Feasibility and Impact of Embedding an Extended DNA and RNA Tissue-Based Sequencing Panel for the Routine Care of Patients with Advanced Melanoma in Spain
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Histological Features
2.3. Somatic Mutations Detected by NGS
2.4. Amplifications and Rearrangements Detected by NGS
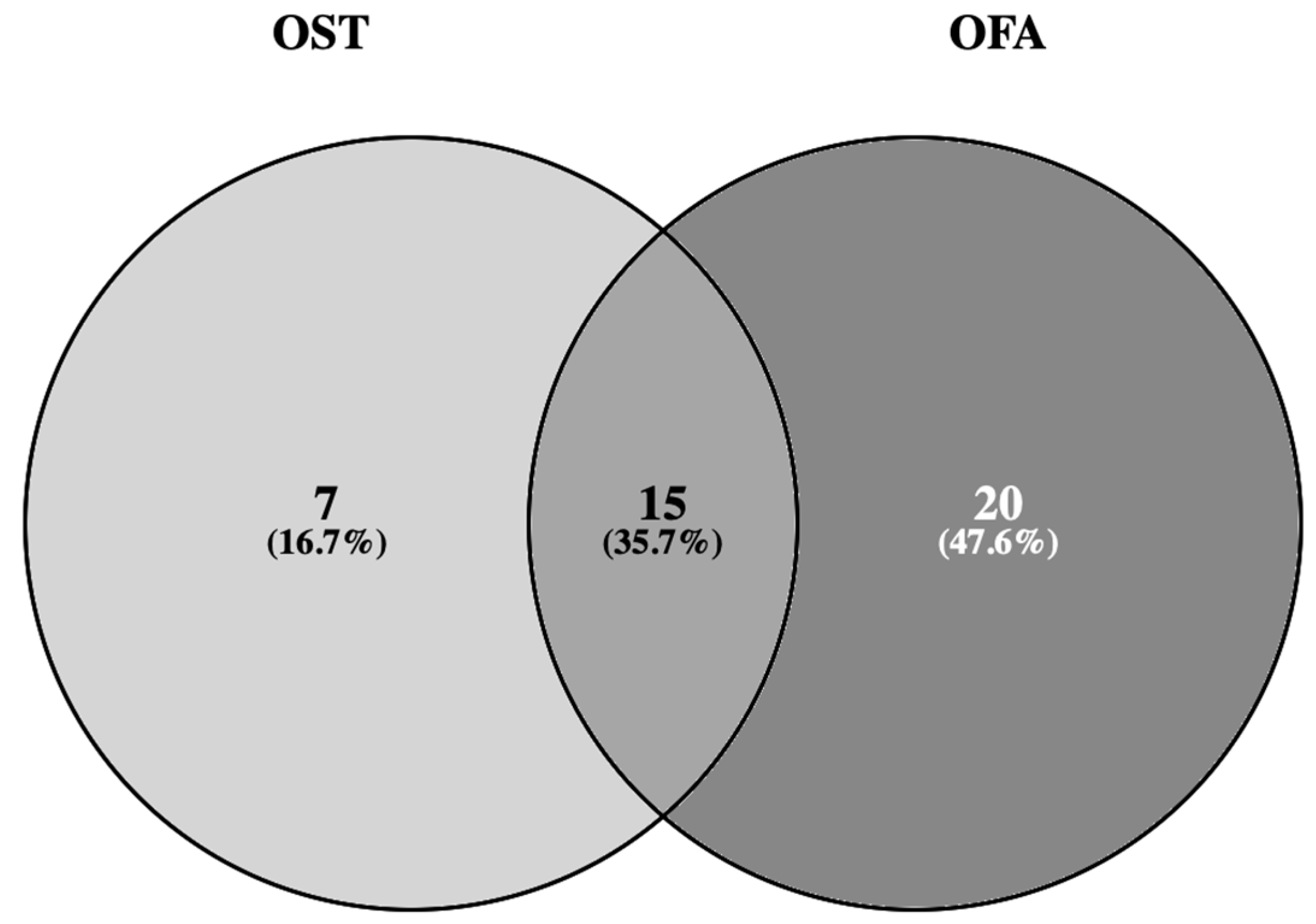
2.5. Concordance between RT-PCR and NGS Techniques for BRAF Mutation
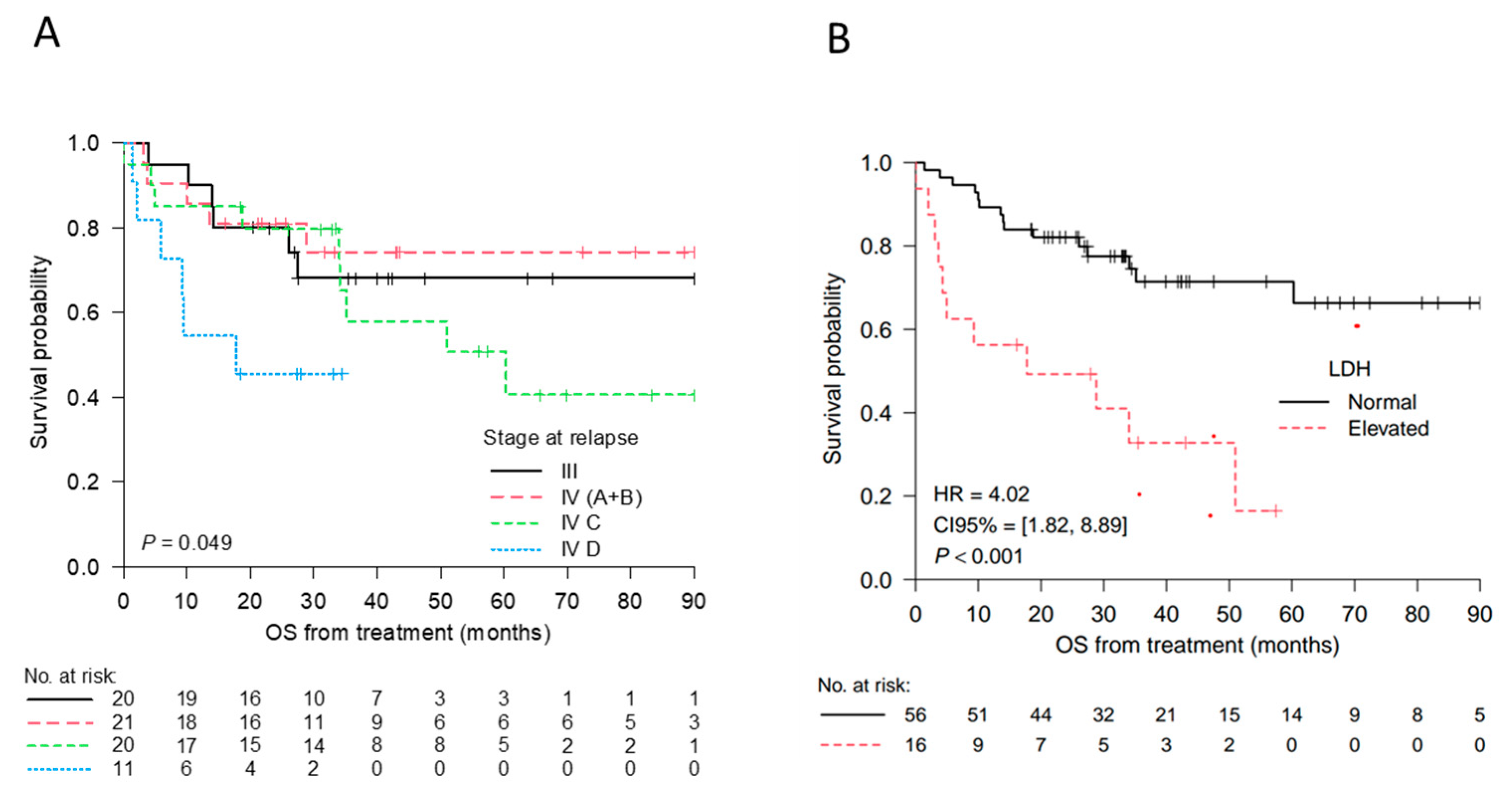
2.6. Treatment Strategies and Overall Survival
3. Discussion
4. Materials and Methods
4.1. Patient and Samples
4.2. Targeted NGS
4.3. RT-PCR for BRAF V600 Mutational Status Determination
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; del Marmol, V.; Dréno, B.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 2: Treatment—Update 2022. Eur. J. Cancer 2022, 170, 256–284. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; del Marmol, V.; Dréno, B.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics: Update 2022. Eur. J. Cancer 2022, 170, 236–255. [Google Scholar] [CrossRef]
- Whiteman, D.C.; Green, A.C.; Olsen, C.M. The Growing Burden of Invasive Melanoma: Projections of Incidence Rates and Numbers of New Cases in Six Susceptible Populations through 2031. J. Invest. Dermatol. 2016, 136, 1161–1171. [Google Scholar] [CrossRef]
- REDECAN. SEOM. La Incidencia del Melanoma Aumenta en España y Llegará a 7.474 Casos Nuevos en 2022. Available online: https://seom.org/images/14_07_2022_NP_Melanoma_cutaneo_infografia.pdf (accessed on 23 December 2023).
