui, R. Inhibition of PAX3 by TGF-β modulates melanocyte viability. Mol. Cell 2008, 32, 554–563. [Google Scholar] [CrossRef]
- Huber, M.A.; Schubert, R.D.; Peter, R.U.; Kraut, N.; Park, J.E.; Rettig, W.J.; Garin-Chesa, P. Fibroblast activation protein: Differential expression and serine protease activity in reactive stromal fibroblasts of melanocytic skin tumors. J. Investig. Dermatol. 2003, 120, 182–188. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Passeron, T.; Watabe, H.; Yasumoto, K.; Rouzaud, F.; Hoashi, T.; Hearing, V.J. The effects of Dickkopf 1 on gene expression and Wnt signaling by melanocytes: Mechanisms underlying its suppression of melanocyte function and proliferation. J. Investig. Dermatol. 2007, 127, 1217–1225. [Google Scholar] [CrossRef]
- Slominski, A.T.; Slominski, R.M.; Raman, C.; Chen, J.Y.; Athar, M.; Elmets, C. Neuroendocrine signaling in the skin with a special focus on the epidermal neuropeptides. Am. J. Physiol. Cell Physiol. 2022, 323, C1757–C1776. [Google Scholar] [CrossRef]
- Kawakami, A.; Fisher, D.E. The master role of microphthalmia-associated transcription factor in melanocyte and melanoma biology. Lab. Investig. 2017, 97, 649–656. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Charrier, A.; Chen, R.; Chen, L.; Kemper, S.; Hattori, T.; Takigawa, M.; Brigstock, D.R. Exosomes mediate intercellular transfer of pro-fibrogenic connective tissue growth factor (CCN2) between hepatic stellate cells, the principal fibrotic cells in the liver. Surgery 2014, 156, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.T.; Melo, S.A.; Özdemir, B.C.; Kato, N.; Revuelta, I.; Miller, C.A.; Gattone, V.H.; LeBleu, V.S.; Kalluri, R. TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J. Am. Soc. Nephrol. 2013, 24, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.S.; Kim, D.H.; Joo, S.Y.; Cho, Y.S.; Kim, J.B.; Seo, C.H. Exosomes derived from human hypertrophic scar fibroblasts induces smad and TAK1 signaling in normal dermal fibroblasts. Arch. Biochem. Biophys. 2022, 722, 109215. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.S.; Joo, S.Y.; Lee, S.Y.; Cho, Y.S.; Kim, D.H.; Seo, C.H. Effect of hypertrophic scar fibroblast-derived exosomes on keratinocytes of normal human skin. Int. J. Mol. Sci. 2023, 24, 6132. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yan, J.; Tong, L.; Liu, S.; Zhang, Y. The role of exosomes from BALF in lung disease. J. Cell. Physiol. 2022, 237, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jiang, T.; Hu, X.; Liu, Z.; Zhao, L.; Liu, H.; Liu, Z.; Ma, L. Downregulation of microRNA-30a in bronchoalveolar lavage fluid from idiopathic pulmonary fibrosis patients. Mol. Med. Rep. 2018, 18, 5799–5806. [Google Scholar] [CrossRef]
- Carney, B.C.; Chen, J.H.; Luker, J.N.; Alkhalil, A.; Jo, D.Y.; Travis, T.E.; Moffatt, L.T.; Simbulan-Rosenthal, C.M.; Rosenthal, D.S.; Shupp, J.W. Pigmentation diathesis of hypertrophic scar: An examination of known signaling pathways to elucidate the molecular pathophysiology of injury-related dyschromia. J. Burn Care Res. 2019, 40, 58–71. [Google Scholar] [CrossRef]
- Dutta, S.; Panda, S.; Singh, P.; Tawde, S.; Mishra, M.; Andhale, V.; Athavale, A.; Keswani, S.M. Hypopigmentation in burns is associated with alterations in the architecture of the skin and the dendricity of the melanocytes. Burns 2020, 46, 906–917. [Google Scholar] [CrossRef]
- Yang, L.; Wei, Y.; Sun, Y.; Shi, W.; Yang, J.; Zhu, L.; Li, M. Interferon-gamma inhibits melanogenesis and induces apoptosis in melanocytes: A pivotal role of CD8+ cytotoxic T lymphocytes in vitiligo. Acta Derm. Venereol. 2015, 95, 664–670. [Google Scholar] [CrossRef]
- Silke, J.; Meier, P. Inhibitor of apoptosis (IAP) proteins-modulators of cell death and inflammation. Cold Spring Harb Perspect Biol. 2013, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Arnheiter, H.; Pavan, W.J. Interspecies difference in the regulation of melanocyte development by SOX10 and MITF. Proc. Natl. Acad. Sci. USA 2006, 103, 9081–9085. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Zheng, X.; Zhang, X.; Wang, Y.; Zhu, S.; Lu, F.; Qu, J.; Hou, L. Sox10 regulates skin melanocyte proliferation by activating the DNA replication licensing factor MCM5. J. Dermatol. Sci. 2017, 85, 216–225. [Google Scholar] [CrossRef]
- Udagawa, T.; Takahashi, E.; Tatsumi, N.; Mutai, H.; Saijo, H.; Kondo, Y.; Atkinson, P.J.; Matsunaga, T.; Yoshikawa, M.; Kojima, H.; et al. Loss of Pax3 causes reduction of melanocytes in the developing mouse cochlea. Sci. Rep. 2024, 14, 2210. [Google Scholar] [CrossRef] [PubMed]
- Medic, S.; Ziman, M. PAX3 expression in normal skin melanocytes and melanocytic lesions (naevi and melanomas). PLoS ONE 2010, 5, e9977. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Qu, B.; Lin, D.; Deng, Y.; Huang, R.; Zhong, Z. Pax3 gene regulated melanin synthesis by tyrosinase pathway in Pteria penguin. Int. J. Mol. Sci. 2018, 19, 3700. [Google Scholar] [CrossRef] [PubMed]
- Bondurand, N.; Pingault, V.; Goerich, D.E.; Lemort, N.; Sock, E.; Le Caignec, C.; Wegner, M.; Goossens, M. Interaction among SOX10, PAX3 and MITF, three genes altered in Waardenburg syndrome. Hum. Mol. Genet. 2000, 9, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.; Boyle, G.M.; Ziman, M.; Medic, S. Mechanisms contributing to differential regulation of PAX3 downstream target genes in normal human epidermal melanocytes versus melanoma cells. PLoS ONE 2015, 10, e0124154. [Google Scholar] [CrossRef]
- Carreira, S.; Goodall, J.; Denat, L.; Rodriguez, M.; Nuciforo, P.; Hoek, K.S.; Testori, A.; Larue, L.; Goding, C.R. Mitf regulation of Dia1 controls melanoma proliferation and invasiveness. Genes Dev. 2006, 20, 3426–3439. [Google Scholar] [CrossRef]
- King, R.; Googe, P.B.; Weilbaecher, K.N.; Mihm, M.C., Jr.; Fisher, D.E. Microphthalmia transcription factor expression in cutaneous benign, malignant melanocytic, and nonmelanocytic tumors. Am. J. Surg. Pathol. 2001, 25, 51–57. [Google Scholar] [CrossRef]