- Villani, A.; Potestio, L.; Fabbrocini, G.; Troncone, G.; Malapelle, U.; Scalvenzi, M. The Treatment of Advanced Melanoma: Therapeutic Update. Int. J. Mol. Sci. 2022, 23, 6388. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.H. Molecular Frontiers in Melanoma: Pathogenesis, Diagnosis, and Therapeutic Advances. Int. J. Mol. Sci. 2024, 25, 2984. [Google Scholar] [CrossRef]
- Sung, W.W.; Chang, C.H. Nevi, dysplastic nevi, and melanoma: Molecular and immune mechanisms involving the progression. Tzu Chi Med. J. 2022, 34, 1–7. [Google Scholar]
- Lauss, M.; Phung, B.; Borch, T.H.; Harbst, K.; Kaminska, K.; Ebbesson, A.; Hedenfalk, I.; Yuan, J.; Nielsen, K.; Ingvar, C.; et al. Molecular patterns of resistance to immune checkpoint blockade in melanoma. Nat. Commun. 2024, 15, 3075. [Google Scholar] [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef]
- Hauschild, A.; Grob, J.-J.; Demidov, L.V.; Jouary, T.; Gutzmer, R.; Millward, M.; Rutkowski, P.; Blank, C.U.; Miller, W.H., Jr.; Kaempgen, E.; et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012, 380, 358–365. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Robert, C.; Hersey, P.; Nathan, P.; Garbe, C.; Milhem, M.; Demidov, L.V.; Hassel, J.C.; Rutkowski, P.; Mohr, P.; et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N. Engl. J. Med. 2012, 367, 107–114. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Casula, M.; Bulgarelli, J.; Pisano, M.; Piccinini, C.; Piccin, L.; Cossu, A.; Mandalà, M.; Ferrucci, P.F.; Guidoboni, M.; et al. Sequential immunotherapy and targeted therapy for metastatic BRAF V600 mutated melanoma: 4-year survival and biomarkers evaluation from the phase II SECOMBIT trial. Nat. Commun. 2024, 15, 146. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Ascierto, M.D.; Long, G.V. Survival Outcomes in Patients With Previously Untreated BRAF Wild-Type Advanced Melanoma Treated With Nivolumab Therapy: Three-Year Follow-up of a Randomized Phase 3 Trial. JAMA Oncol. 2019, 5, 187–194. [Google Scholar] [CrossRef]
- Seth, R.; Agarwala, S.S.; Messersmith, H.; Alluri, K.C.; Ascierto, P.A.; Atkins, M.B.; Bollin, K.; Chacon, M.; Davis, N.; Faries, M.B.; et al. Systemic Therapy for Melanoma: ASCO Guideline Update. J. Clin. Oncol. 2023, 41, 4794–4820. [Google Scholar] [CrossRef]
- Trunzer, K.; Pavlick, A.C.; Schuchter, L.; Gonzalez, R.; McArthur, G.A.; Hutson, T.E.; Moschos, S.J.; Flaherty, K.T.; Kim, K.B.; Weber, J.S.; et al. Pharmacodynamic effects and mechanisms of resistance to vemurafenib in patients with metastatic melanoma. J. Clin. Oncol. 2013, 31, 1767–1774. [Google Scholar] [CrossRef]
- Nazarian, R.; Shi, H.; Wang, Q.; Kong, X.; Koya, R.C.; Lee, H.; Chen, Z.; Lee, M.-K.; Attar, N.; Sazegar, H.; et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 2010, 468, 973–977. [Google Scholar] [CrossRef]