- Giuliano, S.; Cheli, Y.; Ohanna, M.; Bonet, C.; Beuret, L.; Bille, K.; Loubat, A.; Hofman, V.; Hofman, P.; Ponzio, G.; et al. Microphthalmia-associated transcription factor controls the DNA damage response and a lineage-specific senescence program in melanomas. Cancer Res. 2010, 70, 3813–3822. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, C.A.; Moore, K.J.; Nakayama, A.; Steingrímsson, E.; Copeland, N.G.; Jenkins, N.A.; Arnheiter, H. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell 1993, 74, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Álvarez, J.; Dilshat, R.; Fock, V.; Möller, K.; Karl, L.; Larue, L.; Ögmundsdóttir, M.H.; Steingrímsson, E. MITF and TFEB cross-regulation in melanoma cells. PLoS ONE 2020, 15, e0238546. [Google Scholar] [CrossRef] [PubMed]
- Cheli, Y.; Ohanna, M.; Ballotti, R.; Bertolotto, C. Fifteen-year quest for microphthalmia-associated transcription factor target genes. Pigment Cell Melanoma Res. 2010, 23, 27–40. [Google Scholar] [CrossRef] [PubMed]
- McGill, G.G.; Horstmann, M.; Widlund, H.R.; Du, J.; Motyckova, G.; Nishimura, E.K.; Lin, Y.L.; Ramaswamy, S.; Avery, W.; Ding, H.F.; et al. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell 2002, 109, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Carreira, S.; Goodall, J.; Aksan, I.; La Rocca, S.A.; Galibert, M.D.; Denat, L.; Larue, L.; Goding, C.R. Mitf cooperates with Rb1 and activates p21Cip1 expression to regulate cell cycle progression. Nature 2005, 433, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Choi, M.E. TGF-β-activated kinase-1: New insights into the mechanism of TGF-β signaling and kidney disease. Kidney Res. Clin. Pract. 2012, 31, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Ajibade, A.A.; Wang, H.Y.; Wang, R.F. Cell type-specific function of TAK1 in innate immune signaling. Trends Immunol. 2013, 34, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Zehavi, L.; Schayek, H.; Jacob-Hirsch, J.; Sidi, Y.; Leibowitz-Amit, R.; Avni, D. MiR-377 targets E2F3 and alters the NF-kB signaling pathway through MAP3K7 in malignant melanoma. Mol. Cancer 2015, 14, 68. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, D.H.; Cho, K.M.; Kim, K.H.; Kang, N.J. Effect of 3,6-anhydro-l-galactose on α-melanocyte stimulating hormone-induced melanogenesis in human melanocytes and a skin-equivalent model. J. Cell Biochem. 2018, 119, 7643–7656. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Le, Q.; Tong, J.; Wang, H. The IFN-γ-CXCL9/CXCL10-CXCR3 axis in vitiligo: Pathological mechanism and treatment. Eur. J. Immunol. 2024, 54, e2250281. [Google Scholar] [CrossRef]
- Zhou, J.; Ling, J.; Wang, Y.; Shang, J.; Ping, F. Cross-talk between interferon-gamma and interleukin-18 in melanogenesis. J. Photochem. Photobiol. B. 2016, 163, 133–143. [Google Scholar] [CrossRef]
- Tang, L.Y.; Heller, M.; Meng, Z.; Yu, L.R.; Tang, Y.; Zhou, M.; Zhang, Y.E. Transforming growth factor-β (TGF-β) directly activates the JAK1-STAT3 axis to induce hepatic fibrosis in coordination with the SMAD pathway. J. Biol. Chem. 2017, 292, 4302–4343. [Google Scholar] [CrossRef]
- Choi, H.; Choi, H.; Han, J.; Jin, S.H.; Park, J.Y.; Shin, D.W.; Lee, T.R.; Kim, K.; Lee, A.Y.; Noh, M. IL-4 inhibits the melanogenesis of normal human melanocytes through the JAK2-STAT6 signaling pathway. J. Investig. Dermatol. 2013, 133, 528–536. [Google Scholar] [CrossRef]
- Tredget, E.E.; Yang, L.; Delehanty, M.; Shankowsky, H.; Scott, P.G. Polarized Th2 cytokine production in patients with hypertrophic scar following thermal injury. J. Interferon Cytokine Res. 2006, 26, 179–189. [Google Scholar] [CrossRef]
- Mirmohammadsadegh, A.; Hassan, M.; Gustrau, A.; Doroudi, R.; Schmittner, N.; Nambiar, S.; Tannapfel, A.; Ruzicka, T.; Hengge, U.R. Constitutive expression of epidermal growth factor receptors on normal human melanocytes. J. Investig. Dermatol. 2005, 125, 392–394. [Google Scholar] [CrossRef]
- Hassel, J.C.; Winnemöller, D.; Schartl, M.; Wellbrock, C. STAT5 contributes to antiapoptosis in melanoma. Melanoma Res. 2008, 18, 378–385. [Google Scholar] [CrossRef]
- Hushcha, Y.; Blo, I.; Oton-Gonzalez, L.; Mauro, G.D.; Martini, F.; Tognon, M.; Mattei, M. microRNAs in the regulation of melanogenesis. Int. J. Mol. Sci. 2021, 22, 6104. [Google Scholar] [CrossRef]
- Ferracin, M.; Broseghini, E.; Dika, E. Pathophysiology roles and translational opportunities of miRNAs in cutaneous melanoma. In MicroRNA in Human Malignancies, 1st ed.; Negrini, M., Calin, G.A., Croce, C.M., Eds.; Academic Press: London, UK, 2022; Chapter 26; pp. 339–384. [Google Scholar]
- Ma, Y.; Shen, N.; Wicha, M.S.; Luo, M. The roles of the Let-7 family of microRNAs in the regulation of cancer stemness. Cells 2021, 10, 2415. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Gene Symbol | Fold Change |
|---|---|
| Hsa-let-7a-2-3p | 10.2 |
| Hsa-let-7b-3p | 20.3 |
| Hsa-let-7e-3p | 243.3 |
| Hsa-let-7f-1-3p | 33.7 |
| Hsa-let-7i-3p | 13.4 |
| Hsa-miR-31-5p | 6.0 |
| Hsa-miR-342-3p | 8.8 |
| Hsa-miR-125a-5p | 11.5 |
| Hsa-miR-365a-5p | 73.9 |
| Hsa-miR-125b-5p | 5.0 |
| Hsa-miR-138-5p | 3.5 |
| Hsa-miR-605-5p | 64.9 |
| Patients (n = 4) | Location of Specimens (Scar/Normal) | Age (Years) | Sex | Total Burn Surface Area (%) |
|---|---|---|---|---|
| 1 | Arm/arm | 38 | Male | 19 |
| 2 | Leg/leg | 26 | Male | 11 |
| 3 | Trunk/trunk | 30 | Male | 20 |
| 4 | Chest/chest | 40 | Male | 13 |
| Gene | Accession No. | Forward (5′ → 3′) | Reverse (5′ → 3′) |
|---|---|---|---|
| GAPDH | NM_ 002046.7 | CATGAGAAGTATGACAACAGC- CT | AGTCCTTCCACGATACCAA- AGTT |
| BAX | NM_004324.1 | CCTTTTGCTTCAGGGTTTCA | CCATGTTACTGTCCAGTTCG |
| BCL2 | NM_000633.3 | TGCGGCCTCTGTTTGATTT | AGGCATGTTGACTTCATTGT |
| CASP3 | NM_004346.1 | GGAAGCGAATCAATGGACTC- CTGG | GCATCGACATCTGTACCAG- ACC |
| BIRC2 | NM_001166.1 | CAGACACATGCAGCTCGAATG- AG | CACCTCAAGCCACCATCAC- AAC |
| BIRC3 | NM_001165.2 | GCTTTTGCTGTGATGGTGGACTC | CTTGACGGATGAACTCCTGT- CC |
| PAX3 | NM_181459.1 | GGCTTTCAACCATCTCATTCCCG | GTTGAGGTCTGTGAACGGT- GCT |
| SOX10 | NM_006941.1 | ATGAACGCCTTCATGGTGTGGG | CGCTTGTCACTTTCGTTCAGCAG |
| MITF | NM_198159.1 | GGCTTGATGGATCCTGCTTTGC | GAAGGTTGGCTGGACAGGA- GTT |
| TYR | NM_000372.2 | GCACAGATGAGTACATGGGAGG | CTGATGGCTGTTGTACTCCT- CC |
| TYRP1 | NM_000550.1 | TCTCAATGGCGAGTGGTCTGTG | CCTGTGGTTCAGGAAGACG- TTG |
| TYRP2 | NM_001922.2 | CTCAGACCAACTTGGCTACAGC | CAACCAAAGCCACCAGTGT- TCC |
| Target | Host | Dilution | Company (Cat. No.) |
|---|---|---|---|
| GAPDH | Rabbit | 1:1000 | Cell Signaling Technology, Danvers, MA, USA (2118S) |
| GAPDH | Mouse | 1:1000 | Santa Cruz Technology, Dallas, TX, USA (sc-47724) |
| Bax | Rabbit | 1:1000 | Abcam, Cambridge, UK (ab199677) |
| Bcl2 | Rabbit | 1:1000 | Abcam (ab196495) |