- Wagle, N.; Emery, C.; Berger, M.F.; Davis, M.J.; Sawyer, A.; Pochanard, P.; Kehoe, S.M.; Johannessen, C.M.; MacConaill, L.E.; Hahn, W.C.; et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J. Clin. Oncol. 2011, 29, 3085–3096. [Google Scholar] [CrossRef]
- Samarkina, A.; Youssef, M.K.; Ostano, P.; Ghosh, S.; Ma, M.; Tassone, B.; Proust, T.; Chiorino, G.; Levesque, M.P.; Goruppi, S.; et al. Androgen receptor is a determinant of melanoma targeted drug resistance. Nat. Commun. 2023, 14, 6498. [Google Scholar] [CrossRef]
- Castillo, P.; Marginet, M.; Jares, P.; García, M.; Gonzalvo, E.; Arance, A.; García, A.; Alos, L.; Teixido, C. Implementation of an NGS panel for clinical practice in paraffin-embedded tissue samples from locally advanced and metastatic melanoma patients. Explor. Target. Anti-Tumor Ther. 2020, 1, 101–108. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Network, C.G.A. Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [Google Scholar]
- Kazandjian, S.; Rousselle, E.; Dankner, M.; Cescon, D.W.; Spreafico, A.; Ma, K.; Kavan, P.; Batist, G.; Rose, A.A.N. The Clinical, Genomic, and Transcriptomic Landscape of BRAF Mutant Cancers. Cancers 2024, 16, 445. [Google Scholar] [CrossRef] [PubMed]
- Sasson, D.C.; Smetona, J.T.; Parsaei, Y.; Papageorge, M.; Ariyan, S.; Olino, K.; Clune, J. Malignant Melanoma in Older Adults: Different Patient or Different Disease? Cureus 2023, 15, e34742. [Google Scholar] [CrossRef]
- Heppt, M.V.; Siepmann, T.; Engel, J.; Schubert-Fritschle, G.; Eckel, R.; Mirlach, L.; Kirchner, T.; Jung, A.; Gesierich, A.; Ruzicka, T.; et al. Prognostic significance of BRAF and NRAS mutations in melanoma: A German study from routine care. BMC Cancer 2017, 17, 536. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, J.M.; Wang, M.; Vemula, S.S.; Mirza, S.; Weier, J.; Aquino, J.D.; McCalmont, T.H.; LeBoit, P.E.; Bastian, B.C.; Yeh, I. Amplification of Mutant NRAS in Melanocytic Tumors With Features of Spitz Tumors. Mod. Pathol. 2024, 37, 100469. [Google Scholar] [CrossRef]
- Pham, D.D.M.; Guhan, S.; Tsao, H. KIT and Melanoma: Biological Insights and Clinical Implications. Yonsei Med. J. 2020, 61, 562–571. [Google Scholar] [CrossRef]
- Handolias, D.; Salemi, R.; Murray, W.; Tan, A.; Liu, W.; Viros, A.; Dobrovic, A.; Kelly, J.; McArthur, G.A. Mutations in KIT occur at low frequency in melanomas arising from anatomical sites associated with chronic and intermittent sun exposure. Pigment. Cell Melanoma Res. 2010, 23, 210–215. [Google Scholar] [CrossRef]
- Curtin, J.A.; Busam, K.; Pinkel, D.; Bastian, B.C. Somatic activation of KIT in distinct subtypes of melanoma. J. Clin. Oncol. 2006, 24, 4340–4346. [Google Scholar] [CrossRef]
- Sukniam, K.; Manaise, H.K.; Popp, K.; Popp, R.; Gabriel, E. Role of Surgery in Metastatic Melanoma and Review of Melanoma Molecular Characteristics. Cells 2024, 13, 465. [Google Scholar] [CrossRef]
- Van Haele, M.; Vander Borght, S.; Ceulemans, A.; Wieërs, M.; Metsu, S.; Sagaert, X.; Weynand, B. Rapid clinical mutational testing of KRAS, BRAF and EGFR: A prospective comparative analysis of the Idylla technique with high-throughput next-generation sequencing. J. Clin. Pathol. 2019, 73, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Dankner, M.; Rose, A.A.N.; Rajkumar, S.; Siegel, P.M.; Watson, I.R. Classifying BRAF alterations in cancer: New rational therapeutic strategies for actionable mutations. Oncogene 2018, 37, 3183–3199. [Google Scholar] [CrossRef]
- Yao, Z.; Yaeger, R.; Rodrik-Outmezguine, V.S.; Tao, A.; Torres, N.M.; Chang, M.T.; Drosten, M.; Zhao, H.; Cecchi, F.; Hembrough, T.; et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature 2017, 548, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Carvajal, R.D. KIT as an Oncogenic Driver in Melanoma: An Update on Clinical Development. Am. J. Clin. Dermatol. 2019, 20, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Cirenajwis, H.; Lauss, M.; Ekedahl, H.; Törngren, T.; Kvist, A.; Saal, L.H.; Olsson, H.; Staaf, J.; Carneiro, A.; Ingvar, C.; et al. NF1-mutated melanoma tumors harbor distinct clinical and biological characteristics. Mol. Oncol. 2017, 11, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Martin-Deleon, R.; Teixido, C.; Lucena, C.M.; Martinez, D.; Fontana, A.; Reyes, R.; García, M.; Viñolas, N.; Vollmer, I.; Sanchez, M.; et al. EBUS-TBNA Cytological Samples for Comprehensive Molecular Testing in Non-Small Cell Lung Cancer. Cancers 2021, 13, 2084. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, J.C. (2007–2015) Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 23 December 2023).

| Parameter | Number of Cases |
|---|---|
| Mean age (range) (years old) | 61 (8–91) |
| Sex | |
| Female | 45/87 (51.7%) |
| Male | 42/87 (48.3%) |
| Type of melanoma | |
| Superficial spreading melanoma | 24/87 (28%) |
| Nodular melanoma | 15/87 (17%) |
| Acral melanoma | 8/87 (9%) |
| Mucosal melanoma | 8/87 (9%) |
| Lentigo maligna melanoma | 6/87 (7%) |
| Nevoid melanoma | 3/87 (3%) |
| Spitzoid melanoma | 2/87 (2%) |
| Uveal melanoma | 2/87 (2%) |
| Not established * | 19/87 (22%) |
| Melanoma localization | |
| Sun-exposed region | 52/70 (74%) |
| Sun-shielded region | 18/70 (26%) |
| Stage | |
| I | 2/80 (2.5%) |
| II | 2/80 (2.5%) |
| III | 23/80 (28.8%) |
| IV | 53/80 (66.2%) |
| Treatment | |
| Chemotherapy | 2/79 (2.5%) |
| Immunotherapy | 66/79 (83.5%) |
| Targeted therapy | 4/79 (5.1%) |
| No treatment | 7/79 (8.9%) |
| Clinicopathological Characteristics | BRAF (p) | NRAS (p) |
|---|---|---|
| Sex | ||
| Male | 0.222 | 1 |
| Female | ||
| Age | ||
| Median | 0.186 | 0.514 |
| NGS Site of biopsy | ||
| Primary | 0.134 | 0.611 |
| Metastasis | ||
| UV exposure | ||
| Elastosis | 0.013 | 0.758 |
| Absence of elastosis | ||
| Depth of invasion | ||
| Median | 0.286 | 0.454 |
| Ulceration | ||
| Absent | 0.775 | 0.381 |
| Present | ||
| Vascular Invasion | ||
| Absent | 1 | 1 |
| Present |
| OS | PFS | |
|---|---|---|
| LDH Normal (<234U/L) Elevated (≥234 U/L) | 0.0001 | 0.124 |