| Caspase 3 | Rabbit | 1:1000 | Cell Signaling Technology (9662S) |
| c-IAP1 | Mouse | 1:500 | Santa Cruz Technology (sc-271419) |
| c-IAP2 | Rabbit | 1:1000 | Cell Signaling Technology (3130S) |
| Pax3 | Rabbit | 1:1000 | Cell Signaling Technology (12412S) |
| Sox10 | Mouse | 1:500 | Santa Cruz Technology (sc-365692) |
| MITF | Rabbit | 1:1000 | Cell Signaling Technology (12590S) |
| Tyrosinase | Mouse | 1:500 | Santa Cruz Technology (sc-20035) |
| TRP1 | Mouse | 1:500 | Santa Cruz Technology (sc-166857) |
| TRP2 | Mouse | 1:500 | Santa Cruz Technology (sc-166717) |
| Phospho-Smad2 | Rabbit | 1:1000 | Cell Signaling Technology (3108S) |
| Smad2 | Rabbit | 1:1000 | Abcam (ab33875) |
| Phospho-Smad3 | Rabbit | 1:1000 | Invitrogen, Waltham, MA, USA (MA5-14936) |
| Smad3 | Rabbit | 1:1000 | Cell Signaling Technology (9523S) |
| Phospho-TAK1 | Rabbit | 1:1000 | Cell Signaling Technology (9339S) |
| TAI1 | Rabbit | 1:1000 | Cell Signaling Technology (5206S) |
| Phospho-JNK | Rabbit | 1:1000 | Cell Signaling Technology (9251S) |
| JNK | Rabbit | 1:1000 | Cell Signaling Technology (9252S) |
| Phospho-p38 | Mouse | 1:1000 | Cell Signaling Technology (9216S) |
| p38 | Rabbit | 1:1000 | Cell Signaling Technology (8690S) |
| Phospho-ERK | Rabbit | 1:1000 | Cell Signaling Technology (4370S) |
| ERK | Mouse | 1:1000 | Cell Signaling Technology (4696S) |
| Phospho-STAT1 | Rabbit | 1:1000 | Cusabio Technology, Houston, TX, USA (CSB-PA050162) |
| STAT1 | Rabbit | 1:1000 | Cusabio Technology (CSB-PA825331) |
| Phospho-STAT3 | Rabbit | 1:1000 | Abcam (ab76315) |
| STAT3 | Rabbit | 1:1000 | Cusabio Technology (CSB-PA004173) |
| Phospho-STAT5 | Rabbit | 1:1000 | Cell Signaling Technology (9351S) |
| STAT5 | Rabbit | 1:1000 | Cell Signaling Technology (94205S) |
| Phospho-STAT6 | Rabbit | 1:1000 | Cell Signaling Technology (56554S) |
| STAT6 | Rabbit | 1:1000 | Cell Signaling Technology (5397S) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.S.; Joo, S.Y.; Cho, Y.S.; Lee, Y.R.; Ro, Y.M.; Kwak, I.S.; Hur, G.Y.; Seo, C.H. Exosomes Derived from Hypertrophic Scar Fibroblasts Suppress Melanogenesis in Normal Human Epidermal Melanocytes. Int. J. Mol. Sci. 2024, 25, 7236. https://doi.org/10.3390/ijms25137236
Cui HS, Joo SY, Cho YS, Lee YR, Ro YM, Kwak IS, Hur GY, Seo CH. Exosomes Derived from Hypertrophic Scar Fibroblasts Suppress Melanogenesis in Normal Human Epidermal Melanocytes. International Journal of Molecular Sciences. 2024; 25(13):7236. https://doi.org/10.3390/ijms25137236
Chicago/Turabian StyleCui, Hui Song, So Young Joo, Yoon Soo Cho, You Ra Lee, Yu Mi Ro, In Suk Kwak, Gi Yeun Hur, and Cheong Hoon Seo. 2024. "Exosomes Derived from Hypertrophic Scar Fibroblasts Suppress Melanogenesis in Normal Human Epidermal Melanocytes" International Journal of Molecular Sciences 25, no. 13: 7236. https://doi.org/10.3390/ijms25137236
APA StyleCui, H. S., Joo, S. Y., Cho, Y. S., Lee, Y. R., Ro, Y. M., Kwak, I. S., Hur, G. Y., & Seo, C. H. (2024). Exosomes Derived from Hypertrophic Scar Fibroblasts Suppress Melanogenesis in Normal Human Epidermal Melanocytes. International Journal of Molecular Sciences, 25(13), 7236. https://doi.org/10.3390/ijms25137236