| Stage at relapse III IV | 0.048 | 0.368 |
| Type of treatment Anti-PD1 monotherapy Anti-PD1 combination (anti-CTLA4/anti-LAG3) Targeted therapy Other treatments | 0.312 | 0.030 |
| Treatment intention Adjuvant Metastatic | 0.230 | 0.444 |
| BRAF mutation Absent Present | 0.556 | 0.343 |
| NRAS mutation Absent Present | 0.771 | 0.861 |
| BRAF treatment interaction | 0.320 | 0.031 |
| Oncomine Gene Panels | |||
|---|---|---|---|
| Hotspot Genes | Copy Number Variants | Fusion Drivers | |
| AKT1 | JAK3 | ALK | ABL1 |
| ALK | KIT | AR | ALK |
| AR | KRAS | BRAF | AKT3 |
| BRAF | MAP2K1 | CCND1 | AXL |
| CDK4 | MAP2K2 | CDK4 | BRAF |
| CTNNB1 | MET | CDK6 | EGFR |
| DDR2 | MTOR | EGFR | ERBB2 |
| EGFR | NRAS | ERBB2 | ERG |
| ERBB2 | NOTCH1 | FGFR1 | ETV1 |
| ERBB3 | PDGFRA | FGFR2 | ETV4 |
| ERBB4 | PIK3CA | FGFR3 | ETV5 |
| ESR1 | PTEN | FGFR4 | FGFR1 |
| FBXW7 | RAF1 | KIT | FGFR2 |
| FGFR1 | RET | KRAS | FGFR3 |
| FGFR2 | ROS1 | MET | MET |
| FGFR3 | SMAD4 | MYC | NTRK1 |
| GNA11 | SMO | MYCN | NTRK2 |
| GNAQ | STK11 | PDGFRA | NTRK3 |
| HRAS | TP53 | PIK3CA | PDGFRA |
| IDH1 | PPARG | ||
| IDH2 | RAF1 | ||
| JAK1 | RET | ||
| JAK2 | ROS1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castrejon, N.; Martin, R.; Carrasco, A.; Castillo, P.; Garcia, A.; Albero-González, R.; García, M.; Marginet, M.; Palau, N.; Hernández, M.; et al. Feasibility and Impact of Embedding an Extended DNA and RNA Tissue-Based Sequencing Panel for the Routine Care of Patients with Advanced Melanoma in Spain. Int. J. Mol. Sci. 2024, 25, 6942. https://doi.org/10.3390/ijms25136942
Castrejon N, Martin R, Carrasco A, Castillo P, Garcia A, Albero-González R, García M, Marginet M, Palau N, Hernández M, et al. Feasibility and Impact of Embedding an Extended DNA and RNA Tissue-Based Sequencing Panel for the Routine Care of Patients with Advanced Melanoma in Spain. International Journal of Molecular Sciences. 2024; 25(13):6942. https://doi.org/10.3390/ijms25136942
Chicago/Turabian StyleCastrejon, Natalia, Roberto Martin, Antonio Carrasco, Paola Castillo, Adriana Garcia, Raquel Albero-González, Mireia García, Marta Marginet, Núria Palau, Mónica Hernández, and et al. 2024. "Feasibility and Impact of Embedding an Extended DNA and RNA Tissue-Based Sequencing Panel for the Routine Care of Patients with Advanced Melanoma in Spain" International Journal of Molecular Sciences 25, no. 13: 6942. https://doi.org/10.3390/ijms25136942
APA StyleCastrejon, N., Martin, R., Carrasco, A., Castillo, P., Garcia, A., Albero-González, R., García, M., Marginet, M., Palau, N., Hernández, M., Montironi, C., Clot, G., Arance, A., Alos, L., & Teixido, C. (2024). Feasibility and Impact of Embedding an Extended DNA and RNA Tissue-Based Sequencing Panel for the Routine Care of Patients with Advanced Melanoma in Spain. International Journal of Molecular Sciences, 25(13), 6942. https://doi.org/10.3390/ijms25136942








